Abstract
Introduction: Accurate and rapid assessment of the coronary microcirculation has become an important medical challenge. However, reliable and non-invasive quantitative methods to diagnose coronary microvascular disease (CMVD), select treatments for coronary artery disease (CAD), and therefore improve coronary microcirculation are lacking. Current detection methods have limitations. Therefore, we will assess whether a new detection method, the non-invasive index of microcirculatory resistance (IMR), based on computed tomography (CT) perfusion and hydrodynamics (CT-IMR), can effectively evaluate the function of coronary microvessels. Methods: We will conduct a multicenter, randomized, open-label study, including a Phase I single-center and Phase II multicenter trial, to assess the accuracy of the non-invasive CT-IMR coronary measurement of microcirculation function. The study will enroll 295 patients who will undergo coronary CT angiography (CCTA), dynamic CT-myocardial perfusion imaging (CT-MPI), invasive coronary angiography (ICA), and invasive IMR. This study will identify the key influencing factors when calculating myocardial microcirculation perfusion and develop an accurate three-dimensional coronary reconstruction method and a non-invasive coronary IMR calculation method based on computational fluid dynamics (CFD). This will facilitate the development of a non-invasive system to detect and measure coronary microcirculation. Conclusion: The clinical trial for computed tomography myocardial perfusion based non-invasive index of microcirculatory resistance (MPBIMR) will establish the key influencing factors when calculating myocardial microcirculation perfusion and create a non-invasive CT-IMR calculation method based on CFD. This method may diagnose patients with simple coronary microvascular lesions and those with coronary microvascular lesions combined with coronary vascular lesions.
Keywords: Index of microcirculatory resistance, coronary intervention, cardiovascular imaging, hydrodynamics, coronary microvascular disease
Introduction
It is becoming more common for patients to present with symptoms and signs suggesting ischemic heart disease but with no obstructive coronary artery disease (INOCA) [1,2]. Approximately two-thirds of patients with INOCA suffer from microvascular disease (MVD), resulting in recurrent angina symptoms that represent a health care challenge [3]. In addition, the INOCA population has more limiting dyspnea and is more likely to be female, although the cardiovascular risk score of these patients is lower than that of patients with obstructive coronary artery disease (CAD) [4].
It is now possible to diagnose coronary microvascular disease (CMVD) with both invasive and non-invasive methods and accumulating research suggests that CMVD is a contributing factor to a poor prognosis in patients with angina and INOCA [5,6]. Ford et al. described a protocol using invasive coronary angiography (ICA) to identify obstructive CAD and INOCA in 206 (53%) and 185 (47%) participants, respectively. They found that 109 of 151 INOCA participants (72%) had microvascular angina identified by an interventional diagnostic procedure with reference invasive tests [4].
Noninvasive imaging modalities for detecting CMVD has also been validated. Taqueti et al. described a retrospective analysis of 329 patients referred for ICA after stress testing with myocardial perfusion positron emission tomography (PET). Through PET, the coronary flow reserve (CFR) was measured noninvasively, and a low CFR was linked with a higher risk of cardiovascular events [7]. Similarly, Thomson et al. reported a cohort of 118 women with suspected microvascular dysfunction who had undergone invasive coronary reactivity testing (CRT), compared with 21 asymptomatic control participants. They showed that noninvasive cardiac magnetic resonance imaging (CMRI) and myocardial perfusion reserve index (MPRI) can detect coronary microvascular dysfunction (CMD) defined by invasive CRT. Symptomatic women had lower mean pharmacological stress MPRI compared with control participants. A MPRI threshold of 1.84 predicted CRT abnormality with a sensitivity of 73% and a specificity of 74% [8]. However, CMRI appears to be burdensome in terms of economics, as well as the complex and lengthy calculations required for widespread implementation in clinical practice [9,10].
By using the thermodilution technique, the coronary microvascular status can be analyzed quantitatively and invasively using an index of microcirculatory resistance (IMR) measurements from one pressure-temperature sensor wire interrogation [11,12]. For decision-making purposes, values of IMR ≥25 units or CFR <2.0 are indicative of abnormal microcirculatory function [13]. It is imperative to treat microvascular angina by targeting the dominant cause of microcirculatory dysfunction [14]. However, variability in the degree of microvascular obstruction would make it more difficult to demonstrate a difference in IMR between normal and abnormal microcirculatory conditions. Moreover, this method causes some trauma to the human body and increases the risk of coronary complications during the examination.
Our research group has developed a coronary computed tomography angiography (CCTA)-derived fractional flow reserve (FFR) (FFRCT), which has a diagnostic coincidence rate of more than 85% [15]. Our team has established the technologies of computed tomography (CT) scanning, three-dimensional (3D) reconstruction, clinical parameter selection, mathematical model construction, and calculation system construction, which will lay a good foundation for the clinical trial outlined in this article [15]. Furthermore, we have successfully completed relevant preliminary experiments, which will help establish the feasibility of using CT to measure myocardial perfusion.
The myocardial perfusion-based non-invasive index of microcirculatory resistance (MPBIMR) trial is designed to test the non-invasive coronary IMR calculation method based on computational fluid dynamics (CFD) in patients with CAD. MPBIMR has the benefits of being non-invasive, and having a fast computing speed, safe operating parameters, user-friendly interface, and economic advantages, compared with existing methods. In addition, MPBIMR can not only diagnose patients with simple CMVD, but also patients with CMVD combined with coronary macrovascular disease, which has great significance in guiding therapeutic strategies.
Materials and methods
Study aim
This clinical trial will be carried out as a multicenter, randomized, open-label study to test a non-invasive coronary IMR calculation method based on CT perfusion and hydrodynamics, namely, CT-IMR. The invasive IMR acquired during ICA will be the reference standard, and non-invasive CT-IMR will be the comparator diagnostic method. The primary endpoint is the superiority of CT-IMR in identifying CMD by establishing an accurate three-dimensional geometric model of the coronary microcirculation hemodynamics.
Population recruitment and flowchart
The study will enroll 295 patients with angina pectoris who undergo CCTA and dynamic CT-myocardial perfusion imaging (CT-MPI), including 140 participants of single-center clinical validation (phase I) trial and 155 participants of multicenter clinical validation (phase II) trial. Assessment of myocardial microcirculation perfusion, including CFR and IMR, will be performed as part of ICA. The flowchart of how the study will be conducted is shown in Figure 1. The inclusion and exclusion criteria are listed in Table 1. All participants will be asked to provide informed consent before participation in the study. All diagnostic imaging and examinations need to be taken within 5 days after enrolment. Phase II will be enrolled from five medical centers in China.
Figure 1.
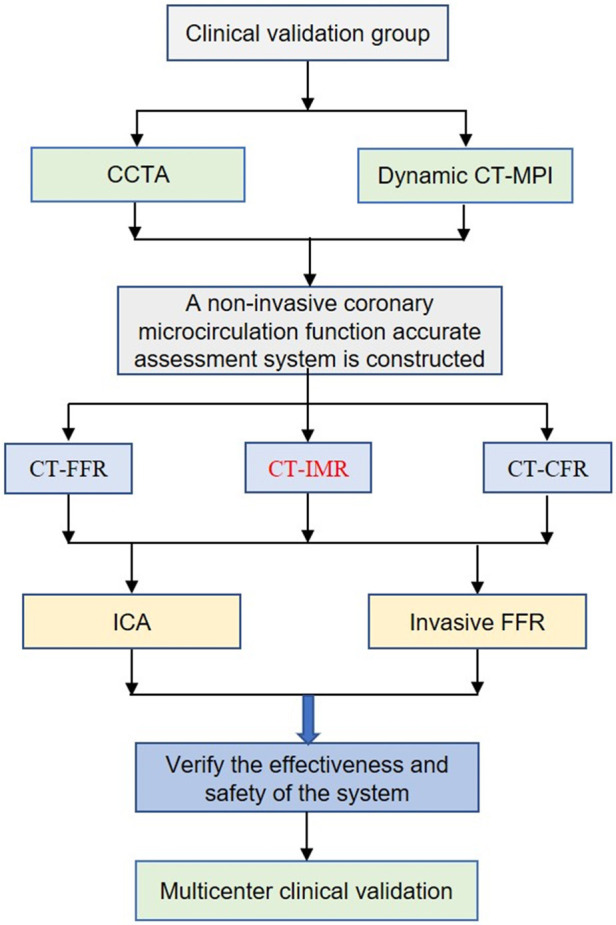
Study flow. CT, computed tomography; CCTA, coronary computed tomography angiography; CT-MPI, CT-myocardial perfusion imaging; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; CFR, coronary flow reserve; ICA, invasive coronary angiography.
Table 1.
Study inclusion and exclusion criteria
| Inclusion criteria |
| 1. Participant must be older than 18 years |
| 2. Patients with angina pectoris, who has risk factors for CMD (diabetes, metabolic syndrome, cardiac hypertrophy, female) |
| 3. Written informed consent available |
| 4. Participant is indicated for invasive coronary angiography |
| 5. Participant needs to be taken within 30 days after enrollment |
| Exclusion criteria |
| 1. Previous PCI or CABG |
| 2. Participant is not eligible for measuring IMR |
| 3. Complicated complex congenital heart disease |
| 4. Artificial pacemaker or internal defibrillator leads implanted |
| 5. Implanted artificial heart valve |
| 6. Severe arrhythmia including complete AV block, ventricular arrhythmia |
| 7. Impaired chronic renal function (serum creatinine >1.5 ULN) |
| 8. Allergic to iodine |
| 9. Pregnancy |
| 10. Body mass index >35 kg/m2 |
| 11. Needs for emergency procedures |
| 12. Left ventricle is markedly thickened |
| 13. Severe distortion of the blood vessel |
| 14. Unstable hemodynamics including abrupt chest pain cardiogenic shock, unstable blood pressure (systolic <90 mmHg), severe congestive heart failure or pulmonary edema |
| 15. Life-threatening diseases (life expectancy <2 months) |
| 16. Tako Tsubo syndrome (TTS) |
| 17. Inappropriate subject judged by clinician |
CMD, coronary microvascular dysfunction; PCI, percutaneous transluminal coronary intervention; CABG, coronary artery bypass grafting; IMR, index of microcirculatory resistance.
Study process
Study clinical and imaging data such as CCTA, CT-MPI, ICA and invasive multimodal physiology assessment including CFR and IMR will be transmitted to the independent core laboratory. Two teams will calculate CT-IMR based on the algorithm and the mathematical model of CT perfusion imaging. Furthermore, they will perform ICA and invasive pressure guidewire inspection to measure the IMR, which will verify the accuracy of CT-IMR, including the specificity and sensitivity of the method. In addition to establishing related algorithms and mathematical models, CT-CFR, and CT-IMR will also be calculated. After completing phase I, the technical requirements and preparation of product specifications will be completed to confirm the accuracy, reliability, sensitivity, and specificity of CT-IMR in phase II.
CCTA and dynamic CT-MPI
CCTA and CT-MPI will be performed using SOMATOM Definition Flash CT (Siemens, Germany). In accordance with the preset scanning protocols by the Society of Cardiac Computed Tomography, CTA scanning protocols will coincide with prospective electrocardiography triggering technology [16]. We will use a paired 3D fully convolutional neural networks (FCNs) with different scales to achieve accurate segmentation of coronary arteries, which is expected to extract both long-range contextual and 3D high-resolution contextual information. Despite having the same architecture, the paired FCNs differ in weights, using a 3D reconstruction of the well-known architecture U-Net [17]. Furthermore, the myocardial blood flow (MBF) value for each segment of the heart will be displayed on the cardiac color map. A measurement of the left ventricular wall thickness, curvature, and volume will be performed in this study. Additionally, the myocardial perfusion (F) and intramyocardial blood volume (Bv) will be calculated by the time attenuation curve (TAC) of contrast agent injection into the peripheral blood. Various clinical factors affecting myocardial perfusion, such as blood pressure, blood viscosity, heart rate, myocardial volume, and degree of coronary vascular lesions, will be comprehensively weighted through constant comparative analysis, and various integrated simulation operations will be carried out. The measurement factors that will have the greatest influence on the accuracy of calculating the myocardial perfusion anomaly caused by microcirculation will be selected.
Noninvasive CT-IMR
We will calculate and obtain CT-IMR using the principle of CFD based on the measurement of CT myocardial perfusion and evaluate the diagnostic accuracy for coronary microcirculation lesions. An interface condition, which keeps the continuity of the velocity and stress on the interface, is implemented on the interface of the fluid and solid domain. A patient-specific time-dependent inflow rate is used as the inlet boundary condition. On the outlet boundaries, a circuit model named the impedance boundary condition is applied.
To solve the continuous Navier-Stokes equations with a computer, we first need to discretize the equation on an unstructured grid which is generated by a mesh generator to obtain a finite dimensional system. The Navier-Stokes equations are discretized by a finite element method in the spatial dimensions, and a fully implicit second-order backward differentiation method is then used for the temporal discretization. The large-scale nonlinear system at each time step is solved by the NKS method. The NKS method is a highly scalable parallel solver for nonlinear partial differential equations, which has three main components, an inexact Newton method with a line search method is used to handle the nonlinear system, a Krylov subspace method is introduced to solve the Jacobian system at each Newton step, and an overlapping Schwarz method is employed as the preconditioner to accelerate the Krylov method [18]. The noninvasive CT-IMR is calculated based on the velocity and pressure obtained in the CFD simulation [19] (Equation 1).
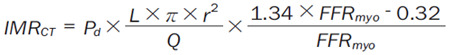 |
Where FFRmyo = Pa/Pd, Pa is the aortic pressure, and Pd is the distal coronary pressure, which are obtained in the CFD simulation. L is the distance of the two sensors on the doppler wire, which is supposed to be a constant 3 cm, r is the diameter of the coronary artery, and Q is the flow rate obtained in the CT perfusion.
Coronary angiography
ICA and invasive IMR will be performed according to the American College of Cardiology/American Heart Association guidelines for coronary angiography and intervention [20]. Multimodal physiological evaluation including CFR and IMR will be measured using the thermodilution technique. Maximal hyperemia will be induced by intravenous administration of adenosine (140 μg kg-1∙min-1). Coronary angiography images will be analyzed at the core laboratory by an experienced technician who is blinded to the study.
Invasive multimodal physiology assessment
Following standard operating procedures, an intracoronary pressure and temperature sensor-tipped guidewire (St. Jude Medical, USA) will be used to measure distal coronary pressure and coronary flow determined by the coronary thermodilution method [12]. The average conduction time (Tmn) will be recorded when the cardiac muscle will reach the maximum hyperemic state at a dose of 160 µg/kg/min of adenosine 5’-triphosphate, and the temperature curve will be sensed by the temperature sensor induced at room temperature with 0.9% sodium chloride solution (3 ml). The mean conduction time (bTmn) will be obtained after three consecutive operations. Meanwhile, Pa, Pd, FFR, and CFR values will be recorded in the resting and hyperemic states. If FFR >0.80 or coronary artery stenosis is mild to moderate, IMR can be calculated using the simplified formula: IMR = Pd × Tmn [21]. However, when there is severe coronary artery stenosis or FFR≤0.80, IMR = Pa × Tmn [(Pd-Pw)/(Pa-Pw)] [22]. Pw represents the wedge pressure of the coronary artery, that is, the mean pressure at the distal end of the lesion after complete coronary stenosis or balloon incarceration.
Primary efficacy analysis
The sensitivity and specificity of CT-IMR in the diagnosis of microcirculation dysfunction will be analyzed.
Secondary efficacy analysis
In order to assess the accuracy and precision of CT-IMR in the diagnosis of microcirculation dysfunction, we will analyze the diagnostic coincidence rate, positive predictive value, and negative predictive value.
Sample size calculation
A major evaluation index of our study is the accuracy of coronary microcirculation parameters calculated by CCTA, that is, the diagnostic compliance rate compared with the IMR of the invasive pressure guidewire. The following formula will be used to estimate the sample size (Equation 2):
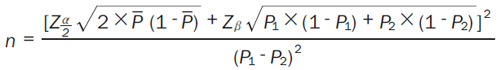 |
P1 is the target value of the diagnostic accordance rate of the noninvasive CT-IMR. P2 is the diagnostic accordance rate of the existing IMR target: β = 0.8, α = 0.05. The diagnostic accordance rate of the noninvasive CT-IMR has no research data for reference, so we will set a target of 0.85 (P1). According to existing research, the diagnostic accordance rate of IMR is 0.95 (P2), so 140 samples will be needed.
The sample size for the non-invasive CT-IMR diagnostic test will be calculated using different formulas. The formula for calculating the sample size based on sensitivity is shown below (Equation 3):
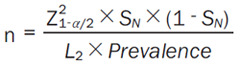 |
The formula for calculating the sample size based on specificity is as follows (Equation 4):
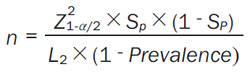 |
Z1-α/2 is the value of Z when the cumulative probability of a normal distribution is equal to α/2. If α is 0.05, Z1-α/2 is 1.96. L is the width of the 95% interval of our allowable sensitivity or specificity, which is artificially specified by the researcher and generally set at 0.03-0.1; here, we will take 0.1. The prevalence rate (prevalence) of the test product will be 0.45 [23] (as a result of certain screening at the early stage, the prevalence rate can be reached).
Based on the required sample size calculated sensitivity in 137 cases and with a 5% increase in the sample size, 144 cases will be recruited. The required sample size calculated based on specificity is 147, but 155 cases will be needed following a 5% increase. Accordingly, we will include 155 participants.
Statistical hypotheses
To perform the statistical analysis, SPSS (version 24, SPSS Inc, USA) for Windows will be used. A normality test and homogeneity of variance will be performed on all measurement data. Normal measurement data will be expressed as mean ± standard deviation. An independent sample t-test or analysis of variance will be used to test for homogeneity of variance. If the variance is heterogeneous, t-test or approximate F test will be used. For non-normal data, the Mann-Whitney U test will be used to represent the median (numerical range). Sensitivity, specificity, and accuracy will be obtained for diagnostic performance evaluation. They are defined as follows (Equation 5):
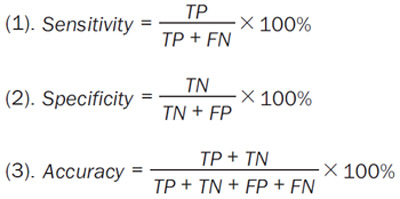 |
where TP and TN represent the number of true-positive and true-negative cases, respectively. FN and FP indicate the number of false-negative and false-positive cases, respectively. McNemar’s test for matched data will be performed to compare the diagnostic performance. The area under the curve (AUC) value of the receiver operating characteristic (ROC) curve will also be calculated to evaluate the performance of CT-IMR. Comparisons of the AUC values will be performed using Delong’s method [24]. In order to analyze correlations between variables, the Spearman rank correlation test will be applied. The chi-square test will be conducted for categorical data, and Fisher’s exact test will be conducted with a number less than 5. P vales <0.05 will be considered statistically significant.
Study outcomes
The primary endpoint will be identified as follows: (i) Several key measurement factors will be screened to establish an accurate 3D geometric model of the coronary microcirculation hemodynamics; (ii) An accurate non-invasive assessment system of the coronary microcirculation function will be constructed to diagnose simple coronary microcirculation disorders and macrovascular lesions combined with microcirculation disorders. The advantages of this system are that it is non-invasive, safe and convenient to operate, and has a fast processing speed, low cost, and intuitive user-friendly interface; and (iii) The core technical indicators of the evaluation system, such as diagnostic compliance, sensitivity, and specificity, will be able to achieve the set goals.
Data collection, management, and analysis
Study sites will assign a unique study ID number to each participant, as applicable. Records of the participant/ID relationship(s) will be maintained by the study site. Medical information about individual participants obtained through this study will be treated confidentially.
The investigator must ensure accuracy, completeness and timeliness of the data reported in the case report forms (CRFs) and in all other required reports. Data reported on the CRFs which are derived from source documents must be consistent with the source documents and discrepancies need to be justified in a documented rationale, signed and dated by the (principal) investigator, and filed in the subject medical file. For this trial, all data will be collected by means of a standard electronic case report form (eCRF).
Data confidentiality
Participant confidentiality will be maintained throughout the clinical study in a way that ensures the information can always be tracked back to the source data. For this purpose, a unique participant identification code (e.g., site number, participant number) will be assigned and used to allow identification of all data reported for each participant.
Discussion
The main purpose of the MPBIMR trial is to identify the key influencing factors when calculating myocardial microcirculation perfusion, establish an accurate 3D coronary reconstruction method, and create a non-invasive CT-IMR calculation method based on CFD. Moreover, the results will provide important insights into the potential benefits of an accurate non-invasive system to detect and assess the function of coronary microcirculation.
Diagnosing CMVD in the cardiac catheterization laboratory remains a challenge, particularly in patients with stable angina without obstructive epicardial CAD. Measurement of CFR or microcirculatory resistance (the inverse of conductance) provides an indication of impaired microcirculatory conductance [14]. Traditionally, CFR, defined as the ratio of hyperemic to resting flow measured with a Doppler wire, has been the standard technique to diagnose CMVD. In patients with microvascular angina, Transthoracic Echocardiography (TTE) Doppler is an accurate and noninvasive way to estimate CFR [25,26]. Furthermore, CFR measured noninvasively by PET is considered a promising and effective noninvasive technique to evaluate CMD [7,27]. CMR-derived perfusion imaging is another promising technique to assess myocardial perfusion because of its high spatial and temporal resolution. In CMR, lesions are identified by analyzing the intensity of the late gadolinium-enhanced signal, and this method has been increasingly utilized for the non-invasive evaluation of myocardial microvascular function [28,29].
However, CFR may be less sensitive and specific for detecting ischemia without obstructive CAD [30]. Due to the fact that CFR does not distinguish between microcirculation and epicardial artery, it may be distorted if there is occult coronary arterial disease [31]. Given its limited diagnostic accuracy, it is still not routinely applied in clinical practice.
In the catheterization laboratory, the microcirculatory resistance can be assessed by combining intracardiac pressure with thermodilution measurements (to calculate the IMR) [14]. Normal and abnormal microcirculatory function can be distinguished by IMR, which is not affected by coronary epicardial stenosis [12]. IMR may provide a quantitative assessment of the coronary microvascular status [11,12], independent of hemodynamic changes, and is more reproducible than CFR [32]. In addition to being precise and reproducible, this method may provide a better understanding of microvascular (dys) function and facilitate the development of new treatment options [33]. However, this method is associated with certain harm to the human body and increases risks of coronary complications during the examination. Moreover, it is difficult for patients to undergo repeated examinations within a certain time period, which is not suitable for evaluating therapeutic effects and drug efficacy. Therefore, there is an urgent need for an effective and affordable non-invasive testing method to diagnose early-stage CMVD and guide treatment selection and screening of effective drugs.
CFD is a relatively new field that combines numerical calculations and classical fluid mechanics theory. The combination of high image quality of CCTA and functional assessment of IMR has the potential to improve diagnostic performance in potential CMVD patients. When viewed from the pathological perspective, coronary microvessel dysfunction results in changes in the blood flow and flow velocity in the dominant region, which appears as local sparsity or defect [34]. We speculated that the abnormal blood flow and flow velocity in coronary microvessels could be reflected in CT perfusion imaging under specific scanning parameters. We also hypothesized that the heterogeneous change in the spatial distribution of the micro-artery perfusion area could be identified on CT images.
In addition, our team has established CT scanning, 3D reconstruction, clinical parameter selection, mathematical model construction, calculation system construction, and other technologies, which lay a good foundation for future research (Figure 2). To establish the feasibility of CT in measuring myocardial perfusion, we conducted relevant preliminary experiments and established a method for measuring myocardial perfusion (Figures 3 and 4).
Figure 2.
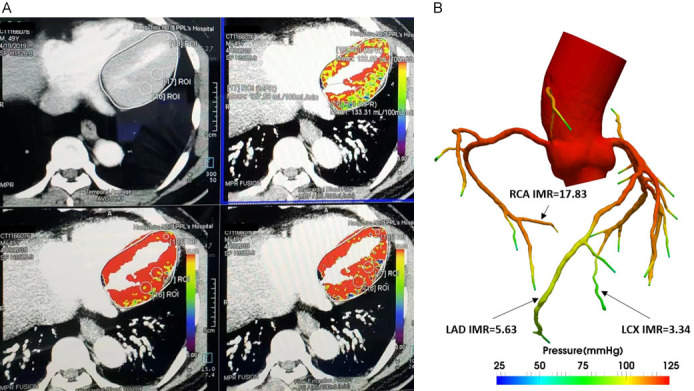
A: An example case of CT-IMR based on CFD. Patients obtaining perfusion information of coronary artery based on load dynamic CT perfusion imaging. B: An example case of CT-IMR based on CFD. CT-IMR value obtained by using the self-developed mathematical model and algorithm. IMR, index of microcirculatory resistance; LAD, left anterior descending; LCX, left circumflex; RCA, right coronary artery.
Figure 3.
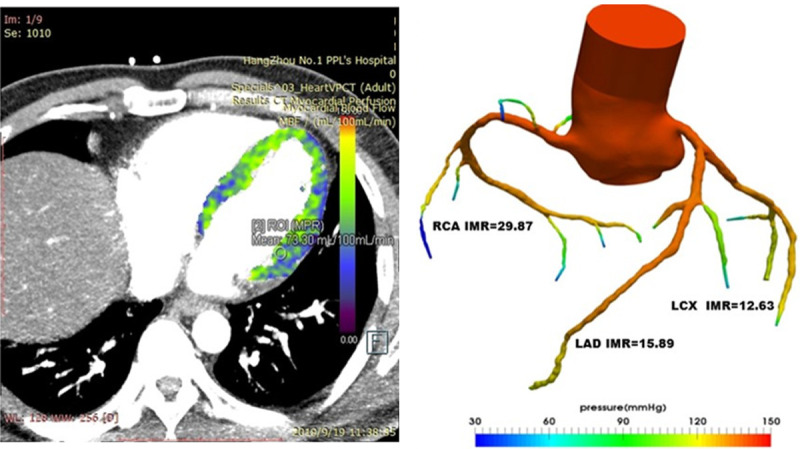
IMR results based on CT perfusion calculation: 15.89 for LAD, 12.63 for LCX and 29.87 for RCA. CT, computed tomography; IMR, index of microcirculatory resistance; LAD, left anterior descending; LCX, left circumflex; RCA, right coronary artery.
Figure 4.
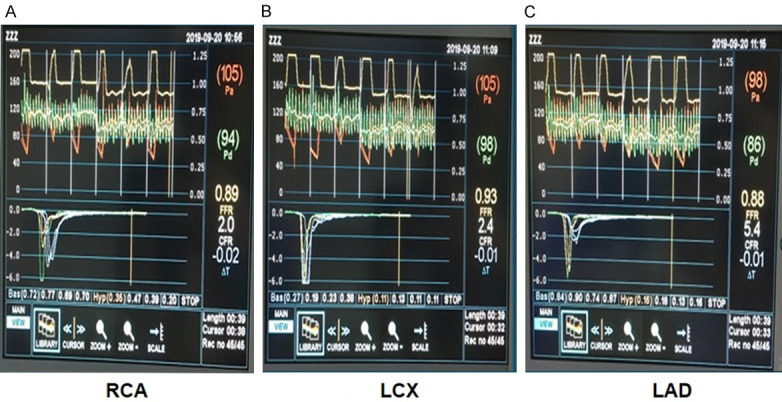
IMR results measured by temperature dilution method: 13.76 for LAD, 10.78 for LCX and 33.84 for RCA. Pa, the aortic pressure; Pd, the distal coronary pressure; CFR, coronary flow reserve; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; LAD, left anterior descending; LCX, left circumflex; RCA, right coronary artery; Tmn, mean transit time.
Acknowledgements
J-YH and G-XT conceived and designed the study. B-BG and G-XT wrote the draft of the paper. D-RZ, YF-Q, L-JZ, P-X, J-MY, L-Z, X-HY, S-SM, J-JG will conduct screening participants. X-ST, J-CX will conduct data collection. Analysis including the calculations for the non-invasive index of microcirculatory resistance by CT imaging will be performed by B-KW, J-L and R-LC. J-YH will be involved in critical revision of the study for important intellectual content. The final version of the manuscript was revised by all authors and approved by them. The authors thank all patients who participated in this study. We also thank Leah Cannon, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn/), for editing the English text of a draft of this manuscript. This research was supported by the Key R&D Project through the Science and Technology Department of Zhejiang Province (grant number: 2020C03018). Regardless of the research group’s involvement, the protocol design, implementation, management, data collection and analysis of the study are completely independent. This study was approved by the ethics committee of Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine (2019-033-01), and informed consent will be obtained for all enrolled patients. The results will be published in a peer-reviewed journal.
Disclosure of conflict of interest
None.
References
- 1.Marinescu MA, Löffler AI, Ouellette M, Smith L, Kramer CM, Bourque JM. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc Imaging. 2015;8:210–220. doi: 10.1016/j.jcmg.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135:1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schindler TH, Dilsizian V. Coronary microvascular dysfunction: clinical considerations and noninvasive diagnosis. JACC Cardiovasc Imaging. 2020;13:140–155. doi: 10.1016/j.jcmg.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 4.Ford TJ, Yii E, Sidik N, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, McCartney P, Corcoran D, Collison D, Rush C, Stanley B, McConnachie A, Sattar N, Touyz RM, Oldroyd KG, Berry C. Ischemia and no obstructive coronary artery disease: prevalence and correlates of coronary vasomotion disorders. Circ Cardiovasc Interv. 2019;12:e008126. doi: 10.1161/CIRCINTERVENTIONS.119.008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jespersen L, Hvelplund A, Abildstrøm SZ, Pedersen F, Galatius S, Madsen JK, Jørgensen E, Kelbæk H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–744. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 6.Michelsen MM, Mygind ND, Frestad D, Prescott E. Women with stable angina pectoris and no obstructive coronary artery disease: closer to a diagnosis. Eur Cardiol. 2017;12:14–19. doi: 10.15420/ecr.2016:33:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27. doi: 10.1161/CIRCULATIONAHA.114.011939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson LE, Wei J, Agarwal M, Haft-Baradaran A, Shufelt C, Mehta PK, Gill EB, Johnson BD, Kenkre T, Handberg EM, Li D, Sharif B, Berman DS, Petersen JW, Pepine CJ, Bairey Merz CN. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A national heart, lung, and blood institute-sponsored study from the women’s ischemia syndrome evaluation. Circ Cardiovasc Imaging. 2015;8:10.1161/CIRCIMAGING.114.002481 e002481. doi: 10.1161/CIRCIMAGING.114.002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation. 2010;121:2317–2325. doi: 10.1161/CIRCULATIONAHA.109.900191. [DOI] [PubMed] [Google Scholar]
- 10.Lanza GA, Buffon A, Sestito A, Natale L, Sgueglia GA, Galiuto L, Infusino F, Mariani L, Centola A, Crea F. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol. 2008;51:466–472. doi: 10.1016/j.jacc.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 11.Chung JH, Lee KE, Park JW, Shin ES. Coronary microvascular disease and clinical prognosis in deferred lesions: the index of microcirculatory resistance. Clin Hemorheol Microcirc. 2019;71:137–140. doi: 10.3233/CH-189403. [DOI] [PubMed] [Google Scholar]
- 12.Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, Yock PG, Yeung AC. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107:3129–3132. doi: 10.1161/01.CIR.0000080700.98607.D1. [DOI] [PubMed] [Google Scholar]
- 13.Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, Yii E, Sidik N, McCartney P, Corcoran D, Collison D, Rush C, McConnachie A, Touyz RM, Oldroyd KG, Berry C. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol. 2018;72:2841–2855. doi: 10.1016/j.jacc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 15.Wu X, Wu B, He W, Wang X, Wang K, Yan Z, Cheng Z, Huang Y, Zhang W, Chen R, Liu J, Wang J, Hu X. Expanding the coronary tree reconstruction to smaller arteries improves the accuracy of FFR(CT) Eur Radiol. 2021;31:8967–8974. doi: 10.1007/s00330-021-08012-7. [DOI] [PubMed] [Google Scholar]
- 16.Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, Nieman K, Pontone G, Raff GL. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8:342–358. doi: 10.1016/j.jcct.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Ronneberger O, Philipp F, Brox T. U-Net: convolutional networks for biomedical image segmentation. Med Image Comput Comput Assist Interv. 2015;9351:234–241. [Google Scholar]
- 18.Chen R, Wu B, Cheng Z, Shiu WS, Liu J, Liu L, Wang Y, Wang X, Cai XC. A parallel non-nested two-level domain decomposition method for simulating blood flows in cerebral artery of stroke patient. Int J Numer Method Biomed Eng. 2020;36:e3392. doi: 10.1002/cnm.3392. [DOI] [PubMed] [Google Scholar]
- 19.De Bruyne B, Pijls NH, Smith L, Wievegg M, Heyndrickx GR. Coronary thermodilution to assess flow reserve: experimental validation. Circulation. 2001;104:2003–2006. doi: 10.1161/hc4201.099223. [DOI] [PubMed] [Google Scholar]
- 20.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American college of cardiology foundation/American heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation. 2011;124:e574–651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 21.Yong AS, Layland J, Fearon WF, Ho M, Shah MG, Daniels D, Whitbourn R, Macisaac A, Kritharides L, Wilson A, Ng MK. Calculation of the index of microcirculatory resistance without coronary wedge pressure measurement in the presence of epicardial stenosis. JACC Cardiovasc Interv. 2013;6:53–58. doi: 10.1016/j.jcin.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Cerrato E, Quirós A, Echavarría-Pinto M, Mejia-Renteria H, Aldazabal A, Ryan N, Gonzalo N, Jimenez-Quevedo P, Nombela-Franco L, Salinas P, Núñez-Gil IJ, Rumoroso JR, Fernández-Ortiz A, Macaya C, Escaned J. PRotective effect on the coronary microcirculation of patients with diabetes by clopidogrel or ticagrelor (PREDICT): study rationale and design. A randomized multicenter clinical trial using intracoronary multimodal physiology. Cardiovasc Diabetol. 2017;16:68. doi: 10.1186/s12933-017-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8:1445–1453. doi: 10.1016/j.jcin.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 24.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 25.Sicari R, Rigo F, Cortigiani L, Gherardi S, Galderisi M, Picano E. Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near-normal coronary arteries. Am J Cardiol. 2009;103:626–631. doi: 10.1016/j.amjcard.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 26.Galiuto L, Sestito A, Barchetta S, Sgueglia GA, Infusino F, La Rosa C, Lanza G, Crea F. Noninvasive evaluation of flow reserve in the left anterior descending coronary artery in patients with cardiac syndrome X. Am J Cardiol. 2007;99:1378–1383. doi: 10.1016/j.amjcard.2006.12.070. [DOI] [PubMed] [Google Scholar]
- 27.Titterington JS, Hung OY, Wenger NK. Microvascular angina: an update on diagnosis and treatment. Future Cardiol. 2015;11:229–242. doi: 10.2217/fca.14.79. [DOI] [PubMed] [Google Scholar]
- 28.Naresh NK, Butcher JT, Lye RJ, Chen X, Isakson BE, Gan LM, Kramer CM, Annex BH, Epstein FH. Cardiovascular magnetic resonance detects the progression of impaired myocardial perfusion reserve and increased left-ventricular mass in mice fed a high-fat diet. J Cardiovasc Magn Reson. 2016;18:53. doi: 10.1186/s12968-016-0273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li R, Yang ZG, Wen LY, Liu X, Xu HY, Zhang Q, Guo YK. Regional myocardial microvascular dysfunction in cardiac amyloid light-chain amyloidosis: assessment with 3T cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2016;18:16. doi: 10.1186/s12968-016-0240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Wessel TR, Arant CB, Pohost GM, Lerman A, Quyyumi AA, Sopko G. Insights from the NHLBI-sponsored women’s ischemia syndrome evaluation (WISE) study: part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47(Suppl):S4–S20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 31.Fearon WF, De Bruyne B. The shifting sands of coronary microvascular dysfunction. Circulation. 2019;140:1817–1819. doi: 10.1161/CIRCULATIONAHA.119.043952. [DOI] [PubMed] [Google Scholar]
- 32.Fearon WF, Kobayashi Y. Invasive assessment of the coronary microvasculature: the index of microcirculatory resistance. Circ Cardiovasc Interv. 2017;10:e005361. doi: 10.1161/CIRCINTERVENTIONS.117.005361. [DOI] [PubMed] [Google Scholar]
- 33.Xaplanteris P, Fournier S, Keulards DCJ, Adjedj J, Ciccarelli G, Milkas A, Pellicano M, Van’t Veer M, Barbato E, Pijls NHJ, De Bruyne B. Catheter-based measurements of absolute coronary blood flow and microvascular resistance: feasibility, safety, and reproducibility in humans. Circ Cardiovasc Interv. 2018;11:e006194. doi: 10.1161/CIRCINTERVENTIONS.117.006194. [DOI] [PubMed] [Google Scholar]
- 34.Kotecha T, Martinez-Naharro A, Boldrini M, Knight D, Hawkins P, Kalra S, Patel D, Coghlan G, Moon J, Plein S, Lockie T, Rakhit R, Patel N, Xue H, Kellman P, Fontana M. Automated pixel-wise quantitative myocardial perfusion mapping by CMR to detect obstructive coronary artery disease and coronary microvascular dysfunction: validation against invasive coronary physiology. JACC Cardiovasc Imaging. 2019;12:1958–1969. doi: 10.1016/j.jcmg.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


