Abstract
Objective: To observe the effect of etomidate on spatial learning and memory and neuronal apoptosis in rats of different ages. Methods: The rats of different ages were divided into 3 age groups: juvenile (21-day old), adult (~3-months old) and elderly (~19-months old). Then, rats with similar age within a group were randomly divided into three subgroups, with 10 rats in each group. The experimental subgroups were intraperitoneally injected with etomidate (emulsion formulated, i.p. injection) at a dose of 5 mg/kg; the solvent control subgroups were given intraperitoneal injection of vehicle emulsion; and blank control subgroups received laparoscopic injection of normal saline. The rats’ learning and memory ability was tested by Morris water maze. The tissue sections of each group’s hippocampus were analyzed by H&E staining. The apoptosis of hippocampal cells was detected by TUNEL staining. MAPK expression in hippocampus was tested by Western blot. Results: Etomidate significantly extended the escape latency and reduced the platform crossings and the swimming time at original platform of juvenile rats, indicating that the spatial learning and memory function of juvenile rats can be affected by etomidate. However, etomidate had no effect on spatial learning and memory in adult and elderly rats. There were no obvious abnormalities in number of neurons and morphology of vertebral cells in the hippocampus of all experimental subgroups when compared with those of corresponding blank control subgroups. There was no statistically significant difference in apoptosis index of the hippocampal tissue between the experimental subgroups and corresponding blank control subgroups (P>0.05). Within juvenile group, the expression of p-ERK in the hippocampus of experimental subgroup was remarkably lower than that of solvent control subgroup and blank control subgroup (P<0.05), while there were no significant differences in p-p38 and p-JNK expressions among the three subgroups of juvenile rats (P>0.05). The expressions of p-ERK, p-p38 and p-JNK in adult and elderly rats were not affected by etomidate. Conclusion: Etomidate may have certain effects on spatial learning and memory in juvenile rats but not in adult and elderly rats. Etomidate affects neither the number of neurons in the CA1 area of the hippocampus nor the morphology of vertebral cells and did not cause the apoptosis of nerve cells. The mechanism of etomidate influence on the spatial learning and memory function of young rats may connect with the inhibition of MAPK/ERK pathway.
Keywords: Etomidate, spatial learning and memory, neuronal apoptosis, MAPK/ERK pathway
Introduction
Postoperative cognitive dysfunction (POCD) refers to a reversible and fluctuating acute mental disorder syndrome that occurred after surgical anesthesia. The main manifestations of POCD are mental confusion, anxiety, personality changes and memory impairment [1,2]. Postoperative cognitive impairment can lead to delayed rehabilitation, prolonged hospitalization and increased medical expenses of patients, and even affect their long-term quality of life [3].
Etomidate, a derivative of imidazole, is a rapid hypnotic intravenous general anesthetic. It can quickly enter brain and other organs with abundant blood flow after intravenous injection [4,5]. Etomidate induces neocortical sleep at the onset of hypnosis, exerts anesthesia by enhancing activity of hippocampal GABA neurons, and is mediated by GABAA receptors [6]. Etomidate has limited hemodynamic effects, and is clinically advocated for neurosurgical anesthesia at risk of cerebral ischemia, as well as in shock and elderly patients [7]. At present, there is still controversy whether etomidate affects the cognitive function of patients after surgery. Several studies have found that etomidate does not increase the damage of hippocampal neurons in rats with brain injuries. However, some studies have found that intravenous application of etomidate (concentration 2-20 μmol/L) inhibits the formation of long-term potentiation in hippocampus in a dose-dependent manner [8].
Accumulating evidence has shown that the hippocampus plays an important role in brain activities including memory and behavior [9-11]. Recent studies indicated that the mitogen-activated protein kinase (MAPK) signal pathway was inhibited by etomidate in CHO cells via binding to the two-pore-domain potassium channel TREK-1. Excessive activation of TREK-1 by etomidate can lead to cell cycle arrest [12]. TREK-1, a protein which controls potassium flux, is widely expressed on the surface of neuron cells and plays important roles in maintaining brain function. MAPK is abundant in the central nervous system and is critical for morphological differentiation and neuronal viability [13]. To further understand the effect of etomidate on cognitive function, this study aimed to explore its effects on spatial learning and memory ability and neuronal apoptosis in rats of different ages, and investigate whether the negative effects of etomidate is related to MAPK pathways.
Materials and methods
Experimental animals
Thirty healthy SPF male SD rats aged 21-22 days were selected as juvenile group, weighing 31-40 g; 30 SPF male SD rats aged 3-4 months were chosen as adult group, weighing 180 to 220 g; and 30 healthy SPF male SD rats aged 18-20 months were enrolled as elderly group, weighing 450-550 g. All animal procedures were carried out in accordance with the guidelines approved by the Institutional Animal Care and Use Committee (IACUC) of Hainan Medical University.
Methods
Experimental grouping
The grouping method is shown in Figure 1. Rats in each of the above 3 groups were stochastically divided into three subgroups, and each subgroup consisted of 10 rats. An experimental subgroup was given intraperitoneal injection of etomidate (5 mg/kg). A solvent control subgroup was given the same volume of fat emulsion as the experimental subgroup. A blank control subgroup received same volume of normal saline as experimental subgroup.
Figure 1.
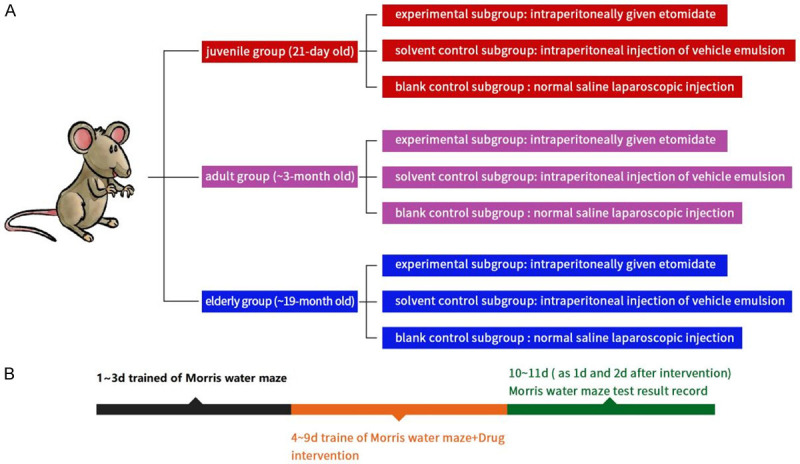
Experimental grouping and schematic diagram of Morris water maze test. A: Experimental grouping and drug intervention. B: Morris water maze test process.
Morris water maze
The spatial learning and memory functions were detected by Morris water maze (Shanghai Ruolong Technology Development Co., LTD.). The positioning navigation experiment was conducted first. Rats in each group were trained for 3 days without drug injection, once a day and once from each quadrant. The experimental conditions of rats were recorded on the third day as pre-intervention results. Then, the drug was injected continuously for 5 d. During the injection period, the rats were still trained normally but not recorded. The experiments were continued and recorded 1 d and 2 d after the injection (recorded as 1 d and 2 d after intervention). The rats were put in the water maze facing the pool wall, and the time they took to find the platform was recorded accordingly (escape latency). If the rats did not reach the platform within 120 s, they would be guided to the platform, and the escape latency was recorded as 120 s. The space exploration experiment was conducted on the second day after the positioning navigation experiment. The platform in the water maze was removed, and the rats were put into the water maze facing the pool wall. The platform crossings and the swimming time at original platform within 120 s were recorded.
H&E staining
After the Morris water maze test, some rats were sacrificed (anesthetized with 30 mg/kg sodium pentobarbital, sacrificed by cervical dislocation), and their brain tissue was collected and fixed with 4% paraformaldehyde (Biosharp, BL539A, Anhui, China). After immersing in 4% paraformaldehyde for 24 h, the tissue was embedded with paraffin and cut into 4-μm-thick slices by using a microtome (Leica, CM 2016, Germany). After dewaxing and dehydration in gradient ethanol, hippocampal tissue sections of each group were stained with HE Staining kits (Abcam, ab245880, US) following the manufactory’s protocol. Pathological changes of hippocampal tissue were observed under an optical microscope (Olympus, CX43, Japan).
TUNEL staining
Paraffin embedded tissue sections were dewaxed, dehydrated and processed with Proteinase K (Biosharp, BL080, Anhui, China) and 3% H2O2 solution (in methanal) routinely.
The labeling reaction was conducted with TUNEL detection solution (Invitrogen™, C10617, USA). Ten fields were randomly selected for each sample to calculate the average number of TUNEL positive cells [11].
Western blot
RIPA lysis buffer was used to homogenize the samples and isolate total proteins from rat hippocampal tissue (Thermo Scientific™, 89901, USA). The concentration was determined by DC assay kit (Bio-rad, 5000112, USA), and 30 μg of total protein was taken for Western blot assay. Proteins were separated by precast gradient SDS-PAGE gels (4-12%, Bio-Rad, 3450123, USA) and transferred onto PVDF membrane (Millipore, ISEQ00010, USA). After blocking the PVDF membrane with 5% non-fat milk (Biosharp, BL102, Anhui, China) for 1 h at room temperature, primary antibodies including anti-p-ERK (abcam, ab229912, USA), anti-p-p38 (abcam, ab178867, USA) and p-JNK (CST, mAb#9255, USA) were added to cover the PVDF membrane at 4°C overnight, followed by incubating with peroxide-horseradish-labeled secondary antibody (Thermo Scientific™, SA5-10288, USA) for 1 h at room temperature. ECL kit (Invitrogen™, WP20005, USA) was used for development. The protein expression was quantified according to the gray value of protein bands (Image J software V.1.52r, Bethesda, USA). β-Actin (Invitrogen™, AM1720, USA) was used as internal reference.
Statistical analysis
The statistical software SPSS 26.0 (IBM, USA) was applied to process and analyze the data in this study. The measurement data were expressed by mean ± SEM (x̅±se) and analyzed by one-way analysis of variance and LSD-T test. P<0.05 was considered as statistically significant.
Results
Etomidate prolongs escape latency in juvenile rats
As shown in Figure 2, no significant difference was found in preintervention escape latency within each age group (P>0.05). The escape latency at 1 day and 2 days after intervention in juvenile-experimental subgroup was significantly higher than that in the corresponding solvent-control subgroup and the blank-control subgroup (P<0.05, Figure 2A). However, in the adult or elderly groups, no significant difference was found in escape latency after intervention (both day 1 and day 2) when compared to that before intervention (P>0.05, Figure 2).
Figure 2.
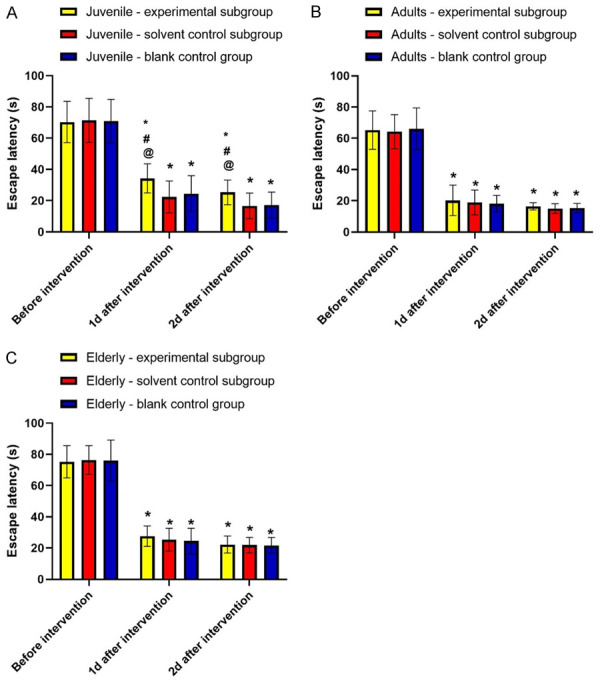
Etomidate prolongs escape latency in juvenile rats. Note: A: Juvenile group; B: Adult group; C: Elderly group. Compared with before intervention, *P<0.05; compared with solvent control subgroup, #P<0.05; compared with blank control group, @P<0.05.
Etomidate reduces platform crossings and swimming time at original platform in juvenile rats
The swimming trajectory image of the rat is shown in Figures 3 and 4. Within the juvenile group, the platform crossings and swimming time at original platform in experimental subgroup were 4.17 times and 58.49 seconds, respectively, which were significantly reduced as compared with those of solvent control subgroup and blank control subgroup (P<0.05). In adult and elderly groups, no significant differences were obtained in platform crossings and the swimming time among experimental subgroup, solvent control subgroup and blank control subgroup (P>0.05).
Figure 3.
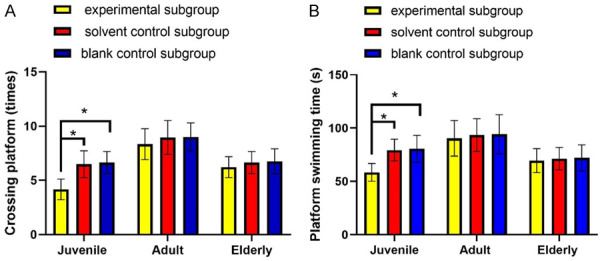
Etomidate reduces the platform crossings and swimming time at original platform in juvenile rats. Note: A: Platform crossings; B: Platform swimming time. *P<0.05.
Figure 4.
Swimming trajectories of rats in each group. A1: Swimming trajectories of juvenile-experimental subgroup; A2: Swimming trajectories of juvenile-solvent control subgroup; A3: Swimming trajectories of juvenile-blank control subgroup; B1: Swimming trajectories of adult-experimental subgroup; B2: Swimming trajectories of adult-solvent control subgroup; B3: Swimming trajectories of adult-blank control subgroup; C1: Swimming trajectories of elderly-experimental subgroup; C2: Swimming trajectories of elderly-solvent control subgroup; C3: Swimming trajectories of elderly-blank control subgroup.
Etomidate had no effect on hippocampus of rats
As shown in Figure 5, no difference was found in the number of neurons in hippocampal CA1 region among the 3 subgroups in the juvenile group. Similar results were observed in the adult and elderly groups. No difference in the number of neurons in hippocampal CA1 region suggested that etomidate did not affect neurons in CA1 region.
Figure 5.
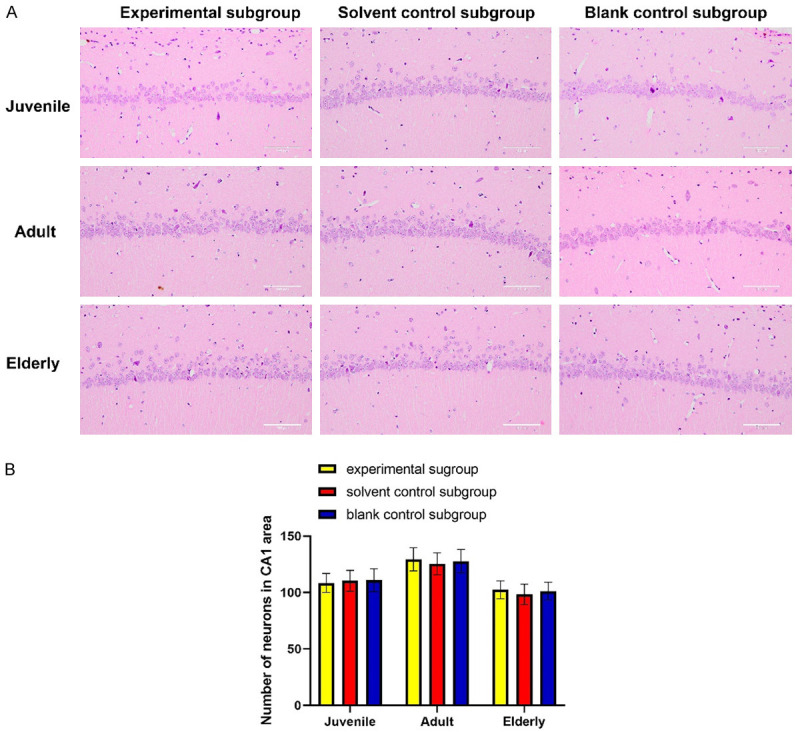
Etomidate had no effect on hippocampus of rats. A: Histogram of number of neurons in CA1 area; B: H&E staining results of each group. Scale bar: 100 μm. There was no significant difference in the number of neurons in CA1 area among the subgroups of the same age (P>0.05).
Moreover, the morphology of neurons in the CA1 area and morphology of vertebral cells were normal in all groups. The density of neurons was uniform, and no obvious degeneration or necrosis of vertebral cells was observed.
Etomidate has no effect on apoptosis in hippocampus of rats
In juvenile group, the apoptosis index of hippocampal tissue in experimental subgroup, solvent control subgroup and blank control subgroup was 3.1%, 2.79% and 2.71%, respectively (P>0.05). Also, no significant difference in apoptosis index was found among the subgroups in adult group and elderly group. See Figure 6.
Figure 6.
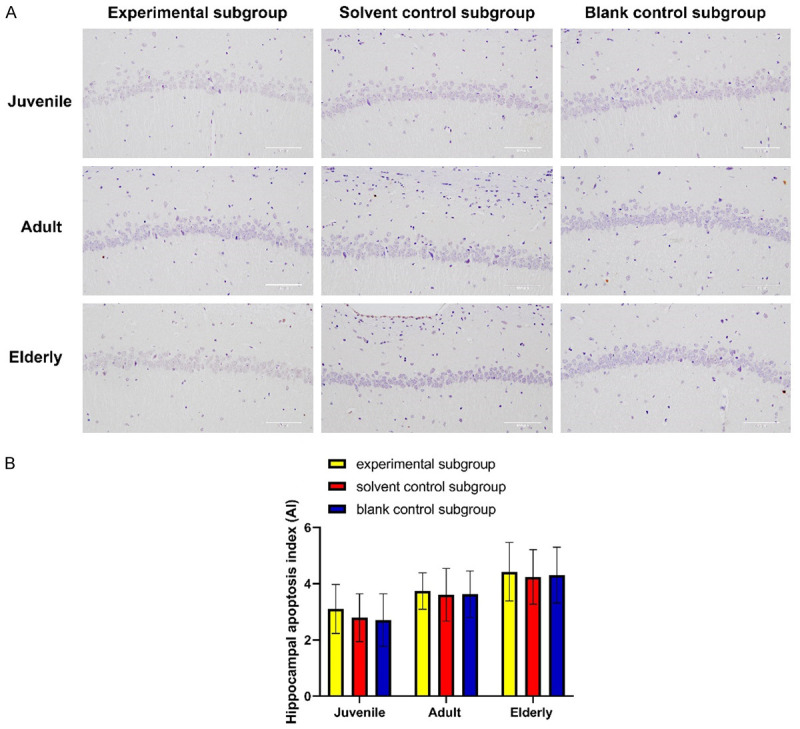
Etomidate has no effect on apoptosis in rat hippocampus. A: Histograms of apoptosis in rat hippocampus. B: TUNEL detection in hippocampus of rats in each group. Scale bar: 100 μm. No significant difference in apoptosis index was found among the subgroups of the same age.
Etomidate inhibits MAPK signaling pathway in hippocampus of juvenile rats
The expression of p-ERK in hippocampus of juvenile experimental subgroup was less than that of juvenile solvent control and blank control subgroups (P<0.05), but no significant differences were found in p-p38 and p-JNK expressions among the three juvenile subgroups (P>0.05). There was no significant difference in the expression of p-ERK, p-p38 and p-JNK among the 3 subgroups in adult and elderly groups (P>0.05, Figure 7).
Figure 7.
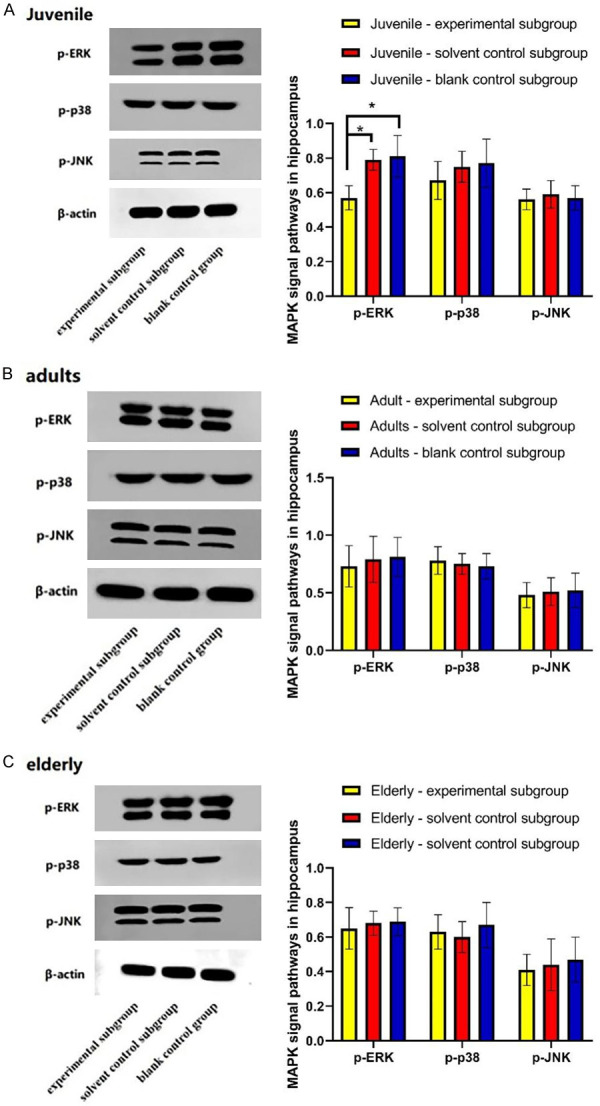
Etomidate inhibits MAPK signaling pathway in hippocampus of juvenile rats. A: Expression of MAPK signaling pathway in juvenile rats. B: Expression of MAPK signaling pathway in adult rats; C: Expression of MAPK signaling pathway in elderly rats. *P<0.05.
Discussion
POCD is a central nervous system complication of patients undergoing anesthesia surgery, and patients usually manifested as cognitive changes in learning, thinking, orientation, attention and other changes in central nervous system [14,15]. Neuronal cell death is one of the most common factors which negatively influence the learning and memory function [16-18]. Various animal studies have reported different results of etomidate effects on neuronal cell viability. Some animal experiments discovered that etomidate could induce immature brain neurotoxicity and even brain neuron apoptosis, which affected the development of cognitive function [19,20]. However, other studies indicated that etomidate in early infant mice at P10 (ten-day old mice) did not elevate the activated caspase 3 level, which played an important role in pro-apoptosis process [21,22]. In this study, no significant difference in numbers of neuron was found between etomidate treated rats and DMSO treated rats, indicating that etomidate anesthesia did not lead to neuronal cell death.
After etomidate treatment, we applied the Morris water maze test to evaluate the spatial learning and memory of rats in each group. It was shown that the escape latency of juvenile rats in the experimental subgroup was higher than those in solvent and blank control subgroups at 1 d and 2 d after intervention (P<0.05). But escape latency in adult and elderly rats was not affected by etomidate treatment (P>0.05). The platform crossings and swimming time at original platform of juvenile experimental subgroup were obviously less than those of juvenile solvent and blank control groups (P<0.05). However, no significant differences were found in platform crossings and swimming time at original platform among the 3 subgroups in adult and elderly groups (P>0.05). These indicated that etomidate may only affect the spatial learning and memory in juvenile rats. This is consistent with previous demonstrated results [23,24] that etomidate may affect neurocognitive function of infants and young mice, but no damages to nerve cells were found.
MAPK signaling pathway plays a vital role in learning and memory. The phosphorylation of MAPK is necessary for memory formation. Using the small molecule MAPK inhibitor UO126 to inhibit MAPK phosphorylation can significantly affect memory and learning in rats. The rat treated with UO126 took longer time to find the targets when compared to the control group (DMSO group) [25]. Similar results were observed by Paul R. Benjamin group. They found that the long-term memory formation in snail was completely blocked after injection of UO126 to the body cavities of snails [26]. In this study, we investigated the expression of MAPK in hippocampus in each group of rats by Western blot. The MAPK signaling pathway in juvenile experimental subgroup was inhibited by etomidate. Etomidate, however, had no effect on adult and elderly rats. As mentioned, etomidate only affected learning and memory behavior in juvenile rats rather than adult rats. Taken together, these indicated that the reduced level of p-ERK, a representative member of the MAPK family, can negatively affect learning and memory ability in juvenile rats. Interestingly, no significant changes in expressions of p-p38 and p-JNK were observed in all groups, indicating that they were not related to the spatial learning and memory ability in rats.
The ERK pathway, p38 pathway and JNK pathway in MAPK family have all been confirmed to be related to apoptosis of hippocampal nerve cells, among which the activation of ERK pathway can inhibit apoptosis and promote proliferation, while the activation of p38 pathway and JNK pathway can promote apoptosis [27-29]. This study suggests that the effect of etomidate on spatial learning and memory ability of juvenile rats may be related to the inhibition of ERK pathway, while not related to p38 and INK pathway. In addition, the TUNEL test results of hippocampal tissue in each group indicated that no significant differences in hippocampal apoptosis index was found among the subgroups group of the same age. Also, etomidate had no significant effect on hippocampal morphology and brain apoptosis in rats at all ages.
However, this study has some limitations. The effect of etomidate on the behavior of Morris water maze in juvenile rats might not be through the ERK pathway. To further demonstrate the effect of etomidate in modulating spatial learning and memory in juvenile rats, more fundamental studies need to be carried out. For example, how the ERK-/- rats perform in Morris water maze is meaningful and can help to fill the fundamental gap between etomidate and spatial learning and memory. We hope to establish an organoid model to explore the underlying mechanism of etomidate and its effects on memory.
In summary, etomidate may have certain negative effects on spatial learning and memory in juvenile rats, but not in adult and elderly rats. Etomidate did not affect the number of neurons and the morphology of vertebral cells in CA1 area of hippocampus. The etomidate treatment does not induce neuronal apoptosis, but its effect in juvenile rats may be connected to the inhibition of MAPK/ERK pathway.
Disclosure of conflict of interest
None.
References
- 1.Alcoverro-Fortuny Ò, Alarcón BG, Usan FV, Ruíz DS, Oscoz-Irurozqui M, Señé GM. Etomidate improves seizure adequacy during electroconvulsive therapy. Psychiatry Res. 2019;273:350–354. doi: 10.1016/j.psychres.2019.01.065. [DOI] [PubMed] [Google Scholar]
- 2.Park HY, Lee Y, Lim CY, Kim M, Park J, Lee T. Effects of etomidate use in ICU patients on ventilator therapy: a study of 12,526 patients in an open database from a single center. Korean J Anesthesiol. 2021;74:300–307. doi: 10.4097/kja.20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devlin RJ, Kalil D. Etomidate as an induction agent in sepsis. Crit Care Nurs Clin North Am. 2018;30:e1–e9. doi: 10.1016/j.cnc.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Choi GJ, Kang H, Baek CW, Jung YH, Ko JS. Etomidate versus propofol sedation for electrical external cardioversion: a meta-analysis. Curr Med Res Opin. 2018;34:2023–2029. doi: 10.1080/03007995.2018.1519501. [DOI] [PubMed] [Google Scholar]
- 5.Xie D, Li M, Yu K, Lu H, Chen Y. Etomidate alleviates cardiac dysfunction, fibrosis and oxidative stress in rats with myocardial ischemic reperfusion injury. Ann Transl Med. 2020;8:1181. doi: 10.21037/atm-20-6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.April MD, Arana A, Schauer SG, Davis WT, Oliver JJ, Fantegrossi A, Summers SM, Maddry JK, Walls RM, Brown CA 3rd NEAR Investigators. Ketamine versus etomidate and peri-intubation hypotension: a national emergency airway registry study. Acad Emerg Med. 2020;27:1106–1115. doi: 10.1111/acem.14063. [DOI] [PubMed] [Google Scholar]
- 7.Flamée P, Varnavas V, Dewals W, Carvalho H, Cools W, Bhutia JT, Beckers S, Umbrain V, Verborgh C, Forget P, Chierchia GB, Brugada P, Poelaert J, de Asmundis C. Electrocardiographic effects of propofol versus etomidate in patients with brugada syndrome. Anesthesiology. 2020;132:440–451. doi: 10.1097/ALN.0000000000003030. [DOI] [PubMed] [Google Scholar]
- 8.Hulsman N, Hollmann MW, Preckel B. Newer propofol, ketamine, and etomidate derivatives and delivery systems relevant to anesthesia practice. Best Pract Res Clin Anaesthesiol. 2018;32:213–221. doi: 10.1016/j.bpa.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Han SJ, Lee TH, Yang JK, Cho YS, Jung Y, Chung IK, Park SH, Park S, Kim SJ. Etomidate sedation for advanced endoscopic procedures. Dig Dis Sci. 2019;64:144–151. doi: 10.1007/s10620-018-5220-3. [DOI] [PubMed] [Google Scholar]
- 10.Malapero RJ, Zaccagnino MP, Brovman EY, Kaye AD, Urman RD. Etomidate derivatives: novel pharmaceutical agents in anesthesia. J Anaesthesiol Clin Pharmacol. 2017;33:429–431. doi: 10.4103/0970-9185.222521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu CN, Wu KC, Chung WS, Zheng LC, Juan TK, Hsiao YT, Peng SF, Yang JL, Ma YS, Wu RS, Chung JG. Etomidate suppresses invasion and migration of human A549 lung adenocarcinoma cells. Anticancer Res. 2019;39:215–223. doi: 10.21873/anticanres.13100. [DOI] [PubMed] [Google Scholar]
- 12.Upchurch CP, Grijalva CG, Russ S, Collins SP, Semler MW, Rice TW, Liu D, Ehrenfeld JM, High K, Barrett TW, McNaughton CD, Self WH. Comparison of etomidate and ketamine for induction during rapid sequence intubation of adult trauma patients. Ann Emerg Med. 2017;69:24–33. doi: 10.1016/j.annemergmed.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Constantinescu SM, Driessens N, Lefebvre A, Furnica RM, Corvilain B, Maiter D. Etomidate infusion at low doses is an effective and safe treatment for severe Cushing’s syndrome outside intensive care. Eur J Endocrinol. 2020;183:161–167. doi: 10.1530/EJE-20-0380. [DOI] [PubMed] [Google Scholar]
- 14.Av Sá LGD, Silva CRD, de A Neto JB, Cândido TM, de Oliveira LC, do Nascimento FB, Barroso FD, da Silva LJ, de Mesquita JR, de Moraes MO, Cavalcanti BC, Júnior HV. Etomidate inhibits the growth of MRSA and exhibits synergism with oxacillin. Future Microbiol. 2020;15:1611–1619. doi: 10.2217/fmb-2020-0078. [DOI] [PubMed] [Google Scholar]
- 15.Smischney NJ, Nicholson WT, Brown DR, Gallo De Moraes A, Hoskote SS, Pickering B, Oeckler RA, Iyer VN, Gajic O, Schroeder DR, Bauer PR. Ketamine/propofol admixture vs etomidate for intubation in the critically ill: KEEP PACE Randomized clinical trial. J Trauma Acute Care Surg. 2019;87:883–891. doi: 10.1097/TA.0000000000002448. [DOI] [PubMed] [Google Scholar]
- 16.Lee JM, Min G, Keum B, Lee JM, Kim SH, Choi HS, Kim ES, Seo YS, Jeen YT, Chun HJ, Lee HS, Um SH, Kim CD. Using etomidate and midazolam for screening colonoscopies results in more stable hemodynamic responses in patients of all ages. Gut Liver. 2019;13:649–657. doi: 10.5009/gnl18514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGrath M, Hoyt H, Pence A, Jayakar SS, Zhou X, Forman SA, Cohen JB, Miller KW, Raines DE. Competitive antagonism of etomidate action by diazepam: in vitro gabaa receptor and in vivo zebrafish studies. Anesthesiology. 2020;133:583–594. doi: 10.1097/ALN.0000000000003403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du X, Zhou C, Pan L, Li C. Effect of dexmedetomidine in preventing etomidate-induced myoclonus: a meta-analysis. Drug Des Devel Ther. 2017;11:365–370. doi: 10.2147/DDDT.S121979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abou Arab O, Fischer MO, Carpentier A, Beyls C, Huette P, Hchikat A, Benammar A, Labont B, Mahjoub Y, Bar S, Guinot PG, Lorne E. Etomidate-induced hypotension: a pathophysiological approach using arterial elastance. Anaesth Crit Care Pain Med. 2019;38:347–352. doi: 10.1016/j.accpm.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Jindal S, Sidhu GK, Kumari S, Kamboj P, Chauhan R. Etomidate versus propofol for motor seizure duration during modified electroconvulsive therapy. Anesth Essays Res. 2020;14:62–67. doi: 10.4103/aer.AER_5_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hua J, Miao S, Shi M, Tu Q, Wang X, Liu S, Wang G, Gan J. Effect of butorphanol on etomidate-induced myoclonus: a systematic review and meta-analysis. Drug Des Devel Ther. 2019;13:1213–1220. doi: 10.2147/DDDT.S191982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Av Sá LG, da Silva CR, S Campos R, de A Neto JB, Sampaio LS, do Nascimento FB, Barroso FD, da Silva LJ, Queiroz HA, Cândido TM, Rodrigues DS, Leitão AC, de Moraes MO, Cavalcanti BC, Júnior HV. Synergistic anticandidal activity of etomidate and azoles against clinical fluconazole-resistant Candida isolates. Future Microbiol. 2019;14:1477–1488. doi: 10.2217/fmb-2019-0075. [DOI] [PubMed] [Google Scholar]
- 23.Gurgel do Amaral Valente Sá L, da Silva CR, Neto JBA, do Nascimento FBSA, Barroso FDD, da Silva LJ, Cabral VPF, Barbosa AD, Silva J, Marinho ES, de Moraes MO, Rios MEF, Cavalcanti BC, Lima ISP, Júnior HVN. Antifungal activity of etomidate against growing biofilms of fluconazole-resistant Candida spp. strains, binding to mannoproteins and molecular docking with the ALS3 protein. J Med Microbiol. 2020;69:1221–1227. doi: 10.1099/jmm.0.001241. [DOI] [PubMed] [Google Scholar]
- 24.Chung M, Santer P, Raub D, Zhao Y, Zhao T, Strom J, Houle T, Shen C, Eikermann M, Yeh RW. Use of etomidate in patients with heart failure undergoing noncardiac surgery. Br J Anaesth. 2020;125:943–952. doi: 10.1016/j.bja.2020.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung JH, Hyun B, Lee J, Koh DH, Kim JH, Park SW. Neurologic safety of etomidate-based sedation during upper endoscopy in patients with liver cirrhosis compared with propofol: a double-blind, randomized controlled trial. J Clin Med. 2020;9:2424. doi: 10.3390/jcm9082424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miao S, Zou L, Wang G, Wang X, Liu S, Shi M. Effect of dexmedetomidine on etomidate-induced myoclonus: a randomized, double-blind controlled trial. Drug Des Devel Ther. 2019;13:1803–1808. doi: 10.2147/DDDT.S194456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavalcanti BC, do Amaral Valente Sá LG, de Andrade Neto JB, de Sousa Silva AA, Rios MEF, Barreto FS, de Oliveira Ferreira JR, da Silva CR, Barroso FD, Magalhães HIF, Nobre HV Jr, de Moraes MO. Etomidate is devoid of genotoxicty and mutagenicity in human lymphocytes and in the Salmonella typhimurium/microsomal activation test. Toxicol In Vitro. 2020;68:104946. doi: 10.1016/j.tiv.2020.104946. [DOI] [PubMed] [Google Scholar]
- 28.McGrath M, Ma C, Raines DE. Dimethoxy-etomidate: a nonhypnotic etomidate analog that potently inhibits steroidogenesis. J Pharmacol Exp Ther. 2018;364:229–237. doi: 10.1124/jpet.117.245332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farrell NM, Killius K, Kue R, Langlois BK, Nelson KP, Golenia P. A comparison of etomidate, ketamine, and methohexital in emergency department rapid sequence intubation. J Emerg Med. 2020;59:508–514. doi: 10.1016/j.jemermed.2020.06.054. [DOI] [PubMed] [Google Scholar]


