Abstract
Introduction
There is an ongoing debate about the existence and effects of Helicobacter pylori (Hp) in adenotonsillar tissue.
Objective
A clinical study was conducted to assess the existence of Hp in the adenoid and/or adenotonsillar tissues, which were surgically excised due to chronic adenotonsillitis.
Methods
Phosphoglucosamine mutase gene for the detection of Hp and cytotoxin-associated gene as virulence gene were examined in 84 adenotonsillar tissues obtained from 64 patients and patients’ serum by using polymerase chain reaction.
Results
Hp IgG was detected in 57 (89%) patients’ serum. A total of seven tissue samples from 64 patients (10.9%) were found positive for Hp DNA, of which five were adenoids and two were tonsil tissues. All polymerase chain reaction positive samples were also positive for the cytotoxin-associated gene, which is a virulence determinant for the organism.
Conclusion
This study suggests that children are exposed to Hp at an early age of their life in this province. Hp may have a role in the pathogenesis of chronic adenotonsillitis, especially in endemic areas.
Keywords: Helicobacter pylori, Reservoirs, Adenoids, Palatine tonsil
Resumo
Introdução
Há um debate atual sobre os efeitos da Helicobacter pylori (HpHp) no tecido adenotonsilar.
Objetivo
Conduzimos um estudo clinico para avaliar a existência de Hp nos tecidos adenoideanos e/ou adenotonsilar, os quais foram removidos cirurgicamente em decorrência de adenotonsilite crônica.
Método
No total, 84 amostras de tecido obtidos de 64 pacientes foram analisadas para o gen fosfoglucosamina mutase para a detecção de Hp. Os casos positivos foram a seguir examinados para o gen associado à citotoxina, relacionado a virulência, usando-se o método de Reação de Polimerase em Cadeia (PCR).
Resultados
A IgG de Hp foi detectado em 57 (89%) soros de pacientes. Sete amostras de tecido de sessenta e quatro pacientes (10.9%) resultou positivo para o DNA de Hp, das quais cinco eram adenóides e duas eram tecido tonsilar. No PCR todas as amostras foram também positivas para o gen associado à citotoxina, o qual é um determinante de virulência.
Conclusão
Esse estudo sugere que as crianças são expostas ao Hp nos primeiros anos de vida nessa província e que o Hp pode ter um papel na patogênese da adenotonsilite crônica, principalmente em áreas endêmicas.
Palavras-chave: Helicobacter pylori, Reservatórios, Adenoides, Tonsila palatina
Introduction
Helicobacter pylori (Hp) is a microaerophilic, Gram-negative spiral microorganism that colonizes the human gastric mucosa. It is the most frequent cause of gastric and duodenal ulcers in humans. The relationship between Hp and some gastrointestinal malignancies has also been demonstrated.1 Particularly, cytotoxin-associated gene (cagA) positive strains are associated with a significantly increased risk for the development of atrophic gastritis, mucosa-associated lymphoid tissue lymphoma (MALToma), and gastric cancer. Hp is generally acquired during childhood (<10 years) via the fecal–oral, oral–oral, and gastric–oral transmission routes,2, 3, 4, 5 and can remain silent for a lifetime.3, 6 The transmission route of Hp infection has not been clearly understood.
The Waldeyer ring is the first step in the mucosal defense against invading pathogens through its MALT content. Adenoid and tonsil tissues are part of the Waldeyer ring. These participate in the immune system, especially in children. On the other hand, adenoidectomy and/or tonsillectomy represent the most common surgical procedures in childhood because of chronic infection and/or hypertrophy.7
Though the stomach is a natural reservoir for Hp, various tissues such as the gall bladder, gingiva, oral lesions, and the dental plate have been demonstrated to be potential extra-gastric reservoirs of this pathogen.8, 9 Furthermore, Hp has been found in nasal polyps, nasal mucosa, and saliva.10 Recent studies have suggested that adenotonsillar tissue could be an extra-gastric reservoir for Hp, but the results have been conflicting.5, 8, 11, 12, 13, 14, 15 Detection of the colonization and accumulation of Hp in different regions of the body is particularly important to better understanding the modes of transmission and progress of infection of this organism. This study aimed to clarify the role of adenotonsillar tissue on Hp infection. Whether adenotonsillar tissue an extra-gastric reservoir for Hp or Hp has a role in the pathogenesis of chronic adenotonsillitis.
Methods
Patients
This clinical study was performed at the University Otorhinolaryngology and Clinical Microbiology Departments between August of 2011 and August of 2012. Surgically excised adenoid and tonsil tissues and serum samples were collected from children who underwent adenoidectomy and adenotonsillectomy due to chronic adenotonsillitis. A total of 64 children (34 males, 30 females) were included in the study. The mean age of the patients was 5.9 years, ranging between 1 and 17 years. A total of 84 clinical specimens were collected. Sixty-two biopsy specimens were adenoid and 22 were tonsillar tissue. Venous peripheral blood samples (5–6 mL) were collected from all patients.
Inclusion criteria
The surgical indications for adenoidectomy and adenotonsillectomy were as follows: chronic or recurrent tonsil infections (six or more tonsillitis episodes per a year) for tonsillectomy, and adenoid enlargement (especially up to 70% blockage of the choana on endoscopic examination), which causes obstructive symptoms for adenoidectomy.7
Exclusion criteria
Patients who had taken antibiotics, proton pump inhibitors, or H2 receptor antagonists and antacids during a four-week period prior to surgery, as well as patients who had a clinical history of dyspeptic or gastroesophageal symptoms on the systematic questionnaire were excluded from the study. All patients were operated under general anesthesia. Tissues and serum samples were stored at −70 °C until laboratory analysis.
Laboratory
Venous peripheral blood samples (5–6 mL) were collected from all patients. Blood samples were then centrifuged at 2500 rpm for 10 min. The serum was separated and stored at −80 °C. Serum samples were analyzed for Hp immunoglobulin G (IgG) with a commercial immune assay kit (Dima GmbH – Germany). Briefly, 250–500 μL of patient serum was dropped onto the test cassette and the results were read visually within 10 min, according to the manufacturer's recommendations. This is a rapid serologic test. The test was used to indicate the higher prevalence of Hp exposure in the childhood in this region.
DNA extraction
Adenoid and tonsil tissue specimens were mechanically disrupted using the Tissue Lyser system (Qiagen – Hilden, Germany), and then incubated overnight in tissue lysis buffer. Bacterial DNA was extracted from these samples after completing digestion of tissues using the QIAamp DNA Mini Kit (Qiagen), according to the manufacturer's instructions.
Polymerase chain reaction (PCR) for detection of Hp DNA and cagA gene
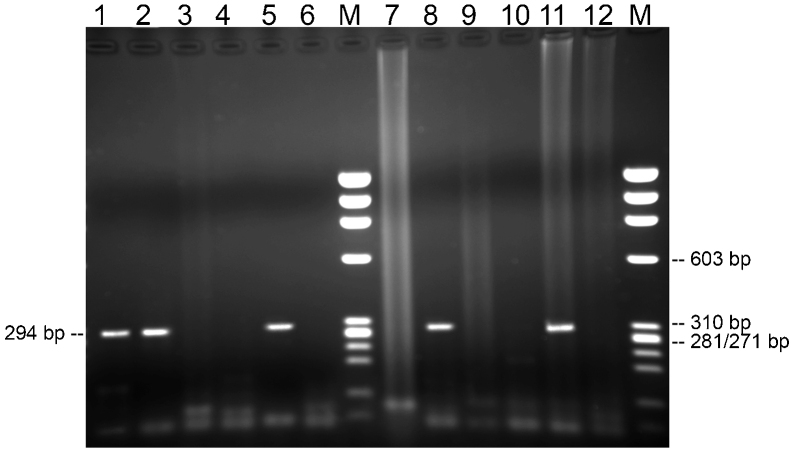
For detection of Hp and its virulence gene in the clinical specimens, phosphoglucosamine mutase gene (glmM) and cytotoxin-associated gene (cagA) genes were studied with an in-house PCR method with previously-reported primers.16, 17 A total 25 μL of amplification mix consisting of 12.5 μL TopTaq DNA PCR Master Mix (QIAGEN – Hilden, Germany), each primers 1 μL (10Ypmol/μL), 8 μL H2O, and 2.5 μL/each sample extraction product was prepared. Amplification conditions and primer sets are shown in Table 1. PCR products were electrophoresed in 2% agarose gel, then ethidium bromide-stained gel was evaluated in a gel imaging system (Gel logic 2200 imaging system [1708 × 1280 pixels; Kodak Company– NY, United States]; Fig. 1).
Table 1.
DNA primers used and the amplification conditions.
| Gene | Primer sequence | PCR product (bp) | PCR conditions | References |
|---|---|---|---|---|
| glmM | 5′-AAGCTTTTAGGGGTGTTAGGGGTTT-3′ | 294 | 94 °C, 1 min; 55 °C, 1 min; 72 °C, 1 min (35 cycles) | 16 |
| 5′-AAGCTTACTTTCTAACACTAACGC-3′ | ||||
| cagA | ATAATGCTAAATTAGACAACTTGAGCGA | 298 | 94 °C, 1 min; 60 °C, 1 min; 72 °C, 1 min (45 cycles) | 17 |
| TTAGAATAATCAACAAACATCACGCCA | ||||
PCR, polymerase chain reaction.
Figure 1.
glmM (294 bp) is illustrated in the agarose gel (2%). Lanes 1, 2, 5, 8, positive samples; lane 11, positive control; lane 12, negative control; lanes 3, 4, 6, 7, 9, 10, negative samples. Lane M, molecular weight markers (DNA molecular Weight Marker IX, Roche).
PCR analysis was performed on randomly selected tonsil and adenoid tissues.
Ethical considerations
This study was preformed after ethical approval from the Human Ethics Committee of the University. Informed consents were signed by parents of the patients before blood sample and tissue collection.
Results
Venous peripheral blood samples (5–6 mL) were collected from all patients. A total of 57 serum samples of 64 patients (89%) were found to be positive for Hp IgG. Mean age was 6.3 years (3–17 years); sex distribution comprised 32 boys and 25 girls.
In the PCR analysis, seven clinical specimens belonging to different patients among the 64 patients (10.9%) were positive for Hp DNA. A representative gel image is shown in Fig. 1. Of these, two were tonsils, and the remaining five were adenoids from different patients. The cagA virulence gene was positive in all Hp positive tissues. It was found that 10.9% of the patients were positive for Hp and 90% of these patients were positive for Hp IgG.
Discussion
Hp infection is widespread throughout the world, and the prevalence of the infection may vary according to patients’ socioeconomic status, geographic area, age, and ethnicity. Its prevalence is about 30–40% in developed countries, whereas the rate is up to 90% in developing or underdeveloped countries.18, 19, 20, 21
The presence of Hp in the upper aero-digestive tract has been reported in different studies. Minocha et al.9 reported a low Hp prevalence in the gastric mucosa of patients with a history of tonsillectomy. It is suspected that Hp colonizes on tonsillar and/or adenoid tissues as an extra-gastric reservoir. Nevertheless, Di Bonoventura did not support this opinion, and claimed that tonsils did not represent a reservoir or sanctuary for Hp.22 However, there are still contradictions in recent investigations. Also, some studies proposed that Hp might have a role in the pathogenesis of chronic adenotonsillitis. The pathogenic process of chronic adenotonsillitis, from single to chronic infections, has not been clearly understood. It has been accepted that tonsillar crypt obstruction due to infection causes differentiation of resident flora to some pathogenic bacteria.2, 23, 24 In the present study, the cagA virulence gene was found positive in all patients who were positive for Hp DNA on PCR analysis. These results support that Hp may have a role in the pathogenesis of chronic adenotonsillitis, especially in endemic regions, such as Turkey.
Bacterial culture is a valuable method of diagnosis for Hp infections. But, the fastidious nature of Hp causes a high frequency of false negative results with this methodology. Therefore, various methods have been used for the diagnosis of Hp infection. Particularly, nested PCR is widely preferred when it comes to detecting Hp in clinical practice because of its high sensitivity and specificity.15 The most accurate methods of diagnosis have been accepted as immunohistochemistry and PCR analysis. There is still no single test that is accepted as the gold standard for Hp detection. The usage of combined tests is still preferred both in pediatric and adult studies.1, 25 The present study used serology for indicating the higher prevalence of Hp exposure in the local pediatric population and the nested PCR method (more sensitive) to detect Hp in tissue samples.
Bitar et al.12 tested 25 adenoid specimens using the rapid urease test (RUT), histological evaluation, and nested PCR methods. They reported an 84% positivity rate for Hp in the adenoids with RUT. On histopathological examination, other bacteria were found 68% in RUT-positive tissues, whereas only 16% were Hp-like organisms. Finally, the authors reported all of their samples to be negative for Hp DNA on nested PCR. Therefore, they concluded that nested PCR was the best way to diagnose Hp. Similarly, Toros et al. tested 84 adenoid and tonsil specimens by RUT and histopathological evaluation.13 They did not detect Hp in any of the specimens by either RUT or histopathology. Hence, they noted that colonization of Hp in tonsillar and/or adenoid tissue was unlikely.
Vilarinho et al.14 aimed to determine whether the adenotonsillar tissue might constitute an extra-gastric reservoir for Hp. They used many diagnostic methods, including RUT, immunohistochemistry with a polyclonal anti–Hp antibody, fluorescence in situ hybridization (FISH) with a specific Hp peptide nucleic acid (PNA) probe, and PCR-DNA ELISA designed for detection of the vacA gene of the organism. Anti–Hp antibodies were detected in 39% of patients. Immunohistochemistry was found positive in three tonsil tissues whose serologies were negative. RUT was positive in two adenoid and two tonsil tissues with positive serology. The PNA-FISH and PCR-DNA ELISA tests, which are considered the most specific and sensitive methods for Hp detection, were found to be negative in all tissues. According to the results, the investigators reported that adenotonsillar tissues did not constitute an extra-gastric reservoir for Hp infection in children.
In contrast to the studies above, Abdel-Monem et al.11 found Hp to be positive in adenotonsillar tissue using PCR with the ureC gene sequence in 16.6% of patients. A total of 30 surgical specimens (20 tonsils and ten adenoids from 20 patients) were evaluated by RUT, blood serology, and PCR. They reported that RUT was positive in 16 specimens (53.3%) and serology was positive for Hp IgG antibodies in four patients (20%). They concluded that the adenotonsillar tissues could be an extra-gastric reservoir area for Hp infection in children with chronic adenotonsillar diseases.
The cagA gene has been shown to be present in 60–70% of Hp strains, and these strains are accepted as virulent.26 The cagA protein interacts with cellular proteins, and consequently, may cause morphological damage in the epithelium and failure in the secretion and signal transmission in human cells. Therefore, these strains are much more potent in causing gastric mucosal damage.26, 27
In this study, it was found that 10.9% of the children with chronic adenotonsillitis were positive for Hp, and 90% of these patients were positive for Hp IgG. Although it was a valuable diagnostic tool in gastric samples, use of RUT was not preferred, as it gives high rates of false positive results due to the intense accumulation of urease-positive present in the normal bacterial flora of the upper aero-digestive tract. Bacterial culture can be a valuable diagnostic tool, but because of the low growth ability of Hp in materials with a high bacterial load in the flora members, it was not used. The detection of the bacterial DNA and its virulence gene were chosen to investigate the bacteria in adenotonsillar tissue. It was observed that Hp was present in 8% and 9% of adenoids and tonsils, respectively. Regarding the previously published studies, a high rate of Hp in the present clinical specimens was found. This was most likely due to the high frequency of the organism in this population. Additionally, it was observed that all Hp positive samples were also simultaneously positive for the cagA gene.
Conclusion
In the light of the study results, it is argued that Hp can colonize the adenoid and tonsillar tissues of children with chronic adenotonsillitis. This pilot study did not classify children separately. Tissues and serum samples were collected from the patients who had undergone adenoidectomy with enlargement and tonsillectomy with chronic or recurrent infections; this represents a limitation of the present study. Further studies are needed to exhibit whether Hp resides in adenotonsillar tissue or has a role in the pathogenesis of chronic adenotonsillitis.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Please cite this article as: Bayindir T, Toplu Y, Otlu B, Yakupogullari Y, Yildirim O, Kalcioglu MT. Prevalence of the Helicobacter pylori in the tonsils and adenoids. Braz J Otorhinolaryngol. 2015;81:307–11.
References
- 1.Lawson A.J. In: Manual of clinical microbiology. Versalovic J., Carroll K.C., Funke G., et al., editors. ASM Press; Washington, DC: 2011. Helicobacter; pp. 900–915. [Google Scholar]
- 2.Cirak M.Y., Ozdek A., Yilmaz D., Bayiz U., Samim E., Turet S. Detection of Helicobacter pylori and its CagA gene in tonsil and adenoid tissues by PCR. Arch Otolaryngol Head Neck Surg. 2003;129:1225–1229. doi: 10.1001/archotol.129.11.1225. [DOI] [PubMed] [Google Scholar]
- 3.Azevedo N.F., Guimaraes N., Figueiredo C., Keevil C.W., Vieira M.J. A new model for the transmission of Helicobacter pylori: role of environmental reservoirs as gene pools to increase strain diversity. Crit Rev Microbiol. 2007;33:157–169. doi: 10.1080/10408410701451922. [DOI] [PubMed] [Google Scholar]
- 4.Azevedo N.F., Huntington J., Goodman K.J. The epidemiology of Helicobacter pylori and public health implications. Helicobacter. 2009;14:1–7. doi: 10.1111/j.1523-5378.2009.00703.x. [DOI] [PubMed] [Google Scholar]
- 5.Vayisoglu Y., Ozcan C., Polat A., Delialioglu N., Gorur K. Does Helicobacter pylori play a role in the development of chronic adenotonsillitis? Int J Pediatr Otorhinolaryngol. 2008;72:1497–1501. doi: 10.1016/j.ijporl.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Bujanover Y., Reif S., Yahav J. Helicobacter pylori and peptic disease in the pediatric patient. Pediatr Clin North Am. 1996;43:213–234. doi: 10.1016/s0031-3955(05)70403-x. [DOI] [PubMed] [Google Scholar]
- 7.Suurna M.V. In: Current diagnosis and treatment in otolaryngology-head and neck surgery. Lalwani A.K., editor. McGraw-Hill; New York: 2012. Management of adenotonsillar disease; pp. 362–376. [Google Scholar]
- 8.Jabbari M.Y., Rafeey M., Radfar R. Comparative assessment of Helicobacter pylori colonization in children tonsillar tissues. Int J Pediatr Otorhinolaryngol. 2009;73:1199–2201. doi: 10.1016/j.ijporl.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Minocha A., Raczkowski C.A., Richards R.J. Is a history of tonsillectomy associated with a decreased risk of Helicobacter pylori infection? J Clin Gastroenterol. 1997;25:580–582. doi: 10.1097/00004836-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen A.M., Engstrand L., Genta R.M., Graham D.Y., el-Zaatari F.A. Detection of Helicobacter pylori in dental plaque by reverse transcription-polymerase chain reaction. J Clin Microbiol. 1993;31:783–787. doi: 10.1128/jcm.31.4.783-787.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel-Monem M.H., Magdy E.A., Nour Y.A., Harfoush R.A., Ibreak A. Detection of Helicobacter pylori in adenotonsillar tissue of children with chronic adenotonsillitis using rapid urease test PCR and blood serology: a prospective study. Int J Pediatr Otorhinolaryngol. 2011;75:568–572. doi: 10.1016/j.ijporl.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Bitar M.A., Soweid A., Mahfouz R., Zaatari G., Fuleihan N. Is Helicobacter pylori really present in the adenoids of children? Eur Arch Otorhinolaryngol. 2005;262:987–992. doi: 10.1007/s00405-005-0926-1. [DOI] [PubMed] [Google Scholar]
- 13.Toros S.Z., Toros A.B., Kaya K.S., Deveci I., Özel L., Naiboğlu B., et al. A study to detect Helicobacter pylori in adenotonsillar tissue. Ear Nose Throat J. 2011;90:E32. doi: 10.1177/014556131109000418. [DOI] [PubMed] [Google Scholar]
- 14.Vilarinho S., Guimaraes N.M., Ferreira R.M., Gomes B., Wen X., Vieira M.J., et al. Helicobacter pylori colonization of the adenotonsillar tissue: fact or fiction? Int J Pediatr Otorhinolaryngol. 2010;74:807–811. doi: 10.1016/j.ijporl.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Eyigor M., Eyigor H., Gultekin B., Aydin N. Detection of Helicobacter pylori in adenotonsiller tissue specimens by rapid urease test and polymerase chain reaction. Eur Arch Otorhinolaryngol. 2009;266:1611–1613. doi: 10.1007/s00405-008-0903-6. [DOI] [PubMed] [Google Scholar]
- 16.Lu J.J., Perng C.L., Shyu R.Y., Chen C.H., Lou Q., Chong S.K., et al. Comparison of five PCR methods for detection of Helicobacter pylori DNA in gastric tissues. J Clin Microbiol. 1999;37:772–774. doi: 10.1128/jcm.37.3.772-774.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamlet A., Thoreson A.C., Nilsson O., Svennerholm A.M., Olbe L. Duodenal Helicobacter pylori infection differs in cagA genotype between asymptomatic subjects and patients with duodenal ulcers. Gastroenterology. 1999;116:259–268. doi: 10.1016/s0016-5085(99)70121-6. [DOI] [PubMed] [Google Scholar]
- 18.Khalifa M.M., Sharaf R.R., Aziz R.K. Helicobacter pylori: a poor man's gut pathogen? Gut Pathog. 2010;2:2–12. doi: 10.1186/1757-4749-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agirdir B.V., Bozova S., Derin A.T., Turhan M. Chronic otitis media with effusion and Helicobacter pylori. Int J Pediatr Otorhinolaryngol. 2006;70:829–834. doi: 10.1016/j.ijporl.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 20.Lukes P., Astl J., Pavlik E., Potuzníková B., Sterzl I., Betka J. Helicobacter pylori in tonsillar and adenoid tissue and its possible role in oropharyngeal carcinogenesis. Folia Biologica. 2008;54:33–39. [PubMed] [Google Scholar]
- 21.Zahedi M.J., Moghadam S.D., Mosavi M.A., Mirshekari T., Hayatbakhsh M. Helicobacter pylori colonization in biopsies of the adenotonsillectomy specimens. Am J Applied Sci. 2009;6:2050–2053. [Google Scholar]
- 22.di Bonaventura G., Neri M., Neri G., Catamo G., Piccolomini R. Do tonsils represent an extragastric reservoir for Helicobacter pylori infection. J Infect. 2001;42:221–222. doi: 10.1053/jinf.2001.0815. [DOI] [PubMed] [Google Scholar]
- 23.Brook I., Yocum P., Foote P.A., Jr. Changes in the core tonsillar bacteriology of recurrent tonsillitis: 1977–1993. Clin Infect Dis. 1995;21:171–176. doi: 10.1093/clinids/21.1.171. [DOI] [PubMed] [Google Scholar]
- 24.Skinner L.J., Winter D.C., Curran A.J., Barnes C., Kennedy S., Maquire A.J., et al. Helicobacter pylori and tonsillectomy. Clin Otolaryngol Allied Sci. 2001;26:505–509. doi: 10.1046/j.1365-2273.2001.00513.x. [DOI] [PubMed] [Google Scholar]
- 25.Ogata S.K., Kawakami E., Patricio F.R., Pedroso M.Z., Santos A.M. Evaluation of invasive and non-invasive methods for the diagnosis of Helicobacter pylori infection in symptomatic children and adolescents. Sao Paulo Med J. 2001;119:67–71. doi: 10.1590/S1516-31802001000200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chomvarin C., Namwat W., Chaicumpar K., Mairiang P., Sangchan A., Sripa B., et al. Prevalence of Helicobacter pylori vacA, cagA, cagE, iceA and babA2 genotypes in Thai dyspeptic patients. Int J Infect Dis. 2008;12:30–36. doi: 10.1016/j.ijid.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Hatakeyama M. SagA of CagA in Helicobacter pylori pahogenesis. Curr Opin Microbiol. 2008;11:30–37. doi: 10.1016/j.mib.2007.12.003. [DOI] [PubMed] [Google Scholar]