Abstract
PomA is thought to be a component of the ion channel in the sodium-driven polar-flagellar motor of Vibrio alginolyticus. We have found that some cysteine substitutions in the periplasmic region of PomA result in a slow-motility phenotype, in which swarming and swimming speeds are reduced even in the presence of high concentrations of NaCl. Most of the mutants showed a sodium ion dependence similar to that of the wild type but with significantly reduced motility at all sodium ion concentrations. By contrast, motility of the D31C mutant showed a sharp dependence on NaCl concentration, with a threshold at 38 mM. The motor of the D31C mutant rotates stably, as monitored by laser dark-field microscopy, suggesting that the mutant PomA protein is assembled normally into the motor complex. Mutational studies of Asp31 suggest that, although this residue is not essential for motor rotation, a negative charge at this position contributes to optimal speed and/or efficiency of the motor.
Flagella are the filamentous organelles responsible for motility of most bacteria. Flagellar rotation is driven by a reversible rotary motor embedded in the cytoplasmic membrane at the base of each flagellar filament. The energy for rotation of the flagellar motors comes from the electrochemical gradient of specific ions across the cytoplasmic membrane (protons in Escherichia coli and Salmonella enterica serovar Typhimurium, or sodium ions in Vibrio spp. and alkalophilic Bacillus strains) (4, 11, 12, 17).
The essential component for energy conversion in the motor is thought to be a force-generating unit that functions as an ion channel and interacts with the coupling ion to produce mechanical force. In the proton-driven motor, the channel is composed of a complex of MotA and MotB (5, 9, 10, 25, 27, 28), membrane proteins with four and one single transmembrane segments, respectively (6, 7, 26, 31). It is believed that the cytoplasmic domain of MotA interacts electrostatically with the C-terminal domain of the rotor protein FliG (19, 32). Four proteins, PomA, PomB, MotX, and MotY, are essential for rotation of the sodium-driven polar-flagellar motors of the marine bacteria Vibrio alginolyticus (1, 8, 23) and Vibrio parahaemolyticus (13, 20, 21). PomA and PomB are homologs of MotA and MotB and are thus thought to form a sodium channel in the motor. The functions of MotX and MotY are unknown, although MotX may also be a sodium channel component of the motor, because overproduction of MotX is lethal in E. coli in proportion to the external sodium ion concentration, and also because this lethality is suppressed by the addition of amiloride, a specific inhibitor of the sodium-driven motor (20). Mutations resistant to phenamil, an amiloride analog, were recently identified in PomA and PomB, and these mutations showed synergistic effects on motility (13, 15). In addition, PomA could be coprecipitated with PomB by anti-PomB antibody, and PomB could be coprecipitated with PomA by anti-PomA antibody (30). These results support the hypothesis that PomA and PomB form a complex. Recently, it has been shown directly that the complex consisting of PomA and PomB catalyzes sodium influx in reconstituted proteoliposomes (24).
Slow-motility mutants caused by mutations in MotA have been isolated in E. coli and Salmonella serovar Typhimurium (5, 29). It was reported that in most of the slow-motility mutants motor torque was normal at low speeds but reduced at high speeds. These mutants generated approximately equal torques in H2O and D2O, but their swimming speeds were significantly reduced in D2O compared with those in H2O (5). These results suggested that motor rotation is kinetically limited in these mutants by a process that might involve proton transfer. In the case of the sodium-driven motor, rates of sodium ion flux are easily altered by changing the NaCl concentration or by using sodium channel-specific inhibitors. This approach allows a direct assessment of the relationship between slow rotation and ion flux through the motor. We have generated a series of cysteine-substituted mutations in the periplasmic residues of PomA and found that some of these mutants show a slow-motility phenotype (2). In this study, one of these slow-motility mutants, which could not swim in a low concentration of NaCl, was characterized.
The PomA mutation D31C causes slow polar flagellar motility.
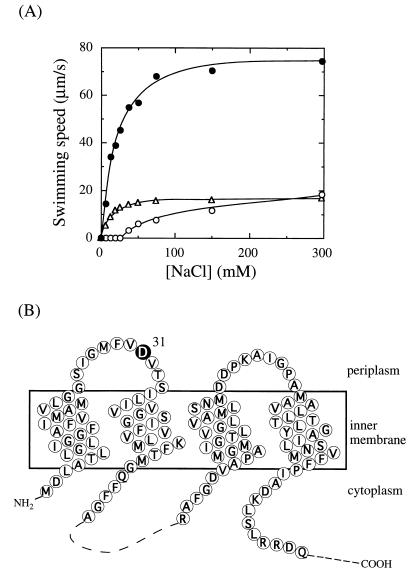
We first examined the relationship between swimming speed and NaCl concentration in the slow-motility pomA mutants isolated previously. The plasmid pYA301 (pomA+ Kmr) (15) containing wild-type or mutant pomA was introduced into the V. alginolyticus strain NMB188 (Laf− PomA− Che−) (15) and cultured to late logarithmic phase at 30°C in VPG medium (1% polypeptone, 0.4% K2HPO4, 3% NaCl, 0.5% glycerol). Swimming speeds were measured in TMN medium containing 50 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 5 mM glucose, and various concentrations of NaCl and KCl to keep the sum of the concentrations of NaCl and KCl at 300 mM. Most of the slow-motility mutants obtained by cysteine substitutions in the periplasmic residues of PomA showed a dependence on sodium ion concentration similar to that of the wild-type strain, but with swimming speeds significantly lower than for the wild-type strain (Fig. 1A; PomA I175C is a typical example). Among the slow-motility mutants, only D31C showed a significantly different dependence on sodium ion concentration (Fig. 1A). This mutant did not swim at low concentrations of NaCl (0 to 19 mM). It could swim in medium containing 38 mM NaCl, and its swimming speed increased gradually as the concentration of NaCl in the medium increased. It swam at about 20 μm/s in 300 mM NaCl, a speed about one-fourth that of cells expressing the wild-type PomA and comparable to the speed of the PomA I175C mutant. As shown in Fig. 1B, Asp31 of PomA is thought to be located in the periplasmic loop region. We found that the swimming speed of the PomA D31C mutant was further reduced by the sulfhydryl modifying reagents dithionitrobenzoic acid and N-ethylmaleimide, although motility of the wild-type strain, which does not contain cysteine in PomA, was not reduced. This result suggests that this residue is exposed to the outside (2).
FIG. 1.
Sodium concentration dependence of slow-motility mutants. (A) NMB188 (PomA− Che−) cells containing wild-type (filled circle) or mutant (I175C [triangle] or D31C [open circle]) pomA cells were harvested in late logarithmic phase and resuspended in TMN medium (pH 7.5) containing various concentrations of NaCl. Motility of the cells was observed at room temperature under a dark-field microscope and recorded on videotape. Swimming speed was determined as described previously (3). The average swimming speed was obtained by measuring more than 20 swimming tracks. (B) The predicted transmembrane regions of PomA. The residue Asp31 is marked by a filled circle and white letter. The topology of PomA was predicted from the hydropathy profile of PomA and the topology of the MotA protein of E. coli (31).
Site-directed mutagenesis of PomA Asp31.
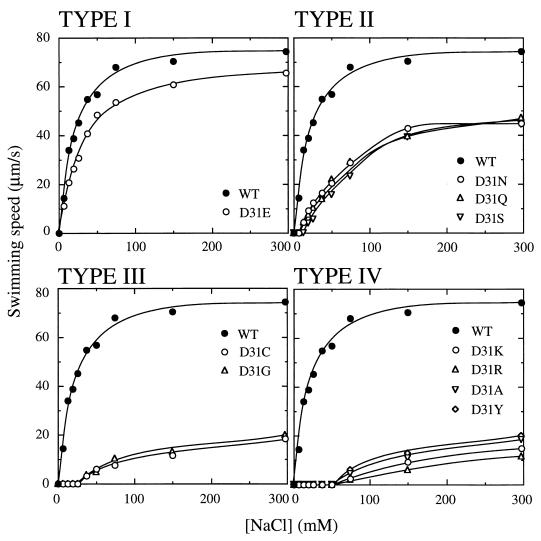
Given the presumed location of Asp31 in the periplasmic space (Fig. 1B), the negative charge of this residue might affect rates of ion influx through the motor. We next carried out site-directed mutagenesis to replace Asp31 of PomA with various residues. Mutations were made in pomA carried in the plasmid pYA301 by the two-step PCR method described previously (15). Plasmids carrying the mutated pomA were transformed to the PomA− Che− strain NMB188, and swimming speeds were measured at various NaCl concentrations (Fig. 2). We generated 10 new mutants, all of which, except the D31F mutant, exhibited motility at 300 mM NaCl. The mutants were classified into four groups according to their NaCl thresholds for motility and their maximal swimming speeds in 300 mM NaCl. The type I mutant D31E showed nearly the same sodium dependence and swimming speed as the wild-type strain. Type II mutants were slightly shifted in their threshold for motility, being motile in 13 to 19 mM NaCl, and they exhibited a reduced maximal swimming speed relative to the wild-type strain. Mutants carrying substitutions with neutral side chains (D31N, D31Q, and D31S) fell into this group. The single type III mutant (D31G) showed the same NaCl dependence and maximum speed as D31C. Type IV mutants had a higher NaCl threshold for motility, requiring more than 75 mM NaCl for motility and showing reduced maximum swimming speeds relative to D31C. Substitution with small (D31A), large (D31Y), or positively charged residues (D31K and D31R) produced this profile. These results show that various mutations in Asp31 of PomA affect the sodium ion concentration dependence of motility. Charge-reversed mutations (D31K and D31R) greatly reduced motility, but the charge-conserved mutation (D31E) did not affect motility very much, suggesting that a negative charge at this position might be important for optimal function of the motor.
FIG. 2.
Sodium-dependent motility of the PomA Asp31 mutants. Mutants were classified into four groups based on the Na+ concentration threshold for motility. NMB188 cells expressing each mutant PomA were harvested at late logarithmic phase and suspended in TMN medium containing various concentrations of NaCl. Swimming speeds were measured as described in the legend to Fig. 1. WT, wild type.
To investigate whether Asp31 is located near ion-binding sites in the channel complex, we examined the inhibition of motility of the D31C mutant by the sodium channel-specific inhibitors phenamil and amiloride. Motility of both the D31C mutant and the wild-type strain was inhibited by 50 μM phenamil or 3 mM amiloride in 100 mM NaCl (data not shown). Thus, Asp31 of PomA might not be directly involved in binding sodium ions. We also performed immunoblotting of whole-cell proteins of the mutants, using a polyclonal anti-PomA peptide antibody. All of the mutant PomA proteins were detected at levels sufficient to support motor function (data not shown).
Analysis of speed fluctuations in the PomA D31C mutant.
It has been shown, by adjusting the sodium ion concentration in the medium, that rotation of the polar flagellar motor of V. alginolyticus is stable as the motor speed is varied over a wide range (from about 50 to about 1,000 revolutions per second [rps]) (22). Thus, if sodium flux is reduced in the D31C motor but other aspects of function are unaffected, the rotation speed of the motor should not fluctuate. However, if the force-generating units of the mutant bind unstably to sites in the motor, this effect should slow the motility and increase fluctuations in rotation speed. To test this idea, we measured flagellar rotation in NMB188 cells containing wild-type or D31C mutant PomA protein by using laser dark-field microscopy (LDM), as described previously (16). In this method, a rapidly rotating flagellum is irradiated by a thin beam of laser light, and its rotation rate is measured from the intensity change of scattered light (18).
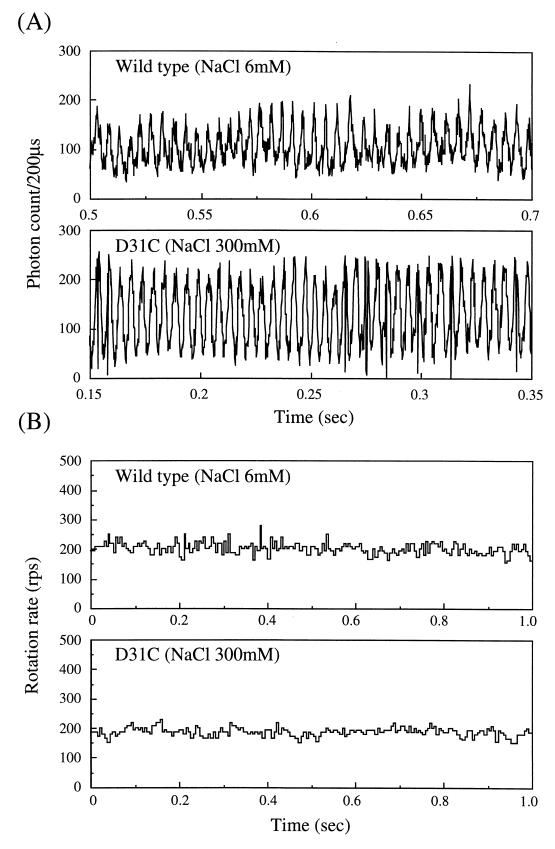
NMB188 cells containing wild-type or D31C PomA protein were harvested at late logarithmic phase, washed once with TMN medium, and resuspended in the same medium. The cell suspension was poured into the space between the slide and the cover glass on which thin spacers were placed. After 10 min, cells not stuck to the cover glass were washed by a flow of TMN medium. To allow speed fluctuations to be compared at the same average rotation speed, wild-type cells were studied in 6 mM NaCl and mutant cells were studied in 300 mM NaCl. Figure 3A shows the intensity of scattered light versus time for both the wild type and the mutant. Both mutant and wild-type flagella rotated steadily at about 200 rps (Fig. 3B). To evaluate speed fluctuations of the motors, we made the following calculation for multiple samples: (standard deviation of the rotation rate)/(average rotation rate) × 100. This value was 10 and 12% for the wild type and the D31C mutant, respectively, suggesting that force-generating units of the mutant D31C motor are stably bound to the motor.
FIG. 3.
Flagellar rotation of wild-type and D31C mutant cells detected by LDM. Rotation of a single polar flagellum of a stuck cell of NMB188 expressing wild-type or D31C PomA protein was measured by using LDM for about 6 s in the presence of 6 mM NaCl (wild type) or 300 mM NaCl (D31C), as described previously (16). (A) Intensity changes of the scattered light from the rotating flagellum. The interval between peaks corresponds to the rotation period. (B) Fluctuations in rotation rate of the wild-type and D31C mutant motors plotted as the reciprocal of the interpeak intervals from the data in panel A. Average rotation rates were 197 ± 20.7 rps (wild type) and 187 ± 16.9 rps (D31C).
Possible role of negative charge of Asp31 for motor rotation.
In this study, we characterized slow-motility mutants that might have defects in essential steps of torque generation by the flagellar motor. Two questions are raised by these slow-motility mutants. First, what slows the rotation of these mutant motors? Second, what causes a threshold for motility? Two factors are likely to be important: the rate of sodium influx through the motors and the efficiency of energy conversion in the motors. That is, ion flux through the mutant motors might be decreased and/or the efficiency of energy coupling in the mutant motors might be reduced by defects in the channel complex. The simplest model for such a defect is that mutation of Asp31 of PomA makes ions pass more slowly through the channel (for example, the pore size might become smaller). Mutations might reduce the rate of flow so much that it results in a threshold for motility. Similarly, reduction in the efficiency of energy conversion in the motor might cause this phenotype.
Based on this study, we cannot conclude which model is more likely to explain the effect on motor rotation imposed by replacements of Asp31. Asp31 is located in the first periplasmic loop (loop1-2) of PomA, and it has been suggested that loop1-2 may be in contact with other proteins, such as PomB, MotX, or MotY, or that it may be embedded in the pore region of the channel (2). In either case, the negative charge of this residue appears to be important. Introduction of a positive charge (D31K or D31R), and also changes in the size of the side chain (D31A and D31Y), caused large increases in the threshold NaCl concentration needed for motility. These mutations (type IV) probably do not cause unstable binding of the force-generating units to the motor, because the motors of D31C mutant rotated steadily. Thus, the mutant phenotype is probably due to defects in the channel complex itself and not in its installation into the motor.
The periplasmic segment of PomA contains two other negative charges, D170 and D171 (in loop3-4). These negative charges, together with D31, may function to recruit sodium ions into the channel complex. An analogous recruitment mechanism for hydrogen ions has been suggested for bacteriorhodopsin (14). The D170C or D171C single mutant reduced motility; however, both of them did not have NaCl thresholds for motility as D31C did. Double mutations of these two residues might affect motility more severely. According to sequence alignment of MotA proteins, these three residues are conserved in some species, but not perfectly. Alignment also shows that MotA proteins have several charged residues in periplasmic domains, and in most cases, their net charges are negative. This might imply that the negatively charged area in MotA proteins can function similarly to recruit protons into MotA/MotB channels.
Recently, our group succeeded in measuring sodium ion flux, in response to a potassium diffusion potential, through purified PomAB channel complexes reconstituted in proteoliposomes (24). Such a direct measurement of the current passed through the channel complex will be needed to clarify the function of residue Asp31. We hope to determine the cause(s) of the Na+ concentration threshold and thus gain insight into the mechanism of torque generation.
Acknowledgments
We thank David F. Blair and Ken Sato for critically reading the manuscript.
This work was supported in part by grants-in-aid for scientific researches (to I.K. and M.H.) from the Ministry of Education, Science and Culture of Japan and the Japan Society for the Promotion of Science (to S.K. and Y.A.).
REFERENCES
- 1.Asai Y, Kojima S, Kato H, Nishioka N, Kawagishi I, Homma M. Putative channel components for the fast-rotating sodium-driven flagellar motor of a marine bacterium. J Bacteriol. 1997;179:5104–5110. doi: 10.1128/jb.179.16.5104-5110.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asai Y, Shoji T, Kawagishi I, Homma M. Cysteine-scanning mutagenesis of the periplasmic loop regions of PomA, a putative channel component of the sodium-driven flagellar motor in Vibrio alginolyticus. J Bacteriol. 2000;182:1001–1007. doi: 10.1128/jb.182.4.1001-1007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atsumi T, Maekawa Y, Yamada T, Kawagishi I, Imae Y, Homma M. Effect of viscosity on swimming by the lateral and polar flagella of Vibrio alginolyticus. J Bacteriol. 1996;178:5024–5026. doi: 10.1128/jb.178.16.5024-5026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blair D F. How bacteria sense and swim. Annu Rev Microbiol. 1995;49:489–522. doi: 10.1146/annurev.mi.49.100195.002421. [DOI] [PubMed] [Google Scholar]
- 5.Blair D F, Berg H C. The MotA protein of E. coli is a proton-conducting component of the flagellar motor. Cell. 1990;60:439–449. doi: 10.1016/0092-8674(90)90595-6. [DOI] [PubMed] [Google Scholar]
- 6.Chun S Y, Parkinson J S. Bacterial motility: membrane topology of the Escherichia coli MotB protein. Science. 1988;239:276–278. doi: 10.1126/science.2447650. [DOI] [PubMed] [Google Scholar]
- 7.Dean G E, Macnab R M, Stader J, Matsumura P, Burks C. Gene sequence and predicted amino acid sequence of the motA protein, a membrane-associated protein required for flagellar rotation in Escherichia coli. J Bacteriol. 1984;159:991–999. doi: 10.1128/jb.159.3.991-999.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furuno M, Nishioka N, Kawagishi I, Homma M. Suppression by the DNA fragment of the motX promoter region on long flagellar mutants of Vibrio alginolyticus. Microbiol Immunol. 1999;43:39–43. doi: 10.1111/j.1348-0421.1999.tb02370.x. [DOI] [PubMed] [Google Scholar]
- 9.Garza A G, Biran R, Wohlschlegel J A, Manson M D. Mutations in motB suppressible by changes in stator or rotor components of the bacterial flagellar motor. J Mol Biol. 1996;258:270–285. doi: 10.1006/jmbi.1996.0249. [DOI] [PubMed] [Google Scholar]
- 10.Garza A G, Bronstein P A, Valdez P A, Harris-Haller L W, Manson M D. Extragenic suppression of motA missense mutations of Escherichia coli. J Bacteriol. 1996;178:6116–6122. doi: 10.1128/jb.178.21.6116-6122.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hase C C, Mekalanos J J. Effects of changes in membrane sodium flux on virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci USA. 1999;96:3183–3187. doi: 10.1073/pnas.96.6.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imae Y, Atsumi T. Na+-driven bacterial flagellar motors. J Bioenerg Biomembr. 1989;21:705–716. doi: 10.1007/BF00762688. [DOI] [PubMed] [Google Scholar]
- 13.Jaques S, Kim Y K, McCarter L L. Mutations conferring resistance to phenamil and amiloride, inhibitors of sodium-driven motility of Vibrio parahaemolyticus. Proc Natl Acad Sci USA. 1999;96:5740–5745. doi: 10.1073/pnas.96.10.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura Y, Vassylyev D G, Miyazawa A, Kidera A, Matsushima M, Mitsuoka K, Murata K, Hirai T, Fujiyoshi Y. Surface of bacteriorhodopsin revealed by high-resolution electron crystallography. Nature. 1997;389:206–211. doi: 10.1038/38323. [DOI] [PubMed] [Google Scholar]
- 15.Kojima S, Asai Y, Atsumi T, Kawagishi I, Homma M. Na+-driven flagellar motor resistant to phenamil, an amiloride analog, caused by mutations in putative channel components. J Mol Biol. 1999;285:1537–1547. doi: 10.1006/jmbi.1998.2377. [DOI] [PubMed] [Google Scholar]
- 16.Kojima S, Atsumi T, Muramoto K, Kudo S, Kawagishi I, Homma M. Vibrio alginolyticus mutants resistant to phenamil, a specific inhibitor of the sodium-driven flagellar motor. J Mol Biol. 1997;265:310–318. doi: 10.1006/jmbi.1996.0732. [DOI] [PubMed] [Google Scholar]
- 17.Kojima S, Yamamoto K, Kawagishi I, Homma M. The polar flagellar motor of Vibrio cholerae is driven by an Na+ motive force. J Bacteriol. 1999;181:1927–1930. doi: 10.1128/jb.181.6.1927-1930.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudo S, Magariyama Y, Aizawa S-I. Abrupt changes in flagellar rotation observed by laser dark-field microscopy. Nature. 1990;346:677–680. doi: 10.1038/346677a0. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd S A, Whitby F G, Blair D F, Hill C P. Structure of the C-terminal domain of FliG, a component of the rotor in the bacterial flagellar motor. Nature. 1999;400:472–475. doi: 10.1038/22794. [DOI] [PubMed] [Google Scholar]
- 20.McCarter L L. MotX, the channel component of the sodium-type flagellar motor. J Bacteriol. 1994;176:5988–5998. doi: 10.1128/jb.176.19.5988-5998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarter L L. MotY, a component of the sodium-type flagellar motor. J Bacteriol. 1994;176:4219–4225. doi: 10.1128/jb.176.14.4219-4225.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muramoto K, Kawagishi I, Kudo S, Magariyama Y, Imae Y, Homma M. High-speed rotation and speed stability of the sodium-driven flagellar motor in Vibrio alginolyticus. J Mol Biol. 1995;251:50–58. doi: 10.1006/jmbi.1995.0415. [DOI] [PubMed] [Google Scholar]
- 23.Okunishi I, Kawagishi I, Homma M. Cloning and characterization of motY, a gene coding for a component of the sodium-driven flagellar motor in Vibrio alginolyticus. J Bacteriol. 1996;178:2409–2415. doi: 10.1128/jb.178.8.2409-2415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato K, Homma M. Functional reconstitution of the Na+-driven polar flagellar motor component of Vibrio alginolyticus. J Biol Chem. 2000;275:5718–5722. doi: 10.1074/jbc.275.8.5718. [DOI] [PubMed] [Google Scholar]
- 25.Sharp L L, Zhou J, Blair D F. Tryptophan-scanning mutagenesis of MotB, an integral membrane protein essential for flagellar rotation in Escherichia coli. Biochemistry. 1995;34:9166–9171. doi: 10.1021/bi00028a028. [DOI] [PubMed] [Google Scholar]
- 26.Stader J, Matsumura P, Vacante D, Dean G E, Macnab R M. Nucleotide sequence of the Escherichia coli motB gene and site-limited incorporation of its product into the cytoplasmic membrane. J Bacteriol. 1986;166:244–252. doi: 10.1128/jb.166.1.244-252.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stolz B, Berg H C. Evidence for interactions between MotA and MotB, torque-generating elements of the flagellar motor of Escherichia coli. J Bacteriol. 1991;173:7033–7037. doi: 10.1128/jb.173.21.7033-7037.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang H, Braun T F, Blair D F. Motility protein complexes in the bacterial flagellar motor. J Mol Biol. 1996;261:209–221. doi: 10.1006/jmbi.1996.0453. [DOI] [PubMed] [Google Scholar]
- 29.Togashi F, Yamaguchi S, Kihara M, Aizawa S-I, Macnab R M. An extreme clockwise switch bias mutation in fliG of Salmonella typhimurium and its suppression by slow-motile mutations in motA and motB. J Bacteriol. 1997;179:2994–3003. doi: 10.1128/jb.179.9.2994-3003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yorimitsu T, Sato K, Asai Y, Kawagishi I, Homma M. Functional interaction between PomA and PomB, the Na+-driven flagellar motor components of Vibrio alginolyticus. J Bacteriol. 1999;181:5103–5106. doi: 10.1128/jb.181.16.5103-5106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J, Fazzio R T, Blair D F. Membrane topology of the MotA protein of Escherichia coli. J Mol Biol. 1995;251:237–242. doi: 10.1006/jmbi.1995.0431. [DOI] [PubMed] [Google Scholar]
- 32.Zhou J, Lloyd S A, Blair D F. Electrostatic interactions between rotor and stator in the bacterial flagellar motor. Proc Natl Acad Sci USA. 1998;95:6436–6441. doi: 10.1073/pnas.95.11.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]