Abstract
Background: The alleviating effects of carbocisteine (S-carboxymethylcysteine, SCMC) have been implicated in chronic obstructive pulmonary disease; however, very little is known about its mechanisms in asthma. In this study, we aimed to investigate the effects of SCMC on airway remodeling in asthmatic mice induced by ovalbumin (OVA). Methods: The asthma mouse model was generated by OVA sensitization and stimulation and subsequently intervened by SCMC or dexamethasone. Bronchoalveolar lavage fluid (BALF) and lung tissues were collected from each group of mice. The TGF-β1 levels in BALF were measured by ELISA. Masson’s staining was used to detect collagen fiber deposition in mouse airway tissues, while immunohistochemistry and RT-qPCR were conducted to examine the protein and mRNA expression of TGF-β1 in mouse lung airway tissues, respectively. The correlation between TGF-β1 mRNA expression and the area of collagen fiber deposition in airway tissues was analyzed by Pearson’s correlation coefficient. Results: The area of collagen fiber deposition in the airway tissues of asthmatic mice was significantly increased, while SCMC alleviated the collagen fiber deposition in the airway tissues. TGF-β1 expression was significantly elevated in BALF and airway tissues of asthmatic mice, while SCMC inhibited TGF-β1 expression. TGF-β1 expression was significantly and positively correlated with collagen fiber deposition in mouse airway tissues. Conclusions: SCMC intervention improves collagen fiber deposition in airway tissues and inhibits TGF-β1 expression in asthmatic mice.
Keywords: Asthma, mouse, carbocistein, TGF-β1
Introduction
Bronchial asthma (hereinafter referred to as asthma) is a global disease, greatly endangering personal health, and the Global Initiative for Asthma in 2020 pointed out that there were approximately 300 million asthma patients in the world and nearly 30 million in China [1]. Asthma is a heterogeneous disease featured with chronic airway inflammation, and its main pathophysiological changes include eosinophil-dominated inflammatory cell infiltration, increased bronchial secretions, bronchial smooth muscle spasm and airway remodeling [2]. Airway remodeling can occur early in the course of asthma and exists throughout the chronic inflammatory process [3]. Its pathological changes are mainly manifested by mucocytosis of airway epithelial cells, subepithelial collagen deposition and fibrosis, smooth muscle hypertrophy/proliferation, as well as vascular hyperplasia [4]. Current asthma treatments aim to alleviate airway inflammation and exacerbations, but none are targeted towards airway remodeling [5]. Therefore, perturbation of airway remodeling or reversing established remodeling has the potential to improve symptoms and disease burden in asthmatic patients. Transforming growth factor-β (TGF-β) exerts a regulatory function during the process of asthma airway remodeling, and TGF-β1 is an important cytokine involved in the process [6]. Therefore, the regulation of TGF-β1 expression might play a significant role in airway remodeling of asthma.
Carbocistein (S-carboxymethylcysteine, hereinafter referred to as SCMC), the side-chain carboxymethyl derivative of the sulfur-containing amino acid, cysteine, has been known and available for nearly 80 years [7]. The structure and mechanism of SCMC differ from other commonly available mucolytic drugs that bear free sulfhydryl (thiol) groups via which they split glycoprotein bonds in mucus [8]. It was found that SCMC inhibited airway TGF-β1 expression after early application, implying that SCMC functions effectively in preventing airway remodeling in chronic obstructive pulmonary disease (COPD) [9]. Taketa et al. discovered that SCMC attenuated the number of eosinophils in bronchoalveolar lavage fluid (BALF) of asthmatic mice, weakened bronchial hyperreactivity and reduced the release of cytokines such as serum IL5 and IL13 [10]. In addition, SCMC has been shown to attenuate hydrogen peroxide-induced inflammatory injury in A549 cells [11]. However, the modulating effect of SCMC in airway remodeling during asthma remains unexplored. The murine models of allergic respiratory diseases induced by ovalbumin (OVA) have been widely used to elucidate immunological and nonimmunological mechanisms involved in the pathogenesis of asthma [12]. In this study, we established an asthmatic mouse model by OVA. Based on this model, we assessed the effectiveness of SCMC in treating airway remodeling, which could provide alternative strategies for the clinical treatment of asthma.
Material and methods
Experimental animals
Thirty-two clean-grade BALB/C female mice (7 weeks, 19 ± 2 g) were provided by the Experimental Animal Center of the Third Military Medical University, Chongqing. The experimental protocol was conducted in accordance with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee of the Third Military Medical University, Chongqing (Approval number: 20210412). All mice were housed in a temperature- (22-24°C) and humidity-controlled (50-60%) room on a 12/12 h light/dark cycle with ad libitum access to chow and drinking water. The mice were acclimatized for one week before the experiments were performed.
Establishment of asthma model and treatments
Mice were classified into the normal saline (NS), asthma (AS), AS + SCMC, and AS + dexamethasone (DEX) groups (n = 8). The method proposed by Kearley et al. [13] was followed. The asthma mouse model was established by intraperitoneal injection combined with subcutaneous injection of OVA. In brief, the AS, AS + SCMC, and AS + DEX groups were sensitized with OVA by intraperitoneal combined subcutaneous injection on day 1 and day 13, respectively, and stimulated by nebulization with 10% OVA solution for 18 days from day 19, once a day for 30 min each time. Besides, the AS group was given 0.2 mL of saline intraperitoneally 30 min before each stimulation, while SCMC (10 mg/kg) was given by gavage, and DEX (2 mg/kg) was administered by intraperitoneal injection (0.2 mL). In the NS group, normal saline was given in each sensitization and intervention. The mice were euthanized 24 h after the last OVA stimulation. BLAF and lung tissues were collected for subsequent experiments. Figure 1 presents the flow chart of the modeling.
Figure 1.
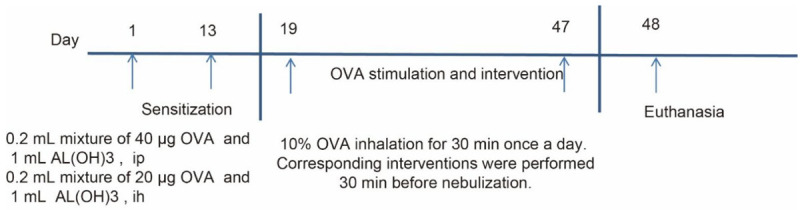
Flow chart of asthma modeling.
Enzyme-linked immunosorbent assay (ELISA)
BALF samples were collected by flushing the lungs of mice with 1 mL phosphate-buffered saline three times. The BALF samples were then centrifuged at 300 g for 10 min at 4°C, and the supernatant was harvested. The mouse TGF-β1 ELISA Kit (ab119557, Abcam, Cambridge, UK) was used to quantify TGF-β1 according to the manufacturer’s instructions.
RT-qPCR
Total RNA was extracted from mouse lung tissues using Trizol (Thermo Fisher Scientific Inc., Waltham, MA, USA). After reverse transcription of total RNA into cDNA by PrimeScript™ RT reagent kit (Takara Biotechnology Ltd., Dalian, Liaoning, China), qPCR reactions were performed on a LightCycler 480 System (Roche Diagnostics, Co., Ltd., Rotkreuz, Switzerland) using TB Green® Fast qPCR Mix (Takara). GAPDH was used as an internal control. The gene expression was analyzed via 2-ΔΔCt method. The primer sequences were: TGF-β1 forward primer: 5’-TGATACGCCTGAGTGGCTGTCT-3’, reverse primer: 5’-CACAAGAGCAGTGAGCGCTGAA-3’; GAPDH forward primer: 5’-CATCACTGCCACCCAGAAGACTG-3’, reverse primer: 5’-ATGCCAGTGAGCTTCCCGTTCAG-3’.
Masson’s staining
Collagen fiber deposition in mouse lung airway tissues was observed using Masson’s staining kit (G1340, Beijing Solarbio Life Sciences Co., Ltd., Beijing, China). Briefly, mouse lung tissues were routinely paraffin-embedded and sectioned (5 μm). The sections were dewaxed, rehydrated and stained with a configured Weigert hematoxylin staining solution for 5 min, followed by 10 s of treatment with ethanol. The sections were treated with Masson staining solution for 5 min and stained with Ponceau 2R for 5 min. After staining with aniline blue staining solution for 2 min, the sections were subjected to conventional dehydration and xylene clearing. After neutral gum fixation, the sections were observed under a light microscope (Olympus Optical Co., Ltd., Tokyo, Japan), and the areas of blue collagen fiber deposition were quantified by ImageJ.
Immunohistochemical staining
Paraffin-embedded lung tissue sections were de-waxed and rehydrated, and heat-mediated antigen retrieval was performed by using Tris/ethylenediaminetetraacetic acid buffer (pH = 9.0). The sections were incubated with 3% H2O2 to block endogenous peroxidase activity and 5% BSA to reduce nonspecific staining. The sections were incubated with the primary antibody to TGF-β1 (1:500, ab215715, Abcam) overnight at 4°C, followed by a 2-h incubation with secondary antibody goat anti-rabbit IgG H&L (HRP) (1:1000, ab6721, Abcam) at room temperature. Color development was performed using DAB, and the nuclei were counter-stained by hematoxylin. The tan TGF-β1 positively stained areas were observed under a light microscope (Olympus).
Statistical methods
All experimental data, which were statistically analyzed with SPSS17.0 software (IBM Corp. Armonk, N.Y., USA), were denoted as mean ± standard deviation (x̅ ± sd). One-way ANOVA was used to compare multiple sample means, within which the comparison between two groups was performed using LSD test. P < 0.05 indicated that the difference was statistically significant.
Results
SCMC inhibited collagen deposition in the airway tissues of asthmatic mice
We induced asthma in mice (AS, with NS as controls) and treated them with SCMC, with DEX serving as a positive control. Masson’s staining was conducted to observe the collagen deposition in the airway tissues of mice. Few collagen fibers were deposited around the airways of mice in the NS group (Figure 2A). In the AS group, the mice showed significant collagen deposition (Figure 2B). The SCMC-treated mice (Figure 2C; Table 1) exhibited less collagen fiber deposition than that in the AS + DEX group (Figure 2D).
Figure 2.
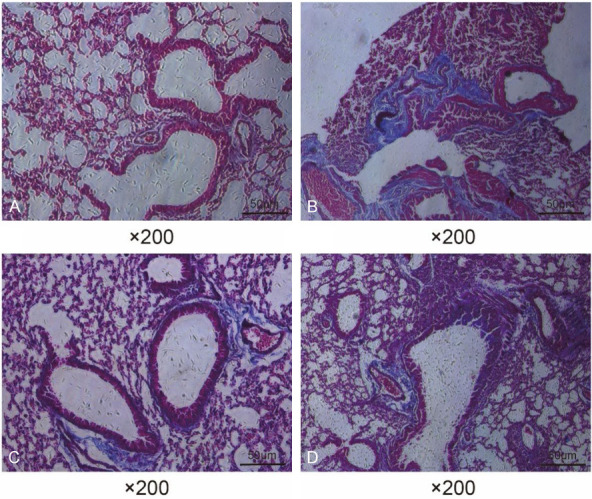
SCMC attenuated collagen deposition in the airways of asthmatic mice. Representative images of Masson staining in the airway tissues of mice in NS group (A), AS group (B), AS + SCMC group (C), and AS + DEX group (D).
Table 1.
Collagen fiber deposition around the airways of mice
| Groups | Relative quantification of peri-airway collagen fibril deposition (µm2/µm) |
|---|---|
| NS Group | 3.36 ± 0.59 |
| AS Group | 20.93 ± 3.05* |
| AS + SCMC Group | 14.68 ± 1.28*,#,Δ |
| AS + DEX Group | 19.01 ± 1.01* |
Note: Data were presented as mean ± SD.
p < 0.01 vs the NS group;
p < 0.01 vs the AS group;
p < 0.01 vs the AS + DEX group.
NS, normal saline; AS, asthma; SCMC, carbocistein; DEX, dexamethasone.
SCMC inhibited the expression of TGF-β1 in asthmatic mice
The levels of TGF-β1 in the BALF of mice were measured by ELISA (Table 2). We found that the TGF-β1 levels in BALF was significantly higher in the AS group compared with the NS group. SCMC or DEX treatment reduced the TGF-β1 content in BALF of asthmatic mice, but SCMC- or DEX-treated mice still showed elevated TGF-β1 levels compared with the NS group.
Table 2.
TGF-β1 content in the BALF of mice
| Groups | TGF-β1 content (ng/L) |
|---|---|
| NS Group | 28.87 ± 4.98 |
| AS Group | 248.86 ± 16.31* |
| AS + SCMC Group | 140.64 ± 10.78*,# |
| AS + DEX Group | 139.95 ± 11.63*,# |
Note: Data were presented as mean ± SD.
P < 0.01 vs the NS group;
P < 0.01 vs the AS group.
NS, normal saline; AS, asthma; SCMC, carbocistein; DEX, dexamethasone.
The expression of TGF-β1 in the airway tissues of mice was analyzed by immunohistochemistry. In the NS group, TGF-β1 protein was expressed in small amounts mainly in airway epithelial cells and not in the rest of the tissues (Figure 3A; Table 3). In contrast, in the AS group, TGF-β1 was largely expressed in the airway wall and lung interstitium (Figure 3B; Table 3). In addition, the mice in the AS + SCMC (Figure 3C; Table 3) and AS + DEX (Figure 3D; Table 3) groups also had a distinct TGF-β1 expression profile, but the expression was significantly lower than that of the AS group.
Figure 3.
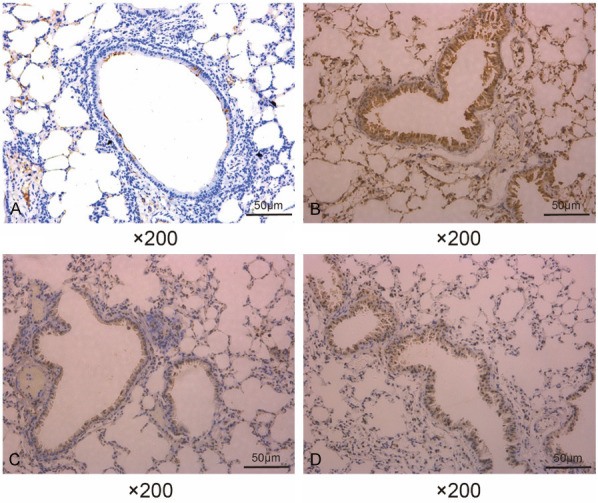
SCMC downregulated TGF-β1 expression in the airways of asthmatic mice. Immunohistochemical staining was performed to detect the expression of TGF-β1 in the airway tissues of mice in the NS group (A), AS group (B), AS + SCMC group (C), and AS + DEX group (D).
Table 3.
TGF-β1 protein expression in lung tissues
| Groups | Corrected mean optical density values |
|---|---|
| NS Group | 0.08 ± 0.01 |
| AS Group | 0.39 ± 0.02* |
| AS + SCMC Group | 0.21 ± 0.01*,# |
| AS + DEX Group | 0.12 ± 0.01*,#,Δ |
Note: Data were presented as mean ± SD.
P < 0.01 vs the NS group;
P < 0.01 vs the AS group;
P < 0.01 vs the AS + SCMC group.
NS, normal saline; AS, asthma; SCMC, carbocistein; DEX, dexamethasone.
Finally, the relative expression of TGF-β1 mRNA in the lung tissues of mice was detected by 2-ΔΔCT values using RT-qPCR assay (Table 4). The results showed that the expression of TGF-β1 mRNA was significantly increased in the airway tissues of asthmatic mice. SCMC and DEX treatment reduced the expression of TGF-β1 mRNA, but the expression was still significantly increased compared with that of mice in the NS group.
Table 4.
TGF-β1 mRNA expression in lung tissues
| Groups | ΔCT | ΔΔCT | 2-ΔΔCT |
|---|---|---|---|
| NS Group | 5.30 ± 0.18 | 0 ± 0.12 | 1.00 ± 0.08 |
| AS Group | 2.71 ± 0.13* | -2.59 ± 0.08* | 6.02 ± 0.25* |
| AS + SCMC Group | 3.35 ± 0.08*,# | -1.95 ± 0.10*,# | 4.23 ± 1.05*,# |
| AS + DEX Group | 4.07 ± 0.14*,#,Δ | -1.23 ± 0.06*,#,Δ | 2.36 ± 0.10*,#,Δ |
| F value | 516.138 | 1198.305 | 129.179 |
Note: Data were presented as mean ± SD.
p < 0.01 vs the NS group;
p < 0.01 vs the AS group;
p < 0.01 vs the AS + SCMC group.
NS, normal saline; AS, asthma; SCMC, carbocistein; DEX, dexamethasone.
Correlation analysis of TGF-β1 expression and collagen deposition
We analyzed whether TGF-β1 expression in airway tissues correlated with collagen fiber deposition (Figure 4). The results showed that the area of Masson-stained collagen deposition in lung tissues of the AS + SCMC group was positively correlated with the level of TGF-β1 mRNA expression (r = 0.82, P < 0.01).
Figure 4.
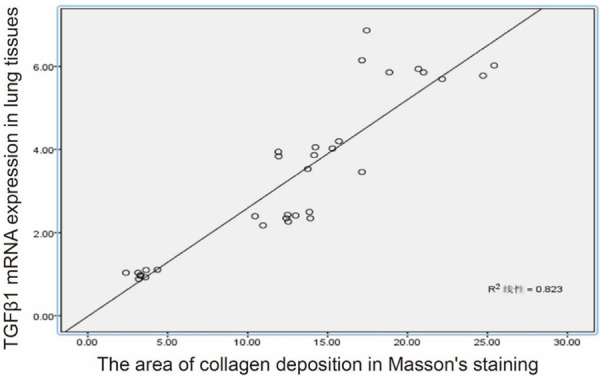
Correlation between the area of collagen deposition of lung tissues and the TGF-β1 mRNA expression.
Discussion
As one of the most vital pathological changes in asthma, airway remodeling is characterized by hypertrophy and hyperplasia of airway smooth muscle, destruction of airway epithelium, thickening of the airway wall, changes in the extracellular matrix, hypertrophy and hyperplasia of mucus glands and infiltration of inflammatory cells [14]. The clinical symptoms and the degree of lung function decline in asthma patients are related to the patients’ airway remodeling to a great extent [15], which highlights the significance of delaying airway remodeling treatment in the management of asthma. In this study, an asthma model with significant airway remodeling was successfully established using combined intraperitoneal and subcutaneous injection of OVA for sensitization and nebulized excitation of BALB/C mice. It was revealed that SCMC can inhibit the expression of TGF-β1 in lung tissues and suppressed airway remodeling in asthmatic mice.
The antitussive and phlegmolytic effects of SCMC are mainly achieved through a series of effects, such as influencing the secretion of bronchial glands, reducing the viscosity of sputum and strengthening the ciliary movement of the respiratory tract [16]. SCMC administration has been previously indicated as a novel therapeutic option for asthmatic patients, especially with cough variant asthma [17]. Actually, adding SCMC to inhaled corticosteroids (ICS) in asthmatic patients can effectively ameliorate the clinical manifestations, such as cough and shortness of breath in asthmatic patients, and the improvement of PEF in patients after the addition of SCMC is more obvious when compared with the application of ICS alone, indicating that SCMC can effectively increase the effect of ICS and play a role in enhancing the lung function of asthmatic patients [18]. Moreover, SCMC is effective in scavenging hypochlorous acid and hydroxyl radicals, thus reducing the release of inflammatory factors (e.g., IL8) and exerting anti-inflammatory effects [19,20]. Besides, it also reduced the release of inflammatory factors such as TGF and VEGF in COPD mice [21]. SCMC ameliorated airway remodeling (thickness of airway epithelium and smooth muscle, airway fibrosis, and the level of TGF-β1) in rats with COPD [22]. More relevantly, Takeda et al. reported that SCMC was effective in reducing airway hyperresponsiveness and airway inflammation at two distinct phases of the response to the secondary allergen challenge in sensitized mice [23]. However, the regulatory effects of SCMC on airway remodeling in asthma remain unclear.
TGF-β regulates airway remodeling through the following actions: (1) causing recurrent epithelial damage and repair of the airway epithelium contributing to increased inflammatory and remodeling responses in the submucosa [24]; in addition, inducing the conversion of airway epithelial cells to mesenchymal cells is also an important role of TGF-β in epithelial cells [25]; (2) promoting subepithelial fibrosis [26]; (3) leading to hypertrophy of airway smooth muscle cells; and (4) resulting in microvascular proliferation and remodeling [27,28]. The severity of asthma and the degree of airway obstruction are positively correlated with the level of TGF-β1 expression [29]. Intramuscular DEX, a glucocorticoid receptor agonist, has been proposed as an equivalent therapy to oral prednisone or prednisolone since its potential advantages include a longer half-life requiring a shorter course and less vomiting [30]. Here, we used DEX as a positive control for SCMC. DEX may antagonize airway remodeling by regulating TGF-β1/Smad3 signaling pathway, which is likely to play a role in the treatment of bronchial asthma [31]. Furthermore, Chung et al. showed that DEX reversed TGF-β1 effects on cAMP levels induced by isoproterenol [32]. Our in vivo observation showed that SCMC showed the consistent inhibitory effects as DEX on TGF-β1 mRNA and protein expression. Even though DEX exhibited superior effects on controlling TGF-β1 levels than SCMC in the present study, up to 30-50% of asthmatics have poor response to corticosteroid treatment [33]. SCMC has been verified to ameliorate steroid sensitivity treatment in COPD as well [34]. Therefore, we believe that SCMC might provide an alternative option for corticosteroid-resistant patients.
In the current experiment, SCMC was found to inhibit airway remodeling by decreasing the expression of TGF-β1 in asthmatic mice, thus providing a theoretical basis for its application in the long-term treatment of asthma. A potential pitfall of this work was the lack of possible mechanistic exploration between SCMC and TGF-β1 downregulation. Future investigation with the examination of airway fibrosis is warranted to validate our data.
Acknowledgements
This study was supported by Master’s Research Start-up Funds of the Affiliated Hospital of Zunyi Medical University (Hospital No. (2013) 23); Science and Technology Project of Guizhou Province (qiankehezhicheng[2020]4Y162).
Disclosure of conflict of interest
None.
References
- 1.Bateman ED, Busse W, Pedersen SE, Bousquet J, Huang S, Zhou X, Gul N, Hollis S, Gibbs M. Global Initiative for Asthma 2016-derived asthma control with fluticasone propionate and salmeterol: a Gaining Optimal Asthma Control (GOAL) study reanalysis. Ann Allergy Asthma Immunol. 2019;123:57–63. e52. doi: 10.1016/j.anai.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Ji HL. Epithelial Sodium and Chloride Channels and Asthma. Chin Med J (Engl) 2015;128:2242–2249. doi: 10.4103/0366-6999.162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antoniu SA. Cytokine antagonists for the treatment of asthma: progress to date. BioDrugs. 2009;23:241–251. doi: 10.2165/11317130-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol. 2011;128:451–462. doi: 10.1016/j.jaci.2011.04.047. quiz 463-454. [DOI] [PubMed] [Google Scholar]
- 5.Joseph C, Tatler AL. Pathobiology of airway remodeling in Asthma: the emerging role of integrins. J Asthma Allergy. 2022;15:595–610. doi: 10.2147/JAA.S267222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang N, Yan D, Liu Y, Liu Y, Gu X, Sun J, Long F, Jiang S. A HuR/TGF-beta1 feedback circuit regulates airway remodeling in airway smooth muscle cells. Respir Res. 2016;17:117. doi: 10.1186/s12931-016-0437-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell SC, Steventon GB. S-carboxymethyl-L-cysteine. Drug Metab Rev. 2012;44:129–147. doi: 10.3109/03602532.2011.631015. [DOI] [PubMed] [Google Scholar]
- 8.Hooper C, Calvert J. The role for S-carboxymethylcysteine (carbocisteine) in the management of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3:659–669. [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Liu J. Intervention of atorvastatin on transforming growth factor-β1 and collagen in COPD rat models. Zhejiang Medical Journal. 2013 [Google Scholar]
- 10.Takeda K, Shiraishi Y, Matsubara S, Miyahara N, Matsuda H, Okamoto M, Joetham A, Gelfand EW. Effects of combination therapy with montelukast and carbocysteine in allergen-induced airway hyperresponsiveness and airway inflammation. Br J Pharmacol. 2010;160:1399–1407. doi: 10.1111/j.1476-5381.2010.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Zheng JP, Zhu SX, Guan WJ, Chen M, Zhong NS. Carbocisteine attenuates hydrogen peroxide-induced inflammatory injury in A549 cells via NF-kappaB and ERK1/2 MAPK pathways. Int Immunopharmacol. 2015;24:306–313. doi: 10.1016/j.intimp.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Aun MV, Bonamichi-Santos R, Arantes-Costa FM, Kalil J, Giavina-Bianchi P. Animal models of asthma: utility and limitations. J Asthma Allergy. 2017;10:293–301. doi: 10.2147/JAA.S121092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kearley J, Buckland KF, Mathie SA, Lloyd CM. Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2-IL-33 pathway. Am J Respir Crit Care Med. 2009;179:772–781. doi: 10.1164/rccm.200805-666OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirota N, Martin JG. Mechanisms of airway remodeling. Chest. 2013;144:1026–1032. doi: 10.1378/chest.12-3073. [DOI] [PubMed] [Google Scholar]
- 15.Panettieri RA Jr, Kotlikoff MI, Gerthoffer WT, Hershenson MB, Woodruff PG, Hall IP, Banks-Schlegel S, National Heart L, Blood I. Airway smooth muscle in bronchial tone, inflammation, and remodeling: basic knowledge to clinical relevance. Am J Respir Crit Care Med. 2008;177:248–252. doi: 10.1164/rccm.200708-1217PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yageta Y, Ishii Y, Morishima Y, Ano S, Ohtsuka S, Matsuyama M, Takeuchi K, Itoh K, Yamamoto M, Hizawa N. Carbocisteine reduces virus-induced pulmonary inflammation in mice exposed to cigarette smoke. Am J Respir Cell Mol Biol. 2014;50:963–973. doi: 10.1165/rcmb.2012-0292OC. [DOI] [PubMed] [Google Scholar]
- 17.Ishiura Y, Fujimura M, Yamamori C, Nobata K, Myou S, Kurashima K, Michishita Y, Takegoshi T. Effect of carbocysteine on cough reflex to capsaicin in asthmatic patients. Br J Clin Pharmacol. 2003;55:504–510. doi: 10.1046/j.1365-2125.2003.01788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Y, Lu HZ, Xu JR, Wang XL, Zhou W, Hou LN, Zhu L, Yu ZH, Chen HZ, Cui YY. Carbocysteine restores steroid sensitivity by targeting histone deacetylase 2 in a thiol/GSH-dependent manner. Pharmacol Res. 2015;91:88–98. doi: 10.1016/j.phrs.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Maccio A, Madeddu C, Panzone F, Mantovani G. Carbocysteine: clinical experience and new perspectives in the treatment of chronic inflammatory diseases. Expert Opin Pharmacother. 2009;10:693–703. doi: 10.1517/14656560902758343. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Gao Y, Yang Q, Fang X, Li Z. Pingchuanning decoction attenuates airway inflammation by suppressing autophagy via phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway in rat models of asthma. J Cell Biochem. 2019;120:3833–3844. doi: 10.1002/jcb.27665. [DOI] [PubMed] [Google Scholar]
- 21.El-Megharbel SM, Hamza RZ, Refat MS. Synthesis, chemical identification, antioxidant capacities and immunological evaluation studies of a novel silver(I) carbocysteine complex. Chem Biol Interact. 2014;220:169–180. doi: 10.1016/j.cbi.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Song Y, Yu P, Lu JJ, Lu HZ, Zhu L, Yu ZH, Chen HZ, Cui YY. A mucoactive drug carbocisteine ameliorates steroid resistance in rat COPD model. Pulm Pharmacol Ther. 2016;39:38–47. doi: 10.1016/j.pupt.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Takeda K, Miyahara N, Kodama T, Taube C, Balhorn A, Dakhama A, Kitamura K, Hirano A, Tanimoto M, Gelfand EW. S-carboxymethylcysteine normalises airway responsiveness in sensitised and challenged mice. Eur Respir J. 2005;26:577–585. doi: 10.1183/09031936.05.00090304. [DOI] [PubMed] [Google Scholar]
- 24.Yeganeh B, Mukherjee S, Moir LM, Kumawat K, Kashani HH, Bagchi RA, Baarsma HA, Gosens R, Ghavami S. Novel non-canonical TGF-beta signaling networks: emerging roles in airway smooth muscle phenotype and function. Pulm Pharmacol Ther. 2013;26:50–63. doi: 10.1016/j.pupt.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, Murray LA, Argentieri R, Kicic A, Stick SM, Bai TR, Knight DA. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med. 2009;180:122–133. doi: 10.1164/rccm.200811-1730OC. [DOI] [PubMed] [Google Scholar]
- 26.Ventura I, Vega A, Chacon P, Chamorro C, Aroca R, Gomez E, Bellido V, Puente Y, Blanca M, Monteseirin J. Neutrophils from allergic asthmatic patients produce and release metalloproteinase-9 upon direct exposure to allergens. Allergy. 2014;69:898–905. doi: 10.1111/all.12414. [DOI] [PubMed] [Google Scholar]
- 27.Lin S, Teng J, Li J, Sun F, Yuan D, Chang J. Association of Chemerin and Vascular Endothelial Growth Factor (VEGF) with diabetic nephropathy. Med Sci Monit. 2016;22:3209–3214. doi: 10.12659/MSM.896781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandaru S, Marri VK, Akka J, Alvala M, Mundluru HP. Association of transforming growth factor-Beta 1 promoter variant -509 c/t with bronchial asthma in South Indian population. Inflammation. 2015;38:409–414. doi: 10.1007/s10753-014-0045-5. [DOI] [PubMed] [Google Scholar]
- 29.Juan LI, Shen Y, Qian Y. Effects of TGF-β_1 type I receptor antagonist on airway inflammation and remodeling in a murine model of chronic bronchial asthma. Chinese Journal of Asthma (Electronic Edition) 2013 [Google Scholar]
- 30.Keeney GE, Gray MP, Morrison AK, Levas MN, Kessler EA, Hill GD, Gorelick MH, Jackson JL. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014;133:493–499. doi: 10.1542/peds.2013-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng Y, Yang C, Yang W, Jiang T. Effect of dexamethasone on TGF-beta1/Smad3 signalling pathway in airway remodelling model of asthmatic rats. J Coll Physicians Surg Pak. 2019;29:537–540. doi: 10.29271/jcpsp.2019.06.537. [DOI] [PubMed] [Google Scholar]
- 32.Chung E, Ojiaku CA, Cao G, Parikh V, Deeney B, Xu S, Wang S, Panettieri RA Jr, Koziol-White C. Dexamethasone rescues TGF-beta1-mediated beta2-adrenergic receptor dysfunction and attenuates phosphodiesterase 4D expression in human airway smooth muscle cells. Respir Res. 2020;21:256. doi: 10.1186/s12931-020-01522-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang AL, Panganiban R, Qiu W, Kho AT, Chupp G, Meyers DA, Bleecker ER, Weiss ST, Lu Q, Tantisira KG. Drug repurposing to treat glucocorticoid resistance in Asthma. J Pers Med. 2021;11:175. doi: 10.3390/jpm11030175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song Y, Chi DY, Yu P, Lu JJ, Xu JR, Tan PP, Wang B, Cui YY, Chen HZ. Carbocisteine improves histone deacetylase 2 deacetylation activity via regulating sumoylation of histone deacetylase 2 in human tracheobronchial epithelial cells. Front Pharmacol. 2019;10:166. doi: 10.3389/fphar.2019.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]


