Abstract
Objectives: Membranous nephropathy (MN) is an autoimmune nephropathy. The incidence of MN is increasing gradually in recent years. Previous studies focused on antibody production, complement activation and podocyte injury in MN. However, the etiology and underlying mechanism of MN remain to be further studied. Methods: GSE104948 and GSE108109 of glomerular expression profile were downloaded from Gene Expression Omnibus (GEO) database, GSE47184, GSE99325, GSE104954, GSE108112, GSE133288 of renal tubule expression profile, and GSE73953 of peripheral blood mononuclear cells (PBMCs) expression profile. After data integration by Networkanalyst, differentially expressed genes (DEGs) between MN and healthy samples were obtained. DEGs were enriched in gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG), and protein-protein interaction (PPI) networks of these genes were constructed through Metascape, etc. We further understood the function of hub genes through gene set enrichment analysis (GSEA). The diagnostic value of DEGs in MN was evaluated by receiver operating characteristic (ROC) analysis. Results: A total of 3 genes (TP53, HDAC5, and SLC2A3) were screened out. Among them, the up-regulated TP53 expression may be closely related to MN renal pathological changes. However, the expression of MN podocyte target antigen was not significantly different from that of healthy controls. In addition, the changes of Wnt signaling pathway in PBMCs and the effects of SLC2A3 on the differentiation of M2 monocyte need further study. Conclusion: It is difficult to unify a specific mechanism for the changes of glomerulus, renal tubules and PBMCs in MN patients. This may be related to the pathogenesis, pathology and immune characteristics of MN. MN podocyte target antigen may not be the root cause of the disease, but a stage result in the pathogenesis process.
Keywords: Membranous nephropathy, molecular mechanism, integrated bioinformatics, key biomarker
Introduction
Membranous nephropathy (MN), an autoimmune disease, is a common pathological type of primary glomerular diseases characterized by diffuse glomerular basement membrane thickening [1]. The main clinical manifestations are nephrotic syndrome with proteinuria, edema and hypoproteinemia [2]. Compared with other common autoimmune nephropathy, such as IgA nephropathy and lupus nephritis, MN is less common in hematuria and almost no gross hematuria [3,4]. Some MN patients could have spontaneous remission [5,6]. At the pathological level, local glomerular proliferation and infiltration of circulating immune cells were rare in this disease [1,2]. At the immunological level, the autoantibodies deposited in the glomeruli were mainly IgG4 [1,7]. The proportion of regulatory B cells in patients was higher than that in healthy controls [8,9]. However, IgG4 and regulatory B cells are generally thought to inhibit inflammation and immune responses [8]. All these phenomena indicate that MN is a unique autoimmune disease.
In recent years, studies on the pathogenesis of MN have emerged in an endless stream, but there are still many unexplained issues. In terms of the discovery of podocyte target antigen, Beck et al. in 2009 detected the presence of M-type phospholipase A2 antibody (PLA2R1) in the serum of patients, but not in the serum of other glomerular diseases and normal persons [7]. Subsequently, a large number of podocyte target antigens, such as THSD7A and NELL1, have been discovered by researchers [10]. But the mechanism of their exposure is not clear. In terms of pathogenic antibodies, immunofluorescence showed that IgG subclasses deposited on capillary walls of MN patients were mainly IgG4, and a small amount of IgG1, IgG2 or IgG3 existed [1]. Normally, IgG4 does not activate complement [11]. However, there is evidence of complement activation in MN patients [12,13]. Therefore, some scholars believe that in MN patients complement is activated by non-IgG4 antibodies [14]. However, other scholars suggested that although IgG4 could not activate classical pathway, it could activate complement through alternative pathway or mannose-lectin pathway [15,16]. In terms of the pathogenic factors of MN, although the target antigen was located in podocyte, Liu etc. believed that exposure of MN target antigen was located in the lung based on the correlation between PM2.5 and MN incidence [17]. This hypothesis has not yet been proven, but it is supported by fellow researchers [18,19].
In recent studies, integrated bioinformatics is becoming more common. The integration of related bioinformatics studies can screen out differential genes more accurately and facilitate the exploration of potential mechanisms of diseases. When the results of multiple bioinformatics analyses are inconsistent or have no statistical significance, statistical analysis results close to the real situation can be obtained by using integrated bioinformatics analysis [20]. Unfortunately, to date, few bioanalyses have been performed specifically for membranous nephropathy. Therefore, we decided to use this approach to investigate the molecular mechanism of membranous nephropathy based on the genetic data of patients’ glomerulus, renal tubules, and peripheral blood mononuclear cells (PBMCs).
Method
Access to GEO datasets
Gene series were screened by GEO database. Series of membranous nephropathy samples and healthy control samples were searched. Finally, GSE47184, GSE73953, GSE99325, GSE104948, GSE104954, GSE108109, GSE108112, and GSE133288 were identified. The glomerular series were GSE104948 and GSE108109. The renal tubules series were GSE47184, GSE99325, GSE104954, GSE108112 and GSE133288. The PBMCs series is GSE73953. The basic information for the selected dataset is shown in Table 1. The probes were transformed into the homologous gene symbol by means of the platform’s annotation information.
Table 1.
Characteristics of the GEO datasets
| Tissue | Series GEO accession | Series type | Number of samples | Group | Organism | Series platform ID |
|---|---|---|---|---|---|---|
| Glomerular | GSE104948 | Expression profiling by array | 25 | MN (n = 21) | Homo sapiens | GPL24120 |
| HC (n = 4) | ||||||
| GSE108109 | Expression profiling by array | 50 | MN (n = 44) | Homo sapiens | GPL19983 | |
| HC (n = 6) | ||||||
| Renal tubule | GSE47184 | Expression profiling by array | 22 | MN (n = 18) | Homo sapiens | GPL14663 |
| HC (n = 4) | ||||||
| GSE99325 | Expression profiling by array | 22 | MN (n = 18) | Homo sapiens | GPL19184 | |
| HC (n = 4) | ||||||
| GSE104954 | Expression profiling by array | 21 | MN (n = 18) | Homo sapiens | GPL24120 | |
| HC (n = 3) | ||||||
| GSE108112 | Expression profiling by array | 48 | MN (n = 43) | Homo sapiens | GPL19983 | |
| HC (n = 5) | ||||||
| GSE133288 | Expression profiling by array | 53 | MN (n = 48) | Homo sapiens | GPL19983 | |
| HC (n = 5) | ||||||
| PBMC | GSE73953 | Expression profiling by array | 10 | MN (n = 8) | Homo sapiens | GPL4133 |
| HC (n = 2) |
HC, Healthy Control.
Data integration and differentially expressed genes identified
Since healthy controls are fewer in these genes series, we use Networkanalyst (http://www.networkanalyst.ca) to integrate glomerular and renal tubular series, and identify differentially expressed genes (DEGs). Through “Multiple Gene Expression Table”, the data were integrated and adjusted study batch effect was used to obtain the gene expression data of the combined glomerular and renal tubule samples. The principal component analysis (PCA) plot and density plot were plotted respectively (Supplementary Figure 1) [21]. “Fisher Method” is used to analyze the integrated data to generate a “CombinedTstat” representing difference and a “CombinedPval” representing combined P value. We compared data from MN patients with healthy controls, and perceived “CombinedPval” < 0.05 and a |CombinedTstat| > 50 to be statistically significant for the DEGs. For samples of PBMC series, we used GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r) for screening DEGs. The values for statistical significance were set as adjusted p value < 0.05 and |Fold change| > 2.5.
Functional annotation and pathway enrichment analysis
To functionally annotate the DEGs identified above, we annotated and visualized the enriched terms using Metascape (http://metascape.org/gp/index.html#/main/step1). For gene ontology (GO) terms and Kyoto encyclopedia of genes and genomes (KEGG) pathway analysis, we also applied web-based gene set analysis toolkit (WebGestalt, http://webgestalt.org/) to process these DEGs.
Protein-protein interaction (PPI) networks and screening of hub genes
We drew PPI network by Metascape, and screened hub genes based on this. Using Venn diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/), we screened the common hub genes in glomerulus, renal tubules and PBMCs data sets.
Receiver operating characteristic (ROC) analysis
We used SPSS20.0 to draw ROC for the above gene. We set MN as 1. Key biomarkers of MN were identified by observing area under curve (AUC). If AUC > 0.7 or < 0.3, it indicates that the abnormal expression of this gene has a certain suggestive effect on MN. If AUC > 0.9 or < 0.1, it suggests that this gene is a key biomarker of MN [22].
Enrichment analysis by gene set enrichment analysis (GSEA)
Gene function was analyzed using GSEA 4.1.0 software from MSIGDB database on GSEA website (http://software.broadinstitute.org/gsea/msigdb). The default weighted enrichment method was used for enrichment analysis. Set 1000 times randomly. GSEA analysis was used to enrich the GO and KEGG pathways with high and low expression of key biomarkers. To avoid false positive results by duplicate or nearly duplicate gene sets, the false discovery rate (FDR) should be less than 0.25. The Nominal (NOM) p-value used to evaluate the statistical significance of the genome enrichment score should be less than 0.05. Normalized enrichment score (NES) refers to the enrichment score of the gene set after normalization among the analyzed gene sets. Its absolute value should be greater than 1.
Result
Bioinformatic analysis of data description
Our flow chart is shown in Figure 1. Belonging to glomerular series, 21 MN samples and 3 healthy control samples were selected from GSE104948, and 44 MN samples and 6 healthy control samples were selected from GSE108109. Belonging to renal tubular series, 18 MN samples and 4 healthy control samples were selected from GSE47184, 18 MN samples and 4 healthy control samples from GSE99325, 18 MN samples and 3 healthy control samples from GSE104954, 43 MN samples and 5 healthy control samples were selected from GSE108112, and 48 MN samples and 5 healthy control samples were selected from GSE133288. 8 MN samples and 2 healthy control samples were selected from GSE73953 belonging to PBMC series. After data integration and analysis, 94 up-regulated DEGs and 9 down-regulated DEGs were screened out from the glomerulus. We screened 312 up-regulated DEGs and 223 down-regulated DEGs from renal tubules. We screened 4 up-regulated DEGs and 389 down-regulated DEGs from PBMC. Specific DEGs were shown in Supplementary Table 1. Interestingly, podocyte target antigens, such as PLA2R1, THSD7A, NELL1, etc., are not DEGs.
Figure 1.
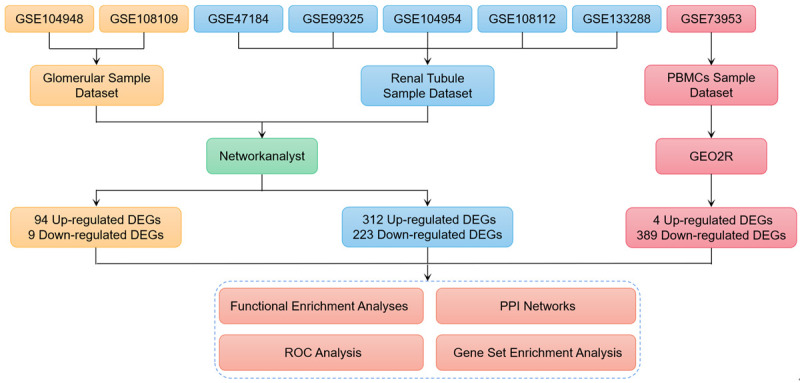
Roadmap of the approach and summarized findings.
Functional enrichment analyses
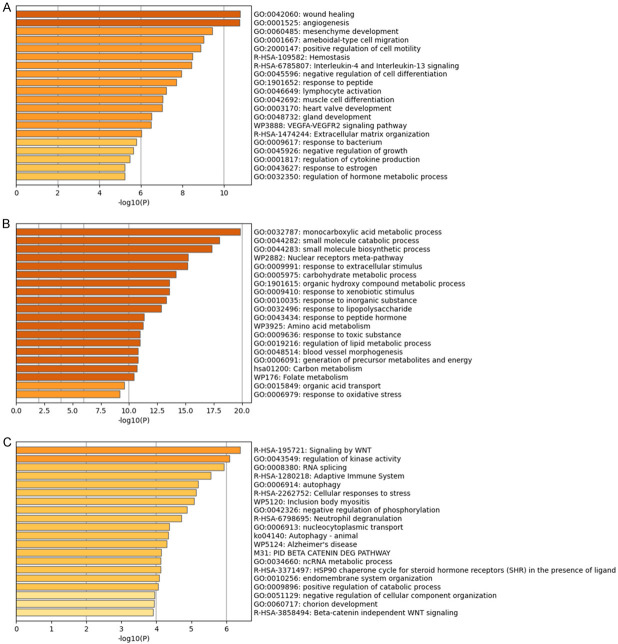
Based on Metascape functional enrichment analysis, it can be seen that the glomerular functions of MN patients are different from those of healthy controls in wound healing, angiogenesis, mesenchyme development, ameboidal-type cell migration, positive regulation of cell motility, hemostasis, interleukin-4 and interleukin-13 signaling, negative regulation of cell differentiation, response to peptide, lymphocyte activation, etc. (Figure 2A). The renal tubules of MN patients were different from those of healthy controls in terms of monocarboxylic acid metabolic process, small molecule catabolic process, small molecule biosynthetic process, nuclear receptors meta-pathway, response to extracellular stimulus, carbohydrate metabolic process, organic hydroxy compound metabolic process, response to xenobiotic stimulus, response to inorganic substance, response to lipopolysaccharide, etc. (Figure 2B). The PBMCs of MN patients were different from those of healthy controls in terms of signaling by WNT, regulation of kinase activity, RNA splicing, adaptive immune system, autophagy, cellular responses to stress, Inclusion body myositis, negative regulation of phosphorylation, neutrophil degranulation, nucleocytoplasmic transport, etc. (Figure 2C). Specific GO terms and KEGG pathway enrichment results were found in Supplementary Table 2. Glomerulus, renal tubules and PBMCs of MN patients were different from that of healthy controls in blood vessel development, coagulation, cellular response to peptide, MAPK signaling pathway, lymphocyte activation, etc.
Figure 2.
Detailed information related to changes in the biological function of DEGs was provided in the dataset by enrichment analysis. Using Metascape, we performed functional enrichment analysis of glomerulus (A), renal tubules (B) and PBMCs (C).
PPI networks and hub gene screening
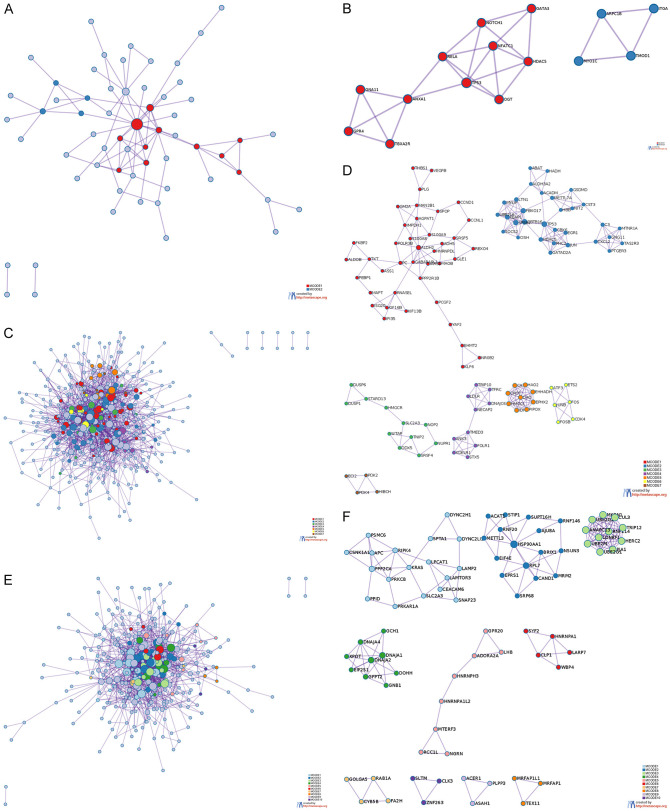
We screened hub genes through PPI network. In the glomerulus, the 2 clusters in the PPI network diagram contain 15 genes (Figure 3A, 3B). The meanings of HDAC5, TP53, ANXA1, GATA3, RELA, TBXA2R, NOTCH1, GNA11, OGT, NFATC1 and GPR4 were response to peptide, regulation of endothelial cell migration, regulation of cell migration involved in sprouting angiogenesis, etc. The meanings of MYO1C, TMOD1, ARPC1B and ITGA5 were regulation of actin filament polymerization, regulation of actin polymerization or depolymerization, regulation of actin filament length, etc.
Figure 3.
PPI network diagram of DEGs in glomerular and tubular. Metascape was used to construct the spatial distribution characteristics of the macroscopic PPI network model of glomerular DEGs (A), renal tubule DEGs (C) and PBMCs DEGs (E). The clusters of glomerular DEGs (B), renal tubule DEGs (D) and PBMCs DEGs (F) were selected.
In renal tubules, the 7 clusters in the PPI network diagram contain 111 genes (Figure 3C, 3D). The meanings of HAO2, CAT, EPHX2, CROT, EHHADH, DAO, HMGCL, PIPOX, IDH1 were peroxisomal protein import, peroxisome, etc. The meanings of a total of 31 genes, including ALDH3A2, MYLIP, NIT2, UBE2L6, CST3, JUN, ABAT, METTL7A, MTNR1A, C3, etc., were regulation of PTEN gene transcription, PTEN regulation, PIP3 activates AKT signaling, etc. The meanings of MYLIP, TRIP10, KDELR1, NECAP2, STX5, DNAJC6, TMED3, LDLR, TFRC, ANK3 and FOLR1 were membrane trafficking, vesicle-mediated transport, COPI-mediated anterograde transport, etc. The meanings of a total of 40 genes, including FOLR1, THBS1, S100A8, IFI35, PCGF2, IARS1, KIF16B, S100A9, SRSF5, ALDH2, FKBP2, etc., were tyrosine metabolism, response to ethanol, protein nitrosylation, etc. The meanings of JUNB, FOS, ETS2, ATF3, CDK4 and FOSB were PID ATF2 pathway, PID AP1 pathway, TGF-beta signaling pathway, etc. The meanings of STARD13, SRSF4, HMGCR, DDX5, NOP2, DUSP1, TNIP2, DUSP6, SLC2A3, WTAP, NUPR1 were regulation of mRNA splicing via spliceosome, negative regulation of phosphorylation, negative regulation of phosphate metabolic process, etc. The meanings of PDK4, HIBCH, ECI2 and PDK2 were atty acid metabolic process and monocarboxylic acid metabolic process.
In PBMCs, the 10 clusters in the PPI network diagram contain 80 genes (Figure 3E, 3F). The meanings of UBE2G1, RNF114, HERC2, UBE2M, LONRF1, CUL3, TRIP12, ANAPC13, PJA1, UBE2Q2 and MKRN1 were antigen processing: ubiquitination and proteasome degradation, class I MHC mediated antigen processing and presentation, adaptive immune system, etc. The meanings of EIF2S1, GFPT2, GNB1, DOHH, DNAJA2, DNAJA1, XPOT, GCH1 and DNAJA4 were response to heat, response to temperature stimulus, HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand, etc. The meanings of a total of 16 genes, including CAND1, RNF146, RNF20, HSP90AA1, BRIX1, SRP68, RPL7, AJUBA, NSUN3, SUPT16H, etc., were ncRNA metabolic process, ncRNA processing, VEGFA-VEGFR2 signaling pathway, etc. The meanings of a total of 18 genes, including PRKAR1A, LAMTOR3, SNAP23, PRKCB, APC, SPTA1, KRAS, LPCAT1, PPP2CA, DYNC2LI1, etc., were signaling by WNT, AXIN missense mutants destabilize the destruction complex, signaling by AXIN mutants, etc. The meanings of CLP1, WBP4, HNRNPA1, SYF2, and LARP7 were RNA splicing, mRNA processing, mRNA splicing-major pathway, etc. The meaning of CYB5B, GOLGA5, FA2H, and RAB1A was endomembrane system organization. The meanings of ADORA2A, MTERF3, HNRNPA1L2, HNRNPH3, RCC1L, GPR20, LHB, and NGRN were ADORA2B mediated anti-inflammatory cytokines production, G alpha (s) signaling events, leishmania parasite growth and survival, etc. The meanings of ASAH1, ACER1, and PLPP3 were sphingosine metabolic process, sphingoid metabolic process, diol metabolic process, etc. We found that it was difficult to obtain a unified meaning from the clusters of glomerulus, renal tubules and PBMCs. Therefore, we will re-conduct functional analysis on the intersection of these hub genes.
Diagnose significance of DEGs
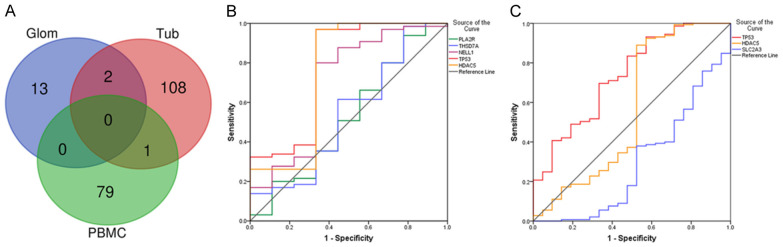
Through the Venn diagram (Figure 4A), we found that the hub genes shared by glomerulus and renal tubules were TP53 and HDAC5. The hub gene shared by PBMCs and renal tubules was SLC2A3. To determine the diagnostic significance of these genes for MN patients, ROC analysis was performed to explore the sensitivity and specificity of these genes for MN diagnosis. Three target antigens, PLA2R1, THSD7A and NELL1, were also included in the analysis. The results showed that TP53 (AUC = 0.776) and HDAC5 (AUC = 0.750) had certain suggestive effects on MN in glomerulus (Figure 4B). However, the expression levels of PLA2R1, THSD7A and NELL1, as podocyte target antigens, showed low accuracy in suggesting MN (0.5 < AUC < 0.7). In renal tubules, TP53 (AUC = 0.734) and SLC2A3 (AUC = 0.293) had certain suggestive effects on MN (Figure 4C). HDAC5 had low accuracy in suggestive effects on MN (AUC = 0.567). Since there were only 2 healthy samples of PBMCs, the results lacked accuracy. In conclusion, the target antigens might not have very good diagnostic significance at the gene expression level. Comparatively, TP53 may have a certain suggestive effect on MN renal pathological changes.
Figure 4.
Diagnostic properties of genes. We filtered these DEGs using Venn diagrams (A). ROC analysis was performed on the screened genes. The diagnostic performance of these genes in glomerulus (B) and renal tubules (C) was calculated according to the gene expression levels. AUC > 0.7 indicates that the gene is suggestive of MN.
TP53, HDAC5 or SLC2A3 associated gene set enrichment analysis (GSEA)
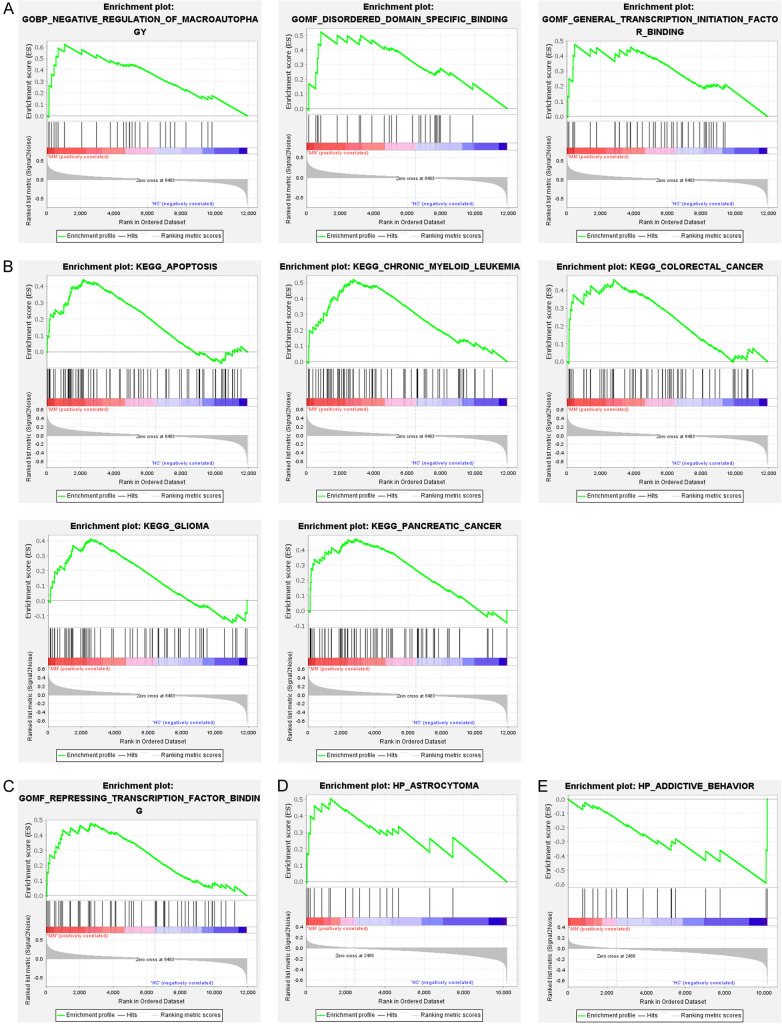
Since there were only 2 samples in the HC group of PBMC, we only performed GSEA analysis for the above TP53, HDAC5 or SLC2A3 related functions in the series of glomerulus and renal tubules. In the glomerulus, we found that the GO terms associated with TP53, negative regulation of macro-autophagy, disordered domain specific binding, and general transcription initiation factor binding were up-regulated (Figure 5A). TP53-related KEGG pathways, including apoptosis, chronic myeloid leukemia, glioma, and pancreatic cancer, were up-regulated (Figure 5B). In the GO terms related to HDAC5, the function of repressing transcription factor binding was up-regulated (Figure 5C). In renal tubules, astrocytoma function was up-regulated in the GO terms related to TP53 (Figure 5D). In the GO terms related to SLC2A3, the function of addictive behavior was reduced (Figure 5E). Furthermore, it was interesting to note that glomerular and tubules did not share the same up-regulation function or pathway in the enrichment analysis. In conclusion, the related functions of TP53 are closely related to kidney injury.
Figure 5.
Gene set enrichment analysis (GSEA) was used to analyze functional enrichment in MN. In glomerulus, GSEA was used to validate the gene signatures of TP53 in GO term (A) and KEGG pathway (B), and the gene signatures of HDAC5 in GO term (C). In renal tubules, GSEA was used to validate the gene signatures of TP53 in GO term (D), and the gene signatures of SLC2A3 in GO term (E). Normalized enrichment score (NES) indicated the analysis results across gene sets.
Discussion
We made three significant observations. First of all, there are certain differences in gene expression in different parts of MN patients, so in the detection of protein expression, the glomerulus and renal tubules should be separated as far as possible, so as not to cause errors in the results. Second, TP53, HDAC5 and SLC2A3 may play a crucial role in the pathogenesis of MN. Third, the exposure of MN autoantigen is not necessarily the cause of the disease, but a staged result in the progression of the disease.
Glomerulus and tubules are difficult to unify the same mechanism because of the different causes of pathological injury. MN is also called membranous glomerulonephritis [1,23,24]. The glomerulus of the lesion is diffuse. The basement membrane is thickened because the antigen-antibody complex is mainly deposited on the capillary wall of the glomerular [12,25]. The glomerular epithelial cells present diffuse fusion of foot processes [26,27]. The mesangial cells in the glomerulus were only slightly proliferated in the early stage, but the mesangial matrix gradually increased in the later stage, leading to glomerulosclerosis [6,28]. In the renal tubules, the pathological changes are mostly vacuolar or granular degeneration caused by proteinuria [29-31]. Endoplasmic reticulum dilated, and phagocytic vesicles and lysosomes increased in renal tubular epithelial cells [31,32]. There were no obvious changes in renal interstitium and arterioles. Thus, the pathological changes of glomerulus and renal tubules in MN are not identical, which makes it difficult to find the similarities between the two in terms of functional enrichment. This also explains why the three core genes we identified are not of great diagnostic value. Of course, our conclusions are not without suggestive implications. At the level of molecular mechanism, VEGF is closely related to kidney injury, and the correlation between MN and VEGF-related pathways has gradually come into view [33-35]. Therefore, attention should be paid to the role of VEGF-related pathways in mN-associated podocytes. In addition, MN podocyte showed upregulation of Wnt signaling pathway, which resulted in a series of aggravation of injury [36], and our results also showed that PBMCs of MN patients had abnormal Wnt signaling pathway. The Wnt pathway itself also has many connections with autoimmunity, and has a regulatory effect on a variety of dendritic cells, B cells, T cells and other immune cells [37-39]. Therefore, whether the control of Wnt pathway can play a role in the prevention and treatment of MN is one of the directions of our next stage of research. Of course, the hub genes screened by us also have guiding significance for the pathogenesis and treatment of MN. This is because TP53, HDAC5 and SLC2A3 are all linked tenderly to autoimmune or kidney disease.
TP53, or tumor protein p53, plays an important role in cellular responses to DNA damage and other genomic aberrations. Activation of TP53 leads to cell cycle arrest, DNA repair or apoptosis. The expression level of TP53 will be up-regulated when the cells are exposed to genotoxic stress, hypoxia, carcinogenic stress and oxidative stress [40]. Meanwhile, TP53 can inhibit mTOR to enhance autophagy. TP53 is also associated with aging and cell senescence [41]. And these abnormalities are also present in MN kidney intrinsic cells [18,27,31]. In addition, TP53 is associated with the susceptibility to diffuse cutaneous systemic sclerosis, lichen sclerosis, in autoimmune disease [42,43]. In renal disease, TP53 is also a major mediator of renal tubular cell injury and death [44].
HDAC5, also known as histone deacetylase 5, changes the structure of chromosomes and affects the access of transcription factors to DNA by histone deacetylation. In diabetes, HDAC5 leads to epithelial-mesenchymal transformation in renal tubular epithelial cells [45]. Blocking HDAC5 inhibits the development and progression of renal fibrosis in a mouse model of unilateral ureteral obstruction (UUO) [46]. HDAC5 also plays an important role in polycystic kidney disease [47]. Therefore, HDAC5 is also a key biomarker of renal tubular injury. In addition, on the immune side, the loss of HDAC5 impedes the differentiation of regulatory T cells (Tregs) [48,49]. And there is a study that shows that the mRNA expression levels of Foxp3 and TGF-β in the kidneys of MN patients are compared to that of anti-neutrophil cytoplasmic antigen-associated pauci-immune crescentic glomerulonephritis and membranoproliferative glomerulonephritis were up-regulated [50]. Foxp3 and TGF-β are the markers that reflect the number and function of Treg [51]. Therefore, the upregulation of HDAC5 may be the key reason that differentiates MN kidney pathology from other autoimmune glomerular diseases.
SLC2A3, solute carrier family 2 member 3, also known as glucose transporter 3 (GLUT3), promotes glucose across mammalian cell membranes. In addition to being expressed in neurons, this protein is also found in sperm, embryos, leukocytes, carcinoma cells and so on [52-55]. In kidney, high glucose induces upregulation of SLC2A3 expression in human glomerular epithelial cells [56]. In immune cells, downregulation of SLC2A3 affects α-granule release and post-activation functions in platelets [57]. At the same time, the down-regulation of this protein may be influenced by hypoxia inducible factor (HIF) [58]. Interestingly, SLC2A3 can induce the kupffer cell, or hepatic macrophage, to secrete cytokines such as IL-10 and TGF-β [59]. The number of M2 monocytes also happened to increase in PBMCs of MN patients [60]. The M2 monocyte is a monocyte that secretes IL-10, and the number of the monocytes is positively correlated with the 24-hour urinary protein of MN and the serum anti-PLA2R1 antibody concentration. This may indicate that SLC2A3 plays an important role in immune abnormalities of MN.
Moreover, the diagnostic value of podocyte target antigen for MN is limited. The mechanism we have discovered may shed light on the underlying causes of antigen exposure. We found that there was no statistically significant difference in the expression levels of these autoantigens in MN patients compared with healthy controls. On the contrary, the above three factors that seem to be unrelated to MN have more significant differences. PLA2R1 is the most common autoantigen in MN patients, with a detection rate of about 70-80% [61]. Although no article has directly proved the relationship between TP53 and MN, activation of p53 pathway can increase PLA2R expression [62]. It has been documented that PLA2R1 inhibits inflammatory response by binding to PLA2 protein, and the increased expression of PLA2R1 is associated with inflammatory stimulation and TP53-related aging pathways [17,62,63]. These also prove that MN is more common in the elderly [1]. The incidence of the disease is associated with environmental pollution or the patient’s history of pneumonia in adolescence [4,64]. However, it has been reported that about 7% of patients are positive for anti-PLA2R1 antibodies in serum but negative for PLA2R1 in renal tissue [65]. There are also many cases of MN relapse after transplantation [66]. Cuarental et al. showed increased PLA2R1 expression in renal podocytes by injecting tweaking to 14-week-old C57/BL mice, but proteinuria was not reported in this study [67]. Moreover, PLA2R1 is not a protein specifically expressed in the kidney. In addition to glomerular epithelial cells, PLA2R1 is also expressed in alveolar macrophages, neutrophils, placenta, liver and skeletal muscle [17,61,68]. This may indicate that exposure to PLA2R1 as an antigen in kidney is not the root cause of MN morbidity. Antigen exposure is only a consequence of abnormal bodily function in the patient. Even if the autoimmune response is controlled with immunosuppressants, if the cause of antigen exposure is not ameliorated, the disease will still recur when the drug is discontinued [69-71]. In addition, THSD7A was originally described as an endothelial protein expressed in the placental vascular system that promotes endothelial cell migration during angiogenesis [72]. NELL1 is highly expressed in osteoblasts and promotes bone regeneration, as well as facilitating bone formation through the regenerative ability of stem cells [73,74]. SEMA3B and PCDH7 are newly discovered antigens in recent years, but our understanding of them is not perfect [10]. And, SEMA3B accounts for only 1% of MN patients [75]. Therefore, we do not discuss these two autoantigens here. Meanwhile, this is also the reason why SEMA3B and PCDH7 were not selected for analysis. Taken together, these autoantigens are functionally different. There may be many autoantigens with different functions in the future. So, the exact mechanism of this phenomenon is unclear. Clinically, we did find increased expression of PLA2R and THSD7A on glomerular epithelial cells by immunohistochemical staining. This may indicate that the glomerular epithelial cells of MN patients have insufficient clearance capacity for PLA2R or THSD7A. However, the more different their functions are, the more it indicates that the root cause of MN is not these autoantigens. So, the genes for these podocyte target antigens are not enough by themselves to explain why some people have MN. Perhaps the disease itself is caused by a combination of toxic, inflammatory or infectious factors. In addition, these common podocyte target antigens are not expressed only in the kidney [61], but evidence of extrarenal disease is lacking. This oligo-inflammatory autoimmune glomerular disease may be the result of a combination of factors that lead to podocyte target antigen exposure and immune dysfunction in patients (Figure 6).
Figure 6.
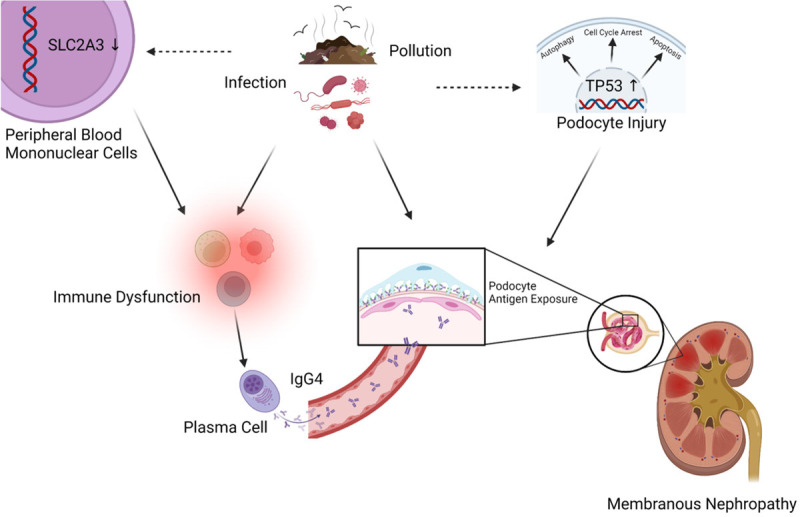
Schematic diagram of MN mechanism hypothesis. Environmental factors (toxicity, inflammation, infection, etc.) lead to up-regulation of renal TP53 and down-regulation of SLC2A3 in PBMCs. Changes in the kidney expose podocyte target antigens. Changes in peripheral immune cells allow the persistence of a humoral immune response with IgG4 as the main antibody. Created with BioRender.com. Agreement number: SO245P2AW5.
Of course, there are some limitations to our study. First, the study is analyzed based on GEO database. We can only observe differences in gene expression, which can not accurately reflect changes in protein structure. This makes our conclusions not entirely accurate. Therefore, further basic experiments are needed to confirm our results. Second, we can’t categorize the data. The severity of MN affects the gene expression in glomerulus and renal tubules. This is the problem that online database research cannot solve. Third, there was only one series related to PBMCs, and there were only two healthy controls. This results in a lack of accuracy in our analysis of the patient’s autoimmune environment. Therefore, PBMC of MN patients will be proactively collected for bioinformatics study.
Conclusions
As an autoimmune glomerular disease, the pathogenesis of MN remains to be further studied, especially the cause of antigen exposure, as well as strange pathological and immune characteristics. After completing the bioassay study of patients, we will conduct studies on these core genes to observe their effects on antigen exposure, Treg cells, M2 monocytes, and other immune cells.
Acknowledgements
We would like to thank the academic editor and reviewers for their important contributions that improved the quality of this article. We would like to acknowledge funding from the National Key Research and Development Program of China (grant no. 2019YFC1709402 to B.L.), and Horizontal Subject (HX-DZM-202017 and HX-DZM-202018 to WJ. L.).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12:983–997. doi: 10.2215/CJN.11761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keri KC, Blumenthal S, Kulkarni V, Beck L, Chongkrairatanakul T. Primary membranous nephropathy: comprehensive review and historical perspective. Postgrad Med J. 2019;95:23–31. doi: 10.1136/postgradmedj-2018-135729. [DOI] [PubMed] [Google Scholar]
- 3.Floege J, Barbour SJ, Cattran DC, Hogan JJ, Nachman PH, Tang SCW, Wetzels JFM, Cheung M, Wheeler DC, Winkelmayer WC, Rovin BH Conference Participants. Management and treatment of glomerular diseases (part 1): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;95:268–280. doi: 10.1016/j.kint.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Wang G, Chen N, Lu T, Nie S, Xu G, Zhang P, Luo Y, Wang Y, Wang X, Schwartz J, Geng J, Hou FF. Long-term exposure to air pollution and increased risk of membranous nephropathy in China. J Am Soc Nephrol. 2016;27:3739–3746. doi: 10.1681/ASN.2016010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bomback AS, Fervenza FC. Membranous nephropathy: approaches to treatment. Am J Nephrol. 2018;47(Suppl 1):30–42. doi: 10.1159/000481635. [DOI] [PubMed] [Google Scholar]
- 6.Lu W, Gong S, Li J, Wang Y. Clinicopathological features and prognosis in patients with idiopathic membranous nephropathy with hypertension. Exp Ther Med. 2020;19:2615–2621. doi: 10.3892/etm.2020.8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong Z, Liu Z, Dai H, Liu W, Feng Z, Zhao Q, Gao Y, Liu F, Zhang N, Dong X, Zhou X, Du J, Huang G, Tian X, Liu B. The potential role of regulatory B cells in idiopathic membranous nephropathy. J Immunol Res. 2020;2020:7638365. doi: 10.1155/2020/7638365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantarelli C, Jarque M, Angeletti A, Manrique J, Hartzell S, O’Donnell T, Merritt E, Laserson U, Perin L, Donadei C, Anderson L, Fischman C, Chan E, Draibe J, Fulladosa X, Torras J, Riella LV, La Manna G, Fiaccadori E, Maggiore U, Bestard O, Cravedi P. A comprehensive phenotypic and functional immune analysis unravels circulating anti-phospholipase A2 receptor antibody secreting cells in membranous nephropathy patients. Kidney Int Rep. 2020;5:1764–1776. doi: 10.1016/j.ekir.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sethi S. New ‘antigens’ in membranous nephropathy. J Am Soc Nephrol. 2021;32:268–278. doi: 10.1681/ASN.2020071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lilienthal GM, Rahmoller J, Petry J, Bartsch YC, Leliavski A, Ehlers M. Potential of murine IgG1 and human IgG4 to inhibit the classical complement and Fcgamma receptor activation pathways. Front Immunol. 2018;9:958. doi: 10.3389/fimmu.2018.00958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ronco P, Debiec H. Molecular pathogenesis of membranous nephropathy. Annu Rev Pathol. 2020;15:287–313. doi: 10.1146/annurev-pathol-020117-043811. [DOI] [PubMed] [Google Scholar]
- 13.Brglez V, Boyer-Suavet S, Seitz-Polski B. Complement pathways in membranous nephropathy: complex and multifactorial. Kidney Int Rep. 2020;5:572–574. doi: 10.1016/j.ekir.2020.02.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma H, Sandor DG, Beck LH Jr. The role of complement in membranous nephropathy. Semin Nephrol. 2013;33:531–542. doi: 10.1016/j.semnephrol.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haddad G, Lorenzen JM, Ma H, de Haan N, Seeger H, Zaghrini C, Brandt S, Kolling M, Wegmann U, Kiss B, Pal G, Gal P, Wuthrich RP, Wuhrer M, Beck LH, Salant DJ, Lambeau G, Kistler AD. Altered glycosylation of IgG4 promotes lectin complement pathway activation in anti-PLA2R1-associated membranous nephropathy. J Clin Invest. 2021;131:e140453. doi: 10.1172/JCI140453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Wang C, Jin L, He F, Li C, Gao Q, Chen G, He Z, Song M, Zhou Z, Shan F, Qi K, Ma L. IgG4 anti-phospholipase A2 receptor might activate lectin and alternative complement pathway meanwhile in idiopathic membranous nephropathy: an inspiration from a cross-sectional study. Immunol Res. 2016;64:919–930. doi: 10.1007/s12026-016-8790-1. [DOI] [PubMed] [Google Scholar]
- 17.Liu W, Gao C, Dai H, Zheng Y, Dong Z, Gao Y, Liu F, Zhang Z, Liu Z, Liu W, Liu B, Liu Q, Shi J. Immunological pathogenesis of membranous nephropathy: focus on PLA2R1 and its role. Front Immunol. 2019;10:1809. doi: 10.3389/fimmu.2019.01809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Logt AE, Fresquet M, Wetzels JF, Brenchley P. The anti-PLA2R antibody in membranous nephropathy: what we know and what remains a decade after its discovery. Kidney Int. 2019;96:1292–1302. doi: 10.1016/j.kint.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Zhang P, Huang W, Zheng Q, Tang J, Dong Z, Jiang Y, Liu Y, Liu W. A novel insight into the role of PLA2R and THSD7A in membranous nephropathy. J Immunol Res. 2021;2021:8163298. doi: 10.1155/2021/8163298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao X, Zhang L, Wang J, Zhang M, Song Z, Ni B, You Y. Identification of key biomarkers and immune infiltration in systemic lupus erythematosus by integrated bioinformatics analysis. J Transl Med. 2021;19:35. doi: 10.1186/s12967-020-02698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou G, Soufan O, Ewald J, Hancock REW, Basu N, Xia J. NetworkAnalyst 3.0: a visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019;47:W234–W241. doi: 10.1093/nar/gkz240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzucchi S, Frosini D, Costagli M, Del Prete E, Donatelli G, Cecchi P, Migaleddu G, Bonuccelli U, Ceravolo R, Cosottini M. Quantitative susceptibility mapping in atypical Parkinsonisms. Neuroimage Clin. 2019;24:101999. doi: 10.1016/j.nicl.2019.101999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lotan D, Kaplan BS, Fong JS, Goodyer PR, de Chadarevian JP. Reduction of protein excretion by dimethyl sulfoxide in rats with passive heymann nephritis. Kidney Int. 1984;25:778–788. doi: 10.1038/ki.1984.90. [DOI] [PubMed] [Google Scholar]
- 24.Gartner HV, Watanabe T, Ott V, Adam A, Bohle A, Edel HH, Kluthe R, Renner E, Scheler F, Schmulling RM, Sieberth HG. Correlations between morphologic and clinical features in idiopathic perimembranous glomerulonephritis. A study on 403 renal biopsies of 367 patients. Curr Top Pathol. 1977;65:1–29. doi: 10.1007/978-3-642-66703-9_1. [DOI] [PubMed] [Google Scholar]
- 25.Lai WL, Yeh TH, Chen PM, Chan CK, Chiang WC, Chen YM, Wu KD, Tsai TJ. Membranous nephropathy: a review on the pathogenesis, diagnosis, and treatment. J Formos Med Assoc. 2015;114:102–111. doi: 10.1016/j.jfma.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Yang LY, Wu YS, Dai BB, Lin SH, Chen H, Li GP, Tao X, Wan JX, Pan YB. sPLA2-IB level correlates with hyperlipidemia and the prognosis of idiopathic membranous nephropathy. Curr Med Sci. 2020;40:683–690. doi: 10.1007/s11596-020-2246-5. [DOI] [PubMed] [Google Scholar]
- 27.Motavalli R, Etemadi J, Kahroba H, Mehdizadeh A, Yousefi M. Immune system-mediated cellular and molecular mechanisms in idiopathic membranous nephropathy pathogenesis and possible therapeutic targets. Life Sci. 2019;238:116923. doi: 10.1016/j.lfs.2019.116923. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Chen B, Gao C, Huang J, Wang Y, Zhang S, Xu Y, Guo W, Wang R. Clinical and pathological features of idiopathic membranous nephropathy with focal segmental sclerosis. BMC Nephrol. 2019;20:467. doi: 10.1186/s12882-019-1641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Tu Q, Jin J, Hu X, Ren Y, Zhao L, He Q. Curcumin improves the renal autophagy in rat experimental membranous nephropathy via regulating the PI3K/AKT/mTOR and Nrf2/HO-1 signaling pathways. Biomed Res Int. 2020;2020:7069052. doi: 10.1155/2020/7069052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakatsue T, Koike H, Han GD, Suzuki K, Miyauchi N, Yuan H, Salant DJ, Gejyo F, Shimizu F, Kawachi H. Nephrin and podocin dissociate at the onset of proteinuria in experimental membranous nephropathy. Kidney Int. 2005;67:2239–2253. doi: 10.1111/j.1523-1755.2005.00328.x. [DOI] [PubMed] [Google Scholar]
- 31.Bhatia D, Choi ME. Autophagy in kidney disease: advances and therapeutic potential. Prog Mol Biol Transl Sci. 2020;172:107–133. doi: 10.1016/bs.pmbts.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Cybulsky AV. Endoplasmic reticulum stress in proteinuric kidney disease. Kidney Int. 2010;77:187–193. doi: 10.1038/ki.2009.389. [DOI] [PubMed] [Google Scholar]
- 33.Hou Y, Li Y, Wang Y, Li W, Xiao Z. Screening and analysis of key genes in miRNA-mRNA regulatory network of membranous nephropathy. J Healthc Eng. 2021;2021:5331948. doi: 10.1155/2021/5331948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donderski R, Szczepanek J, Domagalski K, Tretyn A, Korenkiewicz J, Marszalek A, Szymanski A, Wolski Z, Odrowaz-Sypniewska G, Manitius J. Analysis of relative expression level of VEGF (vascular endothelial growth factor), HIF-1alpha (hypoxia inducible factor 1alpha) and CTGF (connective tissue growth factor) genes in chronic glomerulonephritis (CGN) patients. Kidney Blood Press Res. 2013;38:83–91. doi: 10.1159/000355754. [DOI] [PubMed] [Google Scholar]
- 35.Sivridis E, Giatromanolaki A, Touloupidis S, Pasadakis P, Vargemezis V. Platelet endothelial cell adhesion molecule-1 and angiogenic factor expression in idiopathic membranous nephropathy. Am J Kidney Dis. 2003;41:360–365. doi: 10.1053/ajkd.2003.50044. [DOI] [PubMed] [Google Scholar]
- 36.Dong Z, Dai H, Gao Y, Feng Z, Liu W, Liu F, Zhang Z, Ma F, Xie X, Zhu Z, Liu W, Liu B. Inhibition of the Wnt/beta-catenin signaling pathway reduces autophagy levels in complement treated podocytes. Exp Ther Med. 2021;22:737. doi: 10.3892/etm.2021.10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi J, Chi S, Xue J, Yang J, Li F, Liu X. Emerging role and therapeutic implication of wnt signaling pathways in autoimmune diseases. J Immunol Res. 2016;2016:9392132. doi: 10.1155/2016/9392132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cici D, Corrado A, Rotondo C, Cantatore FP. Wnt signaling and biological therapy in rheumatoid arthritis and spondyloarthritis. Int J Mol Sci. 2019;20:5552. doi: 10.3390/ijms20225552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osugui L, de Roo JJ, de Oliveira VC, Sodre ACP, Staal FJT, Popi AF. B-1 cells and B-1 cell precursors prompt different responses to Wnt signaling. PLoS One. 2018;13:e0199332. doi: 10.1371/journal.pone.0199332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pietrocola F, Izzo V, Niso-Santano M, Vacchelli E, Galluzzi L, Maiuri MC, Kroemer G. Regulation of autophagy by stress-responsive transcription factors. Semin Cancer Biol. 2013;23:310–322. doi: 10.1016/j.semcancer.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Levine AJ. P53 and the immune response: 40 years of exploration-a plan for the future. Int J Mol Sci. 2020;21:541. doi: 10.3390/ijms21020541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zmorzynski S, Wojcierowska-Litwin M, Kowal M, Michalska-Jakubus M, Styk W, Filip AA, Walecka I, Krasowska D. NOTCH3 T6746C and TP53 P72R polymorphisms are associated with the susceptibility to diffuse cutaneous systemic sclerosis. Biomed Res Int. 2020;2020:8465971. doi: 10.1155/2020/8465971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran DA, Tan X, Macri CJ, Goldstein AT, Fu SW. Lichen sclerosus: an autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int J Biol Sci. 2019;15:1429–1439. doi: 10.7150/ijbs.34613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He L, Wei Q, Liu J, Yi M, Liu Y, Liu H, Sun L, Peng Y, Liu F, Venkatachalam MA, Dong Z. AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017;92:1071–1083. doi: 10.1016/j.kint.2017.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Z, Jia K, Wang H, Gao F, Zhao S, Li F, Hao J. METTL14-regulated PI3K/Akt signaling pathway via PTEN affects HDAC5-mediated epithelial-mesenchymal transition of renal tubular cells in diabetic kidney disease. Cell Death Dis. 2021;12:32. doi: 10.1038/s41419-020-03312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiong C, Guan Y, Zhou X, Liu L, Zhuang MA, Zhang W, Zhang Y, Masucci MV, Bayliss G, Zhao TC, Zhuang S. Selective inhibition of class IIa histone deacetylases alleviates renal fibrosis. FASEB J. 2019;33:8249–8262. doi: 10.1096/fj.201801067RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oprisan SA, Clementsmith X, Tompa T, Lavin A. Dopamine receptor antagonists effects on low-dimensional attractors of local field potentials in optogenetic mice. PLoS One. 2019;14:e0223469. doi: 10.1371/journal.pone.0223469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao H, Jiao J, Wang L, O’Brien S, Newick K, Wang LC, Falkensammer E, Liu Y, Han R, Kapoor V, Hansen FK, Kurz T, Hancock WW, Beier UH. HDAC5 controls the functions of Foxp3(+) T-regulatory and CD8(+) T cells. Int J Cancer. 2016;138:2477–2486. doi: 10.1002/ijc.29979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia Q, Zhang J, Han Y, Zhang X, Jiang H, Lun Y, Wu X, Gang Q, Liu Z, Bockler D, Duan Z, Xin S. Epigenetic regulation of regulatory T cells in patients with abdominal aortic aneurysm. FEBS Open Bio. 2019;9:1137–1143. doi: 10.1002/2211-5463.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ifuku M, Miyake K, Watanebe M, Ito K, Abe Y, Sasatomi Y, Ogahara S, Hisano S, Sato H, Saito T, Nakashima H. Various roles of Th cytokine mRNA expression in different forms of glomerulonephritis. Am J Nephrol. 2013;38:115–123. doi: 10.1159/000353102. [DOI] [PubMed] [Google Scholar]
- 51.Lee GR. The balance of Th17 versus Treg cells in autoimmunity. Int J Mol Sci. 2018;19:730. doi: 10.3390/ijms19030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hermo L, Oliveira RL, Smith CE, Au CE, Bergeron JJM. Dark side of the epididymis: tails of sperm maturation. Andrology. 2019;7:566–580. doi: 10.1111/andr.12641. [DOI] [PubMed] [Google Scholar]
- 53.Kramer AC, Steinhauser CB, Gao H, Seo H, McLendon BA, Burghardt RC, Wu G, Bazer FW, Johnson GA. Steroids regulate SLC2A1 and SLC2A3 to deliver glucose into trophectoderm for metabolism via glycolysis. Endocrinology. 2020;161:bqaa098. doi: 10.1210/endocr/bqaa098. [DOI] [PubMed] [Google Scholar]
- 54.Korgun ET, Demir R, Sedlmayr P, Desoye G, Arikan GM, Puerstner P, Haeusler M, Dohr G, Skofitsch G, Hahn T. Sustained hypoglycemia affects glucose transporter expression of human blood leukocytes. Blood Cells Mol Dis. 2002;28:152–159. doi: 10.1006/bcmd.2002.0504. [DOI] [PubMed] [Google Scholar]
- 55.Kim E, Jung S, Park WS, Lee JH, Shin R, Heo SC, Choe EK, Lee JH, Kim K, Chai YJ. Upregulation of SLC2A3 gene and prognosis in colorectal carcinoma: analysis of TCGA data. BMC Cancer. 2019;19:302. doi: 10.1186/s12885-019-5475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moutzouris DA, Kitsiou PV, Talamagas AA, Drossopoulou GI, Kassimatis TI, Katsilambros NK. Chronic exposure of human glomerular epithelial cells to high glucose concentration results in modulation of high-affinity glucose transporters expression. Ren Fail. 2007;29:353–358. doi: 10.1080/08860220601184126. [DOI] [PubMed] [Google Scholar]
- 57.Fidler TP, Middleton EA, Rowley JW, Boudreau LH, Campbell RA, Souvenir R, Funari T, Tessandier N, Boilard E, Weyrich AS, Abel ED. Glucose transporter 3 potentiates degranulation and is required for platelet activation. Arterioscler Thromb Vasc Biol. 2017;37:1628–1639. doi: 10.1161/ATVBAHA.117.309184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lauer V, Grampp S, Platt J, Lafleur V, Lombardi O, Choudhry H, Kranz F, Hartmann A, Wullich B, Yamamoto A, Coleman ML, Ratcliffe PJ, Mole DR, Schodel J. Hypoxia drives glucose transporter 3 expression through hypoxia-inducible transcription factor (HIF)-mediated induction of the long noncoding RNA NICI. J Biol Chem. 2020;295:4065–4078. doi: 10.1074/jbc.RA119.009827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tao L, Pang Y, Wang A, Li L, Shen Y, Xu X, Li J. Functional miR-142a-3p induces apoptosis and macrophage polarization by targeting tnfaip2 and glut3 in grass carp (ctenopharyngodon idella) Front Immunol. 2021;12:633324. doi: 10.3389/fimmu.2021.633324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hou J, Zhang M, Ding Y, Wang X, Li T, Gao P, Jiang Y. Circulating CD14(+)CD163(+)CD206(+) M2 monocytes are increased in patients with early stage of idiopathic membranous nephropathy. Mediators Inflamm. 2018;2018:5270657. doi: 10.1155/2018/5270657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu W, Gao C, Liu Z, Dai H, Feng Z, Dong Z, Zheng Y, Gao Y, Tian X, Liu B. Idiopathic membranous nephropathy: glomerular pathological pattern caused by extrarenal immunity activity. Front Immunol. 2020;11:1846. doi: 10.3389/fimmu.2020.01846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Augert A, Payre C, de Launoit Y, Gil J, Lambeau G, Bernard D. The M-type receptor PLA2R regulates senescence through the p53 pathway. EMBO Rep. 2009;10:271–277. doi: 10.1038/embor.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Griveau A, Wiel C, Le Calve B, Ziegler DV, Djebali S, Warnier M, Martin N, Marvel J, Vindrieux D, Bergo MO, Bernard D. Targeting the phospholipase A2 receptor ameliorates premature aging phenotypes. Aging Cell. 2018;17:e12835. doi: 10.1111/acel.12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bally S, Debiec H, Ponard D, Dijoud F, Rendu J, Faure J, Ronco P, Dumestre-Perard C. Phospholipase A2 receptor-related membranous nephropathy and mannan-binding lectin deficiency. J Am Soc Nephrol. 2016;27:3539–3544. doi: 10.1681/ASN.2015101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med. 2011;364:689–690. doi: 10.1056/NEJMc1011678. [DOI] [PubMed] [Google Scholar]
- 66.Passerini P, Malvica S, Tripodi F, Cerutti R, Messa P. Membranous nephropathy (MN) recurrence after renal transplantation. Front Immunol. 2019;10:1326. doi: 10.3389/fimmu.2019.01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cuarental L, Valino-Rivas L, Mendonca L, Saleem M, Mezzano S, Sanz AB, Ortiz A, Sanchez-Nino MD. Tacrolimus prevents TWEAK-induced PLA2R expression in cultured human podocytes. J Clin Med. 2020;9:2178. doi: 10.3390/jcm9072178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ancian P, Lambeau G, Lazdunski M. Multifunctional activity of the extracellular domain of the M-type (180 kDa) membrane receptor for secretory phospholipases A2. Biochemistry. 1995;34:13146–13151. doi: 10.1021/bi00040a028. [DOI] [PubMed] [Google Scholar]
- 69.Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, Jefferson JA, Gipson PE, Rizk DV, Sedor JR, Simon JF, McCarthy ET, Brenchley P, Sethi S, Avila-Casado C, Beanlands H, Lieske JC, Philibert D, Li T, Thomas LF, Green DF, Juncos LA, Beara-Lasic L, Blumenthal SS, Sussman AN, Erickson SB, Hladunewich M, Canetta PA, Hebert LA, Leung N, Radhakrishnan J, Reich HN, Parikh SV, Gipson DS, Lee DK, da Costa BR, Juni P, Cattran DC MENTOR Investigators. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381:36–46. doi: 10.1056/NEJMoa1814427. [DOI] [PubMed] [Google Scholar]
- 70.Cremoni M, Brglez V, Perez S, Decoupigny F, Zorzi K, Andreani M, Gerard A, Boyer-Suavet S, Ruetsch C, Benzaken S, Esnault V, Seitz-Polski B. Th17-immune response in patients with membranous nephropathy is associated with thrombosis and relapses. Front Immunol. 2020;11:574997. doi: 10.3389/fimmu.2020.574997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dong Z, Dai H, Gao Y, Jiang H, Liu M, Liu F, Liu W, Feng Z, Zhang X, Ren A, Li X, Rui H, Tian X, Li G, Liu B. Effect of Mahuang Fuzi and Shenzhuo decoction on idiopathic membranous nephropathy: a multicenter, nonrandomized, single-arm clinical trial. Front Pharmacol. 2021;12:724744. doi: 10.3389/fphar.2021.724744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang CH, Su PT, Du XY, Kuo MW, Lin CY, Yang CC, Chan HS, Chang SJ, Kuo C, Seo K, Leung LL, Chuang YJ. Thrombospondin type I domain containing 7A (THSD7A) mediates endothelial cell migration and tube formation. J Cell Physiol. 2010;222:685–694. doi: 10.1002/jcp.21990. [DOI] [PubMed] [Google Scholar]
- 73.Hasebe A, Tashima H, Ide T, Iijima M, Yoshimoto N, Ting K, Kuroda S, Niimi T. Efficient production and characterization of recombinant human NELL1 protein in human embryonic kidney 293-F cells. Mol Biotechnol. 2012;51:58–66. doi: 10.1007/s12033-011-9440-4. [DOI] [PubMed] [Google Scholar]
- 74.Lee JH, Song YM, Min SK, Lee HJ, Lee HL, Kim MJ, Park YH, Park JU, Park JB. NELL-1 increased the osteogenic differentiation and mRNA expression of spheroids composed of stem cells. Medicina (Kaunas) 2021;57:586. doi: 10.3390/medicina57060586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sethi S, Debiec H, Madden B, Vivarelli M, Charlesworth MC, Ravindran A, Gross L, Ulinski T, Buob D, Tran CL, Emma F, Diomedi-Camassei F, Fervenza FC, Ronco P. Semaphorin 3B-associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int. 2020;98:1253–1264. doi: 10.1016/j.kint.2020.05.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.