Abstract
Objectives: We researched the effect and mechanism of acupuncture treatment for visceral pain in rats with diarrhea-predominant irritable bowel syndrome (IBS-D). Methods: We set up a rat model of IBS-D with chemical and chronic- and acute-pressure stimulations. Then, the IBS-D rats were treated with acupuncture or 5-BDBD, and the therapeutic efficacy of acupuncture in IBS-D rats was assessed by means of the Bristol scale, diarrhea index, abdominal withdrawal reflex (AWR) score, mast cell count and histologic staining. Results: Acupuncture significantly decreased clinical symptoms in IBS-D rats after a 14 day-treatment. Furthermore, significant down-regulation of P2X4, OX42, BDNF (brain-derived neurotrophic factor) and IRF-5 (interferon regulatory factor 5) expressions were observed in the IBS-D rats, along with the decreased inflammatory factors [interleukin 1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), and interleukin 6 (IL-6)], chemokines [monocyte chemoattractant protein-1 (MCP-1), regulated on activation, normal T cell expressed and secreted (RANTES), and C-X-C motif chemokine ligand 1 (CXCL1)], and neurotransmitters [substance P (SP), 5-hydroxytryptamine (5-HT), and calcitonin gene-related peptide (CGRP)]. 5-BDBD treatment had a similar effect on IBS-D rats. Conclusions: Acupuncture can effectively alleviate abdominal pain by decreasing visceral hypersensitivity and controlling the expression of P2X4 and spinal microglial inflammation in IBS rats.
Keywords: P2X4, acupuncture, visceral pain, IBS-D rat
Introduction
Irritable bowel syndrome (IBS) causes abdominal pain, which is related to stool irregularity and consistency changes [1,2]. Diarrhea predominant IBS (IBS-D) accounts for about 1/3 of affected IBS patients [3]. Antispasmodics for abdominal pain, antidiarrheal agents, psychotherapy, and nutritional interventions are used for treatment of IBS-D [2,4]. Pharmacologic treatments are primarily employed. However, these are ineffective in addressing the key reason for IBS. In addition, long-term drug treatment has side effects. Therefore, it is difficult to treat IBS-D because of an absence of efficient treatment.
IBS-D is a chronic condition with complicated pathophysiology. Recently, studies on the effects of visceral hypersensitivity on IBS-D pathogenesis have gained interest [5]. Visceral hypersensitivity means an enhanced sense of pain and discomfort in the gut resulting from physiologic stimulation [6].
Acupuncture is widely used for treating IBS [7]. Its impact on controlling IBS symptoms showed statistical and clinical significance [8]. Acupuncture lowers the incidence of diarrhea, abdominal distension, and pain and increases the quality of life of patients with IBS [9]. Acupuncture can help symptoms of IBS, but its influence on outcomes is unknown.
The brain-gut axis (BGA) affects the spinal cord, central nervous system (CNS) and outer periphery. The involvement of different neurologic neurotransmitters is also observed [10]. As the enteric nervous system is related to the CNS, the CNS is able to regulate gastrointestinal activity. Reciprocal associations are called brain-gut interactions [11,12]. Dorsal root ganglion (DRG) neurons link the external and internal environments. The spinal cord connects the transmission of peripheral sensitization and central sensitization. Adenosine triphosphate (ATP), a neurotransmitter, can regulate visceral pain signal transduction with P2X receptor binding [13]. Purinergic (P2X) receptors are disseminated in the body. They act in the regulation, formation and transduction of inflammatory pain, neuropathic pain and visceral pain [14-16]. P2X receptors participate in the pain signal transmission of IBS. The P2X3 receptor functions in IBS visceral pain are involved in IBS-induced visceral hyperalgesia development [17-20].
Microglia are resident macrophages of the CNS. After nerve damage, microglia instantly transform from a “resting” stage to an “activated” stage [21]. After the damage, P2X4 receptor (P2X4R) causes tactile allodynia. Knockout of P2X4R reduces the hypersensitivity to pain [21,22]. Activation of the P2X4Rs of spinal microglia helps induce neuropathic pain [23]. There are P2X4Rs in microglia [24]. The expression level of complement receptor type 3 (OX42) is detected to determine whether microglia are activated [25].
The P2X4R’s function in IBS-D visceral pain was examined in our study. Also, the impacts of acupuncture on the transmission of the DRG were examined in IBS-D rats. The results of this study improve our understanding of the scientific basis for the acupuncture treatment of IBS-D visceral pain from the P2X4 regulation perspective, thus providing evidence on the functional mechanism of acupuncture.
Materials and methods
Animals
We used seven-week-old rats (SPF Sprague-Dawley; 180-220 g of body weight) as research animals and fed the rats appropriately for one week. Then, we divided the rats into 4 groups, including control group: healthy rats (n = 16), IBS-D group: IBS-D model rats (n = 16), IBS-D + A group: IBS-D rats with acupuncture treatment (n = 16), and IBS-D + 5-BDBD group: IBS-D rats with a potent inhibitor of P2X4R (5-BDBD) treatment (n = 16). The rats were kept in cages (groups of 2 to 3 per cage) at 22 ± 1°C and a photoperiod of 12-h L/D cycle. Enough water and food were given. The research procedures were permitted by the Animal Ethics Committee [AHUCM-rats-2021124].
Construction of IBS-D model
The rectal dilation method was used to inject physiologic saline with a syringe. The insertion depth is 6-8 cm proximal to the anus. The rats in the model groups received 1 mL of acetic acid (4%) for 30 seconds at 8 cm proximal to the anus after intraperitoneal anesthesia with 0.04 ml/10 g pentobarbital (2%) [26,27]. One mL phosphate-buffered saline (PBS) was used for the dilution of acetic acid. After that, the colon was washed out. In the Control group, 1 mL physiologic saline was used. We immobilized forelimbs with medical tape after acetic acid enema every day so that the rats could not move freely and were restrained for 2 hours every day. The rats were housed for 2 weeks after stimulation. We performed behavior testing at 8.00 a.m. and 12.00 a.m. Also, we counted fecal pellet numbers over 1 h to examine the defecation and examined stool characteristics in accordance with the Bristol scale [28]. Abdominal withdrawal reflex (AWR) was recorded for the verification of the model induction access. We used the Bristol scale to categorize feces into 7 types: grade 1 = normal stool (Bristols 1-4); grade 2 = soft stool (Bristols 5-6); grade 3 = liquid stool; no shape (Bristol 7). Stools from each group were examined for the characteristics of diarrhea during the experiment. The diarrhea index was calculated as follows:
Diarrhea index = loose stool rate × average stool grade
Loose stool rate = number of loose stool particles ÷ total number of loose stool particles
Average loose stool level = total loose stool levels ÷ loose stool particle number
Fecal scores were determined by feces diameter on filter paper in accordance with a previous study [29], grade 1: < 1 cm; grade 2: 1-1.9 cm; grade 3: > 3 cm.
AWR scores
We analyzed AWR in response to colorectal distention (CRD) according to a previous report [30]. Twelve hours before the experiment, the rats were fasted for water and anesthetized before the operation. The round rubber balloon fixed at one end of the catheter (diameter: 2.7 mm; F8, Huayue) was lubricated with paraffin oil, and was injected into the rat anus to 4.5 cm depth. The catheter and the tail root of the rats were wrapped together with tape to fix the balloon. Each dilation lasted for 20 seconds. We repeated the dilation 3 times at a 10 min-interval. We took the average value as the AWR score.
Acupuncture intervention
Acupuncture treatment (20 min/day for 2 weeks) was received by some of the rats. The treatment was started on the 2nd day by means of a paradigm after the exposure. Acupuncture points, ST25 (Tianshu) and BL25 (Dachangshu), were chosen according to the rats’ acupoint map [31,32]. We fixed the position of rats under restrained conditions to proceed with acupuncture easily.
Drug treatment
5-BDBD (Cat. SML0450) was obtained from Sigma-Aldrich, St. Louis, USA). 5-BDBD has no obvious influence on other P2X receptors [33]. The 5-BDBD solution (1 mg/kg; daily dose) was made. We administered 5-BDBD by gavage once daily for 3 days, which was started after 4 hours of IBS-D in rats.
Sample collection
Samples were collected after 14-day acupuncture treatment. Abdominal aortic blood (10 mL) was sampled utilizing a syringe and injected into 4 test-tubes (2 test-tubes with anticoagulant and 2 test-tubes without an anticoagulant) and centrifuged for 15 min at 3000 rpm. We put plasma and serum in anticoagulated and non-anticoagulated test-tubes, respectively. Then, we injected supernatant into an Eppendorf tube and kept it at -20°C for storage.
We collected the samples after sacrificing the rats. Pentobarbital sodium (Sigma, St. Louis, MO, USA) was used as an anesthetized agent for rats. DRG (L6-S2) and distal colon were removed and kept at -80°C. We fixed the distal colon in neutral formalin for paraffin embedding and then for immunofluorescence detection, hematoxylin and eosin (H&E) staining, and microscopic examination.
Enzyme-linked immunosorbent assay (ELISA)
We analyzed pro-inflammatory cytokines [interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), and interleukin 1 beta (IL-1β)], chemokines [monocyte chemoattractant protein-1 (MCP-1), C-X-C motif chemokine ligand 1 (CXCL1), and regulated on activation, normal T cell expressed and secreted (RANTES)], substance P (SP), calcitonin gene-related peptide (CGRP) and 5-hydroxytryptamine (5-HT) from the DRG and distal colon tissues by an ELISA kit (Nanjing Jiancheng Corp., Nanjing, China).
Quantitative real-time polymerase chain reaction (qRT-PCR)
We applied TRIZOL (Ambion, Shanghai, China) to get total RNA from the DRG. We employed the PrimeScript RT reagent Kit (TaKaRa, Dalian, China) to reverse-transcribe total RNA (500 ng) from each sample into complementary DNA (cDNA). cDNA was examined by qRT-PCR (EDC-810, Eastwin, Beijing, China) applying SYBR Green Master Mix (VAZYME, Nanjing, China). We obtained primers from Tsingke (Tsingke, Beijing, China). The primers used are as follows: P2X4 (F: 5’-GGGACTGCAACCTGGATAGA-3’) and R: 5’-GCCTTTCCAAACACGATGAT-3’; OX42 (F: 5’-CATCACCGTGAGTTCCACAC-3’ and R: 5’-GAGAACTGGTTCTGGCTTGC-3’); BDNF (F: 5’-CCATAAGGACGCGGACTTG-3’ and R: 5’-GAGCAGAGGAGGCTCCAAAG-3’); IRF-5 (F: 5’-GAGGAATTTCCAGATCCACAGAGACAGCGA-3’ and R: 5’-TGAACCTTTGATCCCCCAAACACATCCAGC-3’); GAPDH (F: 5’-ACAGCAACAGGGTGGTGGAC-3’ and R: 5’-TTTGAGGGTGCAGCGAACTT-3’). The thermocycling conditions were as follows: 95°C for 10 min, 40 cycles of 95°C for 15 s, 60°C for 60 s, and 95°C for 15 s. We performed assays in triplicate. The expression of relative messenger RNA (mRNA) was quantified by the 2-ΔΔCT method [34]. GAPDH was used as the internal control.
Western blot (WB)
Total protein was prepared by extraction utilizing RIPA lysis buffer (Beyotime, Shanghai, China). We examined the protein level with BCA Protein Assay Kit (Beyotime, Shanghai, China). Then, protein (50 μg) was run on SDS-PAGE (10%, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). A protein marker (M00521, Genscript, Jiangsu, China) was added to every gel. Then, we transferred the protein band from the agarose gel onto a PVDF membrane. We did PVDF membrane activation using methanol. We used non-fat milk (5%) in TBST (Tris-buffered saline with Tween 20; Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) for 2 hours to block nonspecific interactions with the membrane. We cultured the membranes with mouse anti-GAPDH (1:1000, Hanzhou goodhere, Hanzhou, China), anti-P2X4 (1:1000, Bioss, Beijing, China), anti-OX42 (1:1000, Santa, Guangzhou, China), anti-BDNF (1:1000, Abcam, UK), anti-IRF-5 (1:1000, Proteintech Group, Inc, Wuhan, China) for 24 h at 4°C. After that, we incubated the membranes in anti-mouse IgG (1:2000, BOSTER Biological Technology Co. Ltd., Wuhan, China) for 2 hours after washing. The protein band was imaged using the ECL system (P1050; Applygen, Beijing, China). We analyzed intensities by a gel imaging device (Hanzhong Photographic Materials, Tianjin, China).
Immunofluorescence detection
We dissected out the DRG (L6-S2) of rats, fixed it in the same reagent for 3 hours and immersed for 24 hours at 4°C in 0.15 and 0.3 g/mL of sucrose solution. We embedded each tissue in an optimal cutting medium, chilled it, and sliced it into sections (14 μm). We mounted the sections on glass slides, washed them in TBST, and blocked them in 0.1 g/mL goat serum along with TritonX-100 (0.3%) at 37°C for an hour. We incubated slides with anti-mouse P2X4, anti-mouse OX42 and anti-mouse C-FOS for 24 hours at 4°C. Incubation of these was done with the Alexa Fluor 488-conjugated secondary antibody (1:500, Abcam, Cambridge, UK) for an hour at 37°C after washing 3 times in PBS. Staining was conducted by means of DAPI for an hour at 37°C. Finally, we identified each signal with an ECL system (P1050, Applygen, Beijing, China).
Histologic staining and mast cell counts
We sacrificed rats, collected colonic tissues, and then washed the tissues using PBS. Colonic tissue was fixed in formaldehyde buffer (4%) and sliced into sections (4 µm). H&E staining was performed. Then, we observed the sections using a light microscope.
Data analysis
We analyzed the data by using SPSS 22.0 (Chicago, USA) and GraphPad Prism 7.0 (San Diego, USA) and displayed the data as mean ± SD (standard deviation). All tests were carried out in triplicate. The data were evaluated by employing the Student’s t-test. We analyzed the differences with the use of one-way analysis of variance (ANOVA) and Tukey’s multiple comparison test. Statistical significance was set at P < 0.05.
Results
Acupuncture improved clinical symptoms in IBS-D rats
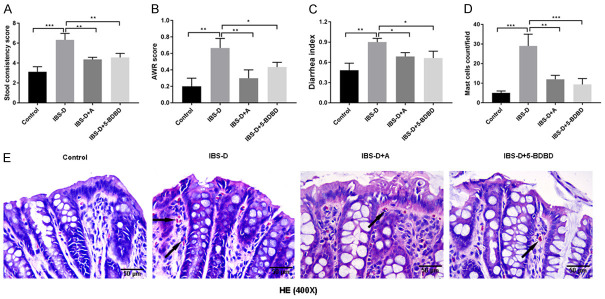
The stool consistency score was markedly improved in the IBS-D group compared to the Control group (P < 0.001) (Figure 1A). Acupuncture or 5-BDBD treatment reduced the stool consistency score when compared to the results of the IBS-D model group (P < 0.01). After 14-day acupuncture treatment at the ST25 (Tianshu) and BL25 (Dachangshu) acupoints, the AWR and the diarrhea index of IBS-D rats were significantly decreased. IBS-D + 5-BDBD group also showed a decreased AWR level and the diarrhea index as compared to those in the IBS-D group (P < 0.05, P < 0.01; Figure 1B and 1C). In addition, the IBS-D group presented more mast cells (Figure 1E) than the Control group. However, the numbers of mast cells diminished significantly in the IBS-D + A and IBS-D + 5-BDBD groups when compared to the number of the IBS-D group (P < 0.01, P < 0.001; Figure 1D). Histologic staining was done to assess whether acupuncture influenced colonic micro-inflammation. The intestinal mucosal barrier in the Control group demonstrated integrity facilitated by colonic epithelial cells (CECs). Significant edema was observed in CECs of the IBS-D group. The colonic tissues of the IBS-D group showed noticeable changes in intestinal mucosal epithelium, such as the influx of inflammatory cells and impaired intestinal mucosal integrity, which were increased (Figure 1E).
Figure 1.
Acupuncture alleviated the pain in diarrhea predominant irritable bowel syndrome (IBS-D) rats. A. Acupuncture’s influence on the stool consistency score of IBS-D rats. B. Impact of acupuncture on the AWR scores of IBS-D rats. C. Effects of acupuncture on the IBS-D rats’ diarrhea indexes. D. Effects of acupuncture on IBS-D rats’ mast cell counts. E. H&E stained IBS-D rats’ colonic epithelial tissues (magnification: 400×, scale bar: 50 µm) (mast cells: black arrows). ***P < 0.001; **P < 0.01; *P < 0.05.
Acupuncture decreased inflammation in IBS-D rats
MCP-1, IL-6, TNF-α, IL-1β, RANTES, and CXCR3 levels in serum were substantially enhanced in the IBS-D group (P < 0.001, Figure 2A). After 14-day acupuncture treatment or 5-BDBD treatment, the levels were diminished compared to those in the Control group (P < 0.001). We found that the IBS-D rats had greater 5-HT, CGRP, and SP contents in the colon and the DRG (P < 0.05 vs. Control group). Acupuncture or 5-BDBD treatment induced the contents of 5-HT, CGRP, and SP in the distal colon and DRG tissue of IBS-D rats (P < 0.05, vs. IBS-D group, Figure 2B).
Figure 2.
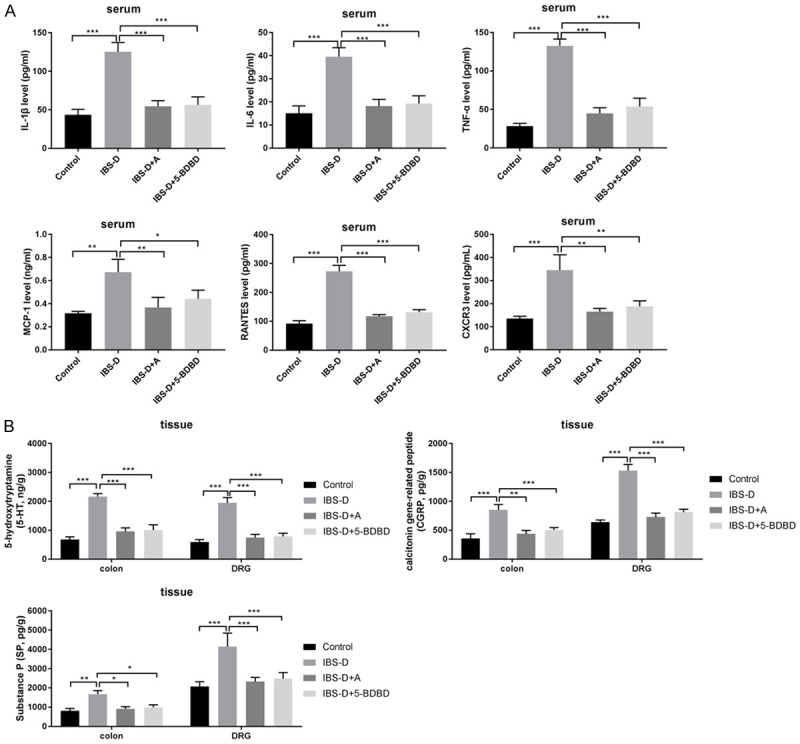
Acupuncture decreased inflammation in IBS-D rats. A. Serum TNF-α, IL-1β, MCP-1, IL-6, RANTES and CXCR3 levels measured by ELISA. B. 5-HT, CGRP and SP levels in the colon and the DRG tissues evaluated by ELISA. ***P < 0.001; **P < 0.01; *P < 0.05.
Acupuncture reduced P2X4, OX42, BDNF, and IRF-5 expressions in IBS-D rats
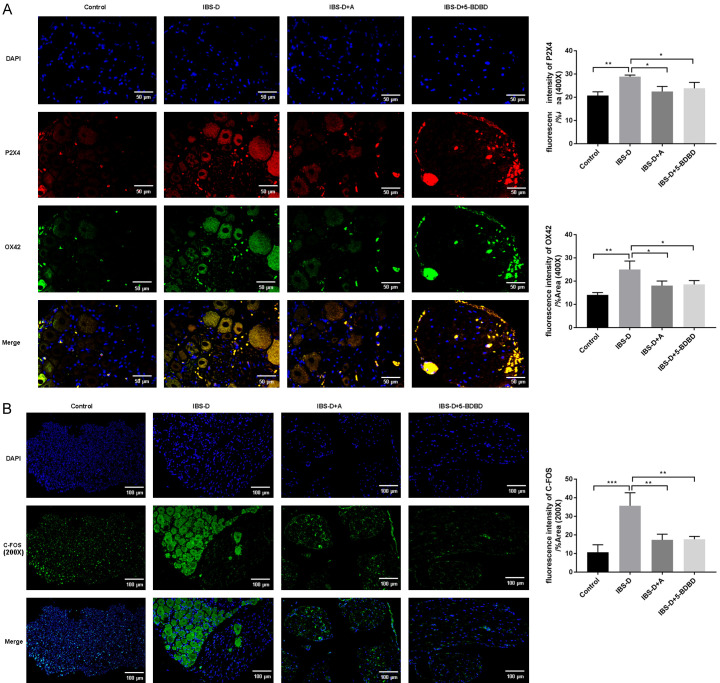
Based on the qRT-PCR assay, there was a prominent increase in P2X4, OX42, BDNF, and IRF-5 expression levels in the IBS-D group (P < 0.001). These expression levels were significantly lowered after acupuncture or 5-BDBD treatment (P < 0.01, P < 0.001, Figure 3A). We observed similar results by WB analysis (Figure 3B). The P2X4 and OX42 staining in the DRG of IBS-D group was substantially improved versus that of the Control group (Figure 4A). After acupuncture or 5-BDBD treatment, OX42 and P2X4 stains were reduced in the IBS-D + A and IBS-D + 5-BDBD groups. 5-BDBD is a specific P2X4R antagonist. Our results observed that the impacts of acupuncture on P2X4 were similar to 5-BDBD treatment results. Acupuncture could decrease P2X4 expression in the microglia of IBS-D rats. The immunofluorescence assay results also showed significantly inhibited C-FOS-positive cell number in DRG of the IBS-D group upon acupuncture or 5-BDBD treatment, suggesting that acupuncture could inhibit the abnormal increase of visceral neuron excitability in the spinal dorsal horn of the IBS-D group (Figure 4B).
Figure 3.
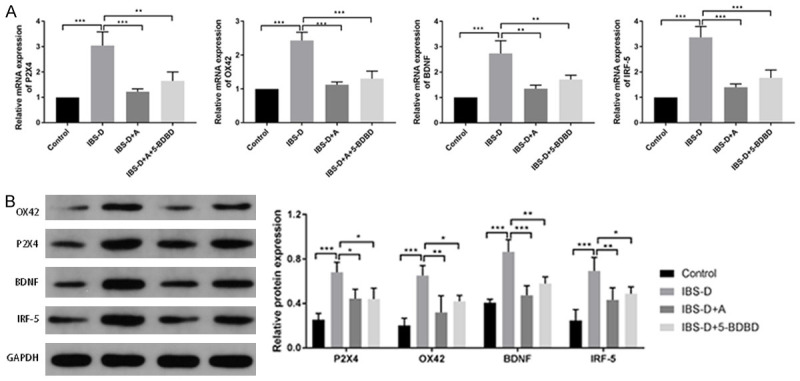
Acupuncture reduced mRNA and protein expressions of P2X4, OX42, BDNF and IRF-5 in IBS-D rats. A. Relative mRNA levels of OX42, P2X4, IRF-5 and BDNF determined by qRT-PCR. B. Protein levels of OX42, BDNF, P2X4 and IRF-5 determined by western blot. ***P < 0.001; **P < 0.01.
Figure 4.
Acupuncture reduced P2X4, OX42 and C-FOS expressions by immunofluorescence. A. Photomicrographs of OX42 (red) and P2X4 (green) immunofluorescence (Original magnification 400×, scale bar: 50 µm) and its fluorescence intensity. B. Representative photomicrographs of immunofluorescence of C-FOS (red) (Original magnification 200×, scale bar: 100 µm) and its fluorescence intensity.
Discussion
IBS with predominant diarrhea (IBS-D) is characterized by abdominal pain. Hypersensitivity in the viscera plays a key role in the discomfort and abdominal pain [35]. However, the visceral hypersensitivity process in IBS-D is complicated. Thus, the effects of acupuncture on visceral hypersensitivity were observed in IBS-D rats here.
Acupuncture is a widely used technique for treating functional gastrointestinal illnesses, including IBS. It has several advantages, including satisfactory effects, convenience and few adverse reactions. Acupuncture reduces visceral hyperalgesia and improves IBS-D symptoms [29,36,37]. In this study, we applied the stool consistency and AWR scores to assess the intestinal transit and visceral hypersensitivity using a rat model. Significant increases in stool consistency and AWR scores were observed in IBS-D rats in comparison to the Control group. IBS patients had an increased pain sensitivity and decreased pain threshold versus the general population [38]. Our data are consistent with these findings. Decreased stool consistency and AWR scores were observed in IBS-D rats after acupuncture or 5-BDBD treatment, showing that acupuncture and 5-BDBD could alleviate abdominal pain by decreasing visceral hypersensitivity in the IBS rats.
The enteric immune system includes many immune cells, sensitizing and triggering the inflammatory cascade against extrinsic (bacteria, parasites, viruses and food) and intrinsic factors (hormones and neurotransmitters from the CNS). 5-HT is one of the main transmitters of BGA. 5-HT receptor antagonists are utilized for managing IBS-D [39] and reducing visceral sensitivity and gastrointestinal motility [40]. 5-HT receptor antagonists with other neurotransmitter-targeted agents in BGA are a promising method [41]. CGRP and the receptors of CGRP are enhanced in DRG. These are related to visceral hypersensitivity. SP acts as a neurotransmitter and modulator for neurogenic inflammation and pain sensitization. In our results, IBS-D rats showed increased concentrations of IL-6, TNF-α, RANTES, IL-1β, MCP-1 and CXCR3 in serum and enhanced CGRP, 5-HT and SP contents. However, the two paradigms (acupuncture and 5-BDBD treatment) decreased the CGRP, 5-HT, and SP contents in the colon and spinal cord. Acupuncture treatment lessened the IBS-D-like symptoms and reduced 5-HT, CGRP and SP changes when compared to the Control group. These bioactive substances are widely involved in regulating gastrointestinal functions at the peripheral and central levels. The acupuncture treatment for IBS-D involves many links of intestinal motility and the nerve immune endocrine network system.
Activated microglia’s P2X4Rs of the spinal cord participate in neuropathic pain pathogenesis [42]. The expression of the P2X4R was enhanced by spinal microglia in neuropathic pain [43]. The expression of OX42 and P2X4 was studied in the IBS-D group in our study, but their expression levels were substantially suppressed after acupuncture treatment. This study found that BDNF expression in the DRG of IBS-D visceral pain rats increased when compared to the Control group. Acupuncture treatment could inhibit the BDNF expression in the IBS-D group. BDNF release after microglia activation in IBS-D visceral pain is likely to be one of the above bioactive factors. This is consistent with a study, that showed that peripheral injury may increase BDNF synthesis by activating the P2X4R on microglia and thereby induce abnormal pain sensitivity [44]. Acupuncture treatment could decrease the C-FOS expression level in the DRG of IBS-D. C-FOS is widely expressed in the spinal cord’s dorsal horn after nociceptive stimulation in the peripheral body and viscera [45]. Therefore, as a marker of enhanced neuronal excitability, C-FOS is widely used to study the pharmacological and physiological mechanism of nociceptive stimulation transmission at the spinal cord [46]. The P2X4R is expressed only in microglia [44]. Therefore, the P2X4R may mediate microglia to participate in the production of IBS abnormal visceral pain, and its overexpression leads to chronic visceral pain sensitivity in IBS. According to these data, acupuncture can relieve the chronic visceral pain sensitivity of IBS-D via regulating P2X4 in spinal microglia.
After inflammatory stimulation, visceral nociceptors form afferent nerve impulses by means of primary sensory DRG transmitting to the CNS [47]. Microglia are the major sources of TNF-α, IL-1β and IL-6 [48]. Spinal microglia are stimulated upon peripheral nerve damage simulation, leading to the secretion of inflammatory factors. Glycosylated myelin is destroyed by inflammatory agents, which results in nerve demyelination, pain and central sensitization [48]. IRF-5, belonging to the transcription factor family, regulates the activities of the immune system. In addition, IRF-5 regulates the inflammatory immune response of various cell types by regulating the expression of cytokines and chemokines [49]. IRF-5 was found to guide monocytes toward a CD11c+ macrophage phenotype to promote inflammation of the intestines [50]. Compared with the those of the IBS-D group, the inflammatory factor and IRF-5 expression levels were lowered in the IBS-D + A group. Inflammatory factor release in the IBS-D model group can be caused by a change in IRF-5 expression. Moreover, acupuncture treatment can reduce this effect.
Acupuncture is an accepted alternative treatment for different types of infection. Acupuncture-induced analgesia is extensively used to lessen different types of pain (e.g., chronic pain) [51,52]. The Dachangshu and Tianshu acupuncture points are conventional acupoint combinations to deal with intestinal diseases [17,20]. 5-BDBD treatment protects against ischemic injury by decreasing the number of infiltrated pro-inflammatory myeloid cells and surface P2X4R expression [33]. We also selected 5-BDBD treatment as the control, and found that 5-BDBD treatment had a similar effect on IBS-D rats as acupuncture.
These results indicate that acupuncture can relieve the chronic visceral pain sensitivity of IBS-D, regulating P2X4 in spinal microglia. There were some limitations of this study. First, we chose 5-BDBD as the control in the current research. We should pay attention to whether 5-BDBD + acupuncture has a synergistic effect compared to acupuncture alone. Due to the limitations of time and experimental environment, this will be verified in the future. In addition, the main process of acupuncture for visceral pain involves a combined procedure at different levels in the body, from the outer periphery to the center. Acupuncture treatment for visceral pain requires various complicated neurotransmitters and neural structures. This treatment’s effects appear through the correlation between the thalamus, cortex spinal cord and brain stem, and through the neuroendocrine-immune network. Studies on the pathophysiology and acupuncture treatment of IBS-D visceral discomfort have consistently focused on P2X receptors. This study provides only the latest target for treating visceral pain in IBS-D. The mechanism of signal transduction of acupuncture needs more in-depth studies. Moreover, IBS-D shows comorbidity with anxiety and depression [53]. The effect of acupuncture on emotional anxiety in IBS-D rats can also be explored.
In summary, acupuncture treatment can relieve IBS-D-like symptoms in rats. These effects could be from decreased inflammatory cytokines and chemokines. Acupuncture can also cause the downregulation of P2X4, OX42, BDNF, IRF-5, and C-FOS expression levels in microglia of DRG, thereby alleviating IBS-D. These results support acupuncture therapy for IBS-D management.
Acknowledgements
The research was funded by the Open fund project acupuncture and Moxibustion Clinical Medicine Research Center of Anhui (2021zjzx07), the Major Research Project of Natural Sciences (81973936) and National Natural Science Foundation of China (Youth Program) (81904095).
Disclosure of conflict of interest
None.
References
- 1.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721. e714. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet. 2020;396:1675–1688. doi: 10.1016/S0140-6736(20)31548-8. [DOI] [PubMed] [Google Scholar]
- 3.Habba SF. Diarrhea predominant irritable bowel syndrome (IBS-D): fact or fiction. Med Hypotheses. 2011;76:97–99. doi: 10.1016/j.mehy.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 4.Hadjivasilis A, Tsioutis C, Michalinos A, Ntourakis D, Christodoulou DK, Agouridis AP. New insights into irritable bowel syndrome: from pathophysiology to treatment. Ann Gastroenterol. 2019;32:554–564. doi: 10.20524/aog.2019.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oświęcimska J, Szymlak A, Roczniak W, Girczys-Połedniok K, Kwiecień J. New insights into the pathogenesis and treatment of irritable bowel syndrome. Adv Med Sci. 2017;62:17–30. doi: 10.1016/j.advms.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka Y, Kanazawa M, Fukudo S, Drossman DA. Biopsychosocial model of irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:131–139. doi: 10.5056/jnm.2011.17.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouyang H, Chen JD. Review article: therapeutic roles of acupuncture in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2004;20:831–841. doi: 10.1111/j.1365-2036.2004.02196.x. [DOI] [PubMed] [Google Scholar]
- 8.Chao GQ, Zhang S. Effectiveness of acupuncture to treat irritable bowel syndrome: a meta-analysis. World J Gastroenterol. 2014;20:1871–1877. doi: 10.3748/wjg.v20.i7.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun JH, Wu XL, Xia C, Xu LZ, Pei LX, Li H, Han GY. Clinical evaluation of soothing Gan and invigorating Pi acupuncture treatment on diarrhea-predominant irritable bowel syndrome. Chin J Integr Med. 2011;17:780–785. doi: 10.1007/s11655-011-0875-z. [DOI] [PubMed] [Google Scholar]
- 10.Tang HY, Jiang AJ, Wang XY, Wang H, Guan YY, Li F, Shen GM. Uncovering the pathophysiology of irritable bowel syndrome by exploring the gut-brain axis: a narrative review. Ann Transl Med. 2021;9:1187. doi: 10.21037/atm-21-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O’Sullivan GC, Kiely B, Collins JK, Shanahan F, Quigley EM. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 12.Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000;95:2698–2709. doi: 10.1111/j.1572-0241.2000.03177.x. [DOI] [PubMed] [Google Scholar]
- 13.Burnstock G. The journey to establish purinergic signalling in the gut. Neurogastroenterol Motil. 2008;20(Suppl 1):8–19. doi: 10.1111/j.1365-2982.2008.01107.x. [DOI] [PubMed] [Google Scholar]
- 14.Xu GY, Shenoy M, Winston JH, Mittal S, Pasricha PJ. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut. 2008;57:1230–1237. doi: 10.1136/gut.2007.134221. [DOI] [PubMed] [Google Scholar]
- 15.Soares-Bezerra RJ, Calheiros AS, da Silva Ferreira NC, da Silva Frutuoso V, Alves LA. Natural products as a source for new anti-inflammatory and analgesic compounds through the inhibition of Purinergic P2X receptors. Pharmaceuticals (Basel) 2013;6:650–658. doi: 10.3390/ph6050650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnstock G, Nistri A, Khakh BS, Giniatullin R. ATP-gated P2X receptors in health and disease. Front Cell Neurosci. 2014;8:204. doi: 10.3389/fncel.2014.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weng ZJ, Wu LY, Zhou CL, Dou CZ, Shi Y, Liu HR, Wu HG. Effect of electroacupuncture on P2X3 receptor regulation in the peripheral and central nervous systems of rats with visceral pain caused by irritable bowel syndrome. Purinergic Signal. 2015;11:321–329. doi: 10.1007/s11302-015-9447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CBA, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Keating C, Pelegrin P, Martínez CM, Grundy D. P2X7 receptor-dependent intestinal afferent hypersensitivity in a mouse model of postinfectious irritable bowel syndrome. J Immunol. 2011;187:1467–1474. doi: 10.4049/jimmunol.1100423. [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Shi Q, Zhu Q, Zou T, Li G, Huang A, Wu B, Peng L, Song M, Wu Q, Xie Q, Lin W, Xie W, Wen S, Zhang Z, Lv Q, Zou L, Zhang X, Ying M, Li G, Liang S. P2X7 receptor of rat dorsal root ganglia is involved in the effect of moxibustion on visceral hyperalgesia. Purinergic Signal. 2015;11:161–169. doi: 10.1007/s11302-014-9439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen XM, Xu J, Song JG, Zheng BJ, Wang XR. Electroacupuncture inhibits excessive interferon-γ evoked up-regulation of P2X4 receptor in spinal microglia in a CCI rat model for neuropathic pain. Br J Anaesth. 2015;114:150–157. doi: 10.1093/bja/aeu199. [DOI] [PubMed] [Google Scholar]
- 22.Beggs S, Trang T, Salter MW. P2X4R+ microglia drive neuropathic pain. Nat Neurosci. 2012;15:1068–1073. doi: 10.1038/nn.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue K. Role of the P2X4 receptor in neuropathic pain. Curr Opin Pharmacol. 2019;47:33–39. doi: 10.1016/j.coph.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Tsuda M, Inoue K. Neuropathic pain and ATP receptors in spinal microglia. Brain Nerve. 2007;59:953–959. [PubMed] [Google Scholar]
- 25.DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- 26.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 27.Hou Q, Huang Y, Zhu S, Li P, Chen X, Hou Z, Liu F. MiR-144 increases intestinal permeability in IBS-D rats by targeting OCLN and ZO1. Cell Physiol Biochem. 2017;44:2256–2268. doi: 10.1159/000486059. [DOI] [PubMed] [Google Scholar]
- 28.Amarenco G. Bristol stool chart: prospective and monocentric study of “stools introspection” in healthy subjects. Prog Urol. 2014;24:708–713. doi: 10.1016/j.purol.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Zheng H, Li Y, Zhang W, Zeng F, Zhou SY, Zheng HB, Zhu WZ, Jing XH, Rong PJ, Tang CZ, Wang FC, Liu ZB, Wang SJ, Zhou MQ, Liu ZS, Zhu B. Electroacupuncture for patients with diarrhea-predominant irritable bowel syndrome or functional diarrhea: a randomized controlled trial. Medicine (Baltimore) 2016;95:e3884. doi: 10.1097/MD.0000000000003884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao YY, Guo YT, Gao JP, Liu JJ, Chang ZP, Feng XJ, Xu D, Deng GF, Hou RG. Shaoyao-Gancao decoction relieves visceral hyperalgesia in TNBS-induced postinflammatory irritable bowel syndrome via inactivating transient receptor potential vanilloid type 1 and reducing serotonin synthesis. Evid Based Complement Alternat Med. 2020;2020:7830280. doi: 10.1155/2020/7830280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao ZM, Liu CL, Zhang QY, Zhang BB, Guo JH, Yuan AH, Cai H. Acupuncture treatment reduces body weight possibly by down-regulating insulin and leptin resistance, and up-regulating soluble leptin receptor level in prediabetic patients. Zhen Ci Yan Jiu. 2018;43:506–511. doi: 10.13702/j.1000-0607.180184. [DOI] [PubMed] [Google Scholar]
- 32.Tao Wu. Clinical observation on the treatment of type 2 diabetes by combined acupuncture and medicine. J Clin Med Literat. 2016 [Google Scholar]
- 33.Srivastava P, Cronin CG, Scranton VL, Jacobson KA, Liang BT, Verma R. Neuroprotective and neuro-rehabilitative effects of acute purinergic receptor P2X4 (P2X4R) blockade after ischemic stroke. Exp Neurol. 2020;329:113308. doi: 10.1016/j.expneurol.2020.113308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Barshop K, Staller K. New pathways, new targets: visceral hypersensitivity pathogenesis in irritable bowel syndrome. Clin Transl Gastroenterol. 2016;7:e146. doi: 10.1038/ctg.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu JC, Ziea ET, Lao L, Lam EF, Chan CS, Liang AY, Chu SL, Yew DT, Berman BM, Sung JJ. Effect of electroacupuncture on visceral hyperalgesia, serotonin and fos expression in an animal model of irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16:306–314. doi: 10.5056/jnm.2010.16.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pei L, Chen H, Guo J, Chen L, Wu X, Xu W, Weng S, Yang E, Hammer T, Sun J. Effect of acupuncture and its influence on visceral hypersensitivity in IBS-D patients: study protocol for a randomized controlled trial. Medicine (Baltimore) 2018;97:e10877. doi: 10.1097/MD.0000000000010877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keszthelyi D, Troost FJ, Masclee AA. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Methods to assess visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G141–154. doi: 10.1152/ajpgi.00060.2012. [DOI] [PubMed] [Google Scholar]
- 39.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegert F, Nieber K. New therapeutical approaches for treatment of irritable bowel syndrome. Med Monatsschr Pharm. 2010;33:285–292. quiz 293-284. [PubMed] [Google Scholar]
- 41.Asagarasu A, Matsui T, Hayashi H, Tamaoki S, Yamauchi Y, Minato K, Sato M. Discovery of a novel 5-HT(3) antagonist/5-HT(1A) agonist 3-amino-5,6,7,8-tetrahydro-2-{4-[4-(quinolin-2-yl)piperazin-1-yl]butyl}quinazolin-4(3H)-one (TZB-30878) as an orally bioavailable agent for irritable bowel syndrome. J Med Chem. 2010;53:7549–7563. doi: 10.1021/jm1002292. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z, Zhang ZY, Fauser U, Schluesener H. Mechanical allodynia and spinal up-regulation of P2X4 receptor in experimental autoimmune neuritis rats. Neuroscience. 2008;152:495–501. doi: 10.1016/j.neuroscience.2007.12.042. [DOI] [PubMed] [Google Scholar]
- 43.Sweitzer SM, Schubert P, DeLeo JA. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. J Pharmacol Exp Ther. 2001;297:1210–1217. [PubMed] [Google Scholar]
- 44.Long T, He W, Pan Q, Zhang S, Zhang D, Qin G, Chen L, Zhou J. Microglia P2X4R-BDNF signalling contributes to central sensitization in a recurrent nitroglycerin-induced chronic migraine model. J Headache Pain. 2020;21:4. doi: 10.1186/s10194-019-1070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Traub RJ, Pechman P, Iadarola MJ, Gebhart GF. Fos-like proteins in the lumbosacral spinal cord following noxious and non-noxious colorectal distention in the rat. Pain. 1992;49:393–403. doi: 10.1016/0304-3959(92)90247-9. [DOI] [PubMed] [Google Scholar]
- 46.Sun YN, Luo JY. Effects of tegaserod on Fos, substance P and calcitonin gene-related peptide expression induced by colon inflammation in lumbarsacral spinal cord. World J Gastroenterol. 2004;10:1830–1833. doi: 10.3748/wjg.v10.i12.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Latorre R, Sternini C, De Giorgio R, Greenwood-Van Meerveld B. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol Motil. 2016;28:620–630. doi: 10.1111/nmo.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang D, Couture R, Hong Y. Activated microglia in the spinal cord underlies diabetic neuropathic pain. Eur J Pharmacol. 2014;728:59–66. doi: 10.1016/j.ejphar.2014.01.057. [DOI] [PubMed] [Google Scholar]
- 49.Fabié A, Mai LT, Dagenais-Lussier X, Hammami A, van Grevenynghe J, Stäger S. IRF-5 promotes cell death in CD4 T cells during chronic infection. Cell Rep. 2018;24:1163–1175. doi: 10.1016/j.celrep.2018.06.107. [DOI] [PubMed] [Google Scholar]
- 50.Corbin AL, Gomez-Vazquez M, Berthold DL, Attar M, Arnold IC, Powrie FM, Sansom SN, Udalova IA. IRF5 guides monocytes toward an inflammatory CD11c(+) macrophage phenotype and promotes intestinal inflammation. Sci Immunol. 2020;5:eaax6085. doi: 10.1126/sciimmunol.aax6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berman BM, Langevin HM, Witt CM, Dubner R. Acupuncture for chronic low back pain. N Engl J Med. 2010;363:454–461. doi: 10.1056/NEJMct0806114. [DOI] [PubMed] [Google Scholar]
- 52.Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85:355–375. doi: 10.1016/j.pneurobio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 53.Kapeller J, Houghton LA, Mönnikes H, Walstab J, Möller D, Bönisch H, Burwinkel B, Autschbach F, Funke B, Lasitschka F, Gassler N, Fischer C, Whorwell PJ, Atkinson W, Fell C, Büchner KJ, Schmidtmann M, van der Voort I, Wisser AS, Berg T, Rappold G, Niesler B. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet. 2008;17:2967–2977. doi: 10.1093/hmg/ddn195. [DOI] [PubMed] [Google Scholar]