Abstract
Background:
South Africa (SA) has the greatest HIV prevalence in the world, with rates as high as 40% among pregnant women. Depression is a robust predictor of nonadherence to antiretroviral therapy (ART) and engagement in HIV care; perinatal depression may affect upwards of 47% of women in SA. Evidence-based, scalable approaches for depression treatment and ART adherence in this setting are lacking.
Methods:
Twenty-three pregnant women with HIV (WWH), ages 18–45 and receiving ART, were randomized to a psychosocial depression and adherence intervention or treatment as usual (TAU) to evaluate intervention feasibility, acceptability, and preliminary effect on depressive symptoms and ART adherence. Assessments were conducted pre-, immediately post-, and three months post-treatment, and included a qualitative exit interview.
Results:
Most (67.6%) eligible individuals enrolled; 71% completed at least 75% of sessions. Compared to TAU, intervention participants had significantly greater improvements in depressive symptoms at post-treatment, β = −11.1, t(24) = −3.1, p < 0.005, 95% CI [−18.41, −3.83], and three months, β = −13.8, t(24) = −3.3, p < 0.005, 95% CI [−22.50, −5.17]. No significant differences in ART adherence, social support, or stigma were found. Qualitatively, perceived improvements in social support, self-esteem, and problem-solving adherence barriers emerged as key benefits of the intervention; additional sessions were desired.
Conclusions:
A combined depression and ART adherence intervention appears feasible and acceptable, and demonstrated preliminary evidence of efficacy in a high-need population. Additional research is needed to confirm efficacy and identify dissemination strategies to optimize the health of WWH and their children.
Keywords: South Africa, HIV, depression, pregnancy, adherence
Introduction
Women of childbearing age bear a significant proportion of the HIV burden in South Africa (SA) [1, 2], with 40% of pregnant women in the KwaZulu-Natal (KZN) province living with HIV in 2017 [3]. While most pregnant women with HIV (WWH) worldwide have access to antiretroviral therapy (ART) [4], adherence to ART and other behaviors critical for maintaining maternal health and preventing mother-to-child transmission (PMTCT) of HIV are suboptimal in SA [2]. Evaluating and addressing the risk factors for vertical HIV transmission and poor maternal HIV health is therefore essential for combatting the HIV epidemic [5].
Depression during pregnancy (i.e., perinatal depression), a robust predictor of poor maternal and infant health [6, 7], is more prevalent among pregnant WWH in SA (affecting up to 47% [8–11]) than among women without HIV (with prevalence rates around 21%, a pooled estimate from a meta-analysis of studies, including several conducted in SA) [12]. Pregnant women with comorbid HIV and depression may be at greater relative risk for falling out of HIV care during the postpartum period [13, 14]. Suboptimal engagement in HIV care among pregnant and postpartum women has been linked to a number of adverse clinical outcomes, including greater maternal mortality [15, 16]; higher rates of vertical HIV transmission [17]; and virologic rebound [18], which can lead to advanced disease and subsequently infant morbidity and mortality [16, 19]. Untreated maternal depression is therefore especially consequential in pregnant WWH.
Psychosocial challenges that co-occur with perinatal depression may also adversely impact engagement in HIV care during the postpartum period. For example, underutilization or lack of social support may prohibit or discourage women from seeking medical treatment after pregnancy, particularly amidst intersecting psychosocial disadvantages (e.g., poverty, geographic isolation [13, 20–22]. Low levels of social support have also been associated with HIV-related stigma among pregnant WWH in SA [23], and the presence of stigma and depressive symptoms have been associated with less social support utilization [24]. Indeed, HIV stigma is highly prevalent across SA [25], and has been widely documented as a barrier to ART uptake, adherence, and retention among pregnant and postpartum WWH [26, 27], therefore representing an important factor influencing the PMTCT cascade in this locale [23, 28, 29].
While effective psychosocial treatments for depression exist, delivery of these treatments to pregnant WWH in resource-limited settings is challenging [30, 31]; the vast majority of studies included in a review of mental health-related issues among pregnant and postpartum WWH were from high-income countries [31]. Of the small number of studies testing interventions aimed at improving mental health outcomes in this population, only a few have demonstrated adequate feasibility and acceptability [30, 32]. A combination peer mentoring program and group cognitive behavioral intervention designed to augment PMTCT services in Cape Town, SA did demonstrate increases in HIV knowledge and reductions in depressive symptoms in pregnant WWH [30]. However, levels of depressive symptoms across the sample were relatively mild, and the intervention had no significant effect on adherence to PMTCT behaviors [30]. Another study examined the effect of a 10-session structured support group program for pregnant women in SA who were newly diagnosed with HIV; while depression was assessed at multiple time points, it focused primarily on HIV disclosure, self-esteem, and coping, and did not evaluate PMTCT adherence [33]. Further, no intervention has attempted to treat both diagnostic levels of depression [34, 35], which cause more distress and interference than depressive symptoms alone, and adherence to ART among pregnant WWH.
Reviews of the literature highlight myriad factors that may impact retention in HIV care, suggesting that multifaceted interventions focusing on multiple areas of concern are likely to be most effective [36, 37]. Thus, innovative interventions that address concurrent and intersecting health challenges, such as diagnostic levels of depression, ongoing postpartum engagement in HIV care for mother and child, and ART adherence are needed [38–40]. This study sought to develop and pilot a scalable counseling intervention, delivered by lay counselors, to reduce clinically significant depression and improve ART adherence in perinatal WWH in KZN, SA. Intervention feasibility and acceptability were the primary study outcomes; changes in depression symptom severity and ART adherence were also explored to assess for signals of clinically meaningful improvement. Changes in stigma and social support post-treatment and at three months, and qualitative exit data that informs future modifications to the intervention and contextualizes study outcomes, are also reported. Intervention participants were hypothesized to have lower levels of depressive symptoms and increased ART adherence at post-treatment, and in the early postpartum period, compared to treatment as usual (TAU).
Methods
Participants & Procedures
Participants were recruited between November 2016 and March 2017 from a district antenatal clinic in eThekwini, KZN, SA. All potential participants were screened for eligibility by a female research assistant fluent in both isiZulu and English. Eligibility criteria included: (1) cisgender female; (2) aged 18–45; (3) currently pregnant and in the late third trimester (gestation ≥ 28 weeks); (4) living with HIV and diagnosed during the index pregnancy, as women who are diagnosed with HIV during pregnancy are more likely to experience depression symptoms than pregnant women who had prior knowledge of their HIV status [41]; (5) meeting criteria for a current major depressive episode (based on data from a structured clinical interview, self-report measure, and team consensus based on clinical impressions); (6) currently on ART and eligible for lifetime treatment; (7) receiving antenatal care at the recruitment site; (8) primary language of English or isiZulu; (9) access to a phone; and (10) resident of the catchment area of the antenatal clinic where participants were recruited. Written informed consent was obtained from all eligible and interested women following a consent discussion conducted by a trained research assistant or other study team member. Enrolled participants were randomized via simple randomization in a parallel design, using a 1:1 allocation ratio facilitated by an online randomization tool, to either the intervention or TAU (e.g., referral to mental health services at the recruitment site via a letter from the study team). Control participants were offered one session of problem-solving around depression and adherence at the end of the study.
The intervention content was based on problem-solving therapy [42, 43] and the LifeSteps adherence intervention [44], with the primary goals of improving (1) depressive symptoms and (2) adherence to ART during pregnancy and the postpartum period. Planning for contraceptive use postpartum was also addressed in the program because of its importance in the PMTCT cascade (please see Table 1). The intervention was delivered by a trained lay counselor, who completed a five-day training on counseling skills and intervention content, and received bi-monthly clinical supervision (via Skype) throughout the study. Finally, although originally conceptualized as a weekly group intervention, sessions were conducted with participants individually, due to participants’ scheduling challenges and concerns around HIV stigma.
Table 1.
Intervention Content
| # | Session Title | Session Content |
|---|---|---|
|
| ||
| 1 | Introduction, Education on Depression, PMTCT and Other Important Health Behaviors | Introduction to counselor and structure and content of intervention; ice breaker activities to build participant rapport and share histories; education about depression, PMTCT, and other health behaviors (e.g. sexual health, disclosure, alcohol and drug use, violence) |
| 2 | Introduction to Problem-Solving and LifeSteps for PMTCT, Part I (Pregnancy) | Review of prior session; introduction to problem-solving; information about taking ART during pregnancy and tuberculosis; develop plans for ART reminder strategies, attending clinic appointments, communicating with providers, coping with ART side effects, managing ART storage, refills, and missed doses |
| 3 | Problem-Solving for Depression: Stigma | Review of prior session; discussion of adherence; review of problem-solving; problem-solving of challenges related to HIV stigma |
| 4 | Problem-Solving for Depression: Social Support | Review of prior session; discussion of adherence; review of problem-solving; problem-solving of challenges related to social support |
| 5 | Problem-Solving for Depression: General Concerns, Part I | Review of prior session; discussion of adherence; review of problem-solving; problem-solving of general challenges related to depression |
| 6 | Problem-Solving for Depression: General Concerns, Part II | Review of prior session; discussion of adherence; review of problem-solving; problem-solving of general challenges related to depression |
| 7 | LifeSteps for PMTCT, Part II (Postpartum) | Review of prior session; discussion of adherence; discussion of ART during the postpartum period; develop plans for ART reminder strategies, attending clinic appointments, communicating with providers, coping with ART side effects, managing ART storage, refills, and missed doses |
| 8 | LifeSteps for Contraception and Study Termination Messages | Review of prior session; discussion of adherence; education on contraception types, use, and pro/cons; develop plans and goals for contraception use during postpartum period; study termination messages |
Participants completed a structured questionnaire at the baseline, post-treatment, and three-month follow-up assessments, as well as a qualitative interview at the post-treatment visit. Participants were compensated ZAR100.00 ($7.17 USD) for each assessment visit or qualitative interview, and R50.00 (approximately $3.59 USD) for transportation costs for each session. Ethics approvals were obtained from the Wits University Human Research Ethics Committee (Durban, SA; M160132), Department of Health Province of KZN (HRKM: 283/16, NHRD: KZ_2016RP3_539), the hospital where recruitment occurred (Durban, SA; 23/RESH/2106), and from the Partners Healthcare Human Research Committee (Boston, USA; 2016P001887). The reporting of this study followed the Consolidated Standards of Reporting Trials (CONSORT) guidelines [45] (see checklist in Electronic Supplemental Material).
Measures
Feasibility and Acceptability.
Intervention feasibility was assessed by tracking the number of potential participants screened, the number determined to be eligible, the number of enrolled participants, and the number of participants who completed at least 75% of sessions. Reasons for ineligibility, as well as reasons for and patterns of drop out, were tracked. Acceptability was assessed via qualitative interviews at post-treatment, which were designed to explore perceived usefulness of the intervention and elicit feedback on participation in the study.
Sociodemographic Information and Reproductive History.
Participants self-reported sociodemographic variables, including age, ethnicity, education level, employment status, and income. Clinical information, including approximate due date and number of lifetime pregnancies, was also collected.
HIV Characteristics.
Participants self-reported HIV disclosure status, year of diagnosis and ART regimen.
Depression.
Current and past major depressive disorder (MDD) was measured using the 17-item Major Depressive Episode module of the Mini International Neuropsychiatric Interview (MINI) [46]. Additionally, the 10-item Edinburgh Postnatal Depression Scale (EPDS) [47, 48] was used to measure depressive symptoms at baseline, post-treatment, and three-month follow-up; higher scores indicate more severe depressive symptoms. The EPDS has been validated among SA women during the perinatal and postpartum periods [49]. A standard clinical cutoff of 13 on the EPDS was used to distinguish women with and without probable depression [47]. Participants were considered to meet criteria for major depression if they met requisite number of symptoms on the MINI and/or scored ≥ 13 on the EPDS [50]. For the latter, the team reviewed baseline data and came to consensus on eligibility.
ART Adherence.
Adherence to ART during the previous month was measured through a composite score developed by Lu and colleagues [51]. The composite score combines the scores of three items into a single self-reported adherence score; the three items assess medication use frequency, percentage of time that the medication was taken as prescribed, and a rating of one’s ability to take prescribed medication. The percent item had 11 response categories (0, 10, 20…100%), whereas the frequency and ability items had six response categories that were assigned scores of 0, 20, 40, 60, 80, and 100, with 100 considered to be perfect adherence. Responses to the three items were averaged to generate a combined one-month ART adherence outcome. Additionally, participants provided reasons for missed doses.
Objective ART adherence data were also collected using the Medication Event Monitoring System (MEMS) [52], which comprises a pill bottle with a digitized cap that measures and records when the bottle was opened each day. MEMS caps were brought to each session and assessment visit and read for an “objective” adherence value. Intervention participants had their caps read regularly throughout the course of the study (e.g., at intervention sessions and assessment visits), whereas control participants had caps read only at assessment visits. For the current analyses, MEMS timepoints were defined as average MEMS use across the two-week period prior to either the first intervention session or to a scheduled assessment visit. As we and others have done in past studies [53–55], we used the two week MEMS monitoring period to have a comparison point for pre-post analyses. Therefore, there were three MEMS timepoints: baseline (two weeks prior to the first session), two weeks prior to post-treatment, and two weeks prior to three-month follow-up. The first two weeks of MEMS data for all participants was discarded to account for potential increases in adherence that could occur as a result of monitoring, thus, the baseline MEMS reading was comprised of the data from the two-week period after the first two weeks of consistent use. Notably, due to scheduling challenges in just a few cases, the first two weeks of consistent use overlapped with the first intervention session at the end of the monitoring period. Three participants were excluded from the final MEMS analyses due to broken MEMS caps or consistent cap non-use over the three measurement periods.
Stigma.
HIV-related stigma was measured at baseline, post-treatment, and 3-month follow-up using the HIV/AIDS Stigma Instrument – People Living with AIDS (HASI-P) [56]. The HASI-P was developed and has been broadly tested in five African countries, including SA, where it was validated with a sample of 1,477 PLWH. The full measure contains 33 items across six subscales, from which we utilized the following three validated subscales: negative self-perception (five items, alpha = 0.91), social isolation (five items, alpha = 0.89), and verbal abuse (eight items, alpha = 0.89). Participants are asked to rate the frequency of stigmatizing events in the prior three months on a four-point Likert scale from “never” to “most of the time.” [56]
Social Support.
A modified version of the Duke-UNC Functional Social Support Questionnaire [57] was used to assess participants’ emotional, informational, and tangible support networks at baseline, post-treatment, and 3-month follow-up. The measure has previously been validated among PLWH in sub-Saharan Africa (SSA; alpha = 0.91 [58]). The scale contains 10 items, on which participants are asked to rate the availability of these types of social support on a four-point Likert scale from “never” to “as much as I would like.” [57]
Pregnancy Intention.
Intentions and attitudes about current pregnancy were measured via the Pregnancy Assessment Monitoring System (PRAMS) [59], which gauges feelings, before, during, and shortly after pregnancy, as well as whether the pregnancy was intended.
Quantitative Analyses
Normality testing of continuous variables was conducted using the Kolmogorov-Smirnov statistic [60]. Descriptive statistics were generated using SPSS v. 21 [61] to summarize sample characteristics and examine key outcome variables at baseline. Linear mixed effects modeling, following an intent to treat approach, was used to assess for differences between the intervention arm and the control arm in the two primary outcomes (clinically significant depression and adherence) and in the two secondary outcomes (stigma and social support). In the R software environment [62], we ran four linear mixed models, one for each outcome variable, with the nlme package [63], which fits linear and non-linear multilevel or hierarchical regression models and adjusts for missing data by removing single time points for which data is absent (rather than dropping participants who have missing data at some time points but not others). Each model included a random effect for participant ID to account for individual variability, and unstandardized coefficients are reported.
Qualitative Analyses
Optional, in-depth qualitative exit interviews were conducted with intervention participants to collect feedback on the intervention. Participants were interviewed by a trained, female, isiZulu-speaking study staff member. All interviews took place in a private, confidential setting. Interviewers explored participants’ experiences with intervention content and structure, as well as their ART adherence. Interviews were digitally recorded, translated, and transcribed by a study staff member fluent in both English and isiZulu. Transcripts were independently reviewed for accuracy and consistency by a study staff member who did not conduct the interview. Using a grounded theory approach [64], content analysis was conducted and resulting conceptual categories, and emergent themes were discussed by the research team. Using techniques described by Miles and Huberman [65] and Strauss and Corbin [66], an overarching thematic framework for data interpretation was developed. Data were then coded into content areas and salient messages were extracted. To ensure reliability and validity, coding and analyses (facilitated by NVivo 12 [67]) were performed by two independent coders, who discussed findings and resolved discrepancies at each stage. An audit trail of coding schemes and coded material was maintained for ongoing reference and comparison.
Results
Feasibility
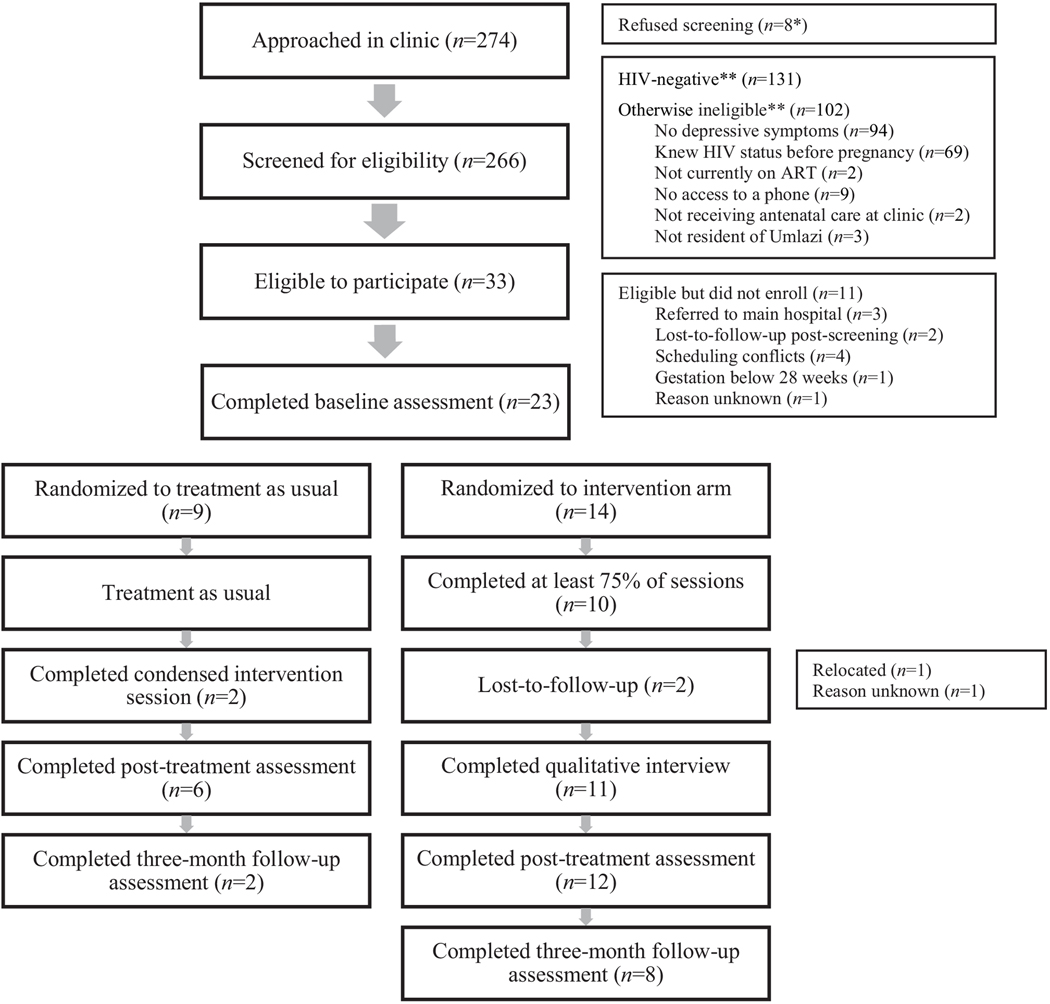
A participant flow diagram is provided in Figure 1. A total of 274 patients were approached in clinic, and 266 were screened for eligibility. Of these, 34 (13%) were eligible and interested in participating, and 23 of those 34 (67.6%) enrolled. Fourteen participants were randomized to the intervention arm, and nine were randomized to the control arm. Among intervention participants, 10 (71%) completed at least 75% of sessions and two (9%) were ultimately lost-to-follow-up (one after having attended one session, and one after having attended zero sessions). Eleven intervention participants (79%) completed a qualitative interview. Of the nine control participants, two (22%) completed the condensed, one-session version of the intervention. Mean time between each intervention session was nearly two weeks (M = 13.95 days), due primarily to inadequate transportation, childcare responsibilities, and scheduling conflicts. Scheduling challenges coordinating participant schedules also hindered the possibility of group sessions (only one session included more than one participant). Post-treatment assessments were conducted between January 2017 and June 2017, and three-month follow-up assessments were completed between March 2017 and June 2017.
Figure 1.
Recruitment Flow
* Reasons for refusing screening not collected.
** Most ineligible individuals were found to be ineligible for more than one reason.
Qualitative Results: Acceptability
Of the 14 intervention participants, 10 (71%) completed an optional qualitative exit interview. Overall, participants expressed satisfaction with the intervention, most notably the perceived positive impact on their ART adherence, depressive symptoms, and other psychosocial challenges. Suggestions for future interventions included expanded eligibility criteria, preference for individual treatment over a group format, and increased number and duration of sessions.
Intervention facilitated increased self-efficacy, self-worth, and hope for the future.
Participants discussed feeling more empowered and equipped to make decisions about their ART adherence and other health care choices as a result of the intervention. Some also expressed a stronger sense of self-efficacy, as well as increased problem-solving abilities and comfort advocating for behaviors that might minimize risk to their own or their child’s health.
“There is a lot [that helped], there really is a lot I benefited [from], because I was able to devise my own choice as well as take care of my child…” (Age 35; diagnosed 5 months)
“She said you need to look at the problem first, then when you have realized the problem, what can I do to solve the problem… then choose the one that you feel would not be destroying anything… It was not easy at the beginning, but… since I started attending these sessions, I now have the courage as well as that impetus to take medication. And I always make sure that I do not miss any.” (Age 37; diagnosed 4 months)
Many participants reported decreased feelings of internalized stigma related to living with HIV and increased self-esteem. Specifically, women reported that the opportunity to discuss their fears and assumptions about their HIV diagnoses facilitated acceptance of their diagnosis, increased their hope for the future, and minimized fears of dying from HIV.
“I have a problem of low self-esteem, as I have this disease and I am failing to accept [it], so I saw that it will help me [to] talk about it, so that I will be able to accept the situation I currently face.” (Age 28; diagnosed 2 months)
“The more I understand and by the time I start realising it is not my fault… I must put myself forward, because if I do not take them [medications], it is me who is going to suffer at the end, no another person.” (Age 28; diagnosed 2 months)
“I was able to talk freely and was comfortable, as compared to before where I would just break into tears because I would just think it is over.” (Age 37; diagnosed 4 months)
“I did not know if you are positive you can live a normal life like other people. I had this [when] I found out that I am positive, that I will be not the same as other people… I did not know that I will be able to talk and do everything as normal, I thought life would change.” (Age 23; diagnosed 2 months)
Participants also reported that positive social connections and a nonjudgmental atmosphere contributed to a reduction in depressive symptoms. Effective communication strategies positively impacted their degree of comfort with disclosing their status to others, and also increased social support in some cases.
“Discovering that I am positive, I told myself that I will be alright, but as time went by I discovered that I was not… It is rare to find someone to talk to, so it helps, and [now] I can tell [others] to try it and see she will get help as well.” (Age 28; diagnosed 2 months)
“I feel very happy and I have pride in me for having been here, and I feel I am a born again. Thank you very much for everything, you have a huge and supportive role to us as mothers who have been pregnant and who have also been hearing for the very first time that they are HIV positive, it is not easy when you find out but eventually you feel you have people supporting you.” (Age 35; diagnosed 5 months)
“What was troubling me a lot was solving a problem that I had, to disclose my status… I was scared to get [baby] tested, but eventually [counselor] advised me to test him so that I will know what is going on… I was crying not knowing what to say if my baby was positive, and the baby was all right. And I managed [to] disclose my status to my sister and there was no problem and I had that relief.” (Age 23; diagnosed 2 months)
Suggestions for the future.
Participants were asked to provide feedback and suggest modifications for future interventions. Although originally conceptualized as a group intervention, due to stigma concerns and logistics, most sessions were conducted individually. Overall, participants reported being satisfied with the individual format due to confidentiality concerns, although some were open to a group format. Participants were generally satisfied with the number and duration of sessions, but recommended expanding the timeline so as to make interventions available earlier in pregnancy and later in the postpartum period. Participants also suggested broadening the study locations and eligibility criteria, noting that providing access to the intervention in other clinics, including in more rural areas, and to other individuals, including non-pregnant WWH and those whose HIV status is unknown, would be particularly beneficial.
“It is better coming here alone. Because sometimes it is scary to speak while there are other people you know.” (Age 24; diagnosed 4 months)
“I was getting to a point where I was starting to feel better but then I was not ready for them [sessions] to finish. I would like them to increase perhaps a bit. Because they are very helpful I can say that.” (Age 24; diagnosed 2 months)
“I would suggest that you begin with people early as early as being three months pregnant until the ninth month… even those that are not pregnant could also benefit, as well as… those that have not yet tested, who don’t know their status… if this could be done perhaps everyone would be motivated… if they could start attending these sessions perhaps this would change their mind set and start taking treatment according[ly] because many people do not take their treatment.” (Age 24; diagnosed 2 months)
“I can say [go] forward with the study, [it] must be available even in other clinics because I only see it here… only, [and] in other clinics it’s not there. I want it to be developed forward so that South Africa will… remain safe.” (Age 31; diagnosed 4 months)
Quantitative Results
Descriptive statistics for the 23 enrolled participants are presented in Table 2.
Table 2.
Baseline Sociodemographic Characteristics
| Variable | Median |
|---|---|
|
| |
| Age | 24 years |
| Time since HIV diagnosis | 2.7 months |
| Time to approximate due date | 3.4 months |
| Length of relationship with father of current pregnancy | 3 years |
| Number of prior pregnancies | 2 |
| Time spent traveling to clinic | 30 minutes |
|
| |
| n (%) | |
|
| |
| Race* | |
| Black South African | 23 (100.0) |
| Monthly income (in South African Rand) | |
| 0–999 ZAR | 5 (21.7) |
| 1000–2999 ZAR | 4 (17.4) |
| 3000–4999 ZAR | 6 (26.1) |
| 5000+ ZAR | 4 (17.4) |
| Unknown | 4 (17.4) |
| Current employment status | |
| Not employed | 16 (69.6) |
| Full-time employed | 4 (17.4) |
| Part-time employed | 3 (13.0) |
| Highest level of schooling | |
| University/vocational school | 1 (4.3) |
| Some university/vocational school | 9 (39.2) |
| Grade 12 | 6 (26.2) |
| Less than grade 12 | 7 (30.3) |
| Relationship to father of current pregnancy | |
| Married | 1 (4.3) |
| Engaged | 2 (8.7) |
| Long-term partner | 17 (73.9) |
| Widowed (and has new partner) | 1 (4.3) |
| Boyfriend | 1 (4.3) |
| Casual partner | 1 (4.3) |
| Reaction upon learning of current pregnancy | |
| Very unhappy or unhappy to be pregnant | 12 (52.1) |
| Very happy or happy to be pregnant | 7 (30.4) |
| Not sure, didn’t mind | 4 (17.4) |
| Had been trying to get pregnant | |
| No | 20 (87.0) |
| Yes | 3 (13.0) |
| Still in relationship with father of current pregnancy | |
| No | 1 (4.3) |
| Yes | 22 (95.7) |
| Partner’s HIV status | |
| Positive | 8 (34.8) |
| Negative | 1 (4.3) |
| Don’t know | 14 (60.9) |
All participants identified as Black South African, and one additionally identified as Indian.
At baseline, participants also provided descriptive information on their perceived ability to take their ART. Over 65% of participants reported no missed doses. Among those who did indicate missed doses (n = 8 out of 23), primary reasons for challenges in maintaining consistent ART use were forgetting (37.5%), traveling or being away from pills for a certain period of time (25%), falling asleep (12.5%), nausea (12.5%), and fear of HIV status disclosure (12.5%). The plurality of participants described reported their ability to take their medications as “very good” (47.8%), followed by “good” (34.8%), “excellent” (13%), and “fair” (4.3%).
Primary Outcome: Clinically Significant Depression.
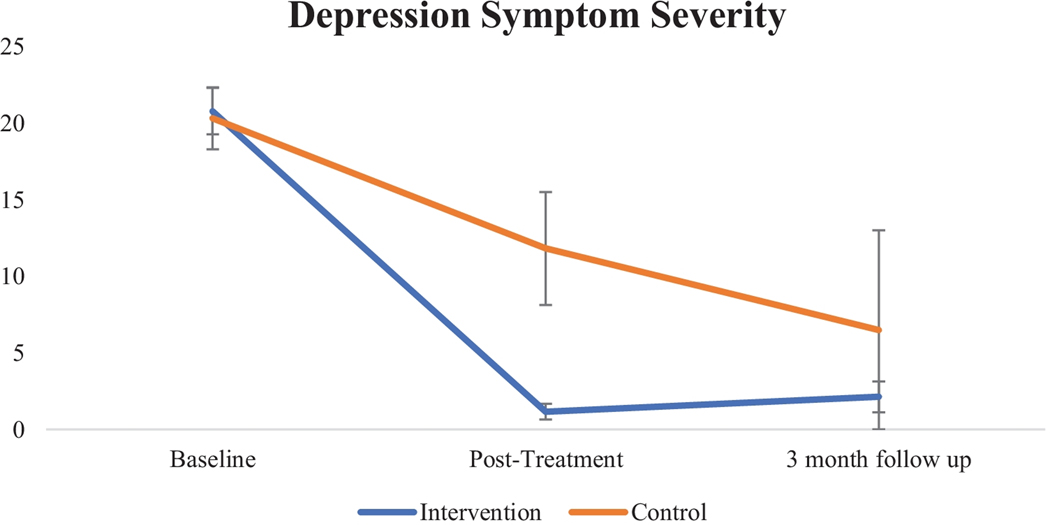
In the linear mixed model with EPDS total scores as the outcome variable, there was a significant interaction between condition and visit at post-treatment, β = −11.1, t(24) = −3.1, p < 0.005, 95% CI [−18.41, −3.83], such that participants in the intervention arm had significantly lower depression symptom severity than participants in the control arm. Treatment gains were maintained at three-month follow-up, evidenced by a significant main effect for this time point, β = −13.8, t(24) = −3.3, p < 0.005, 95% CI [−22.50, −5.17]. See Table 3 for EPDS means by timepoint across the two study arms.
Table 3.
Depression, ART Adherence, Stigma, and Social Support Across Timepoints, By Condition
| Baseline | Post-treatment | Three-month follow-up | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Intervention (n = 14) | Control (n = 9) | Intervention (n = 12) | Control (n = 6) | Intervention (n = 8) | Control (n = 2) | |
|
| ||||||
| Depression | ||||||
| EPDS | 20.8 (5.7) | 20.3 (6.1) | 1.2 (1.7) | 11.8 (9.0) | 2.1 (2.9) | 6.5 (9.2) |
| MINI | n = 12 | n = 7 | n = 0 | n = 2 | n = 0 | n = 1 |
| ART adherence | ||||||
| Composite score | 85.3 (12.9) | 79.0 (14.4) | 92.8 (8.7) | 77.8 (15.4) | 88.7 (9.3) | 89.2 (5.9) |
| MEMS1 | 99.4 (2.2) | 92.9 (14.3) | 98.6 (3.0) | 100 (0) | 92.8 (6.4) | 85.7 (N/A) |
| Stigma | 14.3 (10.2) | 24.3 (12.6) | 6.3 (10.2) | 12.8 (10.9) | 2.5 (6.7) | 12.5 (17.7) |
| Social support | 33.9 (5.8) | 27.7 (9.6) | 35.3 (5.6) | 28 (4.5) | 36.4 (5.5) | 36.5 (3.5) |
Three participants were excluded from this analysis due to broken MEMS caps or cap non-use. At baseline, 15 participants (11 intervention, 4 control) were included in the MEMS analysis, 12 participants (10 intervention, 2 control) were included at post-treatment, and 7 participants (6 intervention, 1 control) were included at three-month follow-up.
Nineteen of 23 participants (82.6%; 7 control, 12 intervention) met criteria for MDD on the MINI at baseline. At post-treatment, two of the 17 participants who completed the study (11.8%) met for MDD on the MINI, and both were in the control arm. At three-month follow-up, one participant in the control arm (10% of all participants who completed this final assessment) and none of the participants in the intervention arm met MINI criteria for MDD (see Table 3). Formal chi-square analyses were not conducted on these data due to small cell sizes.
Primary Outcome: ART Adherence.
In the model that used the adherence composite score as the outcome variable, no significant interactions were found between study condition and visit at either time point (post-treatment or three-month follow-up). The intervention was therefore not associated with increases in ART adherence compared to control. No significant main effects emerged.
In the model predicting MEMS-assessed adherence, there were also no significant interactions between condition and visit at either time point (post-treatment or three-month follow-up). However, there was a significant main effect for time at the three-month follow up visit, such that MEMS-assessed adherence decreased in both study arms, β = −12.7, t(15) = −2.8, p < 0.05, 95% CI [−22.29, −3.15]. Given the small sample sizes in the intervention and the control arm at the three-month follow up, these results should be interpreted with caution. See Table 3 for mean adherence scores by study arm.
Secondary Outcome: Stigma.
There were no significant interactions between condition and visit at either time point (post-treatment or three-month follow-up), indicating that the intervention was not associated with decreases in stigma relative to the control arm. There were, however, significant main effects for condition, β = −10.1, t(21) = −2.2, p < 0.05, 95% CI [−19.58, −0.67], in that, overall, the intervention arm consistently had significantly lower self-reported stigma relative to TAU. There were also significant main effects for time at post-treatment, β = −12.8, t(23) = −1.4, p < 0.01, 95% CI [−22.14, −3.37], such that participants in both conditions experienced significant decreases in stigma from baseline to post-treatment.
Secondary Outcome: Social Support.
There were no significant interactions between condition and visit at either time point (post-treatment or three-month follow-up). Therefore, the intervention did not lead to increases in social support relative to the control arm. There was a significant main effect for condition, β = 6.2, t(21) = 2.3, p < 0.05, 95% CI [0.50, 11.88], indicating that participants randomized to the intervention had, on average, higher but relatively stable levels of social support compared to participants in the control arm.
Discussion
This evidenced-based, combined depression and adherence intervention was likely feasible, though recruitment was challenging due to the nature of the eligibility criteria, and acceptable to the participants who were able to complete qualitative interviews. Quantitative findings suggest potential evidence of efficacy with respect to clinically significant depression symptom reduction but no clinically meaningful trends indicating improvement in ART adherence. Intervention participants demonstrated decreases in depression symptom severity relative to control participants, and they noted an array of additional benefits from the intervention. The present study is among only a few structured, cognitive behavioral therapy-based interventions to provide integrative depression and adherence treatment in general, but also one of even fewer interventions for this population of women presenting with high depression symptom severity who received an HIV diagnosis during pregnancy.
With respect to feasibility, only 12.8% of screened women reported clinically significant levels of depression, far below the expected prevalence based on published prevalence data upwards of 30% [1, 31, 68–70], yet in line with another study of pregnant WWH in SA, in which 11% of participants had elevated EPDS scores using a clinical threshold of 13 [23]. This lower prevalence rate ultimately increased the restrictiveness of our eligibility criteria, which made recruitment challenging. It is possible that women underreported depressive symptoms or that women engaged in antenatal care (where recruitment occurred) were less likely to experience clinically significant levels of depression than women who were not engaged in care. Future studies for this population will likely need to adjust eligibility criteria to ensure that women with more mild symptom presentations who might benefit from the intervention will not be excluded from participation. Among those who were eligible, half of the women screened ultimately participated, and most participants completed at least 75% of sessions. Time between sessions was longer than anticipated (two weeks on average), mainly due to inadequate transportation and/or childcare, or scheduling difficulties. Challenges coordinating participant schedules and participant concerns around stigma also precluded group sessions (only one meeting included more than one attendee).
Qualitative findings suggest that the intervention may be acceptable. Participants who completed qualitative interviews reported enjoying and benefiting from their time with the interventionist, and many expressed a desire for additional (and ongoing) sessions. Women also suggested that future interventions start earlier in pregnancy (e.g., first trimester) and be made available to anyone living (or newly diagnosed) with HIV, notwithstanding pregnancy. Peer-based intervention models have successfully promoted health education and support among WWH across SA (e.g., mothers2mothers [30]), and the use of a lay counselor who is also a peer may have contributed to positive study findings. However, it must be acknowledged that participants who did not partake in qualitative interviews because they were lost to follow up did not have the opportunity to offer feedback on the acceptability of the intervention content. For these participants, it is possible that lack of acceptability may have contributed to attrition.
Intervention participants showed significant improvements in depressive symptoms relative to those in the control arm. Qualitative data from intervention participants at post-treatment support the quantitative findings; women who completed the intervention reported feeling supported by the lay counselors and valued the opportunity to voice and receive support around their HIV-and pregnancy-related concerns. Intervention sessions provided space to process one’s HIV diagnosis and ask questions, perhaps in a way that is not readily available in routine clinic care. Some participants noted increases in self-esteem and acceptance of their HIV diagnosis due to the counselors’ nonjudgmental approach. Many also expressed a newfound sense of hope and self-efficacy for living well with HIV; learning general HIV education and problem-solving techniques for managing HIV (e.g., taking daily ART) were identified as major drivers of these improvements. Indeed, prior to their participation, many women associated HIV with poor quality of life and imminent death, as has been documented in other studies [71–73].
Although the intervention led to decreases in depression symptoms, it was not associated with significant increases in ART adherence. The lack of quantitative differences in ART adherence between treatment arms may be explained by high baseline objective adherence (i.e., MEMS caps: 99.4% and 92.9% in the intervention and control arms, respectively) and relatively high self-reported (85.3%) adherence among intervention participants, leaving little room for improvement. The small sample size, particularly at post-treatment and three-month follow-up, also substantially restricted our power to detect significant differences or make conclusive determinations. In qualitative interviews, intervention participants expressed appreciation for educational content on ART and its effects on the body, as well as problem-solving skills for addressing adherence barriers. Further, intervention participants reported feeling more motivated to take their ART, as their outlook on living with HIV improved and depressive symptoms abated. Prior data similarly found strong inverse associations between depression and perinatal ART adherence [14, 31], and postpartum engagement in HIV care [13, 14], in pregnant WWH. This is particularly notable, as the women in this study were diagnosed with HIV during their current pregnancy and were therefore at the greatest risk of falling out of HIV care [74, 75].
There were no significant differences in perceived social support and stigma by condition, though both study arms experienced significant decreases in stigma from baseline to post-treatment. In qualitative interviews, intervention participants reported successfully disclosing their HIV status to loved ones with the support of the interventionist and problem-solving skills. Some confided solely in the interventionist, rather than with family or friends, but nonetheless reported significant benefit from that support, as in other studies [54, 76–78]. Control participants demonstrated similar improvements in these areas on quantitative measures. Participation in a study tailored to pregnant WWH may be beneficial more generally, leading women (regardless of study arm) to feel more accepted. For example, the baseline visit alone, which occurred in a semi-structured interview format, may have been a meaningful opportunity to share difficult experiences without fear of judgment. This unintentional intervention may have disrupted women’s sense of shame, a construct that is widespread construct and strongly linked with depression and underutilization of healthcare services among PLWH [77].
The primary limitation of our study is the small sample size, which limited our ability to detect reliable statistically significant differences between arms. The preliminary relationships identified in these analyses will need to be replicated and confirmed in larger samples, as regression models and statistical tests are generally not reliable in samples of this size. Recognizing the inherent limitations of this approach, we chose to pursue these analyses to assess indicators of clinically meaningful changes that considered all time points, setting the stage for larger scale efficacy testing if the data indeed looked promising. Our use of simple randomization rather than block randomization further limited the sample in that that the groups were relatively uneven. Though simple randomization is easy to implement and reliably generates similar numbers of participants across larger groups, it can lead to unequal numbers in smaller groups [79]. In addition, because we were unable to conduct interviews with women who did not remain engaged in the program, the qualitative data reflect the acceptability and feasibility of the intervention for only a subset of women. Though the content of the intervention may have been acceptable to women who were lost to follow up, there were likely a number of structural and other barriers that made it difficult to attend sessions, which we could not thoroughly assess. Session scheduling was an additional and substantial challenge. Understanding the specific barriers to scheduling and attendance will enable improve the feasibility of the intervention for future testing on a larger scale. While women were largely able to complete most planned sessions, this required flexibility on the part of the interventionist, and all but precluded a group meeting format. It should be noted that some women reported appreciation for individual meetings, feeling more comfortable sharing their experiences one-on-one. Furthermore, the baseline MEMS readings may be limited in that, in a few cases, the two-week periods that we used to assess adherence at baseline overlapped with the first intervention session. As we have documented above, scheduling sessions often proved difficult, and we wanted to ensure that we met the timing and scheduling needs of the participants as much as possible. Relatedly, high baseline adherence likely impacted our ability to detect differences between study arms. When planning for future efficacy testing, considerations must be made as to which groups of pregnant women (e.g., women with low self-reported adherence, high viral loads, or resistance to first-line antiretroviral therapies) might benefit most from the adherence content included in this intervention. Finally, because the study was designed to support adherence during a relatively narrow window of time, women at any stage of the ART initiation process were deemed eligible to participate; that is, we did not include an ART stabilization period prior to the start of the intervention. Since ART initiation has been associated with decreased depression at onset in SSA [80], it will be important to distinguish between gains due to ART use and gains due to the intervention in future studies.
Given the psychological factors that mediate the ways in which maternal depression can impact infant health, existing and future interventions must fully address this complexity by incorporating new findings and adjusting to meet these needs [81, 82]. More women may be reached with a peer-based model inclusive of female community workers and prior intervention graduates, or integration with programs like mothers2mothers, in an effort to bolster existing systems [83]. Additionally, a stepped care approach can be useful for screening and identifying appropriate levels of care for women in resource-limited settings [84–86]. This can also help determine who might benefit most from specific interventions (for both depression and adherence challenges), and further clarify effective and feasible intervention levels and types. Future studies may also consider broadening inclusion criteria to include any recently diagnosed WWH, who may benefit from problem-solving skills and HIV self-care even if not currently depressed or pregnant, which was a finding from our qualitative data as well. WWH who were not recently diagnosed with HIV and are pregnant may also be included in future studies, as peripartum depression has negative effects on engagement in postpartum HIV among women who just received an HIV diagnosis and women who have been living with HIV for a long time. For some women, depression during this period may be associated with unplanned pregnancies, which are common in SA [87], in addition to HV status. Additionally, future studies could incorporate male partner involvement, which has been found to positively affect engagement in care and PMTCT behaviors [88–91].
Overall, this study demonstrates that an intervention to treat depression and improve medication adherence among pregnant WWH is likely feasible and acceptable, though changes will need to be made to increase eligibility, assess barriers to participation, and reduce those barriers to whatever extent possible. The data hint at a possible robust effect on depression in eight or fewer sessions, which needs to be substantiated with further study. Future adjustments are warranted to ensure that the program has clinically meaningful effects on ART adherence, which may mean focusing on women who have self-reported adherence challenges and/or high viral loads. Importantly, the intervention can successfully be conducted in a low-resource setting, and the use of lay counselors as interventionists enhances the potential for broader dissemination to other low-resource areas in SSA.
Supplementary Material
Figure 2.
Depression Symptom Severity, Assessed via the EPDS
Acknowledgments
Funding: This work was supported by the National Institute of Mental Health (NIMH) under grant K23MH096651 (PI Psaros), with some of the investigator effort supported by 9K24DA040489 (Safren) and T32MH116140 (Stanton). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Footnotes
Informed Consent: Informed consent was obtained from all participants included in the study.
Ethical Approval: All procedures were in accordance with the ethical standards of all institutional and/or national research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Welfare of Animals: No studies were performed with animals.
Author Note: Dr. Greer Raggio and Elsa S. Briggs were affiliated with the Department of Psychiatry at Massachusetts General Hospital in Boston, MA at the time the research was conducted. Dr. Raggio is now primarily affiliated with the National Center for Weight and Wellness in Washington, DC, and Ms. Briggs is now primarily affiliated with the Department of Health Systems & Population Health at the University of Washington in Seattle, WA.
Conflict of Interest: Steven Safren receives royalties from Oxford University Press, Guilford Publications, and Springer/Humana Press for book royalties related to cognitive behavioral therapy and other evidenced-based treatments. The other authors of this manuscript have declared no conflicts of interest.
Trial Registration: ClinicalTrials.gov identifier: NCT03069417
Protocol Available at: https://clinicaltrials.gov/ct2/show/NCT03069417
References
- [1].Kharsany ABM, Cawood C, Khanyile D, et al. Community-based HIV prevalence in KwaZulu-Natal, South Africa: results of a cross-sectional household survey. The Lancet HIV 2018; 5: e427–e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].South African National AIDS Council. 2018 Global AIDS Monitoring Report, https://sanac.org.za/wp-content/uploads/2019/08/Global-AIDS-Report-2018.pdf (2018).
- [3].South Africa National Department of Health. The 2015 National Antenatal Sentinel HIV & Syphilis Survey. October 2017.
- [4].UNAIDS. Global HIV & AIDS Statistics — 2019 Fact Sheet, https://www.unaids.org/en/resources/fact-sheet (2019).
- [5].Ramoshaba R, Sithole SL. Knowledge and awareness of MTCT and PMTCT post-natal follow-up services among HIV infected mothers in the Mankweng region, South Africa. TOAIDJ 2017; 11: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kingston D, Tough S, Whitfield H. Prenatal and postpartum maternal psychological distress and infant development: a systematic review. Child Psychiatry Hum Dev 2012; 43: 683–714. [DOI] [PubMed] [Google Scholar]
- [7].Rogers A, Obst S, Teague SJ, et al. Association between maternal perinatal depression and anxiety and child and adolescent development: a meta-analysis. JAMA Pediatr 2020; 174: 1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brittain K, Myer L, Koen N, et al. Risk factors for antenatal depression and associations with infant birth outcomes: results from a South African birth cohort study. Paediatr Perinat Epidemiol 2015; 29: 505–514. [DOI] [PubMed] [Google Scholar]
- [9].Heyningen van T, Myer L, Onah M, et al. Antenatal depression and adversity in urban South Africa. Journal of Affective Disorders 2016; 203: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rochat TJ, Tomlinson M, Bärnighausen T, et al. The prevalence and clinical presentation of antenatal depression in rural South Africa. Journal of Affective Disorders 2011; 135: 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Manikkam L, Burns JK. Antenatal depression and its risk factors: An urban prevalence study in KwaZulu-Natal. S Afr Med J 2012; 102: 940. [DOI] [PubMed] [Google Scholar]
- [12].Zhu Q-Y, Huang D-S, Lv J-D, et al. Prevalence of perinatal depression among HIV-positive women: a systematic review and meta-analysis. BMC Psychiatry 2019; 19: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Buchberg MK, Fletcher FE, Vidrine DJ, et al. A mixed-methods approach to understanding barriers to postpartum retention in care among low-income, HIV-infected women. AIDS Patient Care and STDs 2015; 29: 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sheth SS, Coleman J, Cannon T, et al. Association between depression and nonadherence to antiretroviral therapy in pregnant women with perinatally acquired HIV. AIDS Care 2015; 27: 350–354. [DOI] [PubMed] [Google Scholar]
- [15].Osler M, Hilderbrand K, Goemaere E, et al. The continuing burden of advanced HIV disease over 10 years of increasing antiretroviral therapy coverage in South Africa. Clinical Infectious Diseases 2018; 66: S118–S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chilongozi D, Wang L, Brown L, et al. Morbidity and mortality among a cohort of human immunodeficiency virus type 1-infected and uninfected pregnant women and their infants from Malawi, Zambia, and Tanzania. Pediatric Infectious Disease Journal 2008; 27: 808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Peltzer K, Weiss SM, Soni M, et al. A cluster randomized controlled trial of lay health worker support for prevention of mother to child transmission of HIV (PMTCT) in South Africa. AIDS Res Ther 2017; 14: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sha BE, Tierney C, Cohn SE, et al. Postpartum viral load rebound in HIV-1-infected women treated with highly active antiretroviral therapy: AIDS Clinical Trials Group Protocol A5150. HIV Clinical Trials 2011; 12: 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kuhn L, Kasonde P, Sinkala M, et al. Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants? Clinical Infectious Diseases 2005; 41: 1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ashaba S, Kaida A, Coleman JN, et al. Psychosocial challenges facing women living with HIV during the perinatal period in rural Uganda. PLoS ONE 2017; 12: e0176256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Choi KW, Smit JA, Coleman JN, et al. Mapping a syndemic of psychosocial risks during pregnancy using network analysis. IntJ Behav Med 2019; 26: 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Psaros C, Remmert JE, Bangsberg DR, et al. Adherence to HIV care after pregnancy among women in sub-Saharan Africa: falling off the cliff of the treatment cascade. Curr HIV/AIDS Rep 2015; 12: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brittain K, Mellins CA, Phillips T, et al. Social support, stigma and antenatal depression among HIV-infected pregnant women in South Africa. AIDS Behav 2017; 21: 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Psaros C, Smit JA, Mosery N, et al. PMTCT adherence in pregnant South African women: the role of depression, social support, stigma, and structural barriers to care. Annals of Behavioral Medicine 2020; 54: 626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Peltzer K, Ramlagan S. Perceived stigma among patients receiving antiretroviral therapy: a prospective study in KwaZulu-Natal, South Africa. AIDS Care 2011; 23: 60–68. [DOI] [PubMed] [Google Scholar]
- [26].Hodgson I, Plummer ML, Konopka SN, et al. A systematic review of individual and contextual factors affecting ART initiation, adherence, and retention for HIV-infected pregnant and postpartum women. PLoS ONE 2014; 9: e111421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Turan JM, Nyblade L. HIV-related stigma as a barrier to achievement of global PMTCT and maternal health goals: a review of the evidence. AIDS Behav 2013; 17: 2528–2539. [DOI] [PubMed] [Google Scholar]
- [28].Gourlay A, Birdthistle I, Mburu G, et al. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: a systematic review. Journal of the International AIDS Society 2013; 16: 18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Onono M, Owuor K, Turan J, et al. The role of maternal, health system, and psychosocial factors in prevention of mother-to-child transmission failure in the era of programmatic scale up in western Kenya: a case control study. AIDS Patient Care and STDs 2015; 29: 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Futterman D, Shea J, Besser M, et al. Mamekhaya: a pilot study combining a cognitive-behavioral intervention and mentor mothers with PMTCT services in South Africa. AIDS Care 2010; 22: 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kapetanovic S, Dass-Brailsford P, Nora D, et al. Mental health of HIV-seropositive women during pregnancy and postpartum period: a comprehensive literature review. AIDS Behav 2014; 18: 1152–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kaaya SF, Blander J, Antelman G, et al. Randomized controlled trial evaluating the effect of an interactive group counseling intervention for HIV-positive women on prenatal depression and disclosure of HIV status. AIDS Care 2013; 25: 854–862. [DOI] [PubMed] [Google Scholar]
- [33].Mundell JP, Visser MJ, Makin JD, et al. The impact of structured support groups for pregnant South African women recently diagnosed HIV positive. Women & Health 2011; 51: 546–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tsai AC, Tomlinson M, Comulada WS, et al. Intimate partner violence and depression symptom severity among South African women during pregnancy and postpartum: Population-based prospective cohort study. PLoS Med 2016; 13: e1001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tuthill EL, Pellowski JA, Young SL, et al. Perinatal depression among HIV-infected women in KwaZulu-Natal South Africa: Prenatal depression predicts lower rates of exclusive breastfeeding. AIDS Behav 2017; 21: 1691–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Colvin CJ, Konopka S, Chalker JC, et al. A systematic review of health system barriers and enablers for antiretroviral therapy (ART) for HIV-infected pregnant and postpartum women. PLoS ONE 2014; 9: e108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Geldsetzer P, Yapa HMN, Vaikath M, et al. A systematic review of interventions to improve postpartum retention of women in PMTCT and ART care. Journal of the International AIDS Society 2016; 19: 20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dyer TP, Stein JA, Rice E, et al. Predicting depression in mothers with and without HIV: The role of social support and family dynamics. AIDS Behav 2012; 16: 2198–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rotheram-Borus MJ, le Roux IM, Tomlinson M, et al. Philani Plus (+): a Mentor Mother community health worker home visiting program to improve maternal and infants’ outcomes. Prev Sci 2011; 12: 372–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rotheram-Borus MJ, Richter LM, van Heerden A, et al. A cluster randomized controlled trial evaluating the efficacy of peer mentors to support South African women living with HIV and their infants. PLoS ONE 2014; 9: e84867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kwalombota M. The effect of pregnancy in HIV-infected women. AIDS Care 2002; 14: 431–433. [DOI] [PubMed] [Google Scholar]
- [42].Bell AC, D’Zurilla TJ. Problem-solving therapy for depression: A meta-analysis. Clinical Psychology Review 2009; 29: 348–353. [DOI] [PubMed] [Google Scholar]
- [43].Nezu A, Maguth Nezu C, D’Zurilla T. Problem-Solving Therapy: A Treatment Manual. New York, NY: Springer Publishing Company, 2013. [Google Scholar]
- [44].Safren SA, O’Cleirigh C, Tan JY, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychology 2009; 28: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016; i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lecrubier Y, Sheehan D, Weiller E, et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. European Psychiatry 1997; 12: 224–231. [Google Scholar]
- [47].Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 1987; 150: 782–786. [DOI] [PubMed] [Google Scholar]
- [48].Rochat TJ, Tomlinson M, Newell M-L, et al. Detection of antenatal depression in rural HIV-affected populations with short and ultrashort versions of the Edinburgh Postnatal Depression Scale (EPDS). Arch Womens Ment Health 2013; 16: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lawrie TA, Hofmeyr GJ, De Jager M, et al. Validation of the Edinburgh Postnatal Depression Scale on a cohort of South African women. South African Medical Journal 1998; 88: 1340–1344. [PubMed] [Google Scholar]
- [50].Mokwena K, Masike I. The need for universal screening for postnatal depression in South Africa: confirmation from a sub-district in Pretoria, South Africa. IJERPH 2020; 17: 6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lu M, Safren SA, Skolnik PR, et al. Optimal recall period and response task for self-reported HIV medication adherence. AIDS Behav 2008; 12: 86–94. [DOI] [PubMed] [Google Scholar]
- [52].AARDEX Corporation. Medication Event Monitoring System (MEMS). Zug, Switzerland: AARDEX Corporation, https://www.aardexgroup.com/solutions/mems-adherence-hardware/. [Google Scholar]
- [53].Safren SA, O’Cleirigh CM, Bullis JR, et al. Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: A randomized controlled trial. Journal of Consulting and Clinical Psychology 2012; 80: 404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Safren SA, Bedoya CA, O’Cleirigh C, et al. Cognitive behavioural therapy for adherence and depression in patients with HIV: a three-arm randomised controlled trial. The Lancet HIV 2016; 3: e529–e538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Safren SA, Hendriksen ES, Desousa N, et al. Use of an on-line pager system to increase adherence to antiretroviral medications. AIDS Care 2003; 15: 787–793. [DOI] [PubMed] [Google Scholar]
- [56].Holzemer WL, Uys LR, Chirwa ML, et al. Validation of the HIV/AIDS Stigma Instrument—PLWA (HASI-P). AIDS Care 2007; 19: 1002–1012. [DOI] [PubMed] [Google Scholar]
- [57].Antelman G, Smith Fawzi MC, Kaaya S, et al. Predictors of HIV-1 serostatus disclosure: a prospective study among HIV-infected pregnant women in Dar es Salaam, Tanzania. AIDS 2001; 15: 1865–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Epino HM, Rich ML, Kaigamba F, et al. Reliability and construct validity of three health-related self-report scales in HIV-positive adults in rural Rwanda. AIDS Care 2012; 24: 1576–1583. [DOI] [PubMed] [Google Scholar]
- [59].Dietz P, Bombard J, Mulready-Ward C, et al. Validation of self-reported maternal and infant health indicators in the Pregnancy Risk Assessment Monitoring System. Matern Child Health J 2014; 18: 2489–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lilliefors HW. On the Kolmogorov-Smirnov test for normality with mean and variance unknown. Journal of the American Statistical Association 1967; 62: 399–402. [Google Scholar]
- [61].IBM Corp. SPSS. 2012. [Google Scholar]
- [62].R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, http://www.R-project.org/ (2020). [Google Scholar]
- [63].Pinheiro J, Bates D, DebRoy S, et al. nlme: Linear and Nonlinear Mixed Effects Models, https://CRAN.R-project.org/package=nlme (2021).
- [64].Glaser BG, Strauss AL, Strutzel E. The discovery of grounded theory; strategies for qualitative research. Nursing Research 1968; 17: 364. [Google Scholar]
- [65].Miles MB, Huberman AM. Drawing valid meaning from qualitative data: toward a shared craft. Educational Researcher 1984; 13: 20–30. [Google Scholar]
- [66].Strauss A, Corbin J. Basics of qualitative research: Procedures and techniques for developing grounded theory. Thousand Oaks, CA: Sage, 1998. [Google Scholar]
- [67].QSR International. NVivo software. 2018. [Google Scholar]
- [68].Hartley M, Tomlinson M, Greco E, et al. Depressed mood in pregnancy: Prevalence and correlates in two Cape Town peri-urban settlements. Reprod Health 2011; 8: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Peltzer K, Rodriguez VJ, Jones D. Prevalence of prenatal depression and associated factors among HIV-positive women in primary care in Mpumalanga province, South Africa. SAHARA-J: Journal of Social Aspects of HIV/AIDS 2016; 13: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Stellenberg EL, Abrahams JM. Prevalence of and factors influencing postnatal depression in a rural community in South Africa. Afr j prim health care fam med; 7. Epub ahead of print 24 November 2015. DOI: 10.4102/phcfm.v7i1.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Cook JA, Cohen MH, Burke J, et al. Effects of depressive symptoms and mental health quality of life on use of highly active antiretroviral therapy among HIV-seropositive women. JAIDS Journal of Acquired Immune Deficiency Syndromes 2002; 30: 401–409. [DOI] [PubMed] [Google Scholar]
- [72].Koenig LJ, O’Leary A. Improving health outcomes for women with HIV: the potential impact of addressing internalized stigma and depression. AIDS 2019; 33: 577–579. [DOI] [PubMed] [Google Scholar]
- [73].Torres TS, Harrison LJ, La Rosa AM, et al. Quality of life among HIV-infected individuals failing first-line antiretroviral therapy in resource-limited settings. AIDS Care 2018; 30: 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Knettel BA, Cichowitz C, Ngocho JS, et al. Retention in HIV care during pregnancy and the postpartum period in the option B+ era: systematic review and meta-analysis of studies in Africa. JAIDS Journal of Acquired Immune Deficiency Syndromes 2018; 77: 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Phillips TK, Clouse K, Zerbe A, et al. Linkage to care, mobility and retention of HIV-positive postpartum women in antiretroviral therapy services in South Africa. J Intern AIDS Soc 2018; 21: e25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bass J, Neugebauer R, Clougherty KF, et al. Group interpersonal psychotherapy for depression in rural Uganda: 6-month outcomes: randomised controlled trial. Br J Psychiatry 2006; 188: 567–573. [DOI] [PubMed] [Google Scholar]
- [77].Bennett DS, Traub K, Mace L, et al. Shame among people living with HIV: a literature review. AIDS Care 2016; 28: 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Himelhoch S, Medoff DR, Oyeniyi G. Efficacy of group psychotherapy to reduce depressive symptoms among HIV-infected individuals: a systematic review and meta-analysis. AIDS Patient Care and STDs 2007; 21: 732–739. [DOI] [PubMed] [Google Scholar]
- [79].Shibasaki WM, Martins RP. Simple randomization may lead to unequal group sizes. Is that a problem? American Journal of Orthodontics and Dentofacial Orthopedics 2018; 154: 600–605. [DOI] [PubMed] [Google Scholar]
- [80].Nakasujja N, Vecchio AC, Saylor D, et al. Improvement in depressive symptoms after antiretroviral therapy initiation in people with HIV in Rakai, Uganda. J Neurovirol 2021; 27: 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hanlon C. Maternal depression in low- and middle-income countries. International Health 2013; 5: 4–5. [DOI] [PubMed] [Google Scholar]
- [82].Prince M, Patel V, Saxena S, et al. No health without mental health. The Lancet 2007; 370: 859–877. [DOI] [PubMed] [Google Scholar]
- [83].Besser M Mothers 2 Mothers. Search Results Web results South African Journal of Obstetrics and Gynaecology 2006; 12: 122–128. [Google Scholar]
- [84].Barnett ML, Lau AS, Miranda J. Lay health worker involvement in evidence-based treatment delivery: a conceptual model to address disparities in care. Annu Rev Clin Psychol 2018; 14: 185–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Honikman S, van Heyningen T, Field S, et al. Stepped care for maternal mental health: a case study of the perinatal mental health project in South Africa. PLoS Med 2012; 9: e1001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Vythilingum B, Field S, Kafaar Z, et al. Screening and pathways to maternal mental health care in a South African antenatal setting. Arch Womens Ment Health 2013; 16: 371–379. [DOI] [PubMed] [Google Scholar]
- [87].Iyun V, Brittain K, Phillips TK, et al. Prevalence and determinants of unplanned pregnancy in HIV-positive and HIV-negative pregnant women in Cape Town, South Africa: a cross-sectional study. BMJ Open 2018; 8: e019979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Farquhar C, Kiarie JN, Richardson BA, et al. Antenatal couple counseling increases uptake of interventions to prevent HIV-1 transmission. JAIDS Journal of Acquired Immune Deficiency Syndromes 2004; 37: 1620–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hampanda K, Abuogi L, Musoke P, et al. Development of a novel scale to measure male partner involvement in the prevention of mother-to-child transmission of HIV in Kenya. AIDS Behav 2020; 24: 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Matseke MG, Ruiter RAC, Rodriguez VJ, et al. Factors associated with male partner involvement in programs for the prevention of mother-to-child transmission of HIV in rural South Africa. IJERPH 2017; 14: 1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Montgomery E, van der Straten A, Torjesen K. “Male involvement” in women and childrenʼs HIV prevention: challenges in definition and interpretation. JAIDS Journal of Acquired Immune Deficiency Syndromes 2011; 57: e114–e116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.