Abstract
Infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is associated with acute and postacute cognitive and neuropsychiatric symptoms including impaired memory, concentration, attention, sleep and affect. Mechanisms underlying these brain symptoms remain understudied. Here we report that SARS-CoV-2-infected hamsters exhibit a lack of viral neuroinvasion despite aberrant blood–brain barrier permeability. Hamsters and patients deceased from coronavirus disease 2019 (COVID-19) also exhibit microglial activation and expression of interleukin (IL)-1β and IL-6, especially within the hippocampus and the medulla oblongata, when compared with non-COVID control hamsters and humans who died from other infections, cardiovascular disease, uraemia or trauma. In the hippocampal dentate gyrus of both COVID-19 hamsters and humans, we observed fewer neuroblasts and immature neurons. Protracted inflammation, blood–brain barrier disruption and microglia activation may result in altered neurotransmission, neurogenesis and neuronal damage, explaining neuropsychiatric presentations of COVID-19. The involvement of the hippocampus may explain learning, memory and executive dysfunctions in COVID-19 patients.
Keywords: COVID-19, SARS-CoV-2, brain, cytokine, neurogenesis
Soung et al. report that SARS-CoV-2-infected hamsters and patients who died from COVID-19 exhibit increased microglial activation and expression of interleukin (IL)-1β and IL-6 within the medulla oblongata and hippocampus, as well as reduced hippocampal neurogenesis, compared with uninfected controls.

For the podcast associated with this article, please visit https://academic.oup.com/brain/pages/podcast
Introduction
Mounting evidence indicates that infection with severe acute respiratory syndrome beta-coronavirus (SARS-CoV-2) leads to new-onset neuropsychiatric symptoms. During SARS-CoV-2 disease (COVID-19), patients without prior neuropsychiatric history can present with difficulty concentrating, insomnia, fatigue, hallucinations, delusions and behavioural changes, including suicide.1,2 Patients hospitalized in the intensive care unit (ICU) have been reported to show agitation (69%) and corticospinal tract signs (67%) indicative of encephalopathy, and at discharge some patients re-present with a dysexecutive syndrome (33%).3
A number of recent studies show that postacute sequelae of COVID-19 (PASC) at 2 months after discharge include a variety of neuropsychiatric symptoms, including impaired immediate verbal memory and learning, delayed verbal memory, verbal fluency, working memory, anxiety, depression and posttraumatic stress disorder (PTSD) symptoms.1 These symptoms persisted for at least 1 year.4 The neurobiological bases of these symptoms are unknown.
In COVID-19 decedents, our group and others reported post-mortem neuropathological findings of hypoxia, microglial activation, astrogliosis, a mild lymphocytic infiltration and micro and macro haemorrhages, despite finding no detectable viral RNA or protein in CNS tissue.5,6
In this study, we focused on three areas of the brain, the cortex, hippocampus, medulla and olfactory bulb (OB). In addition to its role in memory, the hippocampus is involved in executive functions, processing speed, path integration and spatial processing,7 and thus hippocampal damage might play some role in PASC symptoms. Neuroimaging studies in postacute COVID-19 patients show disruption of fractional anisotropy and diffusivity, suggesting microstructural and functional alterations of the hippocampus.8 These hippocampal functions have been shown to depend on adult hippocampal neurogenesis.9 Importantly, two of the cytokines found elevated in COVID brains, IL-1β and IL-6, have antineurogenic effects in the hippocampus. The medulla appears to be an area of pronounced microglial activation in COVID-19 brains, and contains nuclei regulating autonomic nervous system activity, consciousness and motor coordination. The OB has garnered attention in COVID-19 because of its neuronal link to the olfactory neuroepithelium, where virus has been detected.10
To investigate pathologies in more detail, we investigated CNS changes associated with SARS-CoV-2 infection in Golden Syrian hamsters, validating our findings in humans deceased from severe COVID-19, compared with uninfected hamsters and age- and sex-matched humans (Supplementary Table 1). The Golden Syrian hamster (Mesocricetus auratus) exhibits pathogenesis and transmissibility of SARS-CoV-2 similar to that observed in humans, with upper and lower respiratory symptoms and pathological signs.11,12 We analysed infected hamster and human OB, cortex medulla, and hippocampus, and report increased blood–brain barrier (BBB) permeability, microglial activation, microglial and neuronal expression of IL-1β and IL-6, respectively, and reduced hippocampal neurogenesis compared with uninfected controls. These studies support the notion that COVID-19 cytokine storm may induce neuroinflammation and neuronal damage, resulting in altered neurotransmission and brain function.
Materials and methods
All data generated or analysed during this study are included in this published article and its Supplementary material files.
Results
Hamsters intranasally infected with SARS-CoV-2 do not exhibit viral neuroinvasion
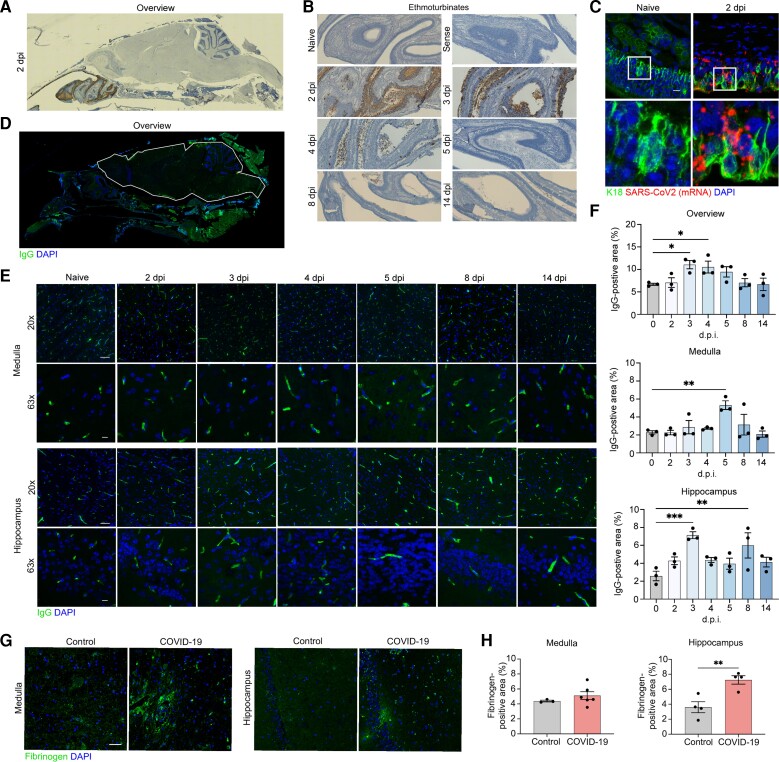
The Golden Syrian hamster is naturally susceptible to SARS-CoV-2 infection. Intranasal inoculation results in mild-to-moderate disease with laboured breathing, ruffled fur, weight loss and hunched posture.12 To examine the utility of the hamster model in defining neuroinflammatory and vasculature alterations that could result in memory, executive function and emotional dysregulation during and after COVID-19, we evaluated olfactory neuroepithelial and brain tissue from 5–6-week-old male hamsters infected intranasally (i.n.) with 2 × 105 plaque forming units (PFU) of a fully infectious SARS-CoV-2 isolate (strain 2019-nCov/USA-WA1/20202) during acute infection and at 7 days after recovery. Whole heads of uninfected and infected hamsters were collected throughout the acute infectious period and at 1 week after viral clearance, which occurs in the lungs at 5–7 days post-infection (dpi).11 High levels of SARS-CoV-2 RNA was detected within the hamster ethmothurbinates at 2–4 dpi, and completely cleared by 8 dpi (Fig. 1A and B). As previously reported,13 viral RNA was detected within K18+ sustentacular cells of the olfactory neuroepithelium (ONE) (Fig. 1C), which might impact olfactory sensory neuron function via loss of calcium signalling.14 SARS-CoV-2-infected sustentacular cells exhibited decreased expression of K18+ compared with uninfected ONE (Supplementary Video 1) and were found sloughed off into the nasal cavities of infected hamsters (Supplementary Video 2). No viral RNA was detectable in the acute and recovered hamster OB, cortex, hippocampus or medulla oblongata at any time point post-infection (Supplementary Fig. 1A).
Figure 1.
SARS-CoV2 infects hamster ONE and induces BBB disruption in hamsters and patients with COVID-19. (A) In situ hybridization for viral RNA at 2 dpi revealed SARS-CoV2 consistently targeted the ethmoturbinates of hamsters, with no infection of the CNS parenchyma. (B) Representative images of viral SARS-CoV2 mRNA in the hamster ethmoturbinates at naïve, 2, 3, 4, 5, 8 and 14 dpi. (C) Co-localization of viral RNA (red) via in situ hybridization and immunodetection of K18+ sustenticular cells (green) of the ONE in naïve or SARS-CoV2-infected hamsters at 7 dpi. Nuclei counterstained with DAPI (blue). (D) Representative image of blood–brain permeability in the hamster brain 2 dpi, showing staining for IgG (green) and DAPI (blue). (E) Representative images of IgG detection (green) within hamster MO and hippocampi at naïve, 2, 3, 4, 5, 8 and 14 dpi, and nuclear stain, DAPI (blue), followed by (F) quantitation of IgG intensity in the total CNS parenchyma (white outline) (top, Overview), medulla (middle, Medulla), and hippocampus (bottom, Hippocampus) at all time points. (G) Representative image of blood–brain permeability in the medulla (left) and hippocampus (right) of control and COVID-19 patient tissue, depicting detection of fibrinogen (green) and DAPI (blue). (H) Quantification of fibrinogen intensity in control versus COVID-19 patient tissues derived from medulla (top) and hippocampus (bottom). Data were pooled from at least two independent experiments. Scale bars = 50 μm (×10), 20 μm (×20) or 10 μm (×63). Data represent the mean ± SEM and were analysed by two-way ANOVA or Student’s t-test.
Hamster and human SARS-CoV-2 infection is associated with widespread BBB disruption
Multiple reports show BBB dysfunction in patients with COVID-19.15,16 To evaluate effects of SARS-CoV-2 on BBB integrity in the hamster model, we assessed brain levels of extravasated serum IgG via immunohistochemistry. At 3–4 dpi there was a significant increase in IgG+ pixels, indicative of BBB disruption, in the OB, cortex, hippocampus and medulla oblongata, but the hippocampus suffered the most significant changes compare to naïve hamsters (Fig. 1D–F and Supplementary Fig. 2). In the medulla oblongata BBB disruption increased from early time points to 5 dpi (Fig. 1E and F, middle). Similar persistent alterations in IgG immunoreactivity were observed in the OB and cortex, albeit to a lesser degree when compared to the hippocampus or medulla (Supplementary Fig. 2A and B).
We next examined BBB permeability in human COVID-19 brain tissue samples via detection of fibrinogen, a blood coagulation protein whose CNS deposition is implicated in a wide range of neurological disease and injuries associated with BBB disruption.17 In a subset of COVID-19 decedents (n = 7) compared with age- and sex-matched controls (Supplementary Table 1) deceased from other infections (n = 3) or cardiovascular disease (n = 2), we observed increased fibrinogen in the hippocampus (Fig. 1G, right and H, right) and a smaller, but significant, increase in medulla (Fig. 1G, left and H, left) and OB (Supplementary Fig. 2C). Together, these data suggest that SARS-CoV-2 infection may lead to region-specific alterations in human BBB integrity.
Hamster SARS-CoV-2 infection results in microglia activation and increased brain cytokines
Interferon (IFN) γ and IL-6 are well-known for altering BBB permeability18 and have been detected in high levels in the serum of SARS-CoV-2-infected hamsters.19 Given that loss of BBB integrity may permit CNS entrance of cytokines or immune cells, we examined cytokine expression by glia and neurons and microglia activation in the same brain regions via immunofluorescence. For cytokine expression studies, we co-localized expression of ionized calcium-binding adapter molecule 1 (IBA1), a marker of microglia, and neuronal nuclear marker (NeuN), a marker of neurons, with IL-1β and IL-6 in hamster and human brain tissue.
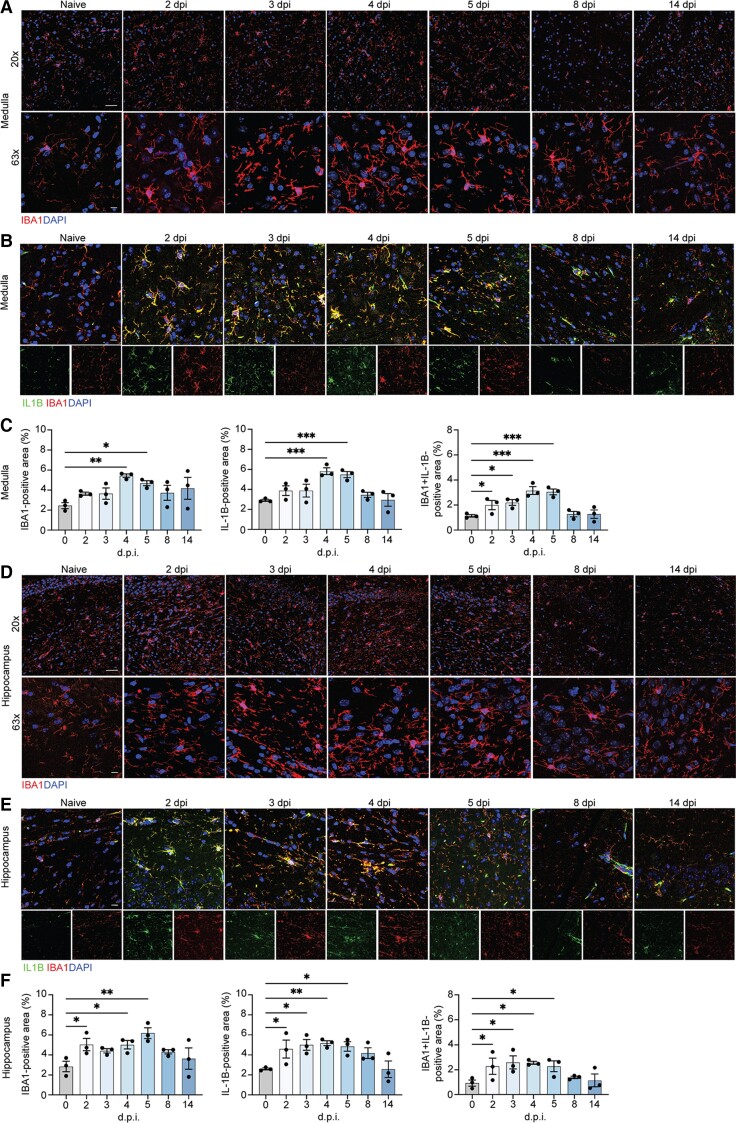
Medulla oblongata from SARS-CoV-2-infected hamsters showed increased levels of IBA1 at 4 dpi, which remained elevated at 14 dpi (Fig. 2A and C, left). Consistent with the increased expression of IBA1, activated microglia were observed, displaying larger cell bodies and thicker processes than those detected in brain tissue from uninfected animals. This finding was particularly pronounced within the inferior olivary nuclei (ION), a region that coordinate signals from the spinal cord to the cerebellum, regulating motor coordination and learning via integration of glutamatergic synaptic inputs.20 IBA1+ activated microglia of SARS-CoV-2-infected hamsters within the ION exhibited increased expression of IL-1β at 2–5 dpi compared to uninfected hamsters, which returned to baseline by 8 dpi (Fig. 2B and C, middle and right). Hippocampal SARS-CoV-2-hamster tissue similarly revealed a gradual increase in IBA1+ activated microglia versus uninfected hamsters, peaking at 5 dpi (Fig. 2D and F, left), and IL-1β levels increased at 2 dpi, peaking at 5 dpi before gradually decreasing to naïve levels by 14 dpi (Fig. 2E and F, middle and right).
Figure 2.
Microglia contribute to neuroinflammation in the medulla oblongata and hippocamus of SARS-CoV-2-infected hamsters. Representative images of IBA1 in the hamster inferior olivary nuclei (ION) (A) and hippocampus (D) at naïve, 2, 3, 4, 5, 8 and 14 dpi, showing staining for IBA1 (red) and DAPI (blue) at ×20 and ×63. Immunostaining for IL-1β and IBA1 in the hamster ION (B) and hippocampi (E) at naïve, 2, 3, 4, 5, 8 and 14 dpi, presented as microscopy with IBA1 (red), IL-1β (green) and DAPI (blue). Quantitation of per cent IBA1+ and IL-1β+ areas, and IL-1β+IBA1+ area, normalized to total IL-1B+ area for ION (C) and hamster (F). Data were pooled from at least two independent experiments. Scale bars = 20 μm (×20) or 10 μm (×63). Data represent the mean ± SEM and were analysed by two-way ANOVA or Student’s t-test.
We also examined the OB and cortex of SARS-CoV-2-infected hamsters (2–5 dpi), which exhibited elevated microglial activation (Supplementary Fig. 3A, B and D) and, in the OB, expression of IL-1β within IBA1+ microglia (Supplementary Fig. 4A and B, top) compared to control tissue. In hamster somatosensory cortex, co-localization of IL-1β within IBA1+ microglia was not significantly elevated (Supplementary Fig. 4B, bottom).
To determine whether astrocytes contribute to neuroinflammation in the CNS of infected hamster, using a SOX9 antibody, we observed no changes in astrocyte cell numbers in OB, medulla and hippocampus of infected hamsters compared to naïve animals (Supplementary Fig. 5). These data suggest that, while astrocytes may be involved in post-infection neuroinflammation in the OB, a region proximal to the location of viral replication, microglia appear to be the main players in more remote brain regions, like the medulla and hippocampus. Taken altogether, our findings show that, despite lack of viral neuroinvasion, SARS-CoV-2-infected individuals develop microglial activation and cytokine expression in brain regions associated with olfactory function, motor coordination, memory and learning.
Human COVID-19 patients exhibit microglial and neuronal expression of interleukins
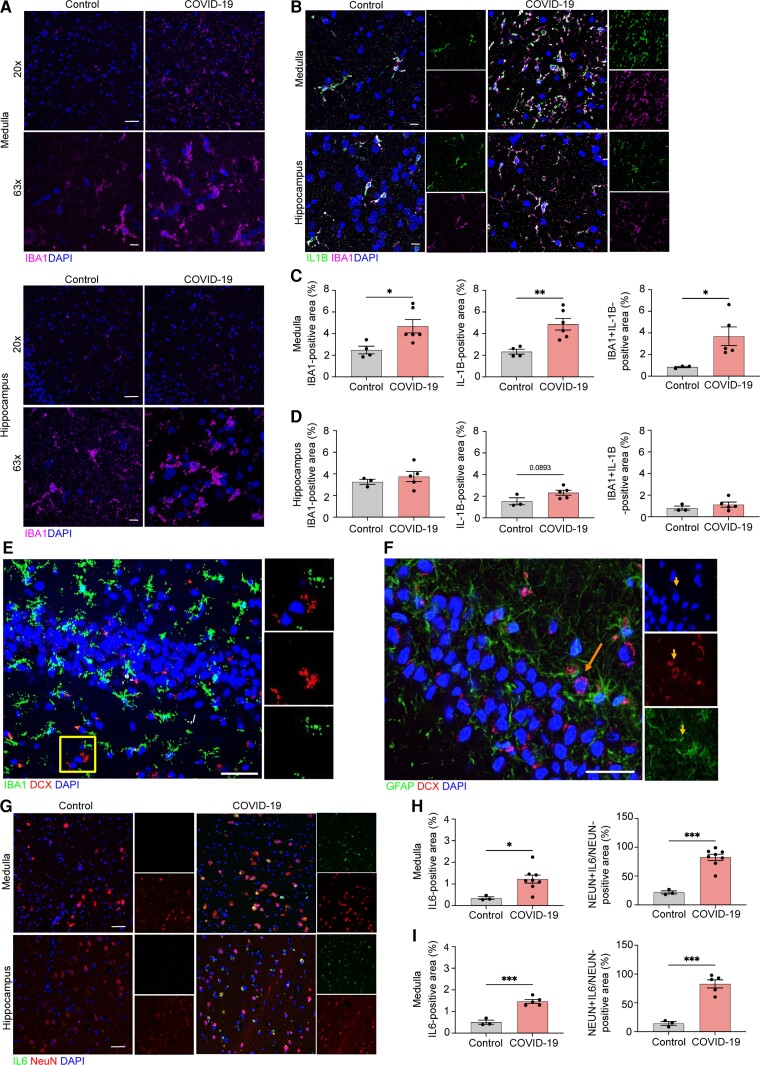
In order to validate our findings in SARS-CoV-2-infected hamsters in human COVID-19 patients, we evaluated microglial activation and cytokine expression in CNS tissues derived from a subset of COVID-19 decedents with CNS inflammatory changes but without neurologic disease,5 using the same methods as described above [Supplementary Table 1, COVID-19 decedents (n = 7) and age- and sex-matched non-COVID-19 controls (n = 5, Supplementary Table 1, Group A)]. As in hamsters, examination of SARS-CoV-2 RNA within the OB, cortex and hippocampus via in situ hybridization did not reveal infection (Supplementary Fig. 1B). However, COVID-19 post-mortem inferior olivary nuclei of the medulla revealed significant microglial activation and increased IL-1β expression, compared with control subjects (Fig. 3A, top, B and C). Elevated expression of IL-6 was also detected in neurons of the inferior olivary nuclei (Fig. 3G, top and H). Similar to hamsters, hippocampal tissue from COVID-19 patients exhibited significantly increased IBA1+ cell numbers and co-localization of IBA1 with IL-1β (Fig. 3A, bottom, B and D) and of NeuN with IL-6 expression (Fig. 3G, bottom and I) compared with control subjects. Hippocampal tissues in non-COVID patients exhibited robust immunodetection of doublecortin (DCX)+/IBA1− and DCX+/glial fibrillary acidic protein (GFAP)− cells (Fig. 3E and F).
Figure 3.
Microglia and neurons contribute to neuroinflammation in the medulla and hippocampus of COVID-19 patients. Representative image of IBA1 in control and COVID-19 patient ION (A, top) and hippocampus (A, bottom), showing staining for IBA1 (magenta) and DAPI (blue) at ×20 and ×63, and quantified for per cent IBA1+ area (C and D, left). Immunostaining for IL-1β and IBA1 in ION and hippocampus of control and COVID-19 patients, presented as microscopy with IBA1 (magenta), IL-1β (green) and DAPI (blue) (B) and per cent IL-1β+ area and IL-1β+IBA1+ area, normalized to total IBA1+ areas for both regions (C and D, middle and right). (E) Representative images of IBA1 in the human adult hippocampus with high-magnification images single channel. Sections stained with DAPI (blue), IBA1 (green) and DCX (red) in non-COVID-19 control. Scale bar = 25 μm. (F) Representative images of GFAP in the human adult hippocampus with high-magnification images single channel. Sections stained with DAPI (blue), GFAP (green) and DCX (red) in non-COVID-19 control. The arrow points to a single DCX+/GFAP− neuron in the subgranular zone (SGZ). Scale bar = 25 μm. Immunostaining for IL-6 and NeuN in ION and hippocampus of control and COVID-19 patients, presented as microscopy with NeuN (red), IL-6 (green) and DAPI (blue) (G) and per cent IL-6+ area and IL-16+NeuN+ area, normalized to total NeuN+ area (H and I). Data were pooled from at least two independent experiments. Scale bars = 20 μm (×20) or 10 μm (×63). Data represent the mean ± SEM and were analysed by two-way ANOVA or Student’s t-test.
OB tissue exhibited elevated expression of IL-1β in IBA1+ microglia, with microglial activation (Supplementary Fig. 4C) compared with uninfected controls. Moreover, OB from COVID-19 patients exhibited elevated neuronal expression of IL-6 compared with controls (Supplementary Fig. 4D).
We also examined whether astrocytes exhibited activate in brain tissues from COVID-19 patients using a GFAP antibody, as done above for hamsters. In COVID-19 human tissue samples, GFAP+ immunostaining was increased in the medulla but not the OB or hippocampus, compared to controls, and significant increases in IL-1β levels in GFAP+ astrocytes were observed in the OB of COVID-19 patients when compared to controls (Supplementary Fig. 6). No differences in astrocytes IL-1β immunostaining were observed between COVID-19 and control patients in the hippocampus and medulla oblongata (Supplementary Fig. 6). Taken together, these results validate findings observed in the CNS of SARS-CoV-2-infected mice in humans with COVID-19.
Loss of hippocampal neurogenesis in hamsters and humans infected with SARS-CoV-2
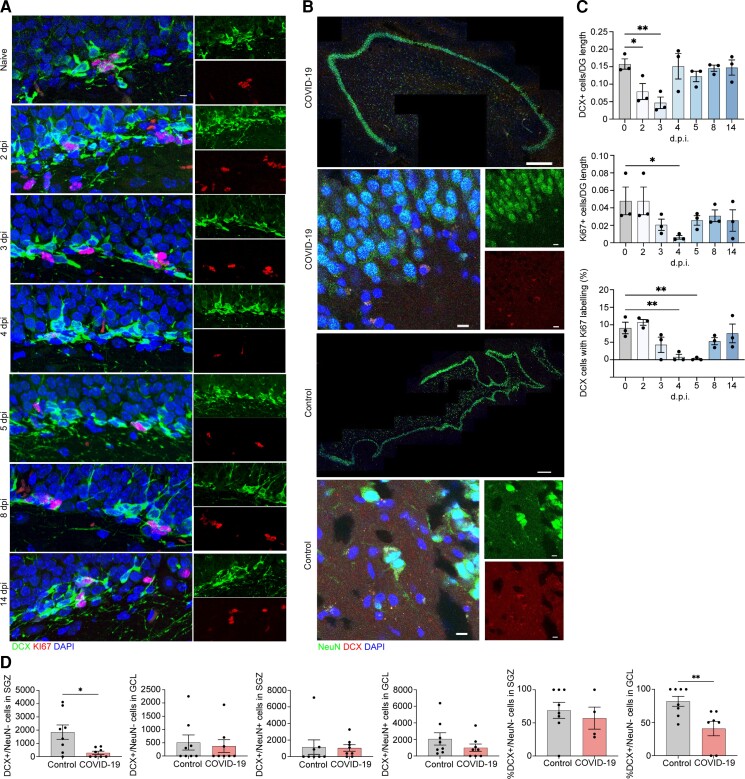
Because adult neurogenesis may be affected by inflammation,21 we hypothesized the cytokine surge following SARS-CoV-2 infection might affect adult neurogenesis. In rodents, adult hippocampal neurogenesis is a robust and well-established phenomenon,22 and IL-1β inhibits neurogenesis during viral encephalitis, both acutely and after recovery,23 and IL-6 represses neurogenesis through DNA demethylation/methylation.24 Unfortunately, there are no commercially available antibodies to detect IL-6 in hamster tissues. However, consistent with the detected increase in IL-1β in the hippocampus of SARS-CoV-2-infected hamsters, we observed a gradual decline in number of cells expressing Ki67, a marker of proliferation, and DCX, a marker of neuroblasts and immature neurons, as well as DCX+/Ki67+ cells, that were almost completely absent in the subgranular zone (SGZ) at 5 dpi (Fig. 4A and C). To determine whether this effect was region-specific, we performed similar analysis of the rostral migratory stream, which displayed no change in DCX+/Ki67+ cell numbers in infected hamsters compared to uninfected controls (Supplementary Fig. 7).
Figure 4.
Neuroblast proliferation in the SARS-CoV2 infected hamster and doublecortin (DCX)-positive cells and neurons in human hippocampus from COVID-19 patient and non-COVID-19 control. (A) Microscopy of the dentate gyrus of hamsters at naïve, 2, 3, 4, 5, 8 and 14 dpi, showing staining of Ki67 (red), neuroblast (green) and DAPI (blue), followed by quantification of per cent Ki67+DCX+ cells, normalized to the total number of DCX+ cells. (C) Quantification of per cent DCX+Ki67+ area, normalized to total DCX+ area in the rostral migratory stream of hamsters at naïve, 2, 3, 4, 5, 8 and 14 dpi. Data were pooled from at least two independent experiments. Scale bars = 50 μm. Data represent the mean ± SEM and were analysed by two-way ANOVA. (B) Select images of whole hippocampus and high-magnification images sections stained with DAPI (blue), NeuN (green) and DCX (red) from COVID-19 patient and non-COVID-19 control. The granule cell layer (GCL), subgranular zone (SGZ) and molecular layer (ML) of the dentate gyrus are visible, combined channels imaged at ×20; scale bar = 500 μm. High-magnification images were captured at ×63, scale bar = 20 μm. (D) In the SGZ, DCX+/NeuN− cells were fewer in COVID-19 patients versus controls [P = 0.026; t(7.794) = 2.731; Welch’s t-test for non-stoichiometric data], with no group differences in DCX+/NeuN+ cell number (P = 0.189; Mann–Whitney). In the GCL, neither DCX+/NeuN− cell count (P = 0.846; Mann–Whitney) nor DCX+/NeuN+ cell count (P = 0.378; Mann–Whitney) differs between COVID-19 and control subjects. Per cent of DCX+/NeuN− cells located in the SGZ versus the GCL did not differ between control and COVID-19 groups (P = 0.453; Mann–Whitney). Per cent of DCX+/NeuN+ cells located in the GCL versus the SGZ was lower in COVID-19 patients versus control subjects (P = 0.009; Mann–Whitney).
Next, we compared numbers of DCX+ neuroblast and DCX+/NeuN+ immature neurons in the SGZ and granule cell layer (GCL) from another subset of COVID-19 decedents who succumbed to pneumonia or stroke (n = 17) and age- and sex-matched non-COVID-19 controls (n = 8, Supplementary Table 1, Group B). Because DCX+ cells migrate from the SGZ into the GCL as they mature and start expressing NeuN,25 DCX+/NeuN− cells in the SGZ are more likely neuroblasts and DCX+/NeuN+ cells located in the GCL are more likely immature neurons. Therefore, to determine numbers of each of these cell populations, we quantified DCX+/NeuN− and DCX+/NeuN+ cells in the SGZ and GCL separately (Supplementary Fig. 8).
We found fewer DCX+/NeuN− neuroblasts in the SGZ of COVID-19 patients compared to non-COVID-19 controls (Fig. 4C and D). Conversely, DCX+/NeuN− cells in the GCL and DCX+/NeuN+ immature neurons in SGZ and GCL were not fewer in COVID-19 versus control subjects (Fig. 4C and D). This suggests that the most immature cell population appears to be the most affected in humans with COVID-19.
We detected no significant effect of age on the number of neuroblasts and immature neurons, DCX+/NeuN− and DCX+/NeuN+ cells in SGZ and GCL in COVID-19 patients or non-COVID-19 controls (Supplementary Fig. 9), in line with previous findings on the persistence of adult hippocampal neurogenesis in older subjects with no chronic neuropsychiatric illness.26
Discussion
This study identifies potential neurobiological mechanisms of CNS damage in SARS-CoV-2 infection, involving BBB disruption, elevated levels of interleukins and blunted hippocampal neurogenesis. Loss of hippocampal neurogenesis may contribute to cognitive and emotional symptoms observed in COVID-19 patients, involving deficits of verbal memory and learning, working memory and executive functions, set-shifting, attention, anxiety, depression and PTSD symptoms.1,4 Limitations of this study include the small sample sizes, and the lack of reagents for detecting additional inflammatory factors in hamsters. Moreover, hamsters develop milder disease without hypoxia, which is different from humans who died from COVID-19.
Cytokines may be the main mediator of BBB disruption and cellular damage as levels in hamsters were highest in areas with more significant BBB disruption. IL-1β, which is elevated in the CSF of COVID-19 patients,27 destabilized the BBB,28 and loss of BBB integrity may permit entrance of cytokines and immune cells into the brain, which, in turn, may activate glial cells. Neural progenitor cell IL-1β receptors have been implicated in reducing neurogenesis in murine models of flavivirus encephalitis.21,23 Fewer DCX+/NeuN− neuroblasts in the SGZ of COVID-19 patients compared with non-COVID-19 controls suggests either decreased progenitor cell maturation or increased neuroblast death.
The time course of human pathological findings remains unknown, and if similar to hamsters, one would expect that after the initial cytokine surge, neurogenesis might recover, as cognitive symptoms and anosmia subside in many patients. Nevertheless, long COVID symptoms have been widely reported. There is the possibility that, in some individuals, the neurogenic niche might not have enough multipotent progenitors for neurogenesis to resume after this insult, as the multipotent progenitor pool is smaller in aging humans.26 If this was the case, some patients might not be able to recover and COVID-19 may result in chronic neuropsychiatric symptoms, as observed in several clinical studies.1,4 Moreover, the hypoxia that results from the severe pulmonary disease of these patients has been associated with activation of microglia in the absence of viral infection, and can also affect DCX+ cells.29
Brain alterations were transient in hamsters, peaking after viral clearance in the nasal cavity. In humans, we do not know how long elevated inflammatory markers persist in subjects who survive the disease. The persistence of neuropsychiatric symptoms in long COVID cases suggests that neuronal damage may be prolonged. Brain imaging studies investigating inflammation markers in post-COVID patients are warranted. Studies using PET radiotracers for the brain translocator protein (TSPO)30 located on microglia and astroglia may reveal useful for gathering data on indices of brain inflammation levels in post-COVID patients.
Given the likely predominant role of neuroinflammation in the mechanism of brain damage in COVID-19, anti-IL-6 and anti-IL-1β therapies, currently under investigation,31 could be useful in limiting a prolonged cytokine storm, possibly preventing motor, cognitive, neurovegetative and emotional dysfunctions.
Supplementary Material
Acknowledgements
The authors thank W. Beatty at the Molecular Microbiology Imaging facility at Washington University School of Medicine and the teams at the New York State Psychiatric Institute for performing psychological autopsy interviews. We dedicate this study to the donors and their families, who contributed to this scientific inquiry despite the extraordinary strain they suffered at the beginning of the pandemic.
Abbreviations
- BBB
blood–brain barrier
- COVID-19
SARS-CoV-2 disease
- dpi
days post-infection
- GCL
granule cell layer
- ION
inferior olivary nuclei
- NeuN
neuronal nuclear marker
- OB
olfactory bulb
- ONE
olfactory neuroepithelium
- SARS-CoV-2
severe acute respiratory syndrome beta-coronavirus
- SGZ
subgranular zone
Contributor Information
Allison L Soung, Center for Neuroimmunology and Neuroinfectious Diseases, Washington University School of Medicine, St. Louis, MO, USA; Department of Medicine, Washington University School of Medicine, St. Louis, MO, USA.
Abigail Vanderheiden, Center for Neuroimmunology and Neuroinfectious Diseases, Washington University School of Medicine, St. Louis, MO, USA; Department of Medicine, Washington University School of Medicine, St. Louis, MO, USA.
Anna S Nordvig, Division of Neurodegenerative Diseases, Department of Neurology, Weill Cornell Medicine, New York, NY, USA.
Cheick A Sissoko, Division of Molecular Imaging and Neuropathology, New York State Psychiatric Institute, New York, NY, USA.
Peter Canoll, Department of Pathology and Cell Biology, Columbia University, New York, NY, USA.
Madeline B Mariani, Department of Psychiatry, Columbia University, New York, NY, USA.
Xiaoping Jiang, Center for Neuroimmunology and Neuroinfectious Diseases, Washington University School of Medicine, St. Louis, MO, USA; Department of Medicine, Washington University School of Medicine, St. Louis, MO, USA.
Traci Bricker, Department of Medicine, Washington University School of Medicine, St. Louis, MO, USA.
Gorazd B Rosoklija, Department of Pathology and Cell Biology, Columbia University, New York, NY, USA; Macedonian Academy of Sciences & Arts, Skopje 1000, Republic of Macedonia.
Victoria Arango, Division of Molecular Imaging and Neuropathology, New York State Psychiatric Institute, New York, NY, USA; Department of Psychiatry, Columbia University, New York, NY, USA.
Mark Underwood, Division of Molecular Imaging and Neuropathology, New York State Psychiatric Institute, New York, NY, USA; Department of Psychiatry, Columbia University, New York, NY, USA.
J John Mann, Division of Molecular Imaging and Neuropathology, New York State Psychiatric Institute, New York, NY, USA; Department of Psychiatry, Columbia University, New York, NY, USA.
Andrew J Dwork, Division of Molecular Imaging and Neuropathology, New York State Psychiatric Institute, New York, NY, USA; Department of Pathology and Cell Biology, Columbia University, New York, NY, USA; Department of Psychiatry, Columbia University, New York, NY, USA; Macedonian Academy of Sciences & Arts, Skopje 1000, Republic of Macedonia.
James E Goldman, Department of Pathology and Cell Biology, Columbia University, New York, NY, USA.
Adrianus C M Boon, Department of Medicine, Washington University School of Medicine, St. Louis, MO, USA.
Maura Boldrini, Division of Molecular Imaging and Neuropathology, New York State Psychiatric Institute, New York, NY, USA; Department of Psychiatry, Columbia University, New York, NY, USA.
Robyn S Klein, Center for Neuroimmunology and Neuroinfectious Diseases, Washington University School of Medicine, St. Louis, MO, USA; Department of Medicine, Washington University School of Medicine, St. Louis, MO, USA; Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, MO, USA; Department of Neurosciences, Washington University School of Medicine, St. Louis, MO, USA.
Funding
This work was supported by National Institutes of Health grants F32 NS128065 (to A.V.), R35NS122310 (to R.S.K.), R21AI164769 and R56AG063372 (to M.B.), U01AI151810 (to A.C.M.B.) and in part by the following: the Stroud Center for Aging Studies at Columbia University (M.B.); NIH grants R01MH83862 (M.B.), U01NS090415 (M.B.), American Foundation for Suicide Prevention SRG-0-129-12 (M.B.), R01MH125030 (A.J.D.), R01MH098786 (A.J.D., G.B.R.), MH064168 (A.J.D.), R01MH040210 (V.A.), P50MH090964 (J.J.M., M.U.).
Competing interests
The Boon laboratory has received unrelated funding support in sponsored research agreements from AI Therapeutics, GreenLight Biosciences Inc. and Nano targeting & Therapy Biopharma Inc. The Boon laboratory has received funding support from AbbVie Inc., for the commercial development of SARS-CoV-2 mAb. A.C.M.B. is a recipient of a licensing agreement with Abbvie Inc., for commercial development of SARS-CoV-2 mAb. J.J.M. receives royalties for commercial use of the C-SSRS from the Research Foundation for Mental Hygiene. Contribution to this article was provided when Dr Arango was employed at Columbia University and the New York State Psychiatric Institute. The opinions expressed in this article are the author’s own and do not reflect the views of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Jaywant A, Vanderlind WM, Alexopoulos GS, Fridman CB, Perlis RH, Gunning FM. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology. 2021;46:2235–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mendez R, Balanza-Martinez V, Luperdi SC, et al. Long-term neuropsychiatric outcomes in COVID-19 survivors: A 1-year longitudinal study. J Intern Med. 2022;291:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thakur KT, Miller EH, Glendinning MD, et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain. 2021;144:2696–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cosentino G, Todisco M, Hota N, et al. Neuropathological findings from COVID-19 patients with neurological symptoms argue against a direct brain invasion of SARS-CoV-2: A critical systematic review. Eur J Neurol. 2021;28:3856–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamamoto J, Suh J, Takeuchi D, Tonegawa S. Successful execution of working memory linked to synchronized high-frequency gamma oscillations. Cell. 2014;157:845–857. [DOI] [PubMed] [Google Scholar]
- 8. Lu Y, Li X, Geng D, et al. Cerebral micro-structural changes in COVID-19 patients—an MRI-based 3-month follow-up study. EClinicalMedicine. 2020;25:100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clelland CD, Choi M, Romberg C, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brann DH, Tsukahara T, Weinreb C, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 2020;6:eabc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bricker TL, Darling TL, Hassan AO, et al. A single intranasal or intramuscular immunization with chimpanzee adenovirus-vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. Cell Rep. 2021;36:109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sia SF, Yan LM, Chin AWH, et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583:834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bryche B, St Albin A, Murri S, et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav Immun. 2020;89:579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hegg CC, Irwin M, Lucero MT. Calcium store-mediated signaling in sustentacular cells of the mouse olfactory epithelium. Glia. 2009;57:634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bellon M, Schweblin C, Lambeng N, et al. Cerebrospinal fluid features in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse transcription polymerase chain reaction (RT-PCR) positive patients. Clin Infect Dis. 2021;73:e3102–e3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alexopoulos H, Magira E, Bitzogli K, et al. Anti-SARS-CoV-2 antibodies in the CSF, blood–brain barrier dysfunction, and neurological outcome: studies in 8 stuporous and comatose patients. Neurol Neuroimmunol Neuroinflamm. 2020;7:e893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petersen MA, Ryu JK, Akassoglou K. Fibrinogen in neurological diseases: Mechanisms, imaging and therapeutics. Nat Rev Neurosci. 2018;19:283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Munoz-Fernandez MA, Fresno M. The role of tumour necrosis factor, interleukin 6, interferon-gamma and inducible nitric oxide synthase in the development and pathology of the nervous system. Prog Neurobiol. 1998;56:307–340. [DOI] [PubMed] [Google Scholar]
- 19. Port JR, Adney DR, Schwarz B, et al. High-fat high-sugar diet-induced changes in the lipid metabolism are associated with mildly increased COVID-19 severity and delayed recovery in the Syrian hamster. Viruses. 2021;13:2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garden DL, Rinaldi A, Nolan MF. Active integration of glutamatergic input to the inferior olive generates bidirectional postsynaptic potentials. J Physiol. 2017;595:1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soung AL, Dave VA, Garber C, Tycksen ED, Vollmer LL, Klein RS. IL-1 reprogramming of adult neural stem cells limits neurocognitive recovery after viral encephalitis by maintaining a proinflammatory state. Brain Behav Immunity. 2022;99:383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leon-Espinosa G, Garcia E, Gomez-Pinedo U, Hernandez F, DeFelipe J, Avila J. Decreased adult neurogenesis in hibernating Syrian hamster. Neuroscience. 2016;333:181–192. [DOI] [PubMed] [Google Scholar]
- 23. Garber C, Vasek MJ, Vollmer LL, Sun T, Jiang X, Klein RS. Astrocytes decrease adult neurogenesis during virus-induced memory dysfunction via IL-1. Nat Immunol. 2018;19:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kong X, Gong Z, Zhang L, et al. JAK2/STAT3 Signaling mediates IL-6-inhibited neurogenesis of neural stem cells through DNA demethylation/methylation. Brain Behav Immun. 2019;79:159–173. [DOI] [PubMed] [Google Scholar]
- 25. Nelson BR, Hodge RD, Daza RA, et al. Intermediate progenitors support migration of neural stem cells into dentate gyrus outer neurogenic niches. Elife. 2020;9:e53777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boldrini M, Fulmore CA, Tartt AN, et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22:589–599.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bodro M, Compta Y, Llanso L, et al. Increased CSF levels of IL-1β, IL-6, and ACE in SARS-CoV-2-associated encephalitis. Neurol Neuroimmunol Neuroinflamm. 2020;7:e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Daniels BP, Holman DW, Cruz-Orengo L, Jujjavarapu H, Durrant DM, Klein RS. Viral pathogen-associated molecular patterns regulate blood–brain barrier integrity via competing innate cytokine signals. mBio. 2014;5:e01476–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kiernan EA, Smith SM, Mitchell GS, Watters JJ. Mechanisms of microglial activation in models of inflammation and hypoxia: Implications for chronic intermittent hypoxia. J Physiol. 2016;594:1563–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meyer JH, Cervenka S, Kim MJ, Kreisl WC, Henter ID, Innis RB. Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry. 2020;7:1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bozzi G, Mangioni D, Minoia F, et al. Anakinra combined with methylprednisolone in patients with severe COVID-19 pneumonia and hyperinflammation: An observational cohort study. J Allergy Clin Immunol. 2021;147:561–566.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.