Abstract
Background
While most large studies on the possible association of COVID-19 and stroke were done in high-income countries, only a few studies consisting of small sample populations have been done in low- to middle-income countries like the Philippines.
Objectives
To determine the risk factors of stroke among hospitalized COVID19 patients in the Philippines; to determine the possible association between these risk factors and stroke among the same cohort; and to determine if there is an association between mortality and stroke in this same group.
Methodology
We obtained relevant clinical and neurological, including stroke data from the Philippine CORONA study, an observational study involving 10,881 patients with COVID-19 admitted in 37 referral hospitals from all over the Philippines.
Results
The incidence of stroke among patients with COVID-19 was 3.4% (n = 367). There were more deaths among patients with stroke and COVID-19 than those without stroke and COVID-19 (42.2% vs 14.7%, p < 0.01). In addition, more patients with stroke were admitted in the ICU (43.3% vs 15.0%, p < 0.01) regardless of cause. Smoking (OR: 1.5, 95% CI: 1.3 to 1.7, p < 0.0001), hypertension (OR:1.75, 95% CI:1.53 to 1.97, p < 0.0001), presence of heart failure (OR: 1.4, 95% CI: 1.07 to 1.86, p = 0.01), presence of any neurologic co-morbidities (OR: 1.4, 95% CI:1.11 to 1.46, p = 0.004), and history of stroke (OR:2.3, 95% CI:1.82 to 2.97, p < 0.0001) had direct significant correlation with stroke; while being a health care worker (OR: 0.5, 95% CI: 0.33 to 0.70, p < 0.0004) had an inverse significant association with stroke.
Conclusion
COVID-19 stroke patients in the Philippines have a higher mortality and ICU admission rates than patients with COVID-19 alone or COVID-19 stroke patients from developed countries. Our cohort has similar cardiovascular and metabolic risk factors to western patients with stroke, highlighting that COVID-19 may only have a small contribution to stroke incidence.
Key Words: Acute ischemic stroke, Acute hemorrhagic stroke, COVID-19, Risk factors, Low-to-middle-income countries
Abbreviation: AHS, acute hemorrhagic stroke; AIS, acute ischemic stroke; CI, confidence interval; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; CT, computed tomography; DM, diabetes mellitus; HR, hazard ratio; ICU, intensive care unit; LMIC, low- to middle-income country; MRI, magnetic resonance imaging; OR, Odd's ratio; RT-PCR, reverse transcription polymerase chain reaction; SARS-COV2, severe acute respiratory syndrome coronavirus 2
Introduction
Coronavirus disease 2019 (COVID-19) has been declared a pandemic for two years already.1 , 2 As of July 2022, more than 575 million people have been infected, of which around 6.39 million have died.3 Although its case fatality rate of ∼2% is lower compared to past influenza pandemics, its highly transmissible nature strains the health care systems leading to significant increases in mortalities and unfavorable morbidities even in highly urbanized countries.1 , 4 With the appearance of delta and other variants, the devastation induced by COVID-19 is not likely to end soon.
While most COVID-19 patients present in the hospital with respiratory symptoms, other organ systems may also be affected.5 , 6 Around 0.8-6% will develop stroke among COVID-19 patients, while 2-3% of admitted stroke patients will harbor severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.7 , 8 These COVID-19 stroke patients have twice the risk of death and they have 20% more risk of having a moderate disability compared to patients with COVID-19 only.9 Nevertheless, COVID-19 stroke patients were usually older and had similar risk factors as those patients with stroke alone, implying that the usual determinants for stroke may be the ones responsible for these increased risks and not COVID-19.1 , 7 , 9 However, in smaller case series and cross-sectional studies, COVID-19 patients with stroke were younger, with a cryptogenic type of stroke, and with no identifiable risk factors compared to those without COVID-19.9 , 10 Since SARS-CoV-2 infection affects organ systems by inducing thrombosis secondary to a hypercoagulable state, the incidence of stroke, especially the ischemic type, may plausibly be increased in COVID-19.10, 11, 12
While most large studies about the possible association of COVID-19 and stroke were done in high-income countries, only one study with a small sample size have been done in low- to middle-income countries (LMIC) like the Philippines.13 Developed countries have more organized and advanced health care systems and reliable national insurance services; hence, the incidence of stroke, its risk factors and mortality rate among COVID-19 patients may not be comparable to the true situation in LMIC.6 , 8
A recently concluded nationwide multicenter, comparative, retrospective cohort study was conducted from February to December 2020 to identify the different neurologic manifestations of COVID-19 in the Philippines.6 A total of 10,881 reverse transcriptase-polymerase chain reaction (RT-PCR) confirmed COVID-19 cases were collected.6 Our main objectives were a) to determine the risk factors of stroke among hospitalized COVID19 patients in the Philippines, b) to determine the possible association between these risk factors and stroke among the same cohort, and c) to determine if there is an association between mortality and stroke in this same group.
Methodology
Study design
The data analyzed in this study were obtained from a previously published nationwide retrospective cohort study that identified the different neurologic manifestations of COVID-19 in the Philippines.6
Inclusion and exclusion criteria
RT-PCR-confirmed adult COVID-19 patients, more than 18 years of age, with final hospital disposition, were included in the study. Those with pneumonia caused by other etiologies other than SARS-CoV-2 were excluded. A complete enumeration of all patients fulfilling these criteria, who were admitted to the hospitals from February until December 2020, was performed. The definition of neurological symptoms was based on the previously published protocol.6 A patient who developed focal sensory or motor deficit confirmed by either cranial computed tomography (CT) scan or magnetic resonance imaging (MRI) were recorded as a stroke patient, as seen on chart review. The imaging was the basis for classifying the patient as either infarct or hemorrhagic stroke. COVID-19 stroke patients comprised the cases while COVID-19 only patients constituted the control group.
Study site
Data collection was done in 37 referral hospitals for COVID-19. Identification of these sites and other information regarding methods for data collection were described in the published protocol.6
Study investigators
This is a part of the Philippine CORONA Study which aimed to determine the incidence of the different neurological diseases and their association with different risk factors and outcomes in a large cohort of COVID-19 patients. This was headed by four steering committee members with 37 study site teams, of which the principal investigators were all neurologists.6
Data collection
The method of data collection has already been published.6 In brief, all COVID-19 confirmed admissions with disposition (discharged or deceased) at the time of data collection were included in the study. A pre-made detailed abstraction form containing the variables of interest was filled out by the field physician by chart review. Possible risk factors for stroke or increased COVID-19 severity such as age, sex, smoking, hypertension, diabetes mellitus (DM), heart failure, coronary artery disease, chronic obstructive pulmonary disease (COPD), bronchial asthma, chronic kidney disease, liver disease, obesity, malignancy, and human immunodeficiency virus infection was obtained. For stroke, the neurologic symptoms and final diagnosis during admission, and different outcome measures like the severity of the disability, intensive care unit (ICU) admission, duration from admission to final disposition, mortality status, and final disposition were included in the form. Abstraction forms were then assessed for validity and inconsistencies before they were de-identified, encoded, and collated per hospital and sent to the Steering Committee of the Philippine CORONA study.
Data analysis
Age was presented as mean, while categorical data were presented as proportions. Standard deviation was used as measure of dispersion. Means and proportions were tested for significance using unpaired t-test and test of two proportions, respectively. Prevalence ratio, defined as the ratio of the proportion of patients with a particular risk factor in COVID-19 stroke patients divided by the proportion of patients with the same risk factor among COVID-19 only patients were computed separately. These were used as estimates of relative risks. The incidence of stroke among patients with COVID-19 was computed by dividing the number of stroke patients by the population (n = 10,881). Likewise, the incidence of different outcome measures like mortality, disability, and intensive care unit (ICU) admission in both COVID-19 stroke patients and COVID-19 patients only were obtained by dividing the number of each of these outcome measures by the number of patients who developed stroke and those who did not, respectively. Subsequently, relative risks were computed by dividing the incidence of each outcome measure among COVID-19 stroke patients by the incidence of outcome measure among those with COVID-19 patients only. To determine the association between stroke and different risk factors; and the different outcome measures and stroke among COVID-19 patients, a univariate logistic regression was done. Stroke and outcome measures were used as dependent variables separately, while risk factors for stroke, and stroke were used as their independent variables, respectively. An extended Cox proportional hazard survival analysis was also done using mortality status as the failure event and the duration from admission to either censoring or failure as survival time. Significant risk factors identified in the logistic regression was used as the predictor variables with presence of stroke as the focus. Since the presence of stroke was a time dependent variable based on the usage of scaled and unscaled Schoenfield residuals, an extended Cox model was used. All data were captured and analyzed using Stata Pro BE 17, with alpha set at < 0.05 as indicator of significance.
Results
Baseline characteristics
There were 10,881 RT-PCR confirmed COVID-19 cases included in the Philippine CORONA Study. The patients were mostly males (n = 5780, 53.1%), with history of neurological disorder (n = 7560, 69.5%), with hypertension (n = 3647, 33.5%), and DM (n = 2191, 20.1%). Only 321 patients with COVID-19 (3%) had a history of stroke.
The overall incidence of stroke among COVID-19 patients was 3.4% (n = 367). Of these, 262 (71.4%) had acute ischemic stroke (AIS) and 101 (27.5%) had acute hemorrhagic stroke (AHS). The incidence of AIS and AHS were 2.4% and 0.9%, respectively.
A total of 1697 COVID-19 patients (15.6%) died due to various etiologies. Most patients who had neurologic symptoms were stable but had persistent deficits at discharge (71.7%). Only 1751 patients (16%) were admitted to the ICU primarily due to acute respiratory failure.
Patients with COVID-19 infection and stroke vs. with COVID-19 infection only
Risk factors
There were significantly more males with stroke and COVID-19 (58.9% vs 52.9%, p < 0.03) and they were significantly older (60 years vs 51 years, p < 0.01) (see Table 1 ). In addition, this cohort had a higher proportion of smokers (25.3% vs 8.9%, p < 0.01), hypertension (69.5% vs 32.2%, p < 0.01), DM (32.2% vs 19.7%, p < 0.01), heart failure (4.4% vs 1.1%, p < 0.01), coronary artery disease (8.4% vs 3.7%, p < 0.01), chronic kidney disease (CKD) (11.7% vs 0.07%, p < 0.01), history of any neurological disorder (77.4% vs 69.2%, p < 0.01), and previous strokes (13.9% vs 2.6%, p < 0.01). In contrast, less health care workers developed stroke (2.0% vs 8.0%, p < 0.01). The difference in proportion of obesity (0.8% vs 0.8%, p = 1), liver disease (0.5% vs 0.5%, p = 1), and chronic obstructive pulmonary disease (1.4 vs 2.4, p = 0.09) between the COVID-19 stroke patients and COVID-19 only patients were not statistically significant.
Table 1.
Baseline characteristics of all patients with COVID-19 (n = 10,881).
| Parameter | Group |
||
|---|---|---|---|
| All patients (n = 10,881) in % | COVID-19 with stroke (n = 367) | COVID-19 only (n = 10,514) | |
| Medical History | |||
| Age, in years | 51 (17.4) | 60 (14.8) | 51 (17.4) |
| Males | 53.12 | 58.9 | 52.9 |
| Smoking | 9.42 | 25.3 | 8.9 |
| Healthcare worker | 8.1 | 2.0 | 8.0 |
| Hypertension | 33.5 | 69.5 | 32.2 |
| Diabetes mellitus | 20.1 | 32.2 | 19.7 |
| Heart failure | 1.2 | 4.4 | 1.1 |
| Coronary artery disease | 3.9 | 8.4 | 3.7 |
| Chronic obstructive pulmonary disease |
1.4 | 2.4 | 1.4 |
| Chronic kidney disease | 5.6 | 11.7 | 0.05 |
| Liver disease | 0.6 | 0.5 | 0.5 |
| Obesity | 0.8 | 0.8 | 0.8 |
| History of any neurological disease | 69.5 | 77.4 | 69.2 |
| Past stroke | 3.0 | 13.9 | 2.6 |
| Acute Symptoms | |||
| With neurological symptoms at the onset |
13.0 | 49.3 | 11.8 |
| With any neurological symptoms | 18.5 | 78.2 | 16.4 |
| With facial sensory complaints | 0.18 | 4.4 | 0.03 |
| With facial weakness | 0.4 | 8.7 | 0.07 |
| With dysarthria | 0.8 | 16.1 | 0.2 |
| With dysphagia | 0.1 | 1.9 | 0.09 |
| With tongue weakness | 0.07 | 1.1 | 0.04 |
| With extremity weakness | 2.3 | 57.8 | 0.3 |
| With extremity sensory complaints | 0.5 | 10.9 | 0.1 |
| With encephalopathy | 5.7 | 34.6 | 4.7 |
| With anoxic brain injury | 0.5 | 2.5 | 0.4 |
| Stroke Incidence | |||
| Stroke | 3.4 | 100% | 0% |
| Acute ischemic stroke (71.4%) | 2.4 | 100% | 0% |
| Acute hemorrhagic stroke (27.5%) | 0.9 | 100% | 0% |
| Outcomes | |||
| Mortality | 15.6 | 42.2 | 14.7 |
| Neurologic outcomes | |||
| 0 – full neurologic recovery | 12.4 | 19.3 | 73.5 |
| 1 – stable with partial improvement of neurological symptom/ disorder | 12.0 | 34.9 | 11.2 |
| 2 – stable with no improvement of neurological disorder | 71.7 | 25.0 | 3.2 |
| 3 – no neurological disorder | 3.9 | 20.7 | 12.1 |
| ICU admission (all causes) | 16.0 | 43.3 | 15.0 |
| ICU admission due to respiratory failure |
8.8 | 29.4 | 10.8 |
| ICU admission due to acute myocardial infarction |
0.7 | 17.7 | 8.5 |
| ICU admission due to acute stroke | 0.5 | 12.8 | 0 |
| ICU admission due to cerebral edema |
0.2 | 1.6 | 0.1 |
COVID-19 – coronavirus disease 2019; ICU – intensive care unit.
Of the patients who developed stroke (n = 367), 186 (50.7%) developed stroke while admitted in the hospital, while 181 (49.3%) had stroke symptoms at the onset and were later diagnosed with COVID-19. Most COVID-19 stroke patients complained of extremity weakness (n = 212, 57.8%), dysarthria (n = 62, 16.9%), and sensory deficits (n = 40, 10.9%). Compared to those with COVID-19 only, the COVID-19 stroke cohort had significantly higher incidences of encephalopathy (34.6% vs 4.7%, p < 0.01) and anoxic brain injury (2.5% vs 0.4%, p < 0.01).
Outcomes
More patients with COVID-19 died in the stroke group (n = 155/367) compared to patients with COVID-19 only (n = 1546/10,514) (42.2% vs 14.7%, p < 0.01). In addition, more COVID-19 stroke patients were admitted to the ICU (43.3% vs 15.0%, p < 0.01) regardless of cause. Notably, more patients who had any stable neurologic deficits were discharged with improved symptoms in patients without stroke (34.9% vs 11.2%). More stroke patients with COVID-19 developed cerebral edema (1.6% vs 0.6%, p < 0.0001).
Relationship between stroke and risk factors among COVID-19 patients
A model using stepwise logistic regression was made to identify the factors associated with the occurrence of stroke among COVID-19 patients, using the presence or absence of stroke as the dependent binary variable and the possible risk factors as predictors. Controlling other predictors, COVID-19 stroke patients were 1.75 times more likely to be hypertensive [Odds Ratio (OR):1.75, 95% Confidence Interval (CI):1.53 to 1.97, p < 0.0001], 2.3 times more likely to have a history of stroke (OR:2.3, 95% CI:1.82 to 2.97, p < 0.0001), 1.5 times more likely to be a smoker (OR: 1.5, 95% CI: 1.3 to 1.7, p < 0.0001), 1.4 times more likely to have past heart failure (OR: 1.4, 95% CI: 1.07 to 1.86, p = 0.01), or any neurological disorders (OR: 1.4, 95% CI:1.11 to 1.46, p = 0.004). In contrast, health care workers were twice less likely to develop stroke when they have COVID-19 (OR: 0.5, 95% CI: 0.33 to 0.70, p < 0.0004).
Survival analysis
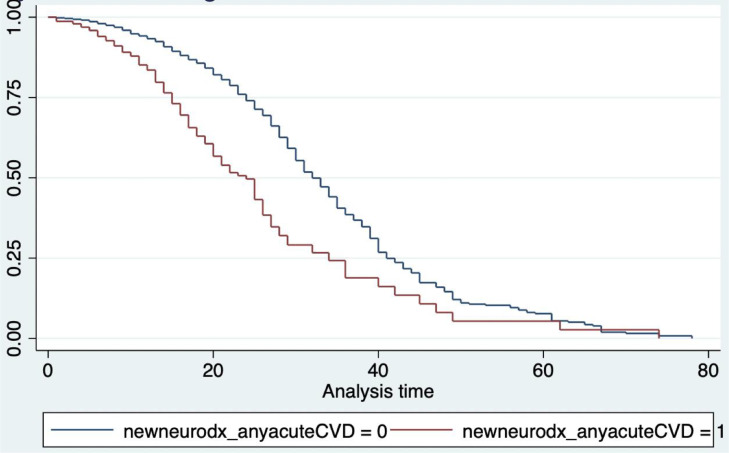
Adjusting for the predictors (smoking, presence of heart failure, history of stroke and being a health care worker), the presence of stroke in a COVID-19 patient increases the hazard of dying due to any cause by 3.45 times [Hazard Ratio (HR) = 3.45, 95% CI: 1.62 to 7.39, p < 0.0001] compared to COVID-19 patients without stroke (Fig. 1). Although the mean ICU length of stay in the COVID-19 without stroke group was longer (18.66 days, 95% CI: 16.79 to 20.54 days) compared to those with stroke (15.72 days, 95% CI: 14.18 to 17.27 days), the difference was not statistically significant (p = 0.33).
Fig. 1.
Kaplan Meyer Curve of COVID-19 patients with (red) vs. without (blue) stroke, adjusted for smoking, presence of heart failure, history of stroke, and being a health care worker. Those without stroke had better survival from admission up to the 60th day. Beyond 60th day, the survival probabilities of both groups were the same. The presence of stroke did not satisfy the proportionality hazard assumption hence the curves were not parallel to one another.
Relationship between different outcome measures and stroke among COVID-19 patients
COVID-19 stroke patients were 2.9 times more likely to die than patients with COVID-19 only. This cohort was twice more likely to die from acute respiratory distress syndrome (p < 0.01), 2.3 times from septic shock (p < 0.01), 2.9 times from heart failure (p < 0.04), 50 times from brain herniation (p < 0.01), and 3 times from multiorgan failure (p < 0.01). Although the same cohort had 30% (p = 0.52) and 42% (p = 0.44) more chance to die from acute coronary syndrome and cardiac arrhythmia, respectively, these were not statistically significant.
Moreover, COVID-19 stroke patients were 2.9 times more likely to be admitted to the ICU. Moreover, they have 2.7 times (p < 0.01) the risk of being admitted to the ICU secondary to respiratory failure, 2.6 times due to shock (p < 0.01), 14 times due to cerebral edema (p < 0.01), 18 times due to impaired consciousness (p < 0.01), and 3.4 times (p < 0.01) due to acute kidney injury. Whereas the same cohort had a 2.4 times chance of being admitted to the ICU due to venous thromboembolism (p = 0.39), 12% (p = 0.85) increased risk due to myocardial infarction, 69% (p = 0.47) increased risk due to cardiac arrhythmia, and 30% (p = 0.80) due to post-cardiac arrest, these were not statistically significant.
Discussion
The incidence of stroke among COVID-19 patients in this study (3.4%) was significantly higher compared to a retrospective study with 8163 confirmed COVID-19 patients (1.3%), and even when only ischemic strokes were considered (2.4% vs. 1.3%).9 Moreover, compared to global (3.4% vs. 1.5%) or smaller cross-sectional local data (3.4% vs. 2.0%), the incidence was still higher in our study.1 , 13 This was despite the decline of stroke admissions in Asia was considerably higher compared to the global average (20.5% vs. 19.2%).1 Although it can be argued that the difference may be explained by the higher incidence of COVID-19 infections across Europe and North America and higher baseline stroke admissions in Asia; our study had a larger RT-PCR confirmed COVID-19 population (n = 10,881) than that of another study (n = 8163).9 In addition, a higher proportion of AHS should be reflected in our data and not AIS since hemorrhagic stroke is the predominant phenotype among Asian people.14
Despite this, our study agrees with Qureshi and colleagues’ findings that the risk factors associated with COVID-19 stroke patients were similar to those that increase the risk of incidence of stroke even in the absence of COVID-19.9 These included age, smoking, hypertension, DM, heart failure, presence of coronary artery disease, presence of CKD, and prior history of stroke.9 , 15 However, when we did a regression analysis to control for predictors, smoking, hypertension, presence of heart failure, presence of past neurologic co-morbidities, and past history of stroke still yielded a significant association with the occurrence of stroke among COVID-19 patients, similar to other studies.9 , 10 , 13
It was previously established that the prevalence of dyslipidemia, DM, central obesity, and others were higher among Asians than Europeans, thus explaining the risk of stroke.14 However, compared to other studies with larger sample size, the proportions of hypertension (69.5% vs. 85%), DM (32.2% vs. 56%), and heart failure (4.4% vs. 33%) in ours were considerably lower.16 , 17 Nevertheless, Asians were twice at risk of developing stroke even in the absence of the above risk factors.14 The lesser incidence of stroke among COVID-19 positive healthcare workers may be due to healthy-worker effect bias, i.e., health care workers or employed people, in general, have better health-seeking behavior or annual mandatory health examinations compared to the unemployed or elderly.18
Similarly, the mortality and hazard rates among COVID-19 stroke patients were higher compared to those with COVID-19 alone.9 , 10 , 19 Compared to western data, COVID-19 stroke patients in our study were 2.17 times more likely and with 3.45 times more likely to die due to several etiologies at a particular time (42.2% vs. 19.4%). Although this may reflect the difference in the health care system, with the United States of America and European countries having better health system delivery than the Philippines, the high number of moderate to severe COVID-19 admissions in the former may also lead to higher mortality, even in stroke cases.9 Nevertheless, a formal study should be conducted to validate this hypothesis. Aside from mortality, COVID-19 stroke patients were 2.9 times more likely to be admitted to the ICU than COVID-19 only patients due to several causes like respiratory failure, septic shock, impaired consciousness, cerebral edema, and acute kidney injury, as seen in previous studies.8 , 19 , 20 Our study showed that the mean length of ICU stay was slightly longer among patients COVID-19 only than COVID-19 stroke patients, although the difference was not statistically significant. This higher mortality rate among COVID-19 stroke patients may have decreased the ICU stay of this cohort.
COVID-19 may increase the risk of AIS and AHS thru several mechanisms. Accordingly, SARS-CoV-2 virus infection may elevate the risk of AIS by generalized hypercoagulability; increased fibrinogen levels through cytokine storm; endotheliitis resulting in inflammation and thrombosis; uncontrolled hypertension from renin-angiotensin-aldosterone-system dysregulation; direct effects on angiotensin-converting enzyme 2 receptors in the central nervous system; and cardiorespiratory strain from hypoxemia and several metabolic derangements.12 , 20 Disseminated intravascular coagulopathy leading to thrombocytopenia and uncontrolled hypertension may lead to AHS.12 In smaller case series and cross-sectional studies, the demography of COVID-19 stroke patients were younger, free from usual stroke risk factors, and with cryptogenic strokes. In this cohort, most strokes were embolic and attributed to cardiac dysfunction and paradoxical embolism from deep venous thrombosis.10 , 21 , 22 However, these were not replicated in larger studies, including ours. Instead, COVID-19 stroke patients were found to have similar risk factors as those with stroke alone, indicating that COVID-19 may not or may only slightly increase the risk of stroke. As DM, HPN, smoking and cardiac diseases increase the risk and severity of COVID- 19, these may confound the association between these two variables. A large study compared the profile of COVID-19 stroke patients and those with stroke alone, no significant differences between the proportions of DM, HPN, cardiac diseases, and other risk factors were found.9 Nevertheless, using a stepwise forward multiple logistic regression model, we found that smoking, hypertension, presence of heart failure, presence of past neurologic co-morbidities, history of stroke, and being a health care worker had a significant association with stroke among patients with COVID-19, controlling for other variables.
The large number of patients included in this study decreases the random error and increases its internal validity and power to detect significant differences between COVID-19 stroke patients and COVID-19 only groups. Several significant differences between variables were seen, with being a male and past history of neurologic diseases deemed insignificant.
There has been a decline in stroke admissions in Asia, including Southeast Asia.14 This, in addition to the unrelenting increase in the number of COVID-19 admissions, make the incidence of stroke underestimated. Fear of going to the hospital, limited mobility and inadequate resources were only a few of the possible reasons. Moreover, COVID-19 patients with mild stroke may opt to stay at home, which can falsely lower stroke admission rates. Since moderate to severe patients are more likely to be admitted, a higher mortality rate or ICU admission is expected. Furthermore, hospitals included in this study were also referral centers for moderate to severe stroke and other diseases. This selection bias limits the external validity of this study. The health worker bias has been mentioned earlier.
Most of the COVID-19 patients included in this study were initially seen by internists or general physicians. Moreover, the National Institute of Health Stroke Scale or the modified Rankin Scale score was not routinely available on chart review. Accordingly, admitted patients with subtle or atypical signs of stroke may not be recognized and lead to misclassification bias in favor of the COVID-19 only group. Limitation in the use of CT scan or MRI for fear of COVID-19 transmission can aggravate this. This can further result in an underestimation of stroke incidence and overestimation of mortality or ICU admission among stroke patients. However, the large sample size may be sufficient to downplay the effect of misclassification. As mentioned, hypertension, DM, and cardiac dysfunction may confound the true relationship between COVID-19 and stroke. Nevertheless, a forward stepwise multiple logistic regression was done to remove the confounding effect.
Ideally, a large prospective observational study with COVID-19 without stroke risk factors as a cohort and non-COVID-19 patients without stroke risk factors as controls and the incidence of stroke as an outcome, should be done to determine the real association between COVID-19 and stroke. However, due to the relatively short duration of the COVID-19 course and with the advent of SARS-CoV-2 vaccination, this might be hard to undertake.
Conclusion
COVID-19 stroke patients in the Philippines have higher mortality and ICU admission rates than patients with COVID-19 alone or COVID-19 stroke patients from developed countries. Our cohort has similar cardiovascular and metabolic risk factors to western patients with stroke, highlighting that COVID-19 may only have a small contribution to stroke incidence.
Declaration of Competing Interest
We declare no competing interests.
Acknowledgments
Acknowledgments
The Philippine CORONA Study Group Investigators and site: Asian Hospital and Medical Center, Muntinlupa City (Corina Maria Socorro A. Macalintal, MD; Joanne B. Robles, MD), Baguio General Hospital and Medical Center, Baguio City (Paulo L. Cataniag, MD; Manolo Kristoffer C. Flores, MD, MBA), Cagayan Valley Medical Center, Tuguegarao City (Noreen Jhoanna C. Tangcuangco-Trinidad, MD), Capitol Medical Center, Quezon City (Dan Neftalie A. Juangco, MD; Giuliani Renz G. Paas, MD), Cardinal Santos Medical Center, San Juan City (Audrey Marie U. Chua, MD, Valmarie Estrada, MD, Philip Rico P. Mejia, MD, Therese Franz B. Reyes, MD), Chong Hua Hospital, Cebu City (Maria Teresa A. Cañete, MD; Ferdinand Renfred A. Zapata, MD), De La Salle University Medical and Health Sciences Institute, Dasmariñas City, (Franko Eugenio B. Castillo, MD; Romulo U. Esagunde, MD; Jean B. Gantioque, MD), Dr. Jose N. Rodriguez Memorial and Sanitarium Hospital, Caloocan City (Maritoni C. Abbariao, MD; Geramie M. Acebuque, MD), Dr. Pablo O. Torre Memorial Hospital, Bacolod City (Evram V. Corral, MD), East Avenue Medical Center, Quezon City (Marian Irene C. Escasura, MD; Marissa T. Ong, MD), Jose B. Lingad Memorial Regional Hospital, City of San Fernando (Khasmeen D. Aradani, MD; Arnold Angelo M. Pineda, MD), Jose R. Reyes Memorial Medical Center, Manila (Joseree-Ann S. Catindig, MD; Mark Timothy T. Cinco, MD; Mark Erving H. Ramos, MD), Lung Center of the Philippines, Quezon City (Romulus Emmanuel H. Cruz, MD; Marita Dantes, MD; Norberto A. Francisco, MD; Rosalia A. Teleg, MD), Makati Medical Center, Makati City (Krisverlyn B. Bellosillo, MD; Jean Paolo M. Delfino, MD; Cid C. Diesta, MD; Julie Anne V. Gamboa, MD; Cara Camille M. Matute, MD; Franzelle P. Padilla, MD; Rosalina E. Picar, MD; John Joshua Q. Punsalan, MD), Manila Doctors Hospital, Manila (Ma. Epifania V. Collantes, MD; Charmaine B. Que, MD; Hanifa A. Sampao, MD; Maxine Camela S. Sta. Maria, MD), Medical Center Manila, Manila (Jennifer Justice F. Manzano, MD; Rizza J. Umali, MD), New Era General Hospital, Quezon City (Marc Conrad C. Molina, MD), Northern Mindanao Medical Center, Cagayan de Oro City (Hazel Claire Minerva-Ang, MD; Arturo F. Surdilla, MD; Loreto P. Talabucon Jr., MD; Natasha F. Wabe, MD), Quirino Memorial Medical Center, Quezon City (Maria Victoria G. Manuel, MD; Al Inde John A. Pajantoy, MD; Josephine Cecilia V. Roque, MD; Paul Emmanuel L. Yambao, MD), Ospital ng Makati, Makati City (Christian Paul B. Banday, MD; Chritopher C. Cipriano, MD; Nehar A. Pangandaman, MD; Avery Gail C. Wasil, MD), Perpetual Succour Hospital, Cebu City (Elrey P. Inocian, MD; Jarungchai Anton S. Vatanagul, MD), Philippine General Hospital, Manila (Almira Doreen Abigail O. Apor, MD; Carissa P. Maligaso, MD), Philippine Heart Center, Quezon City (Prinz Andrew M. dela Cruz, MD; Maricar P. Yumul, MD), Research Institute for Tropical Medicine, Muntinlupa City (Ma. Alma E. Carandang-Concepcion, MD), San Juan De Dios Educational Foundation Inc.– Hospital, Pasay City (Ma. Caridad V. Desquitado, MD; Carl Kevin L. Julao, MD), San Lazaro Hospital, Manila (Dante P. Bornales, MD), Southern Isabela Medical Center, Santiago City (Generaldo D. Maylem, MD; Mark Joseph F. Cuntapay, MD), Southern Philippines Medical Center, Davao City (Annabelle L. Reyes, MD; Aileen Mae B. Lee, MD; Nadia O. Manlegro, MD; Dave Mar L. Pelere, MD), St. Luke's Medical Center - Global City, Taguig City (Lina C. Laxamana, MD; Diana-Lynn S. Que, MD; Jeryl Ritzi T. Yu, MD), St. Luke's Medical Center, Quezon City (Ma. Socorro C. Martinez, MD; Alexandria E. Matic, MD; John Angelo Luigi S. Perez, MD), The Medical City, Pasig City (Glenn Anthony A. Constantino, MD; Aldanica R. Olano, MD; Liz Edenberg P. Quiles, MD; Artemio A. Roxas, MD; Jo Ann R. Soliven, MD; Michael Dorothy Frances M. Tamayo, MD), University of Santo Tomas Hospital, Manila (Ma. Lourdes C. Joson, MD; Jojo R. Evangelista, MD), University of the East Ramon Magsaysay Memorial Medical Center Inc., Quezon City (Ma. Clarissa B. Nuñez, MD; Marietta C. Olaivar, MD; Dominique Q. Perez, MD), Veterans Memorial Medical Center, Quezon City (Mark Deneb O. Armeña, MD; Robert A. Barja, MD), Vicente Sotto Memorial Medical Center, Cebu City (Joshua Emmanuel E. Abejero, MD; Maritzie R. Eribal, MD), Western Visayas Medical Center, Iloilo City (Ryndell G. Alava, MD), Zamboanga City Medical Center, Zamboanga City (Muktader A. Kalbi, MD; Nasheera W. Radja, MD; Mohammad Elshad S. Sali, MD)
Ethical considerations
This research adhered to the Philippine National Ethical Guidelines for Health and Health-related Research (NEGHHRR) 2017 and was carried out in accordance with the Declaration of Helsinki. The protocol was approved by the Single Joint Research Ethics Board of the Department of Health of the Philippines (SJREB–2020–24) and the Research Ethics Board of the University of the Philippines Manila (2020–314–01 SJREB). We also obtained approval from the respective research ethics boards of the 37 participating hospital sites.
Authorship statement
All authors participated in the conceptualization of work, acquisition and analysis of data, drafting and revising, and final approval of the version to be published.
Funding source
None.
Data sharing policy
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Nogueira RG, Abdalkader M, Qureshi MM, et al. Global impact of COVID-19 on stroke care. Int J Stroke. 2021;16(5):573–584. doi: 10.1177/1747493021991652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Overview, global situation. covid19.who.int. Accessed 29 Jul 2022.
- 4.Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12(1):15–22. doi: 10.3201/eid1209.05-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collantes ME, Espiritu AI, Sy MC, Anlacan VM, Jamora RD. Neurological manifestations in COVID-19 infection: a systematic review and meta-analysis. Can J Neurol Sci. 2021;48(1):66–76. doi: 10.1017/cjn.2020.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espiritu AI, Sy MC, Anlacan VM, Jamora RD. The Philippine COVID-19 Outcomes: a retrospective study of neurological manifestations and associated symptoms (The Philippine CORONA study): a protocol study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-040944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jillella DV, Janocko NJ, Nahab F, et al. Ischemic stroke in COVID-19: an urgent need for early identification and management. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0239443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandian JD, Kusuma Y, Kiemas LS, et al. Stroke care during the COVID-19 pandemic: Asian Stroke Advisory Panel Consensus Statement. J Stroke Med. 2021;4(1):7–14. doi: 10.1177/25166085211000915. [DOI] [Google Scholar]
- 9.Qureshi AI, Baskett WI, Huang W, et al. Acute ischemic stroke and COVID-19: an analysis of 27676 patients. Stroke. 2021;52(3):905–912. doi: 10.1161/STROKEAHA.120.031786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaghi S, Ishida K, Torres J, et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51(7):2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pajo AT, Espiritu AI, Apor AD, Jamora RD. Neuropathologic findings of patients with COVID-19: a systematic review. Neurol Sci. 2021;42(4):1255–1266. doi: 10.1007/s10072-021-05068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spence JD, De Freitas GR, Pettigrew LC, et al. Mechanisms of stroke in COVID-19. Cerebrovasc Dis. 2020;49(4):451–458. doi: 10.1159/000509581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jalipa FG, de Castillo LL, Enriquez CA, Collantes ME. Stroke in patients with COVID-19 infection in a tertiary hospital: a retrospective study. Acta Med Philipp. 2022;56(13):20–25. doi: 10.47895/amp.vi0.2854. [DOI] [Google Scholar]
- 14.Espiritu AI, Sy MC, Anlacan VM, Jamora RD. The Philippine CORONA Study Group Investigators. COVID-19 outcomes of 10,881 patients: retrospective study of neurological symptoms and associated manifestations (Philippine CORONA Study) J Neural Trans. 2021;128(11):1687–1703. doi: 10.1007/s00702-021-02400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turana Y, Tengkawan J, Chia YC, et al. Hypertension and stroke in Asia: a comprehensive review from HOPE Asia. J Clin Hypertens. 2021;23(3):513–521. doi: 10.1111/jch.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zakeri A, Jadhav AP, Sullenger BA, Nimjee SM. Ischemic stroke in COVID-19-positive patients: an overview of SARS-CoV-2 and thrombotic mechanisms for the neurointerventionalist. J Neurointerv Surg. 2021;13(3):202–206. doi: 10.1136/neurintsurg-2020-016794. [DOI] [PubMed] [Google Scholar]
- 17.Espiritu AI, Reyes NG, Leochico CF, et al. Body mass index and its association with COVID-19 clinical outcomes: findings from the Philippine CORONA study. Clin Nutr ESPEN. 2022;49:402–410. doi: 10.1016/j.clnesp.2022.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espiritu AI, Chiu HH, Sy MC, et al. The outcomes of patients with diabetes mellitus in The Philippine CORONA Study. Sci Rep. 2021;11(1):24436. doi: 10.1038/s41598-021-03898-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li CY, Sung EC. A review of the healthy worker effect in occupational epidemiology. Occup Med. 1999;49(4):225–229. doi: 10.1093/occmed/49.4.225. [DOI] [PubMed] [Google Scholar]
- 20.Tu TM, Seet CY, Koh JS, et al. Acute ischemic stroke during the convalescent phase of asymptomatic COVID-2019 infection in men. JAMA Netw Open. 2021;4(4):1–12. doi: 10.1001/jamanetworkopen.2021.7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paliwal PR, Tan BY, Leow AS, et al. Impact of the COVID-19 pandemic on hyperacute stroke treatment: experience from a comprehensive stroke centre in Singapore. J Thromb Thrombolysis. 2020;50(3):596–603. doi: 10.1007/s11239-020-02225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Smadi AS, Mach JC, Abrol S, Luqman A, Chamiraju P, Abujudeh H. Endovascular thrombectomy of COVID-19-related large vessel occlusion: a systematic review and summary of the literature. Curr Radiol Rep. 2021;9(4):4. doi: 10.1007/s40134-021-00379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]