Abstract
Various treatments for trigeminal neuralgia (TN) are known to yield initial satisfactory results; however, the surgical treatment has excellent long-term outcomes and a low recurrence rate. Surgical treatment addresses the challenge of vascular compression, which accounts for 85% of the causes of TN. As for surgical treatment for TN, microvascular decompression (MVD) has become the surgical treatment of choice after Peter J. Jannetta reported the results of MVD surgery in 1996. Since then, many studies have reported a success rate of over 90% for the initial surgical treatment. Most MVDs aim to separate (decompress) the culprit vessel from the trigeminal nerve. To increase the success rate of surgery, accurate indications for MVD and management of the offender vessels without complications are critical. In addition, if there is no vascular compression, partial sensory rhizotomy or internal neurolysis can be performed to improve surgical outcomes.
Keywords: Trigeminal neuralgia, Microsurgery, Rhizotomy, Microvasclar decompression, Neurosurgical procedures
HISTORY
Trigeminal neuralgia (TN) is a debilitating neuropathic pain disorder characterized by spontaneous and elicited paroxysms of electric shock-like or stabbing pain in the face region [8,11,17]. It affects basic human psychological, physical, and social needs and activities, such as touching or washing the face, brushing teeth, and doing makeup, resulting in anxiety, depression, and social phobia in patients, thereby affecting their mental health[40,51].
TN is initially treated with drugs, but in medically refractory severe cases or cases where it is difficult to maintain the drug due to the side effect of the drug, other treatment is required. Many studies have recommended surgical treatment. In 1858, Carnochan first attempted surgical treatment for directly targeting the trigeminal ganglion [25]. Until the early 20th century, direct nerve sectioning was considered the most effective surgical treatment [57]. In 1929, Dandy has published the results of 88 patients on nerve sectioning and reported that the arterial loop sometimes obscures the surgical field [18]. Thus, he performed the first microvascular decompression (MVD) in neurosurgery without knowing it and not fully understanding the importance of these vascular loops. However, in his monumental paper in 1932, Dandy reported that TN could be caused by a tumor or an association with blood vessels [19]. He observed compression or alteration of the trigeminal nerve by an artery and indicated this to be the cause of the tic douloureux.
Dandy's idea brought a new wind for TN in Europe, but not in the United States. Taarnhøj, a young Danish neurosurgeon, encountered Dandy's hypothesis in the early 1950s and reported in 1954 that 41 out of 70 TN patients experienced complete pain relief and no permanent facial numbness or facial paralysis following decompression surgery [58,59].
After closely observing Taarnhøj's achievements, Gardner promptly perceived the paradigm shift in treatment that his research would bring. He quickly adopted the surgical technique on nine patients and published the results in 1953 [24]. Thereafter, he continued to practice, and in 1959, he published his famous thesis, including 112 patients. Thus, Gardner and Miklos [23] established the indications for vascular decompression surgery for TN. However, the absence of a surgical microscope made it difficult to fully substantiate the hypothesis.
Jannetta developed classical MVD surgery using a surgical microscope to prove that the compression of the arteries or veins can lead to TN. Over time, Janetta refined his surgical technique and eventually adopted the posterior fossa (retrosigmoid) approach [28]. He used Teflon padding to decompress the culprit vessel and trigeminal nerve because it was easy to handle and produced minimal arachnoid scarring. In addition, using this approach, he re-operated on many of Gardner's old patients, who were operated on by Gardner using a gel-foam that got absorbed over time with symptom recurrence. By reviewing Gardner's cases and honing his ideas, Jannetta was able to present evidence of the effectiveness of MVD. Finally, in 1996, 30 years after the initial MVD surgery, he reported a study with 1185 patients who underwent MVD from 1972 to 1991 at the Presbyterian University Hospital in Pittsburgh [6]. The initial success rate of the study was 82% for complete relief, with an additional 16% experiencing partial relief for a combined initial success rate of 98%. At the 10-year follow-up, 68% of patients reported excellent or good relief, whereas the remaining 32% experienced recurrent symptoms. After his great achievements, long-term follow-up studies were published over time, which showed the durability of MVD surgery. Finally, MVD was the surgical treatment of choice for TN.
SURGICAL OUTCOME
MVD is the first-choice surgery for patients with classical TN. A pooled analysis including 5149 patients showed that the MVD efficacy was generally high, as 62–89% of patients were pain-free at the follow-up (after 3–11 years) [9].
MVD shows excellent pain control results in most cases. Initial pain control after MVD was reported to be 80.3–96% [27,29,52,64]. In a prospective study, 92.5% of patients were pain-free without medication for an average of 28 months after surgery [13]. In another study, 85% of patients reported adequate pain control by an average of 38 months [10]. After 5 years post-surgery, 72–85% of patients showed relatively good results [13,52,64].
In a long-term follow-up study, 68% and 73.4% of patients reported complete pain relief after 10 years and 15 years postsurgery, respectively [6,53]. Postoperative pain relief usually occurs immediately after surgery; however, a delayed effect of 1 month or more is observed in some cases [20]. As a result of the study of 196 patients who underwent MVD for TN in our hospital, 157 patients (80%) had excellent outcome (Barrow Neurological Institute [BNI] pain intensity scale I-II) and 183 patients (93.3%) had good outcome (BNI pain intensity scale I-III) within 1 year after surgery (BNI pain intensity scale criteria; I, no pain; II, occasional pain, not requiring medication; III, some pain, controlled with medication; IV, some pain, not controlled with medication; and V, severe pain/no pain relief).
MVD is usually the most effective classical type of TN (type I). Conversely, surgical treatment for multiple sclerosis-related TN is ineffective, with 50% pain relief after 2 years [10]. Factors predicting a good prognosis after surgical treatment include proper neurovascular compression, trigger points for pain, male sex, absence of venous compression, and shorter disease duration [15,26,43,60]. In addition, some studies reported a low recurrence rate in elderly patients [47], while another study mentioned a shorter time to recurrence in patients over 60 years [4]. The recurrence rate might be related to the duration of the disease; however, this remains a controversial factor.
Moreover, sufficient surgical effect for the appropriate indication for repeated MVD is known to exist [3,16,30]. Although this is not as effective as the initial MVD, the initial pain relief rate is 90.3–93.3%, 67% after 12 months, and 42% after 10 years post-surgery with showing no pain [3,5,62].
COMPLICATIONS
Although MVD is a relatively invasive surgery, the complication rate reported due to the development of surgical techniques and specialized surgical skills is quite low [9]. The rates of serious complications, such as death, and intracerebral hemorrhage and stroke have been reported to be 0.3% and 0.6%, respectively [9]. In addition, complications, such as anesthesia dolorosa (0.02%) and meningitis (0.4%), have rarely been observed [9].
Various reports on cranial nerve-related complications are available. Approximately 1.6–22% of trigeminal nerve deficits have been reported, but about half are transient symptoms [7,52,63,66]. Facial weakness is reported to be 0.6–10.6%, but it improves over time [13,29,40,63]. Hearing loss is variously reported (1.2–6.8%) [53,60,63,64,66]; however, in large-scale studies, 1.8% experienced it [9]. Cerebrospinal fluid (CSF) leaks are found in 1.5–4% [21,32,56]. The relationships of the complications with age of the patients were evaluated to identify the risk factors; however, surgical site infections, cranial nerve disorders, and CSF leaks were not related to age [2,47].
In the case of re-MVD, the incidence of complications was higher than that in the initial MVD. Trigeminal nerve disorder was reported in 8.3–32% after reoperation, and hearing impairment occurred in 6.7% of cases [5,16,62].
DECOMPRESSION TECHNIQUE
Interposition technique
This technique is implemented in a conventional MVD. After a sharp dissection of the arachnoid, the blood vessel area associated with the trigeminal nerve gets clearly exposed. This method separates the culprit vessel from the area in contact with the trigeminal nerve using a decompression material [6]. Teflon is usually used as the decompression material (Fig. 1). However, sometimes, if bleeding occurs at the surgical site or if contact with the dura exists due to excessive Teflon insertion, recurrence of symptoms may occur due to granulation with Teflon after surgery [14,49].
Fig. 1.
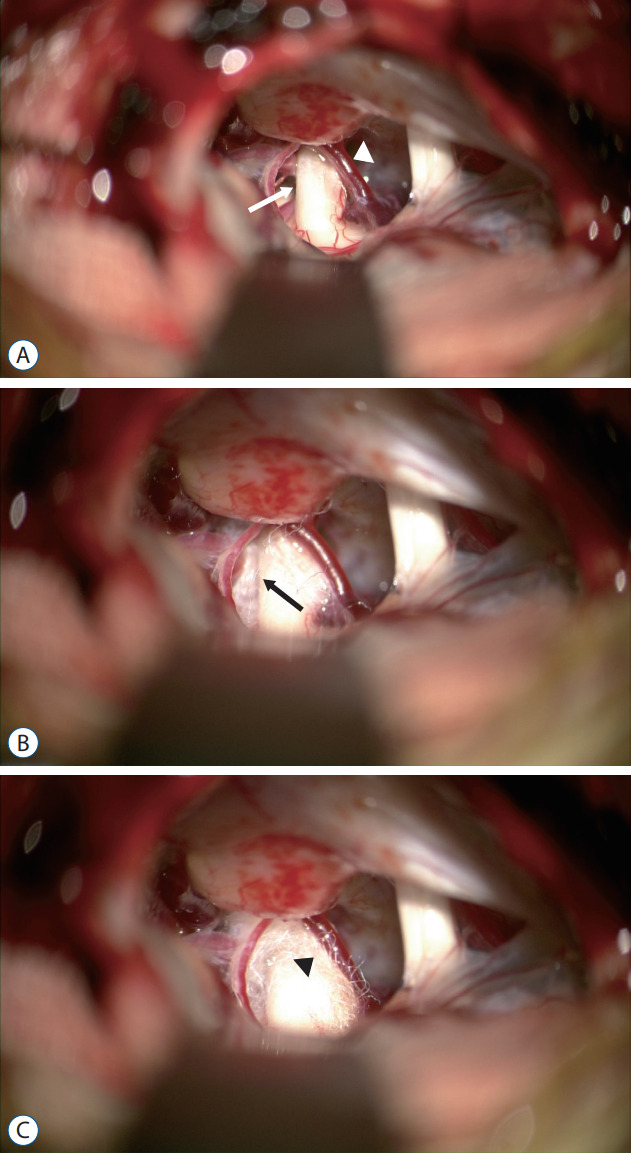
A : The superior cerebellar artery (SCA) is compressing the back side of the trigeminal nerve (TN) (white arrow). The veins also contact the anterior part of the TN (white arrowhead). B : Decompression of SCA was performed using Teflon (black arrow). C : The vein was also decompressed using Teflon (black arrowhead).
Transposition technique
Recently, several studies have suggested that the offending vessel should be removed reliably and thoroughly from the neurovascular compression site rather than inserting a decompression material between the culprit vessel and trigeminal nerve (Fig. 2) [12,35,37,48,67]. This method, first proposed by Fukushima [22], completely separates the trigeminal nerve and the offending vessel. Sindou et al. [54] reported that this non-touching surgical technique had a better long-term prognosis than the touching one. A study suggested suturing of the culprit vessel to the surrounding dura mater with a 5-0 thread [38]. Other study showing good results using the transposition technique in patients who require repeated MVD have also been reported [41]. When the transposition is completely performed, inserting a decompression material is unnecessary and hence, complications related to materials can be prevented [49].
Fig. 2.
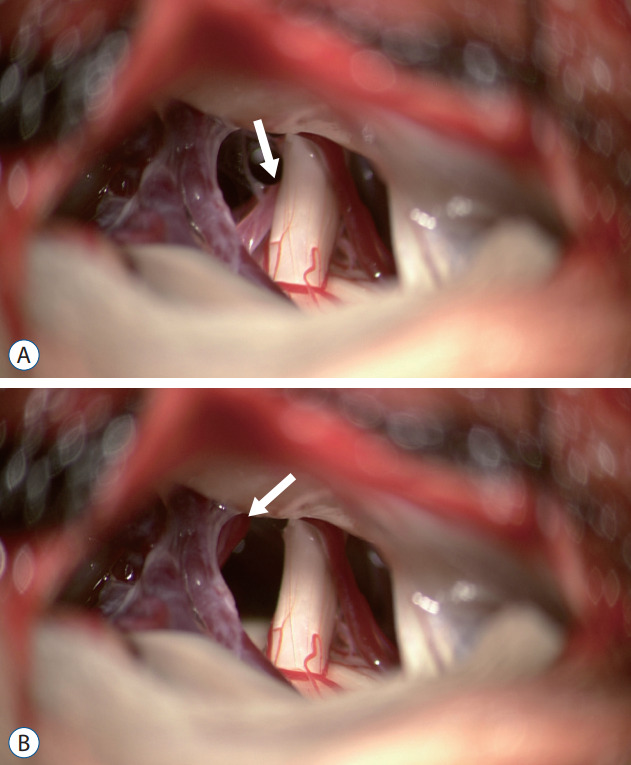
A : The superior cerebellar artery (SCA) was compressed to trigeminal nerve (white arrow). B : Transposition of SCA was performed (white arrow).
However, it is difficult to apply this technique to all TN MVDs because the transposition is too variable due to the diversity of the blood vessels. During transposition, damage to the offending vessels is likely to occur, and sometimes veins must be sacrificed for the workspace. Additionally, excessive movement of the blood vessels can lead to kinking or vasospasm eventually, resulting in stroke [42].
surgical glue or other surgical products, such as tachosil, have recently been used instead of suturing due to the complexity of this surgical technique [1,45,46]. these studies have been recently published, and although the initial effect is acceptable, the durability is controversial because there is not enough follow-up.
Vein offender
Most of the culprit vessels are arteries. However, research on veins has continuously been published over time [31,34,44,50]. A report suggest that in 5–18% of cases, veins are only the offenders [55]. Most studies have suggested the role of veins if symptoms recur or do not disappear after surgery [34]. Another group has suggested that if the culprit vessel of the TN is a vein, the vein must be sacrificed for a complete cure [39]. However, management of veins during MVD surgery is still controversial; many surgeons agree that it is the principle to preserve the veins whenever possible.
Partial sensory rhizotomy (PSR) and internal neurolysis
PSR is performed when no clear offending vessels are observed in the surgical field or preoperative examination. In the past, when anatomical knowledge about somatotrophy was lacking, various means of postoperative pain relief and sensory impairment were suggested [61]. However, these shortcomings have been partially resolved through recent anatomical studies. According to a recent study by Terrier et al. [61], 86.4% of patients reported complete pain relief, and 22.7% exhibited postoperative hypoesthesia following PSR, including secondary TN, in the patient group. In contrast, another study reported a 48% postoperative pain relief. Sensory deficit was reported in 67% of patients [65]. This difference is attributed to the variation in the cross-sectional area of the sensory root and the location of sectioning. As a result, the section of the ventrolateral two-thirds of the pars major of the trigeminal nerve at the pons provides favorable outcomes [61].
If there is no clear offender, internal neurolysis (nerve combing) can be performed. Usually, the trigeminal nerve itself is longitudinally divided along its fibers using a straight blunt-tip probe. Li et al. [36] reported 100% asymptomatic patients immediately after surgery due to internal neurolysis. Thereafter, pain relief was reported in 91.3% of cases. Severe facial numbness immediately after surgery was noted in 11% of patients, and it improved after 6 months post-surgery. Moreover, Ko et al. [33] reported the long-term outcome of internal neurolysis. A 47% pain-free rate was reported after 5 years post-surgery. Most patients (96%) experienced facial numbness or hypesthesia, but these symptoms did not affect the patient's quality of life.
CONCLUSION
The effect of MVD on TN has already been established. However, more advanced research is needed for clearer surgical indications, management of culprit vessels, and durability of the decompression materials. In the future, along with the continuous study on the pathophysiology of TN, a new MVD technique that is safer and can increase the cure rate should be developed.
Footnotes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Informed consent
This type of study does not require informed consent.
Author contributions
Conceptualization : CKP, BJP; Data curation : CKP; Formal analysis : CKP; Methodology : CKP; Project administration : BJP; Visualization : BJP; Writing - original draft : CKP; Writing - review & editing : BJP
Data sharing
None
Preprint
None
References
- 1.Abi-Aad KR, Turcotte E, Patra DP, Welz ME, Maiti T, Hess R, et al. Vascular transposition of the superior cerebellar artery using a fenestrated clip and fibrin glue in trigeminal neuralgia: 2-dimensional operative video. Oper Neurosurg (Hagerstown) 2020;19:E50–E51. doi: 10.1093/ons/opz291. [DOI] [PubMed] [Google Scholar]
- 2.Alford EN, Chagoya G, Elsayed GA, Bernstock JD, Bentley JN, Romeo A, et al. Risk factors for wound-related complications after microvascular decompression. Neurosurg Rev. 2021;44:1093–1101. doi: 10.1007/s10143-020-01296-1. [DOI] [PubMed] [Google Scholar]
- 3.Amador N, Pollock BE. Repeat posterior fossa exploration for patients with persistent or recurrent idiopathic trigeminal neuralgia. J Neurosurg. 2008;108:916–920. doi: 10.3171/JNS/2008/108/5/0916. [DOI] [PubMed] [Google Scholar]
- 4.Ashkan K, Marsh H. Microvascular decompression for trigeminal neuralgia in the elderly: a review of the safety and efficacy. Neurosurgery. 2004;55:840–848. doi: 10.1227/01.neu.0000137660.06337.c5. [DOI] [PubMed] [Google Scholar]
- 5.Bakker NA, Van Dijk JM, Immenga S, Wagemakers M, Metzemaekers JD. Repeat microvascular decompression for recurrent idiopathic trigeminal neuralgia. J Neurosurg. 2014;121:936–939. doi: 10.3171/2014.7.JNS132667. [DOI] [PubMed] [Google Scholar]
- 6.Barker FG, 2nd, Jannetta PJ, Bissonette DJ, Larkins MV, Jho HD. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med. 1996;334:1077–1083. doi: 10.1056/NEJM199604253341701. [DOI] [PubMed] [Google Scholar]
- 7.Bartindale M, Mohamed A, Bell J, Kircher M, Hill J, Anderson D, et al. Neurotologic complications following microvascular decompression: a retrospective study. J Neurol Surg B Skull Base. 2020;81:37–42. doi: 10.1055/s-0039-1677688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayer DB, Stenger TG. Trigeminal neuralgia: an overview. Oral Surg Oral Med Oral Pathol. 1979;48:393–399. doi: 10.1016/0030-4220(79)90064-1. [DOI] [PubMed] [Google Scholar]
- 9.Bendtsen L, Zakrzewska JM, Abbott J, Braschinsky M, Di Stefano G, Donnet A, et al. European academy of neurology guideline on trigeminal neuralgia. Eur J Neurol. 2019;26:831–849. doi: 10.1111/ene.13950. [DOI] [PubMed] [Google Scholar]
- 10.Broggi G, Ferroli P, Franzini A, Servello D, Dones I. Microvascular decompression for trigeminal neuralgia: comments on a series of 250 cases, including 10 patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2000;68:59–64. doi: 10.1136/jnnp.68.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burchiel KJ. Trigeminal neuralgia. J Neurosurg. 2010;112:756–757. doi: 10.3171/2009.8.JNS091061. [DOI] [PubMed] [Google Scholar]
- 12.Chai S, Xu H, Wang Q, Li J, Wang J, Wang Y, et al. Microvascular decompression for trigeminal neuralgia caused by vertebrobasilar dolichoectasia: interposition technique versus transposition technique. Acta Neurochir (Wien) 2020;162:2811–2821. doi: 10.1007/s00701-020-04572-7. [DOI] [PubMed] [Google Scholar]
- 13.Chakravarthi PS, Ghanta R, Kattimani V. Microvascular decompression treatment for trigeminal neuralgia. J Craniofac Surg. 2011;22:894–898. doi: 10.1097/SCS.0b013e31821a07b7. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Lee S, Lui T, Yeh Y, Chen T, Tzaan W. Teflon granuloma after microvascular decompression for trigeminal neuralgia. Surg Neurol. 2000;53:281–287. doi: 10.1016/s0090-3019(00)00169-5. [DOI] [PubMed] [Google Scholar]
- 15.Cheng J, Long J, Hui X, Lei D, Zhang H. Effects of microvascular decompression on depression and anxiety in trigeminal neuralgia: a prospective cohort study focused on risk factors and prognosis. Clin Neurol Neurosurg. 2017;161:59–64. doi: 10.1016/j.clineuro.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Cheng J, Meng J, Lei D, Hui X. Repeat microvascular decompression for patients with persistent or recurrent trigeminal neuralgia: prognostic factors and long-term outcomes. Medicine (Baltimore) 2019;98:e15167. doi: 10.1097/MD.0000000000015167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheshire WP., Jr Trigeminal neuralgia. Curr Pain Headache Rep. 2007;11:69–74. doi: 10.1007/s11916-007-0025-7. [DOI] [PubMed] [Google Scholar]
- 18.Dandy WE. An operation for the cure of tic douloureux: partial section of the sensory root at the pons. Arch Surg. 1929;18:687–734. [Google Scholar]
- 19.Dandy WE. The treatment of trigeminal neuralgia by the cerebellar route. Ann Surg. 1932;96:787–795. doi: 10.1097/00000658-193210000-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Degn J, Brennum J. Surgical treatment of trigeminal neuralgia. results from the use of glycerol injection, microvascular decompression, and rhizotomia. Acta Neurochir (Wien) 2010;152:2125–2132. doi: 10.1007/s00701-010-0840-1. [DOI] [PubMed] [Google Scholar]
- 21.Eseonu CI, Goodwin CR, Zhou X, Theodros D, Bender MT, Mathios D, et al. Reduced CSF leak in complete calvarial reconstructions of microvascular decompression craniectomies using calcium phosphate cement. J Neurosurg. 2015;123:1476–1479. doi: 10.3171/2015.1.JNS142102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukushima T. Posterior cranial fossa neurovascular decompression (Jannetta method) for trigeminal neuralgia and facial spasm. No Shinkei Geka. 1982;10:1257–1261. [PubMed] [Google Scholar]
- 23.Gardner WJ, Miklos MV. Response of trigeminal neuralgia to decompression of sensory root; discussion of cause of trigeminal neuralgia. J Am Med Assoc. 1959;170:1773–1776. doi: 10.1001/jama.1959.03010150017004. [DOI] [PubMed] [Google Scholar]
- 24.Gardner WJ, Pinto JP. The tarrnhoj operation; relief of trigeminal neuralgia without numbness. Cleve Clin Q. 1953;20:364–367. doi: 10.3949/ccjm.20.2.364. [DOI] [PubMed] [Google Scholar]
- 25.Harris W. A history of the treatment of trigeminal neuralgia. Postgrad Med J. 1951;27:18–21. doi: 10.1136/pgmj.27.303.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinskou TB, Maarbjerg S, Wolfram F, Rochat P, Brennum J, Olesen J, et al. Favourable prognosis of trigeminal neuralgia when enrolled in a multidisciplinary management program - a two-year prospective real-life study. J Headache Pain. 2019;20:23. doi: 10.1186/s10194-019-0973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holste K, Chan AY, Rolston JD, Englot DJ. Pain outcomes following microvascular decompression for drug-resistant trigeminal neuralgia: a systematic review and meta-analysis. Neurosurgery. 2020;86:182–190. doi: 10.1093/neuros/nyz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jannetta PJ. Treatment of trigeminal neuralgia by suboccipital and transtentorial cranial operations. Clin Neurosurg. 1977;24:538–549. doi: 10.1093/neurosurgery/24.cn_suppl_1.538. [DOI] [PubMed] [Google Scholar]
- 29.Kabatas S, Karasu A, Civelek E, Sabanci AP, Hepgul KT, Teng YD. Microvascular decompression as a surgical management for trigeminal neuralgia: long-term follow-up and review of the literature. Neurosurg Rev. 2009;32:87–93. doi: 10.1007/s10143-008-0171-3. [DOI] [PubMed] [Google Scholar]
- 30.Kang IH, Park BJ, Park CK, Malla HP, Lee SH, Rhee BA. A clinical analysis of secondary surgery in trigeminal neuralgia patients who failed prior treatment. J Korean Neurosurg Soc. 2016;59:637–642. doi: 10.3340/jkns.2016.59.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasuya H, Tani S, Kubota Y, Yokosako S, Ohbuchi H, Arai N, et al. Characteristics and management of the offending veins in microvascular decompression surgery for trigeminal neuralgia. Neurosurg Rev. 2021;44:2337–2347. doi: 10.1007/s10143-020-01411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan SA, Laulloo A, Vats A, Nath F. Microvascular decompression: incidence and prevention of postoperative CSF leakage in a consecutive series of 134 patients. Br J Neurosurg. 2020;34:416–418. doi: 10.1080/02688697.2020.1749989. [DOI] [PubMed] [Google Scholar]
- 33.Ko AL, Ozpinar A, Lee A, Raslan AM, McCartney S, Burchiel KJ. Long-term efficacy and safety of internal neurolysis for trigeminal neuralgia without neurovascular compression. J Neurosurg. 2015;122:1048–1057. doi: 10.3171/2014.12.JNS14469. [DOI] [PubMed] [Google Scholar]
- 34.Lee SH, Levy EI, Scarrow AM, Kassam A, Jannetta PJ. Recurrent trigeminal neuralgia attributable to veins after microvascular decompression. Neurosurgery. 2000;46:356–361. doi: 10.1097/00006123-200002000-00019. [DOI] [PubMed] [Google Scholar]
- 35.Li GW, Zhang WC, Min Y, Ma QF, Zhong WX. Surgical skills of adhesions and transposition of trigeminal nerve for primary trigeminal neuralgia. J Craniofac Surg. 2014;25:1296–1298. doi: 10.1097/SCS.0000000000000746. [DOI] [PubMed] [Google Scholar]
- 36.Li MW, Jiang XF, Niu C. Efficacy of internal neurolysis for trigeminal =neuralgia without vascular compression. J Neurol Surg A Cent Eur Neurosurg. 2021;82:364–368. doi: 10.1055/s-0041-1723809. [DOI] [PubMed] [Google Scholar]
- 37.Lin CF, Chen HH, Hernesniemi J, Lee CC, Liao CH, Chen SC, et al. An easy adjustable method of ectatic vertebrobasilar artery transposition for microvascular decompression. Clin Neurol Neurosurg. 2012;114:951–956. doi: 10.1016/j.clineuro.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 38.Masuoka J, Matsushima T, Kawashima M, Nakahara Y, Funaki T, Mineta T. Stitched sling retraction technique for microvascular decompression: procedures and techniques based on an anatomical viewpoint. Neurosurg Rev. 2011;34:373–379. doi: 10.1007/s10143-011-0310-0. [DOI] [PubMed] [Google Scholar]
- 39.McLaughlin MR, Jannetta PJ, Clyde BL, Subach BR, Comey CH, Resnick DK. Microvascular decompression of cranial nerves: lessons learned after 4400 operations. J Neurosurg. 1999;90:1–8. doi: 10.3171/jns.1999.90.1.0001. [DOI] [PubMed] [Google Scholar]
- 40.Melek LN, Devine M, Renton T. The psychosocial impact of orofacial pain in trigeminal neuralgia patients: a systematic review. Int J Oral Maxillofac Surg. 2018;47:869–878. doi: 10.1016/j.ijom.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Meybodi AT, Habibi Z, Miri M, Tabatabaie SA. Microvascular decompression for trigeminal neuralgia using the 'stitched sling retraction' technique in recurrent cases after previous microvascular decompression. Acta Neurochir (Wien) 2014;156:1181–1187. doi: 10.1007/s00701-014-2092-y. [DOI] [PubMed] [Google Scholar]
- 42.Mitsos AP, Georgakoulias N, Lafazanos SA, Konstantinou EA. The "hanging technique" of vascular transposition in microvascular decompression for trigeminal neuralgia: technical report of four cases. Neurosurg Rev. 2008;31:327–330. doi: 10.1007/s10143-008-0144-6. [DOI] [PubMed] [Google Scholar]
- 43.Nunta-Aree S, Patiwech K, Sitthinamsuwan B. Microvascular decompression for treatment of trigeminal neuralgia: factors that predict complete pain relief and study of efficacy and safety in older patients. World Neurosurg. 2018;110:e979–e988. doi: 10.1016/j.wneu.2017.11.147. [DOI] [PubMed] [Google Scholar]
- 44.Oishi M, Fukuda M, Noto Y, Kawaguchi T, Hiraishi T, Fujii Y. Trigeminal neuralgia associated with the specific bridging pattern of transverse pontine vein: diagnostic value of three-dimensional multifusion volumetric imaging. Stereotact Funct Neurosurg. 2011;89:226–233. doi: 10.1159/000326778. [DOI] [PubMed] [Google Scholar]
- 45.Otani N, Toyooka T, Fujii K, Kumagai K, Takeuchi S, Tomiyama A, et al. "Birdlime" technique using tachosil tissue sealing sheet soaked with fibrin glue for sutureless vessel transposition in microvascular decompression: operative technique and nuances. J Neurosurg. 2018;128:1522–1529. doi: 10.3171/2017.1.JNS161243. [DOI] [PubMed] [Google Scholar]
- 46.Otani N, Toyooka T, Takeuchi S, Tomiyama A, Wada K, Mori K. Novel technical variations and increased adhesive strength in the "Birdlime" transposition technique for microvascular decompression. World Neurosurg. 2018;116:e460–e468. doi: 10.1016/j.wneu.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Phan K, Rao PJ, Dexter M. Microvascular decompression for elderly patients with trigeminal neuralgia. J Clin Neurosci. 2016;29:7–14. doi: 10.1016/j.jocn.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 48.Pines AR, Butterfield RJ, Turcotte EL, Garcia JO, De Lucia N, Algier EJ, et al. Microvascular transposition without teflon: a single institution's 17-year experience treating trigeminal neuralgia. Oper Neurosurg (Hagerstown) 2021;20:397–405. doi: 10.1093/ons/opaa413. [DOI] [PubMed] [Google Scholar]
- 49.Rzaev DA, Kulikova EV, Moysak GI, Voronina EI, Ageeva TA. Teflon granuloma after microvascular decompression of the trigeminal nerve root in a patient with recurrent trigeminal neuralgia. Zh Vopr Neirokhir Im N N Burdenko. 2016;80:78–83. doi: 10.17116/neiro201680278-83. [DOI] [PubMed] [Google Scholar]
- 50.Sato O, Kanazawa I, Kokunai T. Trigeminal neuralgia caused by compression of trigeminal nerve by pontine vein. Surg Neurol. 1979;11:285–286. [PubMed] [Google Scholar]
- 51.Seo HJ, Park CK, Choi MK, Ryu J, Park BJ. Clinical outcome of percutaneous trigeminal nerve block in elderly patients in outpatient clinics. J Korean Neurosurg Soc. 2020;63:814–820. doi: 10.3340/jkns.2020.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shibahashi K, Morita A, Kimura T. Surgical results of microvascular decompression procedures and patient's postoperative quality of life: review of 139 cases. Neurol Med Chir (Tokyo) 2013;53:360–364. doi: 10.2176/nmc.53.360. [DOI] [PubMed] [Google Scholar]
- 53.Sindou M, Leston J, Decullier E, Chapuis F. Microvascular decompression for primary trigeminal neuralgia: long-term effectiveness and prognostic factors in a series of 362 consecutive patients with clear-cut neurovascular conflicts who underwent pure decompression. J Neurosurg. 2007;107:1144–1153. doi: 10.3171/JNS-07/12/1144. [DOI] [PubMed] [Google Scholar]
- 54.Sindou M, Leston JM, Decullier E, Chapuis F. Microvascular decompression for trigeminal neuralgia: the importance of a noncompressive technique--kaplan-meier analysis in a consecutive series of 330 patients. Neurosurgery. 2008;63:341–350. doi: 10.1227/01.NEU.0000327022.79171.D6. [DOI] [PubMed] [Google Scholar]
- 55.Soni P, Potter T, Soni PP, Estemalik E, Recinos PF, Kshettry VR. Outcomes of microvascular decompression for trigeminal neuralgia with purely venous compression: a systematic review and meta-analysis. Clin Neurol Neurosurg. 2020;198:106230. doi: 10.1016/j.clineuro.2020.106230. [DOI] [PubMed] [Google Scholar]
- 56.Stoker MA, Forbes JA, Hanif R, Cooper C, Nian H, Konrad PE, et al. Decreased rate of CSF leakage associated with complete reconstruction of suboccipital cranial defects. J Neurol Surg B Skull Base. 2012;73:281–286. doi: 10.1055/s-0032-1312709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sweet WH. The history of the development of treatment for trigeminal neuralgia. Clin Neurosurg. 1985;32:294–318. [PubMed] [Google Scholar]
- 58.Taarnhoj P. Trigeminal neuralgia and decompression of the trigeminal root. Surg Clin North Am. 1956:1145–1157. [PubMed] [Google Scholar]
- 59.Taarnhøj P. Decompression of the trigeminal root. J Neurosurg. 1954;11:299–305. doi: 10.3171/jns.1954.11.3.0299. [DOI] [PubMed] [Google Scholar]
- 60.Tang X, Wang Y, Shu Z, Hou Y. Efficacy and prognosis of trigeminal neuralgia treated with surgical excision or gamma knife surgery. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2012;37:616–620. doi: 10.3969/j.issn.1672-7347.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 61.Terrier LM, Amelot A, François P, Destrieux C, Zemmoura I, Velut S. Therapeutic failure in trigeminal neuralgia: from a clarification of trigeminal nerve somatotopy to a targeted partial sensory rhizotomy. World Neurosurg. 2018;117:e138–e145. doi: 10.1016/j.wneu.2018.05.211. [DOI] [PubMed] [Google Scholar]
- 62.Theodros D, Rory Goodwin C, Bender MT, Zhou X, Garzon-Muvdi T, De la Garza-Ramos R, et al. Efficacy of primary microvascular decompression versus subsequent microvascular decompression for trigeminal neuralgia. J Neurosurg. 2017;126:1691–1697. doi: 10.3171/2016.5.JNS151692. [DOI] [PubMed] [Google Scholar]
- 63.Tomasello F, Esposito F, Abbritti RV, Angileri FF, Conti A, Cardali SM, et al. Microvascular decompression for trigeminal neuralgia: technical refinement for complication avoidance. World Neurosurg. 2016;94:26–31. doi: 10.1016/j.wneu.2016.06.097. [DOI] [PubMed] [Google Scholar]
- 64.Xia L, Zhong J, Zhu J, Wang YN, Dou NN, Liu MX, et al. Effectiveness and safety of microvascular decompression surgery for treatment of trigeminal neuralgia: a systematic review. J Craniofac Surg. 2014;25:1413–1417. doi: 10.1097/SCS.0000000000000984. [DOI] [PubMed] [Google Scholar]
- 65.Young JN, Wilkins RH. Partial sensory trigeminal rhizotomy at the pons for trigeminal neuralgia. J Neurosurg. 1993;79:680–687. doi: 10.3171/jns.1993.79.5.0680. [DOI] [PubMed] [Google Scholar]
- 66.Yue Y, Zhao ZR, Liu DC, Liu HJ, Lu DL, Zhang H, et al. Life-threatening complications after microvascular decompression procedure: lessons from a consecutive series of 596 patients. J Clin Neurosci. 2021;86:64–70. doi: 10.1016/j.jocn.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 67.Zhang D, Barata A, Pires P, Soares P, Marques L. Transposition of superior cerebellar artery for microvascular decompression in trigeminal neuralgia using an in situ superior petrosal vein sling technique. World Neurosurg. 2020;134:402–407. doi: 10.1016/j.wneu.2019.11.029. [DOI] [PubMed] [Google Scholar]


