Abstract
The bacterial transposon Tn7 inserts at high frequency into a specific site called attTn7, which is present in the chromosomes of many bacteria. We show here that transcription of a nearby gene, glmS, decreases the frequency of Tn7 insertion into attTn7, thus providing a link between Tn7 transposition and host cell metabolism.
Tn7 and the attTn7 site.
Most transposable elements insert into the bacterial chromosome at multiple DNA sites and only at low frequency. In contrast, Tn7 displays high-frequency, site-specific insertion into a single locus (3, 4). Subsequent molecular analysis revealed that Tn7 inserts in a single orientation into this target site, called an attachment site, or attTn7, at a specific nucleotide position downstream of the glmUS operon, which encodes two proteins involved in cell wall biosynthesis (9, 10, 16) (Fig. 1).
FIG. 1.
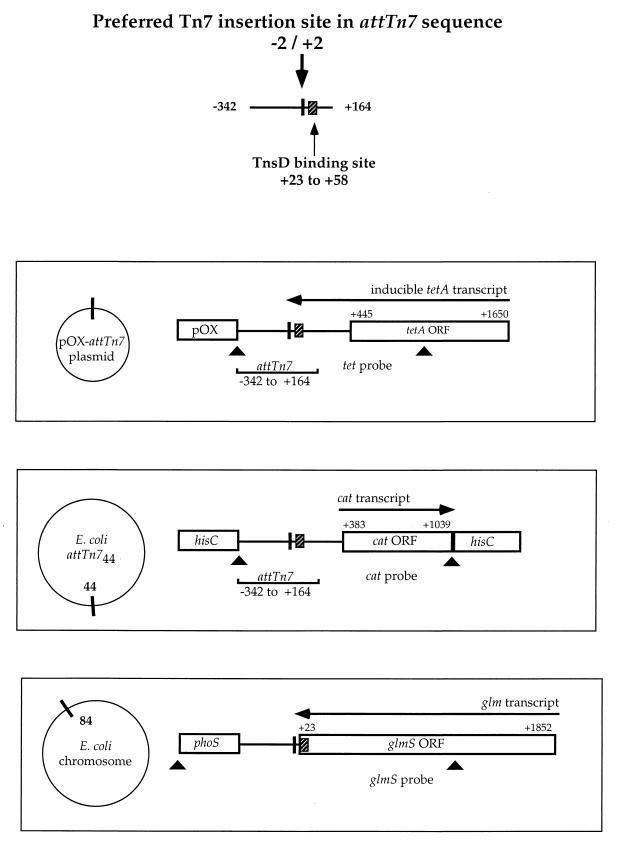
The preferred site for Tn7 insertion in the E. coli chromosome, attTn7. The center of the 5-bp sequence (−2 to +2) duplicated upon Tn7 insertion is designated position 0. Although Tn7 inserts into the transcriptional terminator of the glmUS genes, a small 35-bp DNA segment (+23 to +58) in the coding region of glmS is sufficient for site-specific insertion. The diagram also indicates the preferred orientation of Tn7 insertions in attTn7; the right end (Tn7R) joins proximally to the target sequences near the glmS gene.
The Tn7-encoded protein TnsD is the key component of the transposition machinery which directs site-specific insertion of Tn7 into attTn7. TnsD recognizes a 35-bp DNA segment within the protein-coding region of glmS (2, 15), which is essential for attTn7 target activity (2, 11). Tn7 insertion actually occurs in the transcriptional terminator of the glmUS operon, approximately 25 bp away from the TnsD binding site (8).
Transcription and target activity.
Target activity is the capacity of a particular DNA site to attract a transposon insertion. There are several processes that contribute to the target activity of attTn7, including the positive influence of host factors that augments binding of TnsD to attTn7 (13) and the negative influence of long-range target immunity effects that discourages Tn7 from inserting into attTn7 (7). The specific point of Tn7 insertion and the TnsD binding site are both located within transcribed sequences of the glmUS genes (Fig. 1). We asked whether target site transcription positively or negatively influences target activity of attTn7. We hypothesized that modulation of glmUS expression influences attTn7 target activity and hence Tn7 transposition, thereby connecting Tn7 to host metabolism. Previous experiments using attTn7-containing plasmids showed that the frequency of Tn7 insertion is equivalent in the presence or absence of a strong lac promoter (8). Efficient attTn7 target activity was also found to be independent of transcription orientation (11) and of transcription termination (8). Since some features of these high-copy-number plasmid targets might bypass the effect of transcription on Tn7 insertion, we have reexamined whether transcription in the attTn7 target site modulates Tn7 insertion by using low-copy-number targets.
Transcription of potential target DNAs has been found to block insertion by other transposons (5, 6).
Evaluating the target activity of attTn7 in the chromosome or an F plasmid.
We have previously described a method to evaluate attTn7 target activity by directly measuring “empty” and “filled” attTn7 sites by using a Southern blot assay (7). Insertion frequency is expressed as the percentage of attTn7 sites which are filled by a Tn7 insertion. In addition to its natural location at minute 84 in the Escherichia coli chromosome, the attTn7 site has been introduced at minute 44 in the chromosome (7) and in an F-plasmid derivative, pOX-attTn7 (1, 14). The new chromosomal and plasmid target sites contain ∼500 bp (−342 to +164) of attTn7 (Fig. 2). Thus, we can simultaneously evaluate the target activity for each of these sites in individual cell populations by using the appropriate combination of restriction enzyme digests and DNA probes for Southern blot analysis (Fig. 2).
FIG. 2.
Physical maps and transcriptional activities for the attTn7 target site at the native position in the E. coli chromosome (attTn784), at a different location in the chromosome (attTn744), or in a plasmid target (pOX-attTn7). The target sites in attTn744 and pOX-attTn7 contain sequences from −342 to +164 of attTn784, including the TnsD binding site (shaded box), which is sufficient to direct site specific insertion of Tn7. Each panel shows the combinations of restriction sites (arrowheads) used to generate the hybridization probes used for Southern analysis of Tn7 insertion in that particular target site; the cat probe was used to detect insertion into attTn744 and the tet probe was used to detect insertion into pOX-attTn7.
Inhibition of attTn7 target activity by a high level of transcription in a plasmid target.
We have taken advantage of the pOX-attTn7 plasmid target to reexamine whether target site transcription influences Tn7 insertion. The attTn7 site in this low-copy-number plasmid is flanked by a tightly regulated tetA gene which can be induced by the presence of tetracycline, promoting transcription across the attTn7 site. Cells were grown in conditions that switch “off” or “on” transcription through the attTn7 site, and transposition was examined under these different conditions. As a control, transposition into attTn744, which lacks a tetracycline-inducible promoter, was evaluated simultaneously (Fig. 2).
We found that transposition into pOX-attTn7 was substantially reduced when transcription across attTn7 from the tetracycline promoter occurred and that this is a local effect, not global. We did not observe a significant change in the absolute amount of Tn7 insertion into the control site (attTn744) when cells were grown in the presence or absence of tetracycline (Table 1). In contrast, Tn7 insertion into pOX-attTn7 changed more than fivefold in the same cells in the presence of tetracycline. Therefore, tetracycline inhibits Tn7 only locally, at the attTn7 target site downstream of the tetracycline-inducible promoter.
TABLE 1.
Frequency of Tn7 transposition into pOX-attTn7 or control site when transcription in pOX-attTn7 is off or ona
| Test group | % of target sites with a Tn7 insertion
|
|||||
|---|---|---|---|---|---|---|
| Transcription off
|
Transcription on
|
|||||
| pOX-attTn7 | Control attTn744 site | Ratio | pOX-attTn7 | Control attTn744 site | Ratio | |
| Population 1 | 3.1 | 1.4 | 2 | 0.5 | 1.4 | 0.4 |
| Population 2 | 3.3 | 1.4 | 2 | 0.6 | 1.4 | 0.4 |
| Population 3 | 3.9 | 1.5 | 3 | 0.6 | 1.4 | 0.4 |
| Population 4 | 3.6 | 1.5 | 2 | 0.6 | 1.4 | 0.4 |
Southern blot analysis of Tn7 transposition from attTn784::Tn7 in the chromosome to a control site, attTn744, and a pOX-attTn7 plasmid in cells grown on Luria-Bertani plates without (transcription off in pOX-attTn7) or with (transcription on in pOX-attTn7) tetracycline. Total DNA from ∼108 cells of each clonal population was examined for Tn7 inserts.
Increase of attTn7 target activity by a small (threefold) decrease in transcription from the glmUS genes in the chromosome.
We also examined whether a modest change in the transcriptional activity of the glmUS genes influences the target activity of attTn784. Studies of glmUS have found that gene expression can be experimentally modulated threefold by growing cells in the presence or absence of the amino sugar glucosamine, a GlmS metabolite (12). We have confirmed that growing cells in amino sugars reduces transcription through attTn784; cells containing promoterless lacZY genes in the attTn7 site produced threefold less β-galactosidase activity when grown in M9 minimal media supplemented with 0.4% N-acetylglucosamine (data not shown).
To test if a threefold variation in endogenous transcription through attTn784 influences the activity of that DNA site as a target for Tn7, we compared the levels of Tn7 insertion in cells grown in M9 minimal media in the presence or absence of an amino sugar supplement. To establish that the amino sugar supplement only influences Tn7 insertion into the nearby attTn784 site, we also simultaneously evaluated Tn7 insertion into the attTn744 control site. We have found an inverse correlation between the transcriptional activity of attTn784 and the frequency of Tn7 insertion into that chromosomal target site (Fig. 3). Target activity increases when target site transcription is decreased; that is, Tn7 insertion increases threefold when amino sugars are present. In contrast to the observed effect of amino sugars on the target activity of attTn784, the amino sugar supplement does not increase the level of Tn7 insertion into attTn744 in the same cells. Thus, the effect of the amino sugar supplements on attTn784 target activity reflects a local, rather than global, effect.
FIG. 3.
Tn7 insertion from pOX-attTn7 into the chromosomal attTn784 site increases when target site transcription is reduced by a small (threefold) increment. Tn7 insertion into attTn784 and the control site attTn744 was measured simultaneously by Southern blot analysis of genomic DNA with target site probes. The frequency of transposition into both target sites in each clonal population was calculated as the percentage of DNA molecules that have a Tn7 insertion; columns indicate the mean values, and black bars indicate the range of the highest and lowest values. The figure displays the level of Tn7 insertion (y axis) in cells grown in minimal media with amino sugars (+) (17 populations) relative to the level of Tn7 insertion in cells grown without amino sugars (+) (16 populations).
We do not know yet what kinds of cellular events in actively growing cells might affect the target activity of attTn784 through changes in glmUS transcription. The glmUS genes encode proteins involved in cell wall biosynthesis which are essential for cell growth under laboratory conditions. We propose that glmUS expression, and thus attTn784 target activity, might be linked to cellular growth and the availability of nutrients in the environment.
Acknowledgments
We thank other members of the Craig lab for their useful comments on the experiments and the manuscript. We also thank Patti Eckhoff for her assistance with the manuscript.
Nancy L. Craig is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Arciszewska L K, Drake D, Craig N L. Transposon Tn7 cis-acting sequences in transposition and transposition immunity. J Mol Biol. 1989;207:35–52. doi: 10.1016/0022-2836(89)90439-7. [DOI] [PubMed] [Google Scholar]
- 2.Bainton R J, Kubo K M, Feng J-N, Craig N L. Tn7 transposition: target DNA recognition is mediated by multiple Tn7-encoded proteins in a purified in vitro system. Cell. 1993;72:931–943. doi: 10.1016/0092-8674(93)90581-a. [DOI] [PubMed] [Google Scholar]
- 3.Barth P, Datta N. Two naturally occurring transposons indistinguishable from Tn7. J Bacteriol. 1977;102:129–134. doi: 10.1099/00221287-102-1-129. [DOI] [PubMed] [Google Scholar]
- 4.Barth P T, Datta N, Hedges R W, Grinter N J. Transposition of a deoxyribonucleic acid sequence encoding trimethoprim and streptomycin resistances from R483 to other replicons. J Bacteriol. 1976;125:800–810. doi: 10.1128/jb.125.3.800-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadesus J, Roth J R. Transcriptional occlusion of transposon targets. Mol Gen Genet. 1989;216:204–209. doi: 10.1007/BF00334357. [DOI] [PubMed] [Google Scholar]
- 6.Daniell E, Roberts R, Abelson J. Mutations in the lactose operon caused by bacteriophage Mu. J Mol Biol. 1972;69:1–8. doi: 10.1016/0022-2836(72)90019-8. [DOI] [PubMed] [Google Scholar]
- 7.DeBoy R, Craig N L. Tn7 transposition as a probe of cis interactions between widely separated (190 kilobases apart) DNA sites in the Escherichia coli chromosome. J Bacteriol. 1996;178:6184–6191. doi: 10.1128/jb.178.21.6184-6191.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gringauz E, Orle K A, Waddell C S, Craig N L. Recognition of Escherichia coli attTn7 by transposon Tn7: lack of specific sequence requirements at the point of Tn7 insertion. J Bacteriol. 1988;170:2832–2840. doi: 10.1128/jb.170.6.2832-2840.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtenstein C, Brenner S. Site-specific properties of Tn7 transposition into the E. coli chromosome. Mol Gen Genet. 1981;183:380–387. doi: 10.1007/BF00270644. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein C, Brenner S. Unique insertion site of Tn7 in E. coli chromosome. Nature. 1982;297:601–603. doi: 10.1038/297601a0. [DOI] [PubMed] [Google Scholar]
- 11.McKown R L, Orle K A, Chen T, Craig N L. Sequence requirements of Escherichia coli attTn7, a specific site of transposon Tn7 insertion. J Bacteriol. 1988;170:352–358. doi: 10.1128/jb.170.1.352-358.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plumbridge J. Co-ordinated regulation of amino sugar biosynthesis and degradation: the NagC repressor acts as both an activator and a repressor for the transcription of the glmUS operon and requires two separated NagC binding sites. EMBO J. 1995;14:3958–3965. doi: 10.1002/j.1460-2075.1995.tb00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharpe P, Craig N L. Host proteins can stimulate Tn7 transposition: a novel role for the ribosomal protein L29 and the acyl carrier protein. EMBO J. 1998;17:5822–5831. doi: 10.1093/emboj/17.19.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waddell C S, Craig N L. Tn7 transposition: two transposition pathways directed by five Tn7-encoded genes. Genes Dev. 1988;2:137–149. doi: 10.1101/gad.2.2.137. [DOI] [PubMed] [Google Scholar]
- 15.Waddell C S, Craig N L. Tn7 transposition: recognition of the attTn7 target sequence. Proc Natl Acad Sci USA. 1989;86:3958–3962. doi: 10.1073/pnas.86.11.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker J E, Gay N J, Saraste M, Eberle A N. DNA sequence around the Escherichia coli unc operon. Completion of the sequence of a 17 kilobase segment containing asnA, oriC, unc, glmS and phoS. Biochem J. 1984;224:799–815. doi: 10.1042/bj2240799. [DOI] [PMC free article] [PubMed] [Google Scholar]