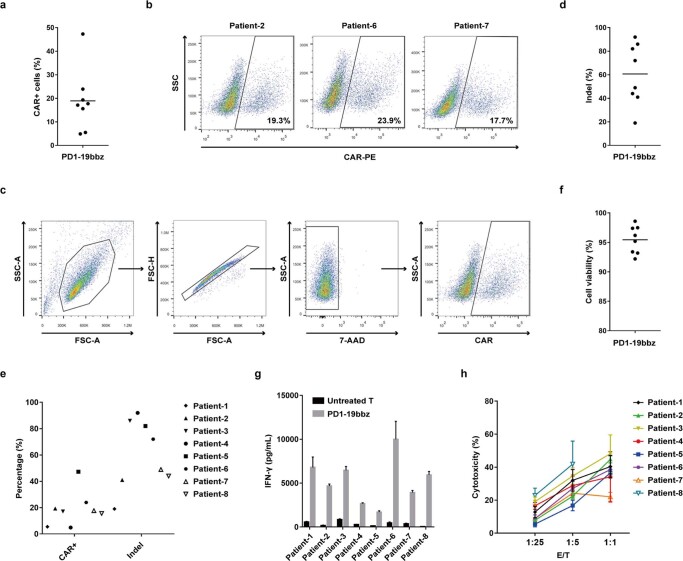
Extended Data Fig. 5. In vitro evaluation of non-viral PD1-targeted CAR-T cell products.
a, Percentage of CAR+ cells in the final products of r/r B-NHL patients (n = 8 patient donors). b, CAR expression determined in three representative patient donors. c, Gating strategy for the detection of CAR expression. d-e, Percentages of CAR integration (e) and PD1 indels (d, e) in the final products (n = 8 patient donors). f, Cell viability of the final products detected by trypan blue staining (n = 8 patient donors). g, IFN-γ secretion measured by ELISA in the supernatant after co-culture with Nalm-6 cells for 18–24 h. Data are mean ± SD (n = 3 technical replicates). h, In vitro cytotoxicity against Nalm-6 cells determined using LDH assay. E/T, effector/target. Data are mean ±SD (n = 3 technical replicates). Mean value is shown in a, d, f.