Abstract
Background/Purpose
The efficacy and safety of coronavirus disease 2019 (COVID-19) booster vaccines remain limited. We investigated the immunogenicity and adverse events of the third dose of mRNA vaccines in healthy adults.
Methods
Volunteers vaccinated with two doses of the adenoviral vaccine (ChAdOx1) 12 weeks before were administered with an mRNA COVID-19 vaccine. These were divided into three groups, full-dose mRNA-1273 (group 1); half-dose mRNA-1273 (group 2); and full-dose BNT-162b2 (group 3). Primary outcomes included serum anti-SARS-CoV-2 spike immunoglobulin G (IgG) titers and neutralizing antibody titers against B.1.1.7 (alpha), B.1.617.2 (delta), and B.1.1.529 (omicron) variants. Secondary outcomes included the evaluation of humoral and cellular immunity and vaccine-associated adverse events after the boost.
Results
Totally 300 participants were recruited, and 298 participants were enrolled. For all three groups, an increase in anti-SARS-CoV-2 spike IgG geometric mean titers (30.12- to 71.80-fold) and neutralizing antibody titers against the alpha variant (69.80- to 173.23-folds), delta variant (132.69- to 324.63-folds), and omicron variant (135.36- to 222.37-folds) were observed on day 28. All groups showed robust T- and B-cell responses after boosting. Adverse events were overall mild and transient but with higher prevalence and severity in group 1 participants than in other groups.
Conclusion
Third dose mRNA COVID-19 vaccines markedly enhanced cellular and humoral responses and were safe. Immunological responses and adverse events were higher in individuals receiving the full-dose mRNA-1273 vaccine, followed by a half-dose mRNA-1273 vaccine and BNT-162b2 vaccine.
Keywords: Messenger RNA vaccine, Coronavirus disease 2019 (COVID-19), Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), Immune response
Introduction
Coronavirus disease 2019 (COVID-19) continues to spread worldwide. As of April 12, 2022, the World Health Organization (WHO) reported 500 million confirmed COVID-19 cases and more than 6 million deaths related to the disease.1 Hand hygiene, physical distancing, wearing masks, contact tracing, and isolation were implemented to reduce transmission and prevent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections. However, mass vaccination against SARS-CoV-2 to provide herd immunity remains the most practical measure to control disease outbreaks and limit severe disease and death.2 , 3
COVID-19 vaccines approved by the WHO under the Emergency Use Listing (EUL) have shown efficacy and effectiveness in preventing infection and disease progression after a standard two-dose vaccination schedule.4, 5, 6 However, waning of the immune response against SARS-CoV-2 has been reported.7 , 8 A systematic review revealed that the effectiveness of vaccines against SARS-CoV-2 infection and symptomatic disease decreases by approximately 20%–30% in six months.9 Population-based data from the United States indicated a decline in vaccine effectiveness upon the increased prevalence of the delta variant.10 Given the decrease in vaccine effectiveness against the variants of concern,11 understanding the effectiveness and efficacy of the third dose of COVID-19 vaccines is important for deciding the vaccination policy to control the spread of SARS-CoV-2 infection.
Heterologous ChAdOx1 (AstraZeneca, UK)/mRNA-1273 (Moderna, USA) and ChAdOx1/BNT162b2 (BioNTech/Pfizer, Germany) vaccination schedules could provide better humoral and cellular immunity than the homologous ChAdOx1 vaccination schedules.12 , 13 Previously, we reported that administering a second dose of the mRNA-1273 vaccine to individuals vaccinated with the ChAdOx1 vaccine resulted in stronger immune responses than those receiving two doses of the ChAdOx1 vaccine.14 We confirmed that neutralizing antibody responses against the B.1.1.7 (alpha) and B.1.617.2 (delta) variants were increased after heterologous mRNA-1273 vaccination compared to that following homologous ChAdOx1 vaccination.14 Recently, the emerging SARS-CoV-2 omicron (B.1.1.529) variant with multiple novel spike protein mutations has raised concerns about escape from naturally acquired immunity and vaccine-elicited protection.15 Vaccination with the mRNA vaccines (BNT162b2 and mRNA-1273) has proven effective in preventing hospital admissions, severe disease, and death caused by the SARS-CoV-2 variants of concern.16 Therefore, it is plausible that administering an mRNA vaccine booster may improve protection against these variants of concern.17 , 18
In this prospective study, we investigated the immunogenicity and adverse events of three different COVID-19 mRNA vaccine regimens in individuals previously vaccinated with two doses of the ChAdOx1 vaccine. Neutralizing antibody titers against the SARS-CoV-2 variants of concern and cellular immune responses were measured.
Materials and methods
Study design and participants
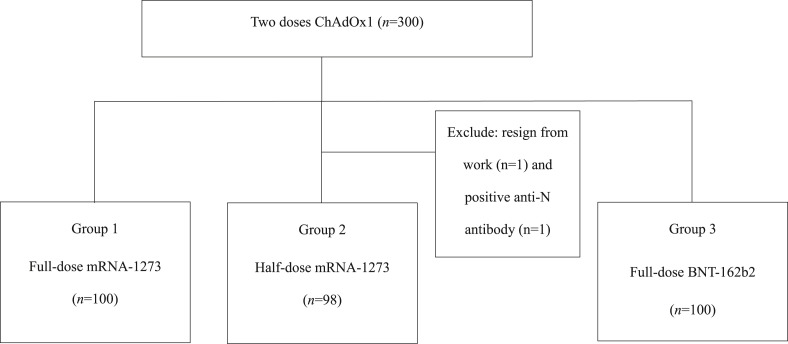
Healthy volunteers who had previously received two ChAdOx1 vaccine doses 12 weeks after the last dose were recruited at the National Taiwan University Hospital. Participants were randomly assigned to three groups: full dose of the mRNA-1273 vaccine (group 1), half dose of the mRNA-1273 vaccine (group 2), or full dose of the BNT-162b2 vaccine (group 3) (Fig. 1 ). There were 100 participants in each group, and blood was drawn from all participants for anti-SARS-CoV-2 spike immunoglobulin G (IgG) antibody titers before and 28 (±3 days) after administering the third vaccine dose. Neutralizing antibody tests were performed on 32 serum samples randomly selected from each group. The cellular immune response was evaluated in 25 participants from each group based on their willingness on the day before and 28 days after administration of the third vaccine dose. A standard diary card was designed to evaluate the safety of the vaccination according to WHO guidelines,19 wherein participants were instructed to record any adverse reactions from the first day after the booster.
Figure 1.
Classification of the three groups included in the study. Abbreviations: mRNA, messenger RNA.
The enrolment criteria and testing procedure here were similar to those outlined in our previous report.14 Adults aged 20–65 years, without underlying illness or well-controlled comorbidities, who had already received two doses of the ChAdOx1 vaccine, were eligible for recruitment. The exclusion criteria included previous laboratory-confirmed SARS-CoV-2 infection, history of other vaccinations within 30 days, pregnancy or breastfeeding, uncontrolled medical illness, and immunosuppression status evaluated by the study investigators. Serum anti-SARS-CoV-2 spike IgG concentrations and neutralizing antibody titers against the alpha, delta, and omicron variants, measured on day 28 after administering the third dose, were considered as the primary outcomes. Secondary outcomes included cellular or humoral immune responses on the 28th day after the boost and adverse reactions during the study period.
Ethics declaration
This study has been approved by the Institutional Review Boards (Ethics Committee) of National Taiwan University Hospital (IRB No. 202111038 MINC).
Laboratory tests
The laboratory test procedure was similar to that in our previous report.14 Briefly, anti-SARS-CoV-2 spike IgG was determined using the Abbott SARS-CoV-2 IgG II Quant assay (Abbott, Chicago, IL). Serum neutralization titers (NT50) were calculated and expressed as the reciprocals of the highest serum dilution that inhibited 50% of the cytopathic effects. The SARS-CoV-2 alpha, delta, and omicron variants of concern were used in the neutralizing antibody test.
Cell isolation, stimulation, and analysis of spike-specific T and B cells were performed. For T helper cell cytokine measurement, peripheral blood mononuclear cells were stimulated with SARS-CoV-2 spike protein-overlapping peptide pools derived from the omicron variant and incubated for 24 h. The levels of secreted cytokines (interleukin-2 [IL-2], IL-6, IL-10, interferon [IFN]-γ, tumor necrosis factor [TNF]-α, IL-5, IL-13, and IL-4) were measured. To detect antigen-specific B cells, the spike protein of the omicron variant (R&D Systems, Minneapolis, MN, USA) was biotinylated. A panel of surface marker antibodies, namely anti-CD3 PE-Cy7, anti-CD19 BV421, anti-CD20 PE-CF594, anti-IgG APC-H7, and anti-IgM BUV395, was used.
Statistical analysis
Categorical variables were compared using the chi-square test or Fisher's exact test. Continuous variables were compared using the Student's t-test. The average values of antibody titers are expressed as geometric means with 95% confidence intervals. The Mann–Whitney U test was performed to compare the antibody responses between the groups. All analyses were set at a 2-tailed significance level of 0.05. All statistical analyses were performed using the Stata software (version 14; StataCorp, College Station, Texas, USA).
Results
A total of 300 participants were recruited, and 298 participants were enrolled. Two participants of group 2 were excluded as one resigned from work and the other was anti-N antibody positive, which indicates SARS-CoV-2 infection. The demographic characteristics and concurrent medications are shown in Table 1 . There were no significant differences in the demographic characteristics, such as age, sex, underlying diseases, and current medication between the three groups.
Table 1.
Baseline demographic characteristics of the three study groups.
| Group 1 Full-dose mRNA-1273 (n = 100) | Group 2 Half-dose mRNA-1273 (n = 98) | Group 3 Full-dose BNT-162b2 (n = 100) | P values | |
|---|---|---|---|---|
| Age (Mean ± SD) | 39.85 ± 9.68 | 37.24 ± 12.43 | 38.98 ± 10.78 | 0.240 |
| Male, n (%) | 35 (35.0%) | 33 (33.7%) | 24 (24.0%) | 0.185 |
| Underlying systemic diseases, n (%) | ||||
| DM under OHA | 2 (2.0%) | 1 (1.0%) | 2 (2.0%) | 0.826 |
| DM under insulin | 1 (1.0%) | 0 (0.0%) | 0 (0.0%) | 0.370 |
| Hypertension | 5 (5.0%) | 6 (6.1%) | 7 (7.0%) | 0.838 |
| Coronary arterial disease | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Congestive heart failure | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Stroke | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Chronic lung disease | 0 (0.0%) | 1 (1.0%) | 0 (0.0%) | 0.359 |
| Chronic viral hepatitis | 3 (3.0%) | 0 (0.0%) | 3 (3.0%) | 0.223 |
| Decompensated hepatic insufficiency | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Chronic kidney disease | 2 (2.0%) | 0 (0.0%) | 1 (1.0%) | 0.370 |
| ESRD under dialysis | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Hyperthyroidism | 3 (3.0%) | 1 (1.0%) | 1 (1.0%) | 0.450 |
| Hypothyroidism | 0 (0.0%) | 1 (1.0%) | 0 (0.0%) | 0.359 |
| Rheumatoid arthritis | 1 (1.0%) | 0 (0.0%) | 1 (1.0%) | 0.611 |
| Ankylosing spondylitis | 2 (2.0%) | 0 (0.0%) | 0 (0.0%) | 0.136 |
| Antiphospholipid syndrome | 2 (2.0%) | 1 (1.0%) | 0 (0.0%) | 0.367 |
| Systemic lupus erythematosus | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Sjogren Syndrome | 1 (1.0%) | 0 (0.0%) | 1 (1.0%) | 0.611 |
| Malignancies | 0 (0.0%) | 3 (3.1%) | 1 (1.0%) | 0.162 |
| Seronegative spondyloarthritis | 0 (0.0%) | 1 (1.0%) | 2 (2.0%) | 0.367 |
| Autoimmune thyroiditis | 4 (4.0%) | 1 (1.0%) | 0 (0.0%) | 0.073 |
| Current medication, n (%) | ||||
| Methotrexate | 0 (0.0%) | 1 (1.0%) | 1 (1.0%) | 0.601 |
| Plaquenil | 2 (2.0%) | 2 (2.0%) | 3 (3.0%) | 0.870 |
| Rituximab | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| NSAID except COX-2 | 0 (0.0%) | 2 (2.0%) | 2 (2.0%) | 0.359 |
| COX-2 inhibitor | 3 (3.0%) | 3 (3.1%) | 3 (3.0%) | 1.000 |
| Sulfasalazine | 2 (2.0%) | 0 (0.0%) | 1 (1.0%) | 0.370 |
| Steroid | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| OPD visit for adverse effect | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
Abbreviation: SD, standard deviation; DM, diabetes mellitus; OHA, oral hypoglycemic agent; ESRD, end stage renal disease; NSAID, non-steroidal anti-inflammatory drug; COX-2, cyclooxygenase-2; OPD, out-patient department.
SARS-CoV-2 anti-spike IgG titers
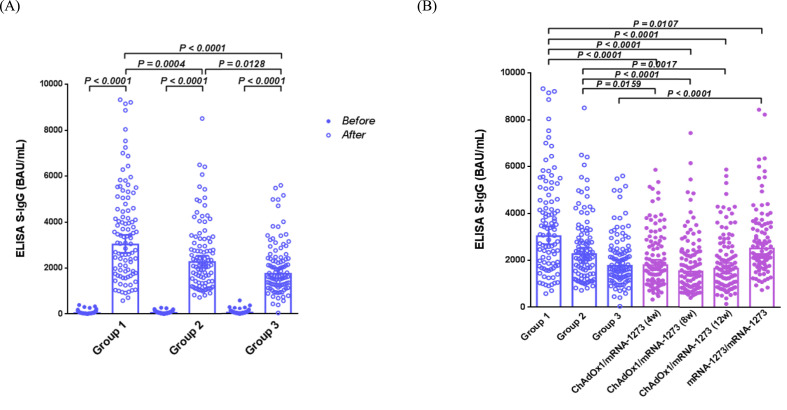
The SARS-CoV-2 anti-spike IgG titers (geometric mean [95% confidence interval] and BAU/mL) of the three groups before and after booster vaccination are shown in Table 2 and Fig. 2 A. The SARS-CoV-2 anti-spike IgG titers increased significantly on day 28 after administration of the third dose compared to those before the booster vaccination (P < 0.001). The anti-spike IgG titers before booster vaccination were significantly lower in group 1 than in groups 2 and 3 (P = 0.0102 and 0.0023, respectively). However, the SARS-CoV-2 anti-spike IgG titers for group 1 (3039 [2675 to 3453] BAU/mL) were significantly higher than that of groups 2 (2253 [2007 to 2529] BAU/mL) and 3 (1764 [1542 to 2018] BAU/mL) on the 28th day after booster vaccination (P = 0.0004 and P < 0.0001, respectively) (Table 2). The fold change in antibody titers on the 28th day after booster vaccination in comparison to before booster vaccination was significantly higher for group 1 (71.80), followed by group 2 (41.11), and then by group 3 (30.12) (Table 2).
Table 2.
Anti-SARS-CoV-2 spike antibody responses of the study subjects before and 28 days after receiving the third-dose of the mRNA vaccine.
| Group 1 Full-dose mRNA-1273 (n = 100) | Group 2 Half-dose mRNA-1273 (n = 98) | Group 3 Full-dose BNT-162b2 (n = 100) | P values |
||||
|---|---|---|---|---|---|---|---|
| Group 1 vs. Group 2 | Group 1 vs. Group 3 | Group 2 vs. Group 3 | |||||
| Day 1 | Anti-S IgG (BAU/mL) Geometric means (95% CI) | 42.32 (35.97–49.79) | 54.80 (47.13–63.71) | 58.57 (50.10–68.48) | 0.0105 | 0.0023 | NS |
| Day 28 | Anti-S IgG (BAU/mL) Geometric means (95% CI) | 3039 (2675–3453) | 2253 (2007–2529) | 1764 (1542–2018) | 0.0004 | <0.0001 | 0.0128 |
| Folds change | 71.80 | 41.11 | 30.12 | <0.0001 | <0.0001 | 0.0043 | |
P values were calculated by Mann Whitney test.
95% CI = 95% confidence interval, NS = not significant.
Figure 2.
Anti SARS-CoV-2 spike IgG antibody responses (BAU/mL) in the study subjects. (A) Antibody responses in the study subjects from the three groups at the day before and 28 days after vaccination with the third dose of the mRNA vaccine. (B) Comparison of the antibody responses between individuals following a three- and two-dose vaccination schedule. ChAdOx1/mRNA-1273 indicates a heterologous mRNA-1273 vaccine boost after priming with the adenoviral vectored ChAdOx1 vaccine. The number in parenthesis indicates the interval (weeks) between the first and second doses of the respective vaccines. mRNA-1273/mRNA-1273 indicates a homologous mRNA-1273 vaccine regimen. Mann–Whitney U test was performed to compare the antibody responses between the groups. P values have been indicated when a statistical significance was determined. Abbreviations: ELISA S-IgG.
Anti-SARS-CoV-2 anti-spike IgG titers on the 28th day after the administration of booster doses to the three groups were compared with those in subjects from our previous study who had received a homologous or heterologous 2nd dose of the COVID-19 vaccine (Fig. 2B).14 We observed that the antibody responses in group 1 participants were significantly higher than those in individuals receiving one or two doses of the mRNA-1273 vaccine (P < 0.0001 to 0.0107). The antibody responses of group 2 participants were significantly higher than those of individuals receiving one dose of the mRNA-1273 vaccine (P < 0.0001 to 0.0159). The antibody responses of group 3 participants were comparable to those of individuals receiving one dose of the mRNA-1273 vaccine but significantly lower than those receiving the full mRNA-1273 vaccine regimen (P < 0.0001).
Neutralizing antibody tests
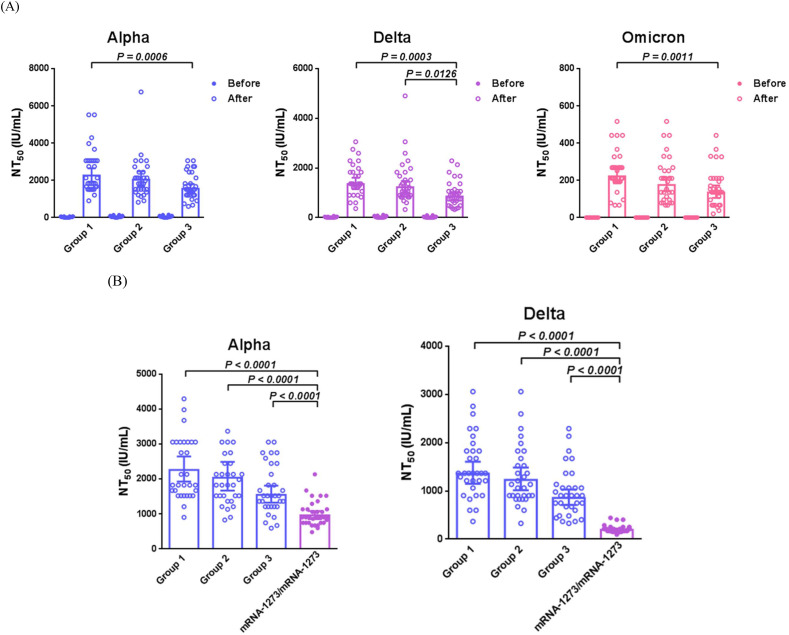
Neutralizing antibody titers against the variants of concern are shown in Table 3 and Fig. 3 A. A significant increase in neutralizing antibody responses (NT50) was detected in all groups on the 28th day after vaccination with the third dose. Group 1 had significantly higher antibody titers against the alpha (P = 0.0006), delta (P = 0.0003), and omicron (P = 0.0011) variants than group 3 on day 28 of booster vaccination. Neutralizing antibody titer against the delta variant was significantly higher in group 2 than in group 3 (P = 0.0126), whereas no significant difference was observed between the alpha and omicron variants groups. The difference in neutralizing antibody titers between groups 1 and 2 was non-significant. We determined the fold change in antibody titers on day 28 after booster vaccination compared to before (Table 3). The fold changes in neutralizing antibody titers against the alpha (P = 0.0008), delta (P = 0.0091), and omicron (P = 0.0011) variants were significantly higher in group 1 than in group 3. Fold changes in neutralizing antibody titers against alpha (P = 0.0028) and delta (P = 0.0029) variants were significantly higher in group 1 than in group 2. The difference in neutralizing antibody fold-change between groups 2 and 3 was non-significant.
Table 3.
Neutralising antibody responses of the study subjects before and 28 days after receiving the third-dose of the mRNA vaccine.
| Variants of concern | Group 1 Full-dose mRNA-1273, NT50 (IU/mL) (n = 100) | Group 2 Half-dose mRNA-1273, NT50 (IU/mL) (n = 98) | Group 3 Full-dose BNT-162b2, NT50 (IU/mL) (n = 100) | P values |
|||
|---|---|---|---|---|---|---|---|
| Group 1 vs. Group 2 | Group 1 vs. Group 3 | Group 2 vs. Group 3 | |||||
| Day 1 | Alpha | 13.06 (7.34–23.22) | 28.35 (18–44.65) | 22.2 (12.81–38.46) | 0.0366 | NS | NS |
| Delta | 4.18 (2.35–7.46) | 11.64 (6.74–20.12) | 6.43 (3.63–11.4) | 0.0186 | NS | NS | |
| Omicron | 1 (1.00–1.00) | 1 (1.00–1.00) | 1 (1.00–1.00) | NS | NS | NS | |
| Day 28 | Alpha | 2262 (1931–2649) | 2041 (1672–2492) | 1549 (1326–1810) | NS | 0.0006 | NS |
| Fold change | 173.23 | 72.00 | 69.80 | 0.0028 | 0.0008 | NS | |
| Delta | 1358 (1148–1606) | 1228 (1015–1486) | 853 (708–1029) | NS | 0.0003 | 0.0126 | |
| Fold change | 324.63 | 105.47 | 132.69 | 0.0029 | 0.0091 | NS | |
| Omicron | 222 (185–267) | 175 (142–217) | 135 (106–173) | NS | 0.0011 | NS | |
| Fold change | 222.37 | 175.30 | 135.36 | NS | 0.0011 | NS | |
Abbreviation: NS, not significant.
Figure 3.
Neutralizing antibody responses (IU/mL) in study subjects. (A) Neutralizing antibody response against the alpha, delta, and omicron variants between the three groups, the day before and 28 days after administration of the third dose of the mRNA vaccine. (B) Comparison of the antibody responses 28 days after administration of the third and the second doses of the vaccines. mRNA-1273/mRNA-1273 indicates a homologous mRNA-1273 vaccination schedule. Mann–Whitney U test was performed to compare the antibody responses between the groups. P values were indicated when a statistical significance was determined. Abbreviations: B.1.1.7, alpha; B.1.617.2, delta; B.1.1.529, omicron; NT50, neutralizing antibody responses.
We compared the neutralizing antibody responses (after the third dose) in the three groups from the present study with those from our previous study (two-dose schedule) (Fig. 3B). The neutralizing antibody titers against the alpha and delta variants were significantly higher in individuals receiving the third dose than in those receiving two doses of the COVID-19 vaccine.
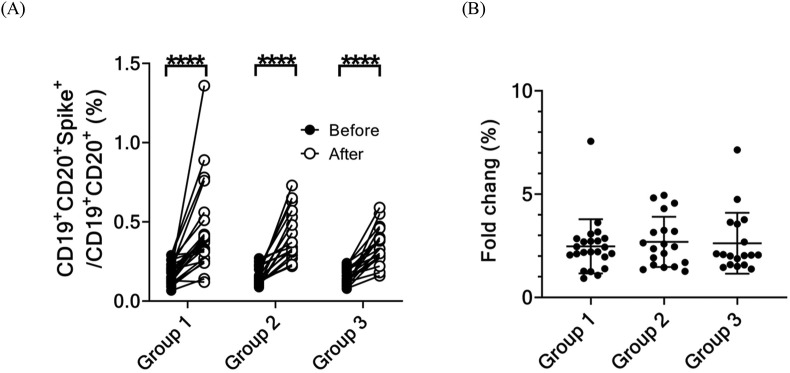
The frequency of memory B cells specific to the spike protein of the omicron variant (Fig. 4 A) was determined by flow cytometry and was significantly higher in all three groups after administration of the third dose of the vaccine than before. However, the fold increase of these cells among the three groups did not differ significantly (Fig. 4B).
Figure 4.
Immunological response of the SARS-CoV-2 spike-specific memory B cells before and 28 days after administration of the booster dose to the three groups. (A) Percentage in and (B) folds change spike-specific memory B cells the day before and 28 days after administration of the booster dose to three groups. (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.005, ∗∗∗∗P < 0.001).
Spike protein-specific T cells
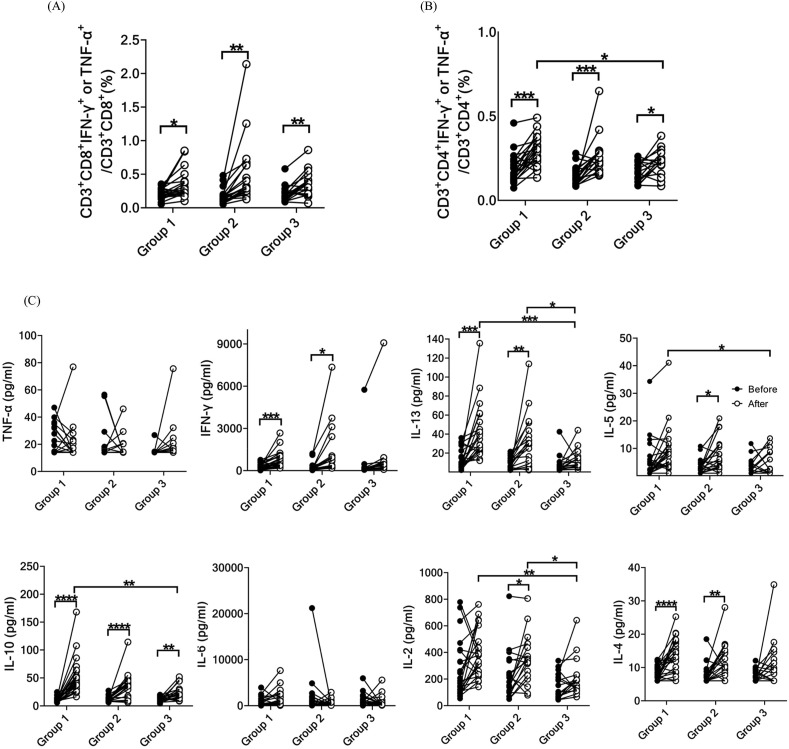
The frequencies and phenotypes of T cells specific to the spike protein of the omicron variant were analyzed. After administration of the third dose, there was a significant increase in the frequencies of spike protein-specific TNF-α- or IFN-γ-secreting CD4+ and CD8+ T cells in all three groups compared to those before vaccination with the booster dose (Fig. 5A and B). The frequencies of spike protein-specific TNF-α- or IFN-γ-secreting CD4+ and CD8+ T cells were not significantly different among the three groups after the booster dose. Group 1 exhibited a significantly higher frequency of spike protein-specific TNF-α- or IFN-γ-secreting CD4+ T cells than group 3 (Fig. 5B). Additionally, we measured the production of Th1/Th2 cytokines by T cells specific to the spike protein of the omicron variant. The booster dose variably enhanced cytokine secretion in all three groups (Fig. 5C). Specifically, there was a significant increase in the production of IFN-γ, IL-13, IL-5, IL-10, IL-2, and IL-4 in groups 1 and 2. In contrast, only IL-10 and IL-2 production significantly increased in group 3. Furthermore, the production of IL-13, IL-5, IL-10, and IL-2 was significantly higher in group 1 than in group 3 following the administration of the third dose.
Figure 5.
Immunological response of SARS-CoV-2 spike-specific memory T cells before and 28 days after administration of the booster dose to three groups. Intracellular staining of cytokines in (A) spike-specific CD8+ T cells and (B) spike-specific CD4+ T cells. (C) Th1/Th2 cytokine production by T cells using bead-based cytokine assay. (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.005, ∗∗∗∗P < 0.001). Abbreviations: IFN-γ, interferon gamma; IL-13, interleukin 13; TNF-α, tumor necrosis factor alpha.
Adverse events
Adverse events after administration of the third-dose vaccine are shown in Table 4 . All adverse events subsided within 14 days of vaccination, and >75% of the participants (191 of 251, 76%) reported full recovery within 1 week. The prevalence of adverse events, such as local injection pain, swelling, fever, chills, headache, and fatigue, was significantly higher in the mRNA-1273 vaccination groups (groups 1 and 2) than in the BNT-162b2 vaccination group (group 3). The severity of the reported adverse events, such as local injection pain, swelling, chills, headache, myalgia, and fatigue, was greater in group 1 participants. No serious adverse events were observed in any of the groups.
Table 4.
Adverse events in the three study groups within 84 days of administration of the third booster dose.
| Group 1 Full-dose mRNA-1273 (n = 81) | Group 2 Half-dose mRNA-1273 (n = 84) | Group 3 Full-dose BNT-162b2 (n = 86) | P values | ||
|---|---|---|---|---|---|
| Pain, n (%) | Yes | 77 (95.1%) | 81 (96.4%) | 75 (87.2%) | 0.042 |
| Grade 2 or 3 | 56 (69.1%) | 37 (44.0%) | 38 (44.2%) | 0.001 | |
| Grade 3 | 22 (27.2%) | 2 (3.6%) | 2 (2.3%) | <0.001 | |
| Erythema, n (%) | Yes | 9 (11.1%) | 10 (11.9%) | 4 (4.7%) | 0.199 |
| Grade 2 or 3 | 2 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0.121 | |
| Swelling, n (%) | Yes | 23 (28.4%) | 14 (16.7%) | 6 (7.0%) | 0.001 |
| Grade 2 or 3 | 6 (7.4%) | 0 (0.0%) | 1 (1.2%) | 0.008 | |
| Fever, n (%) | Yes | 33 (40.7%) | 14 (16.7%) | 10 (11.6%) | <0.001 |
| Grade 2 or 3 | 7 (8.6%) | 3 (3.6%) | 3 (3.5%) | 0.232 | |
| Grade 3 | 3 (3.7%) | 2 (2.4%) | 0 (0.0%) | 0.220 | |
| Chills, n (%) | Yes | 54 (66.7%) | 36 (42.9%) | 17 (19.8%) | <0.001 |
| Grade 2 or 3 | 23 (28.4%) | 11 (13.1%) | 4 (4.7%) | <0.001 | |
| Grade 3 | 5 (6.2%) | 1 (1.2%) | 0 (0.0%) | 0.022 | |
| Headache, n (%) | Yes | 54 (66.7%) | 46 (54.8%) | 40 (46.5%) | 0.031 |
| Grade 2 or 3 | 23 (28.4%) | 12 (14.3%) | 6 (7.0%) | 0.001 | |
| Grade 3 | 4 (4.9%) | 2 (2.4%) | 1 (1.2%) | 0.321 | |
| Myalgia, n (%) | Yes | 67 (82.7%) | 64 (76.2%) | 60 (69.8%) | 0.146 |
| Grade 2 or 3 | 38 (46.9%) | 17 (20.2%) | 16 (18.6%) | <0.001 | |
| Grade 3 | 7 (8.6%) | 2 (2.4%) | 1 (1.2%) | 0.031 | |
| Fatigue, n (%) | Yes | 69 (85.2%) | 63 (75.0%) | 54 (62.8%) | 0.004 |
| Grade 2 or 3 | 33 (40.7%) | 16 (19.0%) | 16 (18.6%) | 0.001 | |
| Grade 3 | 8 (9.9%) | 3 (3.6%) | 1 (1.2%) | 0.025 | |
| Rashes, n (%) | Yes | 5 (6.2%) | 0 (0.0%) | 2 (2.3%) | 0.052 |
| Grade 2 or 3 | 3 (3.7%) | 0 (0.0%) | 2 (2.3%) | 0.226 | |
| Arthralgia/arthritis, n (%) | Yes | 3 (3.7%) | 2 (2.4%) | 0 (0.0%) | 0.220 |
| GI upset (nausea/vomit), n (%) | Yes | 1 (1.2%) | 2 (2.4%) | 1 (1.2%) | 0.779 |
| Swollen lymph nodes in the armpit, n (%) | Yes | 3 (3.7%) | 2 (2.4%) | 5 (5.8%) | 0.513 |
| Othersa, n (%) | Yes | 16 (19.8%) | 25 (29.8%) | 12 (14.0%) | 0.039 |
Others include herpes simplex (n = 1), drowsiness (n = 1), diarrhea (n = 3), acne (n = 1), pain in left armpit (n = 1), poor appetite (n = 4), insomnia (n = 1), soreness (n = 7), powerless (n = 3), swollen and painful finger joints (n = 2), numbness of cheekbones and eye sockets on both sides (n = 1), palpitations (n = 3), chest tightness (n = 6), cough (n = 1), dizziness (n = 4), nasal congestion (n = 2), runny nose (n = 5), bloated chest (n = 1), shortness of breath (n = 2), lethargy (n = 5), bruising at the injection site (n = 1), urticaria (n = 1), painful lymph nodes (n = 6), nerve pain on right side of head (n = 1), sore throat (n = 1), mild abdominal pain (n = 1), menstrual disorders (n = 1), subcutaneous muscle mass tenderness (n = 1) and pain over eye socket (n = 1). GI: gastrointestinal.
Discussion
We demonstrated a marked decline in the anti-SARS-CoV-2 spike IgG antibody titers and neutralizing antibody response three months after administering the two doses of adenoviral vectored ChAdOx1 vaccine. Administering the third dose of an mRNA vaccine 12 weeks after the second dose enhanced protective immune responses. Although participants following a full-dose mRNA-1273 schedule appeared to have a higher prevalence and severity of adverse events than those in the half-dose mRNA-1273 and BNT162b2 vaccination groups, the symptoms were transient and well-tolerated. Our study confirmed the efficacy and safety of third-dose mRNA vaccines.
Using an mRNA vaccine as a booster dose after priming with the adenoviral vectored ChAdOx1 vaccine could induce a stronger immune response than a homologous vaccination strategy consisting of a two-dose ChAdOx1 vaccine.12, 13, 14 Therefore, it is reasonable to use mRNA vaccines as the third dose (booster) in recipients previously vaccinated with two doses of the ChAdOx1 vaccine. In a large open-label clinical trial, 458 adult participants who had completed a two-dose COVID-19 vaccine regimen at least 12 weeks earlier received a booster dose of either mRNA-1273 or adenoviral vectored vaccine Ad26.COV2-S (Johnson & Johnson-Janssen), or the BNT-162b2 vaccine.20 They demonstrated a similar trend in increasing anti-SARS-CoV-2 antibody titers among different vaccine groups.20 Another report investigated immune response following the administration of homologous versus heterologous third dose of COVID-19 vaccines (Ad26.COV2-S, BNT162b2, and ChAdOx1) to individuals vaccinated with two doses of the inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac, Sinovac).21 They observed that a heterologous booster dose induced stronger immune responses than the homologous booster doses.21 In a multicenter randomized controlled trial for the third dose of a COVID-19 vaccine (COV-BOOST), seven different vaccines (ChAdOx1, BNT162b2, mRNA-1273, NVX-CoV2373 [Novavax], Ad26.COV2.S, CVnCoV [Curevac] or VLA2001 [Valneva]) were tested and administered 10–12 weeks after immunization with two doses of the ChAdOx1 or BNT162b2 vaccines.22 All the tested vaccines could effectively boost immunity following an initial course of the ChAdOx1 vaccination.22 A large observational study using Israel's nationwide mass vaccination data indicated that the third dose of the BNT162b2 vaccine effectively prevents severe COVID-19-associated outcomes.23 Our study adds to the growing evidence that immunization with either a full or half-dose of the mRNA-1273 or BNT-162b2 vaccines as a third dose booster induces good neutralizing antibody activities against all the SARS-CoV-2 variants of concern, such as the omicron variant.
T-cell immunity elicited by inactivated vaccines has been reported to contribute to protection from SARS-CoV-2 infection.24 A third dose of the mRNA-1273 vaccine has been shown to improve immunogenicity, including SARS-CoV-2–specific CD4+ T-cell response, in transplant recipients.25 The T-cell assays here demonstrated that full or half third dose of the mRNA-1273 vaccine stimulated more spike-specific cytokine-producing CD4+ T cells and induced higher cytokine secretion than the full or half third doses of the BNT-162b2 vaccine. It is plausible that the increased frequency of spike-specific CD4+ T helper cells may facilitate an increase in antibody responses against SARS-CoV-2.
However, the optimal interval between the second and third dose remains unclear. Neutralizing antibody titers against wild-type SARS-CoV-2 increased by approximately four-fold when a third homologous booster dose was administered six to eight months after a second dose of the BNT162b2 vaccine.26 A study in China reported that the third dose inactivated the CoronaVac vaccine eight months after a second dose of the same vaccine effectively restored immune responses against SARS-CoV-2, despite the substantial decline in neutralizing antibody titers six months after the second dose.28 Based on these results, we concluded that the optimal duration for administering the third dose may be between two and eight months.
Breakthrough infections by the SARS-CoV-2 variants after the standard two-dose COVID-19 vaccine regimen are an emerging public health concern.29 In a clinical trial where subjects were administered a third dose of ChAdOx1vaccine following the initial two doses of the inactivated CoronaVac vaccine, the third dose elicited neutralizing antibody titers and elevated levels of memory T cells against the circulating SARS-CoV-2 variants.30 Additionally, we demonstrated that the levels of neutralizing antibodies against the variants of concern were significantly higher after administering of a third dose of the mRNA vaccine than after two homologous doses of the mRNA-1273 vaccine.
Adverse events after immunization with the third dose of the vaccine were reported to be tolerable and transient.22 Serious adverse events were uncommon, with similar frequencies in the active vaccine and control groups.22 In our study cohort, most adverse reactions were reported within seven days of vaccination and were mild and transient. All subjects recovered within two weeks of vaccination. Although the prevalence and severity of adverse events were higher in participants receiving the full-dose mRNA-1273 vaccine than in those receiving half-dose mRNA-1273 and BNT162b2 vaccines, the symptoms were well tolerated.
Our study has several limitations. First, the enrolled subjects were healthy volunteers aged between 20 and 65 years. Our results may not apply to elderly and immunosuppressed individuals, who are known to respond poorly to COVID-19 vaccines. Second, our results provide in vitro anti-SARS-CoV-2 antibodies, T cell responses, and neutralization tests against emerging variants using a third-dose vaccine booster. Using actual protection against infection with current and emerging variants should be monitored using real-world observational studies.
In conclusion, our study confirms that using mRNA vaccines as the third booster dose (administered 12 weeks after the second dose) is highly effective in restoring SARS-CoV-2-specific immune responses and is well tolerated. However, the duration for which the third dose of the COVID-19 vaccine can protect against the disease requires further investigation.
Funding
This work was supported by the Ministry of Science and Technology, Taiwan [MOST 111-2321-B-002-017, MOST 110-2745-B-002-005] and the National Taiwan University, College of Medicine [109F004T]. The funders had no role in the study design, data collection, data analysis, data interpretation, or manuscript writing.
Declaration of Competing Interest
The authors have no conflicts of interest relevant to this article
Acknowledgement
We acknowledge the services provided by the Biosafety Level-3 Laboratory at the First Core Laboratory of the National Taiwan University College of Medicine, and the Biosafety Level-3 Laboratory at the National Taiwan University Hospital. The authors would like to thank Professor Shin-Ru Shih (Chang-Gung University, Taoyuan, Taiwan) for kindly supporting the WHO reference panel and Ms. Yu-Yun Wu for her help with the statistical analysis. We would also like to express our appreciation to the Central Epidemic Command Centre (CECC) of Taiwan for approval of the heterologous COVID-19 vaccination program in this study.
References
- 1.World Health Organization (WHO) WHO coronavirus (COVID-19) Dashboard. https://covid19.who.int/ Available at: Accessed.
- 2.Singanayagam A., Hakki S., Dunning J., Madon K.J., Crone M.A., Koycheva A., et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. 2022;22:183–195. doi: 10.1016/S1473-3099(21)00648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pouwels K.B., Pritchard E., Matthews P.C., Stoesser N., Eyre D.W., Vihta K.D., et al. Effect of delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021;27:2127–2135. doi: 10.1038/s41591-021-01548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Interim recommendations for use of the ChAdOx1-S [recombinant] vaccine against COVID-19 (AstraZeneca COVID-19 vaccine AZD1222 Vaxzevria™, SII COVISHIELD™) https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-2021.1 Available at: Accessed.
- 5.World Health Organization Interim recommendations for use of the Moderna mRNA-1273 vaccine against COVID-19. https://apps.who.int/iris/bitstream/handle/10665/352124/WHO-2019-nCoV-vaccines-SAGE-recommendation-mRNA-1273-2022.1-eng.pdf Available at: Accessed.
- 6.World Health Organization Interim recommendations for use of the Pfizer-BioNTech COVID-19 vaccine, BNT162b2, under emergency use listing. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-BNT162b2-2021.1 Available at: Accessed.
- 7.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chemaitelly H., Tang P., Hasan M.R., AlMukdad S., Yassine H.M., Benslimane F.M., et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385:e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feikin D.R., Higdon M.M., Abu-Raddad L.J., Andrews N., Araos R., Goldberg Y., et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg E.S., Dorabawila V., Easton D., Bauer U.E., Kumar J., Hoen R., et al. Covid-19 vaccine effectiveness in New York state. N Engl J Med. 2022;386:116–127. doi: 10.1056/NEJMoa2116063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collie S., Champion J., Moultrie H., Bekker L.G., Gray G. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N Engl J Med. 2022;386:494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillus D., Schwarz T., Tober-Lau P., Vanshylla K., Hastor H., Thibeault C., et al. Safety, reactogenicity and immunogenicity of homologous and heterologous prime-boost immunization with ChAdOx1-nCoV19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021;9:1255–1265. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Normark J., Vikstrom L., Gwon T.D., Persson I.L., Edin A., Bjorsell T., et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 vaccination. N Engl J Med. 2021;385:1049–1051. doi: 10.1056/NEJMc2110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheng W.H., Chang S.Y., Lin P.H., Hsieh M.J., Chang H.H., Cheng C.Y., et al. Immune response and safety of heterologous ChAdOx1-nCoV-19/mRNA-1273 vaccination compared with homologous ChAdOx1-nCoV-19 or homologous mRNA-1273 vaccination. J Formos Med Assoc. 2022;121:766–777. doi: 10.1016/j.jfma.2022.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viana R., Moyo S., Amoako D.G., Tegally H., Scheepers C., Althauset C.L., et al. Rapid epidemic expansion of the SARS-CoV-2 omicron variant in southern Africa. Nature. 2022;603:679–686. doi: 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauring A.S., Tenforde M.W., Chappell J.D., Gaglani M., Ginde A.A., McNeal T., et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376 doi: 10.1136/bmj-2021-069761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tseng H.F., Ackerson B.K., Luo Y., Sy L.S., Talarico C.A., Tian Y., et al. Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and delta variants. Nat Med. 2022;28:1063–1071. doi: 10.1038/s41591-022-01753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Accorsi E.K., Britton A., Fleming-Dutra K.E., Smith Z.R., Shang N., Derado G., et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. JAMA Intern Med. 2022;327:639–651. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization WHO Guidelines on clinical evaluation of vaccines: regulatory expectations. https://www.who.int/biologicals/BS2287_Clinical_guidelines_final_LINE_NOs_20_July_2016.pdf Available at: Accessed.
- 20.Atmar R.L., Lyke K.E., Deming M.E., Jackson L.A., Branche A.R., El Sahly H.M., et al. Homologous and heterologous COVID-19 booster vaccinations. N Engl J Med. 2022;386:1046–1057. doi: 10.1056/NEJMoa2116414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clemens S.A.C., Weckx L., Clemens R., Mendes A.V.A., Souza A.R., Silveiraet M.B.V., et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399:521–529. doi: 10.1016/S0140-6736(22)00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munro A.P.S., Janani L., Cornelius V., Aley P.K., Babbage G., Baxter D., et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomized, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barda N., Dagan N., Cohen C., Hernan M.A., Lipsitch M., Kohaneet I.S., et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng Y., Li Y., Yang R., Tan W. SARS-CoV-2-specific T cell immunity to structural proteins in inactivated COVID-19 vaccine recipients. Cell Mol Immunol. 2021;18:2040–2041. doi: 10.1038/s41423-021-00730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall V.G., Ferreira V.H., Ku T., Ierullo M., Majchrzak-Kita B., Chaparroet C., et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falsey A.R., Frenck R.W., Jr., Walsh E.E., Kitchin N., Absalon J., Gurtman A., et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021;385:1627–1629. doi: 10.1056/NEJMc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng G., Wu Q., Pan H., Li M., Yang J., Wang L., et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-center, double-blind, randomized, placebo-controlled phase 2 clinical trials. Lancet Infect Dis. 2022;22:483–495. doi: 10.1016/S1473-3099(21)00681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chau N.V.V., Ngoc N.M., Nguyet L.A., Quang V.M., Ny N.T.H., Khoa D.B., et al. An observational study of breakthrough SARS-CoV-2 delta variant infections among vaccinated healthcare workers in Vietnam. eClinicalMedicine. 2021;41 doi: 10.1016/j.eclinm.2021.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yorsaenga R., Suntronwonga N., Phowatthanasathiana H., Assawakosri S., Kanokudom S., Thongmee T., et al. Immunogenicity of a third dose viral-vectored COVID-19 vaccine after receiving two-dose inactivated vaccines in healthy adults. Vaccine. 2022;40:524–530. doi: 10.1016/j.vaccine.2021.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]