Abstract
To evaluate the outcomes and tolerance of immune check point inhibitors (ICI) for patients with recurrent chordoma.
We reviewed the records of 17 patients with recurrent chordomas who received ICI for progressing disease as part of their treatment between 2016 and 2020. Response was assessed using RECIST 1.1 criteria. The Kaplan-Meier method was used to estimate the duration of response (DOR), progression free survival (PFS), and overall survival (OS). Clinical benefit was defined as having stable disease (SD), a partial response (PR), or complete response (CR).
The median follow-up from the start of ICI was 29 months (interquartile range [IQR], 13–35 m). The majority received pembrolizumab (n=9, 53%), and the median number of cycles delivered was 8 (IQR, 7–12). The 1-year OS was 87%, and the 1-year PFS was 56% with a median PFS of 14 months (95% CI, 5–17 months). Following ICI initiation, most patients (n=15, 88%) had clinical benefit consisting of a CR (n=1, 6%), PR (n=3, 18%), and SD (n=11, 65%). Among all responders (n=15), the median DOR was 12 months. Toxicities were limited: two (12%) patients having grade 3/4 immune-related toxicities (colitis, grade 3; myocarditis, grade 4).
We observed a high rate of clinical benefit and favorable durability from ICI use for patients with recurrent chordoma. These data provide support for the integration of ICI as a standard first-line systemic therapy option for patients with recurrent chordoma. Prospective studies are warranted to further evaluate efficacy and enhance response rates.
Keywords: chordoma, immune checkpoint inhibitors, immunotherapy, bone tumor
INTRODUCTION
Chordomas are rare, locally aggressive neoplasms of bone that arise in the skull base, mobile spine, and sacrum.1,2 Management of these tumors is particularly challenging for multiple reasons. First, their deep-seated location immediately adjacent to the craniospinal axis results in a propensity to encapsulate nearby neural tissue or invade abutting structures makes locoregional treatment difficult and often morbid. Second, chordomas have an indolent growth pattern which often results in patients presenting with locally advanced disease. And thirdly, these tumors are somewhat resistant to traditional systemic therapies.
Surgical extirpation is the current mainstay of treatment with the extent of resection remaining one of the most important prognostic factors.3–7 However, despite macroscopic complete resections, some series suggest that over 50% of patients will eventually develop locoregional recurrence and survival rates at 5 years are around 65–70%.2,8–11 These relatively poor outcomes highlight the need for more effective adjuvant and salvage treatment options.
Currently, medical therapies are somewhat limited. Traditional cytotoxic chemotherapy is inactive against chordomas and thus is not a recommended option.2 Additionally, despite multiple targetable molecular pathways that underlie chordoma pathophysiology, clinical data indicate targeted therapies have only modest activity in patients with recurrent disease.12–14 However, immune checkpoint inhibitors (ICI) are a class of drugs that have revolutionized the treatment approaches across multiple tumor histologies but largely have not been investigated in the treatment of chordomas.
Pre-clinical data indicate that chordomas broadly interact with the immune system. Specifically, data suggests that PD-1/PDL-1 tumor microenvironment interactions may potentially promote the locally aggressive behavior of chordomas by allowing immune evasion and tumor progression.15–17 Based on the pre-clinical data, we hypothesized ICI may have some potential clinical benefit and have selectively integrated it into our practice. Given the paucity of data on this approach, here we present our institutional experience using immune checkpoint inhibition in the management of patients with recurrent chordoma to evaluate associated outcomes and tolerance.
METHODS
We identified 17 consecutive patients with histologically confirmed recurrent chordoma who received ICI for progressing disease as part of their treatment at The University of Texas MD Anderson Cancer Center (MDACC) during the period from 2016 and 2020. After institutional review board approval, medical charts were retrospectively reviewed. Patients underwent a full history, complete physical examination, and baseline staging consisting of CT or PET/CT. Histologic diagnoses of chordoma were confirmed by pathologists at MDACC. When available, PD-L1 staining was categorized as positive (any staining) or negative.
Disease status was categorized into local, regional, or distant at the time of initiation of ICI. Localized was defined as recurrence at the site of the primary tumor. Regional disease was defined as occurring within the paraspinal musculature or bone in close proximity but discontiguous from the primary. Distant disease was classified as that occurring by hematogenous dissemination far from the primary tumor and surgical site.
At the discretion of the treating medical oncologist, several ICI regimens were used for the patients in this study initiated at the time of progressing disease. Most commonly single-agent pembrolizumab at 200 mg delivered intravenous (IV) every 3 weeks for up to 12 cycles dependent on response and tolerance. Durvalumab 1500 mg and tremelimumab 75 mg IV given one day 1 and every 4-week cycle as part of a phase II clinical trial (NCT02815995). Other immune checkpoint inhibitors were used with less frequency.
Most patients at some point during their treatment course received radiation therapy (RT), though often independent from ICI. We defined concurrent treatment with ICI as RT being delivered within 3 months of ICI receipt.
Follow-up
Patients underwent CT or PET/CT imaging at baseline and a serial time points, most commonly after every 2 cycles. Decisions regarding continuation of ICI were based on both clinical and radiographic assessments. If patients demonstrated clinical improvement despite radiographic evidence of mild progression, often ICIs were continued. Patients were categorized as having a clinical benefit to ICI or not; since ICI therapy was initiated for progressing disease, clinical benefit was defined radiographically as achieving stable disease (SD), a partial response (PR), or complete response (CR) by RECIST 1.1 criteria. ICIs were discontinued for clear clinical progression or for immune-related toxicities. Attributable toxicities were retrospectively graded using CTCAE criteria version 5 and categorized as low grade (1 or 2) or high grade (3–5). Reasons for discontinuation of ICI were categorized as completion of intended course, toxicity, clinical progression (i.e.. symptoms), radiographic progression, or currently ongoing in patients who have not had a reason to discontinue to date.
Statistical Analysis
Descriptive statistics were used to evaluate and summarize baseline characteristics, and differences between proportions of categorical data were analyzed by using Fisher’s exact test and chi-squared analyses as appropriate. The distributions of duration of response (DOR), progression-free survival (PFS) and overall survival (OS) were estimated by the Kaplan-Meier method. Duration of response (DOR) was defined as time from the date of the first documented response (i.e., the start date of response) of complete response (CR) or partial response (PR) or stable disease (SD) to the date of the first documented progression or death due to any cause. PFS was defined as the time from ICI initiation to the time of progression or death, whichever occurred first; subsequent systemic therapies were only considered at the time of progression and thus would not confound this time interval. OS was defined as the time from ICI initiation to death. For events that did not occur by the time of data analysis, times were censored at the last contact at which the patient was known to be progression free for DOR, PFS or the last time the patient was known to be alive for OS. A waterfall was used to display change of tumor size from baseline to the time of best response. A Spider plot was used to present the change of tumor size over time. A swimmer plot was used to graphically show the effects of immunotherapy on tumor response. SAS 9.4, TIBCO Spotfire S+ 8.2 for windows and R version 3.5.2 were used for data analysis.
RESULTS
Patient and Tumor Characteristics
Patient and tumor characteristics are listed in Table 1. The median age at initial chordoma diagnosis was 56 years (interquartile range [IQR], 52–65) and 63 years (IQR, 60–69) at the time of ICI initiation. A majority of patients were male (n=13, 76%) and non-Hispanic white (n=13, 76%), and the primary site of involvement was most commonly the sacrum (n=10, 59%; mobile spine, n=5, 29%; base of skull, n=2, 12%). Immunohistochemistry for PD-L1 (clone 22C3) was not performed in all patients (n=8, 47%), but was positive in 4 (44%) and negative in 5 (56%).
Table 1.
Patient and Tumor Characteristics for Patients
| Variable | All Patients (n=17) Value or No. (%) |
|---|---|
| Follow-up time from dx, months | |
| Median | 89 |
| IQR | 62–160 |
| Follow-up time from start of IO, months | |
| Median | 29 |
| IQR | 13–35 |
| Age at initial dx, years | |
| Median | 56 |
| IQR | 52–65 |
| Sex | |
| Female | 4 (24) |
| Male | 13 (76) |
| Race | |
| Black | 1 (6) |
| Asian | 1 (6) |
| Hispanic | 2 (12) |
| Non-Hispanic white | 13 (76) |
| Primary Tumor Location | |
| Sacrum | 10 (59) |
| Mobile Spine | 5 (29) |
| Base of Skull | 2 (12) |
| PD-L1 Staining | |
| Positive | 4 (24) |
| Negative | 5 (29) |
| Not tested | 8 (47) |
| Disease Status Prior to IO | |
| Localized | 5 (29) |
| Regional | 5 (29) |
| Metastatic | 7 (42) |
| Disease Status at LFU | |
| Alive with Disease | 12 (71) |
| Dead with Disease | 4 (24) |
| No evidence of Disease | 1 (6) |
Abbreviations: IQR, interquartile range; IO, immunotherapy; dx, diagnosis
At the time of ICI initiation, disease status was localized for 5 patients (29%), regional for 5 (29%) and metastatic for 7 (42%), and most patients were systemic therapy naïve (n=10, 59%) (Table 2). None were considered to have resectable disease at the time of ICI initiation. Regarding the types of ICI, the majority received pembrolizumab (n=9, 53%). Other ICI regimens included durvalumab/tremelimumab (n=5, 29%), FAZ053 (n=2, 12%), and nivolumab/bempegaldesleukin (n=1, 6%). The median number of cycles delivered was 8 (IQR, 7–12), and the reasons for discontinuation were eventual radiographic progression (n=5, 29%), clinical symptomatic progression (n=2, 12%), completion of the recommended course (n=5, 29%) or immune-related toxicity (n=3, 18%); two patients (12%) have ongoing treatment.
Table 2.
Treatment Characteristics and Responses
| Variable | All Patients (n=17) Value or No. (%) |
|---|---|
| Lines of therapy prior to IO | |
| 0 | 10 (59) |
| 1 | 5 (29) |
| 3–4 | 2 (12) |
| Anti-PD-L1 Agent | |
| Pembrolizumab | 9 (53) |
| Durvalumab/Tremelimumab | 5 (29) |
| Nivolumab/IL-2 | 1 (6) |
| FAZ053 | 2 (12) |
| Number of cycles of IO | |
| Median | 8 |
| IQR | 7–12 |
| Ongoing | 2 |
| Reason for Discontinuation | |
| Completion | 5 (29) |
| Toxicity | 3 (18) |
| Radiographic progression | 5 (29) |
| Clinical progression | 2 (12) |
| Ongoing | 2 (12) |
| Any Clinical Benefit to IO | |
| Yes | 15 (88) |
| No | 2 (12) |
| Best RECIST Response to IO | |
| Progressive disease | 2 (12) |
| Stable disease | 11 (65) |
| Partial response | 3 (18) |
| Complete response | 1 (6) |
Abbreviations: IO, immunotherapy; IL-2, bempegaldesleukin; IQR, interquartile range; RT, radiation therapy
Nearly all (n=16, 94%) patients received radiation therapy (RT) as part of their care at some point, with 13 (76%) receiving RT at the time of recurrence. Of the 13 patients, only 4 (31%) received concurrent RT and ICI therapy, whereas 6 (46%) received RT before ICI and 3 (23%) afterwards as salvage at the time of progression.
Outcomes and Efficacy
Median follow up was 29 months (IQR, 13–35) and most patients were alive with disease at last follow-up (n=12, 71%). With a median OS that has not yet been reached (95% CI, 15-NR), the 1-year OS was 87% (95% CI 56–96%) (Figure 1). The 1-year PFS was 56% (95% CI 29–76%) with a median PFS of 14 m (95% CI, 5–17) (Figure 1).
Figure 1.
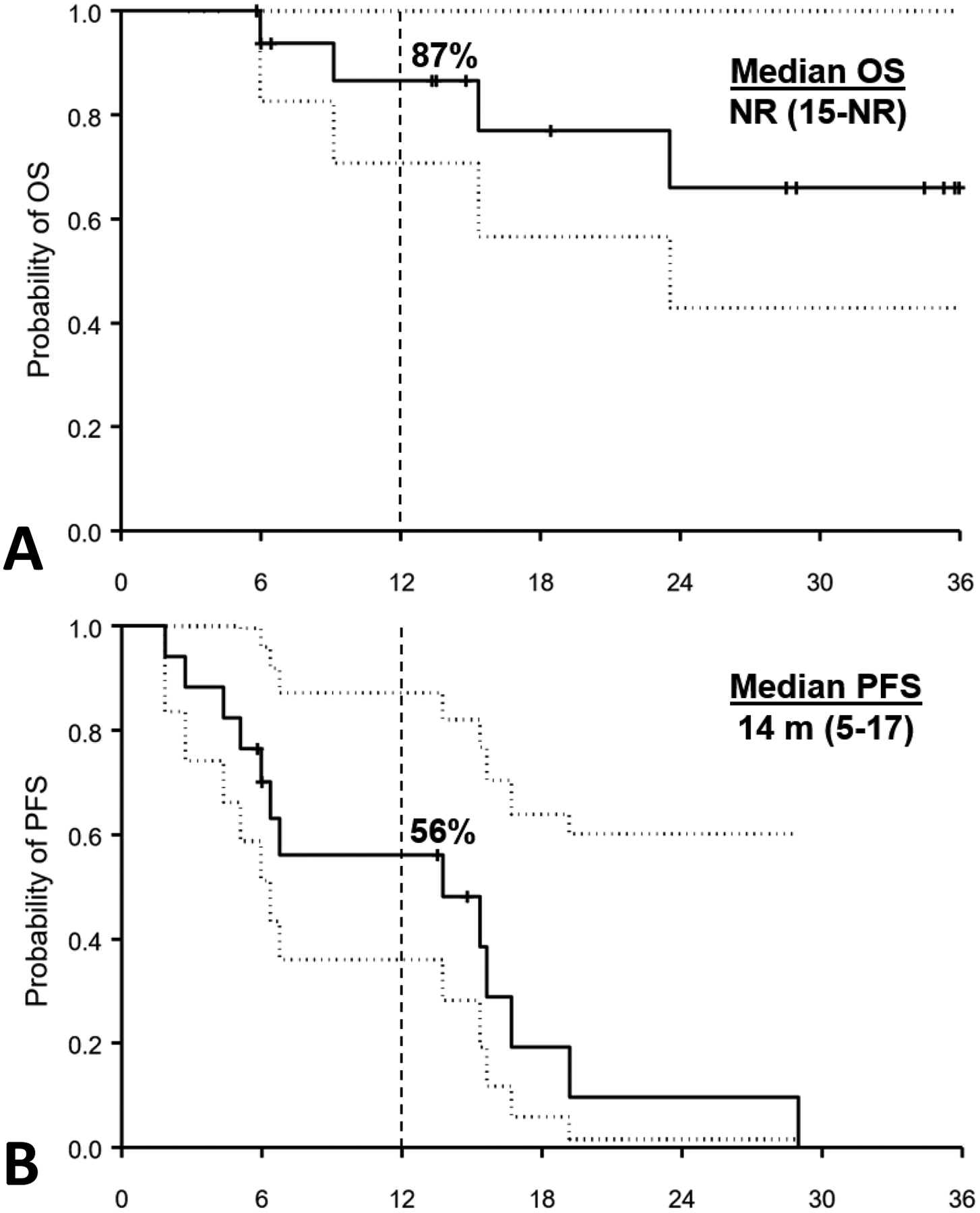
Kaplan-Meier curves showing actuarial outcomes (solid line) and the 95% confidence intervals (dotted line) for patients with chordoma receiving immune checkpoint inhibitors. Panel (A) represents overall survival (1-year OS of 87%) and panel (B) represents progression free survival (1-year PFS of 56%).
Overall, 15 out of the 17 patients (88%) had clinical benefit (CR + PR + SD) to ICI (Table 2, Figure 2). Most patients had SD (n=11, 65%), whereas 3 patients had PR (18%) and 1 a CR (6%) (Figure 2 & 3). Among all responders, the median DOR was 12 months (95% CI 5–15), with an observed shorter median DOR among patients who achieved only SD (6 months; 95% CI, 4–17) compared to a CR/PR (13 months; 95% CI, 11–14) (P=0.7).
Figure 2.
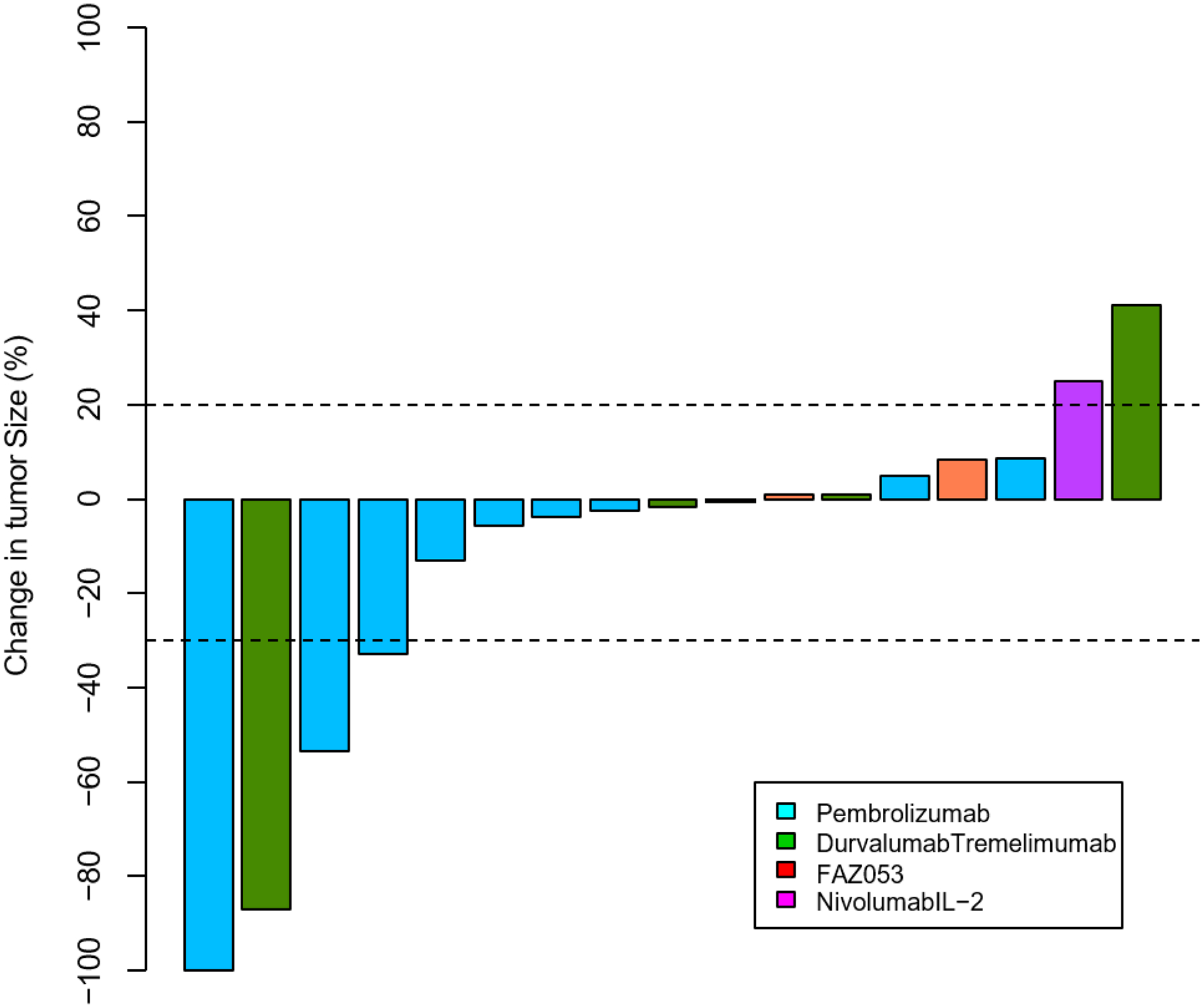
A waterfall plot representing the magnitude of responses as per RECIST 1.1 criteria to immune checkpoint inhibitors for patients with recurrent chordoma.
Figure 3.
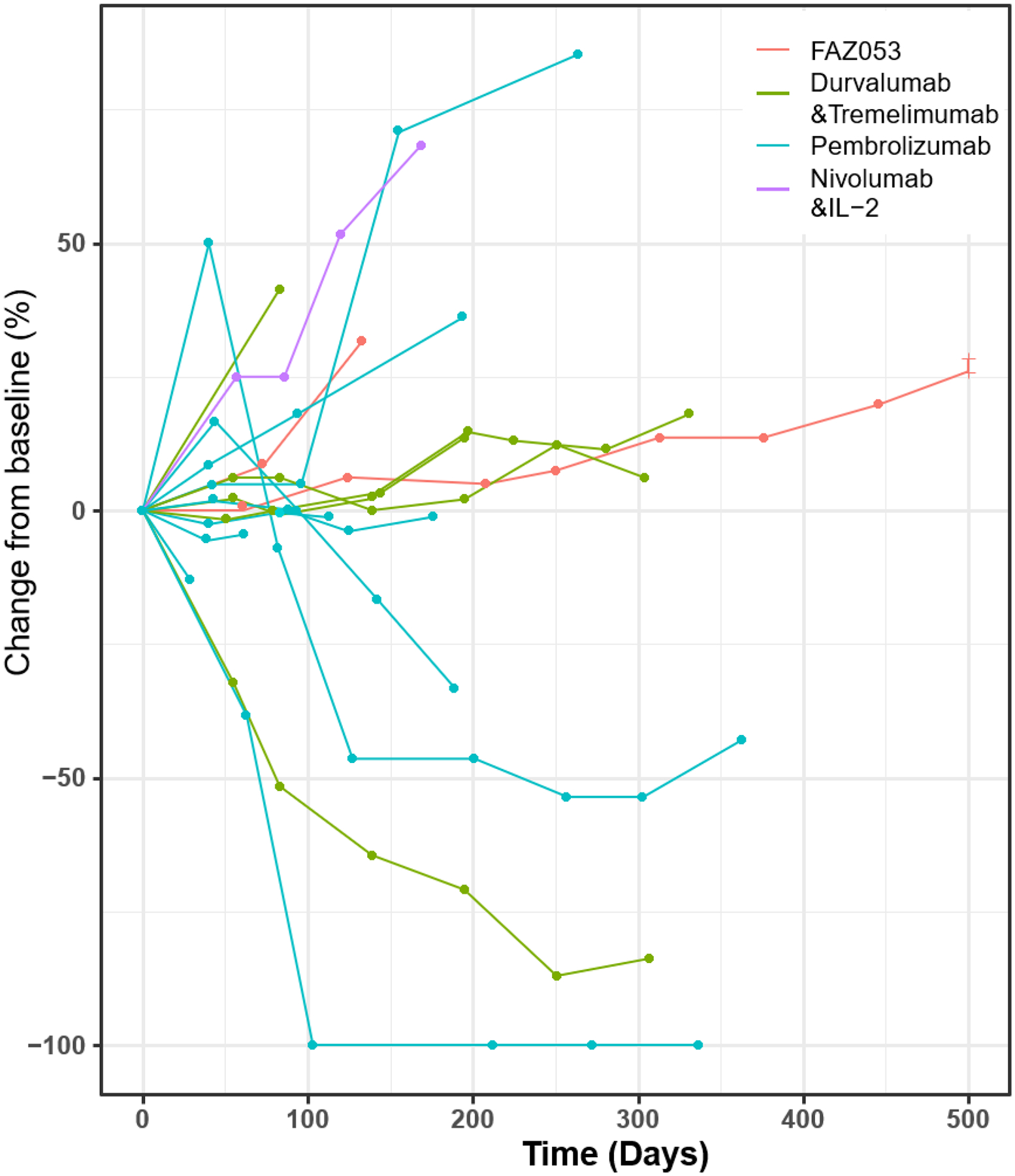
A spider plot representing chordoma tumor change from baseline in patients receiving immune checkpoint inhibitors
Of the 4 patients (24%) who achieved a PR or CR, PD-L1 staining was positive in the complete responder, negative for a partial responder, and not tested in the other two patients. Three received pembrolizumab and 1 durvalumab/tremelimumab, and all received ICI as a first line systemic therapy (Figure 3). Additionally, 2 of the 4 patients received concurrent RT. Interestingly, half of these patients initially had radiographic progression following ICI initiation, but due to a discordance with improved clinical symptoms, ICIs were continued (Figure 3). The median time it took to obtain at least a PR was 5 months (IQR, 3–7), and the median time to maximal response was 8 months (IQR, 5–10). Two patients completed the planned ICI course (12 and 16 cycles), whereas one patient discontinued therapy due to immune-related toxicity (13 cycles) and the other discontinued due to clinical, symptomatic progression (8 cycles) despite a sustained radiographic response.
For the 11 patients (65%) who obtained SD, 6 received pembrolizumab, 3 durvalumab/tremelimumab, and 2 FAZ053. Reasons for discontinuing therapy included: completion of therapy (n=3; median 12 cycles), toxicity (n=2), clinical progression (n=1), and radiographic progression (n=3). For the 3 patients that eventually had PD, the median duration of SD was 4 months (range, 2–14).
Toxicity
Treatment was well-tolerated. Eight (47%) patients experienced some immune-related toxicity - 5 were grade 1/2 and 3 were grade 3/4 – with 3 patients discontinuing ICI because of toxicity. Most grade 1 or 2 toxicities were dermatologic (n=2), endocrine (n=2), or Sicca syndrome-related (n=2). For the higher-grade toxicities, there were two grade 3 and 1 grade 4. One patient experienced a grade 3 myocarditis and myositis after 2 cycles, another had a grade 3 colitis after 6 cycles, and the last had a grade 4 potentially attributable pneumonitis versus infectious reaction.
DISCUSSION
Chordomas are a rare bone malignancy with a high recurrence rate and limited medical therapies. Preclinical data provide rationale for the integration of ICI as a treatment option, but clinical outcomes data are lacking. Here we present one of the largest series of patients with chordoma treated using immune checkpoint inhibitors. We found that nearly all patients had measurable clinical benefit to ICI and treatment was well-tolerated. Additionally, treatment responses were relatively durable, though longer among patients achieving a CR/PR compared to SD. These preliminary data suggest ICI are an effective treatment option for recurrent chordoma.
We observed clinical benefit in 88% of patients receiving ICI, with nearly 25% having a partial or complete response. These findings are notable given the still unmet clinical need for efficacious systemic therapies against recurrent chordoma. Prior reports of ICI use in this disease are limited to case studies and one non-randomized phase II basket study. Nonetheless, similar responses have been observed. Migliorini and colleagues first reported clinical responses to immunotherapy in three cases of relapsed chordoma.18 One patient with a heavily pre-treated cervical spine chordoma received pembrolizumab and had reversal of neurologic symptoms and a marked radiographic response that was sustained at the 6-month follow up. A second patient with a petro-clival chordoma received nivolumab and again a rapid clinical and radiographic was observed that lasted for 9 months. In a more recent case report by Williamson and colleagues, a child with a base of skull poorly-differentiated chordoma was treated with nivolumab and had a partial radiographic response; nivolumab was continued for 14 cycles with ongoing clinical benefit and improved quality of life.19 Finally, only in abstract form by Blay and colleagues, 34 patients with chordoma received pembrolizumab as part of a phase II study; they observed a 1-year PFS of 31%.20 Our data, when taken together with these studies, provide evidence that immunotherapy provides a high rate of clinical benefit, with some patients responding more robustly to ICI.
Tyrosine kinase inhibitors, including imatinib and sorafenib, have the greatest evidence of systemic efficacy in this disease.21 In a phase II study by Stacchiotti and colleagues, 56 patients were prospectively treated with imatinib until progression. They observed a 64% clinical benefit rate and a median PFS of 9 months.12 Similar outcomes were reported with sorafenib use; Bompas and colleagues conducted a phase II study for 27 patients and reported a 12 month PFS of 73%,14 whereas Svoboda and colleagues published a case report of a patient who achieved a PFS of 12 months with sorafenib use.22 While our cohort is smaller than these two reported phase II studies, our preliminary data suggest that ICI may provide a higher rate of clinical benefit with an at least similar, if not more favorable, progression free survival compared to tyrosine kinase inhibitors.
Based on our data, there is a high rate of clinical benefit with a more robust response in a select subset of patients. However, what contributed to those more favorable responses is less certain – type of ICI, tumor checkpoint expression, tumor genetics, the immune microenvironment, or other patient/tumor factors. The higher rate of PR/CR in patients receiving pembrolizumab contrasted against several patients also demonstrating more rapid progression highlights the uncertainties. This conundrum was further highlighted in a study by Scognamiglio and colleagues in which they found that PD-L1 expression in patient-derived chordoma organoids was correlated with TIL presence but did not predict for responses to ICI.23 Additional work is needed in determining how chordomas interact with the immune microenvironment so biomarkers predictive of response can be incorporated into clinical practice.
The integration of our data into the broader chordoma treatment paradigm is complex. In order to achieve optimal patient outcomes, we recommend patients with chordoma receive treatment at specialized centers where expert multidisciplinary care can be coordinated between experienced surgeons, radiation oncologists, and medical oncologists. When systemic therapy is being considered, we consider ICI to be an emerging approach and recommend it as a first-line option in select patients.
This study represents the largest published series to date in the literature of patients with chordoma receiving ICI. However, given the rarity of this tumor and the previous paucity of clinical data, our series is small, which limits the statistical power and analyses performed. Additionally, the cohort is comprised of a heterogenous group of patients with chordoma, and while responses to ICI are encouraging, there are other potential factors that were not controlled for. As with any retrospective series, there is inherent selection bias. Finally, while more consistent PD-L1 staining would have been interesting, prior data suggest it may not have been predictive of response; this should be considered for future studies in addition to other pathologic markers of response to ICI
In conclusion, we observed a high rate of clinical benefit with the use of ICI for patients with chordoma, which provide preliminary data confirming its efficacy in this rare disease. Nearly a quarter of the patients had a more robust partial or complete response, which highlights the need for more predictive biomarkers. These promising data provide support for the integration of ICI as a standard first-line systemic option for patients with chordoma. Prospective studies are warranted to further evaluate efficacy. Additionally, investigation into ways of enhancing response rates (i.e. combination therapies, addition of RT, etc) or the role of incorporating ICI into the paradigm at earlier stages of disease presentation would continue to advance the field.
Conflict of Interest:
Alexander J Lazar reports consulting fees for Merck, Bristol Myers Squibb, and AstraZeneca which are outside the submitted work. Neeta Somaiah reports research funding from AstraZeneca which are outside the submitted work. Anthony P Conley reports consulting fees from Novellus, Inhibrx, Deciphera and ACI. The other authors made no disclosures.
Supported in part by Cancer Center Support (Core) Grant CA016672 to The University of Texas MD Anderson Cancer Center.
REFERENCES
- 1.Barber SM, Sadrameli SS, Lee JJ, et al. : Chordoma-Current Understanding and Modern Treatment Paradigms. J Clin Med 10, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stacchiotti S, Gronchi A, Fossati P, et al. : Best practices for the management of local-regional recurrent chordoma: a position paper by the Chordoma Global Consensus Group. Ann Oncol 28:1230–1242, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varga PP, Szoverfi Z, Fisher CG, et al. : Surgical treatment of sacral chordoma: prognostic variables for local recurrence and overall survival. Eur Spine J 24:1092–101, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Gokaslan ZL, Zadnik PL, Sciubba DM, et al. : Mobile spine chordoma: results of 166 patients from the AOSpine Knowledge Forum Tumor database. J Neurosurg Spine 24:644–51, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergh P, Kindblom LG, Gunterberg B, et al. : Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer 88:2122–34, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Ruggieri P, Angelini A, Ussia G, et al. : Surgical margins and local control in resection of sacral chordomas. Clin Orthop Relat Res 468:2939–47, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stacchiotti S, Casali PG, Lo Vullo S, et al. : Chordoma of the mobile spine and sacrum: a retrospective analysis of a series of patients surgically treated at two referral centers. Ann Surg Oncol 17:211–9, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Karpathiou G, Dumollard JM, Dridi M, et al. : Chordomas: A review with emphasis on their pathophysiology, pathology, molecular biology, and genetics. Pathol Res Pract 216:153089, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Jones PS, Aghi MK, Muzikansky A, et al. : Outcomes and patterns of care in adult skull base chordomas from the Surveillance, Epidemiology, and End Results (SEER) database. J Clin Neurosci 21:1490–6, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Bohman LE, Koch M, Bailey RL, et al. : Skull base chordoma and chondrosarcoma: influence of clinical and demographic factors on prognosis: a SEER analysis. World Neurosurg 82:806–14, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Pan Y, Lu L, Chen J, et al. : Analysis of prognostic factors for survival in patients with primary spinal chordoma using the SEER Registry from 1973 to 2014. J Orthop Surg Res 13:76, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stacchiotti S, Longhi A, Ferraresi V, et al. : Phase II study of imatinib in advanced chordoma. J Clin Oncol 30:914–20, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Hindi N, Casali PG, Morosi C, et al. : Imatinib in advanced chordoma: A retrospective case series analysis. Eur J Cancer 51:2609–14, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Bompas E, Le Cesne A, Tresch-Bruneel E, et al. : Sorafenib in patients with locally advanced and metastatic chordomas: a phase II trial of the French Sarcoma Group (GSF/GETO). Ann Oncol 26:2168–73, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou MX, Peng AB, Lv GH, et al. : Expression of programmed death-1 ligand (PD-L1) in tumor-infiltrating lymphocytes is associated with favorable spinal chordoma prognosis. Am J Transl Res 8:3274–87, 2016 [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Y, Shen J, Gao Y, et al. : Expression of programmed cell death ligand 1 (PD-L1) and prevalence of tumor-infiltrating lymphocytes (TILs) in chordoma. Oncotarget 6:11139–49, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou MX, Guo KM, Lv GH, et al. : Clinicopathologic implications of CD8(+)/Foxp3(+) ratio and miR-574–3p/PD-L1 axis in spinal chordoma patients. Cancer Immunol Immunother 67:209–224, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Migliorini D, Mach N, Aguiar D, et al. : First report of clinical responses to immunotherapy in 3 relapsing cases of chordoma after failure of standard therapies. Oncoimmunology 6:e1338235, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson LM, Rive CM, Di Francesco D, et al. : Clinical response to nivolumab in an INI1-deficient pediatric chordoma correlates with immunogenic recognition of brachyury. NPJ Precis Oncol 5:103, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blay JY, Penel N, Ray-Coquard IL, et al. : High clinical activity of pembrolizumab in chordoma, alveolar soft part sarcoma (ASPS) and other rare sarcoma histotypes: The French AcSe pembrolizumab study from Unicancer. Journal of Clinical Oncology 39, 2021 [Google Scholar]
- 21.Meng T, Jin J, Jiang C, et al. : Molecular Targeted Therapy in the Treatment of Chordoma: A Systematic Review. Front Oncol 9:30, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svoboda RM, Mackay D, Welsch MJ, et al. : Multiple cutaneous metastatic chordomas from the sacrum. J Am Acad Dermatol 66:e246–7, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Scognamiglio G, De Chiara A, Parafioriti A, et al. : Patient-derived organoids as a potential model to predict response to PD-1/PD-L1 checkpoint inhibitors. Br J Cancer 121:979–982, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]


