Abstract
Point‐of‐care ultrasound (POCUS) leads to efficient real‐time diagnosis in a wide range of medical specialties. We describe the use of cardiac, lung and lower limb POCUS to rapidly diagnose massive pulmonary embolism and deep vein thrombosis in a 64‐year‐old patient presenting with acute dyspnea prior to elective bronchoscopy. Left femoral vein thrombus and features of increased right heart pressure on POCUS led to the decision to administer fibrinolytic therapy, with subsequent CT pulmonary angiogram confirming bilateral PE. The use of POCUS allowed for rapid imaging and interpretation leading to a rapid diagnosis of PE, thus fast‐tracking lifesaving anticoagulation, especially in an outpatient setting.
Keywords: bronchoscopy, deep vein thrombosis, POCUS, point‐of‐care ultrasound, pulmonary embolism
A case report of the use of multisystem POCUS to rapidly diagnose massive pulmonary embolism and deep vein thrombosis in a suspected lung cancer patient presenting with acute dyspnoea prior to day‐case bronchoscopy.
INTRODUCTION
Point‐of‐care ultrasound (POCUS) leads to efficient real‐time diagnosis and has wide applicability. 1 Clinical guidelines advocate its use to overcome diagnostic uncertainty in a patient presenting with acute dyspnea in emergency department and inpatient settings. 2 However, its role in the bronchoscopy suite is less frequently reported. One case report describes the use of lung POCUS to diagnose post‐bronchoscopy pneumothorax. 3 In this case report, we describe the use of cardiac, lung and lower limb POCUS to rapidly diagnose massive pulmonary embolism (PE) and deep vein thrombosis (DVT) in a patient presenting with acute dyspnea prior to elective bronchoscopy.
CASE REPORT
A 64‐year‐old Malay lady, non‐smoker with comorbid hypertension and dyslipidemia, presented to our bronchoscopy suite with breathlessness and painless swelling of the left lower limb for 2 days. She was scheduled for elective outpatient bronchoscopy as part of emergent workup for lung malignancy. A month prior, she had been admitted under the Orthopedic unit with paraplegia due to spinal compression from metastatic disease. Further history revealed chronic cough and chest radiograph revealed a left hilar opacity. Computed tomography of the thorax revealed a 6.4 × 5.6 cm left hilar mass, enlarged left supraclavicular lymph node and metastatic lesions in the spine, ribs, and liver consistent with a radiological staging of T3 N3 M1c (Stage IVB). She refused spinal operative intervention, opting instead for excision biopsy of an enlarged cervical lymph node. Histopathological examination of lymph node biopsy was inconclusive. She was then referred to our unit for bronchoscopic biopsy of the left hilar mass.
On further history, she denied hemoptysis, wheezing, chest pain, fever or worsening of her baseline chronic cough. She was hypotensive with a blood pressure of 88/58 mmHg, hypoxic with oxygen saturation of 93% under room air and had a respiratory rate of 34 breaths per minute. Her heart rate was 97 beats/min, and she was apyrexial. Cardiovascular examination revealed a raised jugular venous pressure at 6 cm above the sternal angle and a pansystolic murmur at the lower left sternal edge loudest on inspiration, suggestive of raised right heart pressure. Electrocardiogram revealed right axis deviation and T wave inversion in leads III, V1, V2 and V3. Lower limb examination revealed a non‐tender left lower limb swelling. There were no crepitations or rhonchi on respiratory examination. Nasal prong oxygen was immediately applied, with subsequent arterial blood gas showing pH 7.484, pO2 101.7 mmHg, pCO2 25.5 mmHg, HCO3 18.8 and SaO2 of 98.3. Unfortunately, her blood pressure was unresponsive to fluid boluses and noradrenaline infusion was commenced.
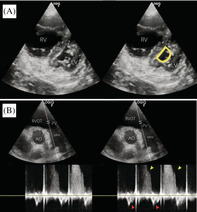
Lower limb POCUS revealed a non‐compressible left femoral vein with an intraluminal hyperechoic lesion with absence of Doppler signal within (Figure 1A). Lung POCUS was unremarkable, demonstrating A‐profile bilaterally. Cardiac POCUS revealed a dilated right ventricle, flattening of the septal wall, and a ‘D’‐shaped left ventricle (Figure 2A). Pulsed wave Doppler placed at the right ventricular outflow tract was suggestive of increased pre‐capillary pulmonary artery pressure (Figure 2B). Tricuspid regurgitation (TR), tricuspid annular plane systolic excursion (TAPSE) of 1.8 mm (abnormality threshold <17 mm), and TR maximal pressure gradient (TR max PG) of 42.5 mmHg was demonstrated.
FIGURE 1.
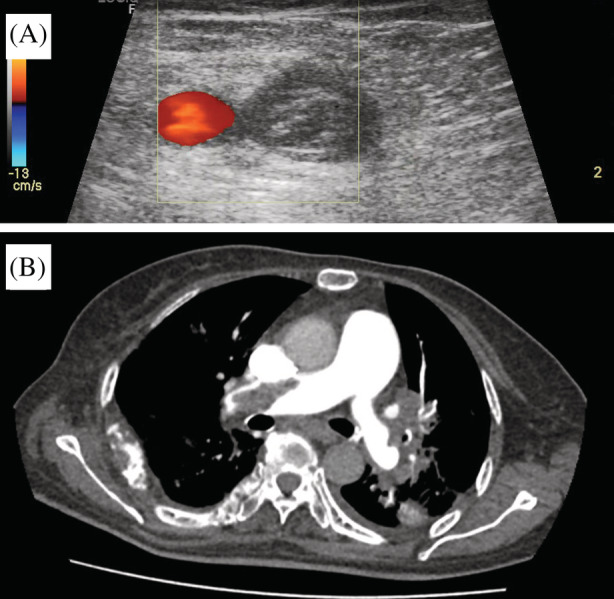
(A) Lower limb POCUS confirmed left lower limb DVT with direct visualization of a thrombus in the left femoral vein. (B) Representative slice of the patient's CTPA (axial view) confirmed a large thrombus in the right main pulmonary artery
FIGURE 2.
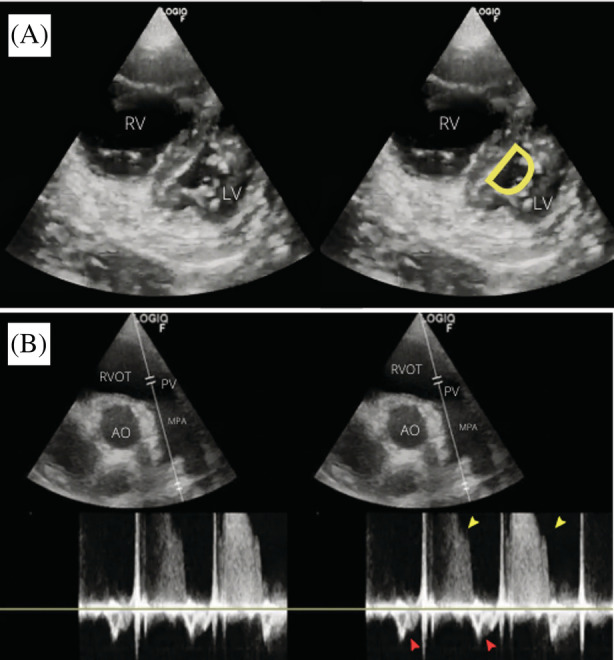
(A) Bedside cardiac POCUS showed flattening of the septal wall with D‐shaped left ventricle and dilated right ventricle in parasternal short axis view (RV = right ventricle, LV = left ventricle). (B) PW Doppler sampled proximal to the pulmonary valve demonstrated raised pre‐capillary pulmonary artery pressure, as evidenced by mid‐systolic notching (red arrows) and pulmonary regurgitation jet (yellow arrows)
The patient's PESI score of 114 points (class IV) indicated high risk of mortality from pulmonary embolism. CTPA was delayed due to the patient's labile blood pressure. Hence, the decision was made to administer thrombolysis prior to CTPA. 70 mg of intravenous alteplase were administered, followed by intravenous heparin infusion. After the patient's blood pressure improved, CTPA was done 30 min post‐thrombolysis, revealing extensive bilateral pulmonary embolism (Figure 1B). The patient was admitted to the medical high dependency ward for close observation. She made significant improvement for the first 2 days but developed STEMI on the third day and eventually succumbed.
DISCUSSION
Lung malignancy is associated with one of the highest venous thromboembolism risks compared to other malignancies, carrying a pooled incidence rate of 45 per 1000‐person years. 4 70% of lung malignancy is metastatic at the time of diagnosis of PE. 5 Our patient was at increased risk of venous thromboembolism due to immobilization and metastatic lung malignancy. 6
This case highlights the time‐efficient nature of POCUS in diagnosing DVT and PE. In our patient, POCUS examination was initiated approximately 10 min after initial history taking and examination. Collectively, lung, cardiac and lower limb POCUS took approximately 15 min to complete. This was followed by a brief discussion lasting 5 min, which culminated in a final decision to administer thrombolytic therapy.
In clinical practice, duplex ultrasonography from thigh to ankle is the diagnostic modality of choice. 7 A positive finding on lower limb POCUS would raise suspicion of PE in a dyspneic patient like ours, as the majority of PE originates from DVT. 8 In the critically ill patient, POCUS can act as a surrogate for ‘formal’ ultrasound. 9 POCUS DVT examination has been extensively validated in various clinical settings. 10 , 11
Combining conventional Wells score with lung and lower limb POCUS leads to increased predictive performance in diagnosing PE. 12 Pulmonary angiography was the historical gold standard in diagnosing PE, but CTPA is the current method of choice. 8 However, CTPA may be delayed due to the need for clinical stabilization, as was the case in our patient. In a hemodynamically unstable patient with suspected PE, specific echocardiography features such as the 60/60 sign, McConnell sign, or right heart thrombi justify emergency reperfusion treatment for PE if immediate CTPA is not feasible. 8
Less commonly, lung ultrasonography (LUS) can diagnose pulmonary embolism with a sensitivity of 74%, specificity of 95% and 95% positive predictive value. 13 Ultrasound findings include characteristic triangular or rounded pleural‐based lesions, however, other causes such as pneumonia, atelectasis and so on must be ruled out as well. 13
The British Thoracic Society guidelines recommend a thorough assessment of fitness for bronchoscopy based on baseline oxygen saturation, lung function, comorbidities, sedation and procedural sampling. 14 Bronchoscopy itself may lead to hypoxaemia, increased cardiac workload and arrhythmias. 15 There are no clear recommendations on the degree of hypoxaemia which precludes bronchoscopy, hence bronchoscopists often decide on a case‐to‐case basis. Our patient's hemodynamic instability and respiratory distress clearly excluded her as a candidate for bronchoscopy, however, POCUS examination added additional value by securing a rapid diagnosis of venous thromboembolism.
CONCLUSION
Multi‐system POCUS proved effective in diagnosing DVT and PE in our patient. Clinicians are reminded of its powerful applicability in assessing acute dyspnoea in a day‐case or outpatient setting.
AUTHOR CONTRIBUTION
Larry Ellee Nyanti initiated the idea for case reporting. Mohammad Amirul Shahril bin Ahmad, Larry Ellee Nyanti, Chan Tha A Hing, Chan Sin Chai, and Siew Teck Tie were involved in the overall management of the patient. Jie Cong Yeoh acquired and interpreted the ultrasonographic data. Mohammad Amirul Shahril bin Ahmad, Larry Ellee Nyanti, Jie Cong Yeoh and Chan Tha A Hing prepared the final manuscript. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
None declared.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and accompanying images.
ACKNOWLEDGMENT
The authors would like to thank the Director General of Health Malaysia for permission to publish this paper, and Universiti Malaysia Sabah for financing the publication fees of this paper.
Ahmad MASb, Nyanti LE, Yeoh JC, A Hing CT, Chai CS, Tie ST. Point‐of‐care ultrasound in pre‐bronchoscopy assessment of acute dyspnea: A case of concurrent massive pulmonary embolism and deep vein thrombosis. Respirology Case Reports. 2022;10:e01029. 10.1002/rcr2.1029
Associate Editor: John Wrightson
Funding information Universiti Malaysia Sabah
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Moore CL, Copel JA. Point‐of‐care ultrasonography. N Engl J Med. 2011;364(8):749–57. 10.1056/NEJMra0909487 [DOI] [PubMed] [Google Scholar]
- 2. Qaseem A, Etxeandia‐Ikobaltzeta I, Mustafa RA, Kansagara D, Fitterman N, Wilt TJ, et al. Appropriate use of point‐of‐care ultrasonography in patients with acute dyspnea in emergency department or inpatient settings: a clinical guideline from the American College of Physicians. Ann Intern Med. 2021;174(7):985–93. 10.7326/M20-7844 Erratum in: Ann Intern Med. 2022;175(3):458–459. [DOI] [PubMed] [Google Scholar]
- 3. Ngo DQ, Aftab G, Frenia D. The utility of point‐of‐care ultrasound for post‐bronchoscopy pneumothorax evaluation. Cureus. 2021;13(5):e15339. 10.7759/cureus.15339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta‐analysis. PLoS Med. 2012;9(7):e1001275. 10.1371/journal.pmed.1001275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shinagare AB, Okajima Y, Oxnard GR, Dipiro PJ, Johnson BE, Hatabu H, et al. Unsuspected pulmonary embolism in lung cancer patients: comparison of clinical characteristics and outcome with suspected pulmonary embolism. Lung Cancer. 2012;78(2):161–6. 10.1016/j.lungcan.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marinho FC, Takagaki TY. Hypercoagulability and lung cancer. J Bras Pneumol. 2008;34(5):312–22. English, Portuguese. 10.1590/s1806-37132008000500011 [DOI] [PubMed] [Google Scholar]
- 7. Mazzolai L, Aboyans V, Ageno W, Agnelli G, Alatri A, Bauersachs R, et al. Diagnosis and management of acute deep vein thrombosis: a joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function. Eur Heart J. 2018;39:4208–18. [DOI] [PubMed] [Google Scholar]
- 8. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543–603. 10.1093/eurheartj/ehz405 [DOI] [PubMed] [Google Scholar]
- 9. Sud S, Mittmann N, Cook DJ, Geerts W, Chan B, Dodek P, et al. Screening and prevention of venous thromboembolism in critically ill patients: a decision analysis and economic evaluation. Am J Respir Crit Care Med. 2011;184(11):1289–98. [DOI] [PubMed] [Google Scholar]
- 10. Mayo PH, Beaulieu Y, Doelken P, Feller‐Kopman D, Harrod C, Kaplan A, et al. American College of Chest Physicians/La Société de Réanimation de Langue Française statement on competence in critical care ultrasonography. Chest. 2009;135(4):1050–60. [DOI] [PubMed] [Google Scholar]
- 11. Pomero F, Dentali F, Borretta V, Bonzini M, Melchio R, Douketis JD, et al. Accuracy of emergency physician‐performed ultrasonography in the diagnosis of deep‐vein thrombosis: a systematic review and meta‐analysis. Thromb Haemost. 2013;109(1):137–45. [DOI] [PubMed] [Google Scholar]
- 12. Nazerian P, Volpicelli G, Gigli C, Becattini C, Sferrazza Papa GF, Grifoni S, et al. Diagnostic performance of wells score combined with point‐of‐care lung and venous ultrasound in suspected pulmonary embolism. Acad Emerg Med. 2017;24(3):270–80. [DOI] [PubMed] [Google Scholar]
- 13. Mathis G, Blank W, Reißig A, Lechleitner P, Reuß J, Schuler A, et al. Thoracic ultrasound for diagnosing pulmonary embolism* a prospective multicenter study of 352 patient. Chest. 2005;128:1531–8. [DOI] [PubMed] [Google Scholar]
- 14. Du Rand IA, Blaikley J, Booton R, Chaudhuri N, Gupta V, Khalid S, et al. British Thoracic Society bronchoscopy guideline group. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax. 2013;68(Suppl 1):i1–i44. 10.1136/thoraxjnl-2013-203618 [DOI] [PubMed] [Google Scholar]
- 15. Davies L, Mister R, Spence DP, Calverley PMA, Earis JE, Pearson MG. Cardiovascular consequences of fibreoptic bronchoscopy. Eur Respir J. 1997;10:695–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


