Abstract
Aim
To describe the efficacy and safety of iStent implantation prior to phacoemulsification in manual as well as femtosecond laser-assisted cataract surgery (FLACS), and highlight this approach as a reasonable, if not necessary, step to advance one's ability and confidence in the use of microinvasive glaucoma surgery (MIGS) technology in phakic patients.
Methods
A retrospective consecutive case series of patients with open angle glaucoma or ocular hypertension who underwent iStent inject implantation followed by cataract surgery (manual or FLACS). All cases underwent postoperative video review and were assessed and classified for intraoperative lens injury and hyphaema. Postoperative data included intraocular pressure (IOP), medication usage and adverse events.
Results
Sixty-three eyes (n = 40 manual, n = 23 FLACS) were analyzed. Preoperatively, the mean IOP was 19.2 ± 4.9 mm Hg on 1.4 ± 0.96 mean medications, with 100% of eyes treated with medication. Intraoperatively, no lens injury was identified, and no significant hyphaema that impeded surgery occurred. At 6 months postoperative, mean IOP was 14.2 ± 1.8 mm Hg (38% reduction: p < 0.001), and >90% of eyes had IOP ≤ 16 mm Hg. The mean number of medications reduced to 0.11 ± 0.3 (92% reduction: p < 0.001), with 89% of eyes medication free. Safety was excellent for both manual and FLACS, with two iStents implanted in all eyes, and no cases of significant hyphaema or lens injury.
Conclusion
Early implantation is safe, maximizes corneal clarity and angle visualization, avoids the risk of non-implantation due to surgical complications, and has a high success rate in both manual cataract surgery and the setting of FLACS.
Clinical significance
The conventional recommended approach of iStent implantation following cataract extraction has been adopted by many, however, with the advent of stand-alone procedures and concern about potential lens injury, there is an opportunity to gain experience with minimal risk in patients undergoing MIGS procedures combined with cataract surgery by implanting iStents at the start of the procedure. There is currently little emphasis or data published in the literature on an early approach to implantation to guide surgeons.
How to cite this article
Manning DK, Haider A, Clement C, et al. Efficacy and Safety of iStent Inject Implantation in Manual and Femtosecond Laser-assisted Cataract Surgery before Lens Extraction. J Curr Glaucoma Pract 2022;16(2):105-110.
Keywords: Femtosecond laser-assisted cataract surgery, Glaucoma, iStent, Microinvasive glaucoma surgery
Introduction
The iStent (Glaukos, Laguna Hills, California, USA) is a trabecular micro-bypass stent that has quickly gained popularity since its approval in Australia in 2012 as a MIGS device. It is typically implanted ab interno in conjunction with cataract surgery to effectively lower intraocular pressure (IOP) more than cataract surgery alone in mild to moderate glaucoma patients. Combined iStent and cataract surgery have been shown to safely reduce both IOP and medication burden up to 5 years postoperatively.1,2
iStent implantation can also be performed as a stand-alone procedure in both phakic and pseudophakic eyes. To date, however, the uptake of this stand-alone approach has been limited by regional regulations as well as uncertainty about the potential safety of such a procedure. Phakic eyes, in particular, are of concern with the potential for lens injury in eyes that are not planned, or required, to undergo lens extraction.
In combined procedures, iStent implantation can be performed at the beginning or the end of the surgery, but there are limited published articles to date highlighting the advantages of one approach over the other. Comparison of the clarity of angle structures before and after cataract surgery has recently been described.3 There was a discernable difference in the observers before or after phacoemulsification on postprocedural video assessment, with 30% of observers commenting that the angle was clearer postlens removal and 27.5% before. This was a small series looking at post hoc still images and may not reflect the view experienced by the surgeon under the operating microscope, however, it suggests that in some cases, the angle structure is significantly clearer before phacoemulsification.
Most surgeons advocate the implantation of the iStent at the end of the case when the anterior chamber (AC) is maximally deep after the crystalline lens has been removed. Modifying one's approach to the beginning of the procedure has several advantages. The cornea is presumed to be of maximal clarity, increasing the likelihood of successful implantation and thereby reduction of IOP and medication usage, bleeding is controlled by intraoperative phacodynamic, and by implanting at the beginning of surgery, there have yet to be any intraoperative complications that would prevent implantation such as occurs if one delays the procedure to the end of the operation.
Additionally, by performing iStent implantation at the start of a combined procedure, there is the ability to attain experience in implantation in phakic eyes. By employing this approach, we can and have evaluated the efficacy and safety of iStent implantation in phakic eyes that are planned to have lens extraction, thereby obviating the ethical concerns about performing stand-alone surgery where a lens may be injured and require unexpected removal.
There are a limited number of surgeons combining FLACS and MIGS with iStent, and once again, to date, there is little published data on the efficacy and safety of this approach.
We describe the safe and successful use of iStent at the start of both manual cataract surgery as well as FLACS and discuss the benefits of this modified approach and its implication in regards to safety for stand-alone iStent surgery.
Methods
Ethical approval for this approach was sought through the Hunter New England Local Health Network research and ethics committee.
This study was undertaken as a retrospective case series of consecutive patients undergoing combined cataract surgery, either manual or FLACS, and iStent implantation in a single center practice. All subjects were diagnosed with open-angle glaucoma or ocular hypertension (OHT), with angle anatomy confirmed by both clinical gonioscopy and anterior segment optical coherence tomography. Subjects underwent full informed consent.
All procedures were performed by a single experienced surgeon with iStent inject implantation performed at the beginning of the combined procedure and were followed for 6 months.
Outcome measures assessed in the study period were IOP reduction from baseline as well as from presurgery treated levels, medication usage, intraoperative complications such as hyphema iris trauma and stent malposition and, in particular, implantation-induced lens injury.
Injury to the lens was assessed by postoperative video review. All cases were digitally recorded via the operating microscope and assessed within 1 week, by the operating surgeon. Lens injury was classified as per Table 1, with no injury recorded as level 0, capsule indentation as level 1, capsule breach as level 2, and lenticular substance injury as level 3. Hyphema was classified as in Table 2, with no bleeding evident as 0, bleeding evident in angle 1, bleeding obscuring view as 2, and bleeding needing washout as 3.
Table 1.
Observed lens injury on video review
| Injury | Level | Number | Percent |
|---|---|---|---|
| No injury | 0 | 63 | 100 |
| Capsule indentation | 1 | 0 | 0 |
| Capsule breach | 2 | 0 | 0 |
| Lens substance injury | 3 | 0 | 0 |
Table 2.
Intraoperative hyphema
| Hyphema | Level | Number | Percent |
|---|---|---|---|
| Nil | 0 | 18 | 28 |
| In angle | 1 | 45 | 72 |
| Obscuring view | 2 | 0 | 0 |
| Needing washout | 3 | 0 | 0 |
Surgical Technique
The iStent is a heparin-coated titanium microstent which weighs less than 0.1 mg. The second generation iStent inject (model GTS 400), a smaller modified version of the original device, is utilized in the below technique. It differs from the original with respect to: (1) Structure: it is a linear device without a snorkel, 360 µm in length with a head of 230 µm diameter containing four sideports positioned within Schlemm's canal (Figs 1 and 2); and (2) A modified injector that allows for simultaneous insertion with a single entry into the eye of two microstents. Surgical technique is similar but considered technically easier in comparison to its predecessor because sideways sliding of the GTS 400 model is not required for positioning into Schlemm's canal.4
Fig. 1.

Second generation GTS 400 iStent inject—four side ports sit within Schlemm's canal with the central lumen facing the AC (Adapted from Richter and Coleman, 2016)
Figs 2A to D.
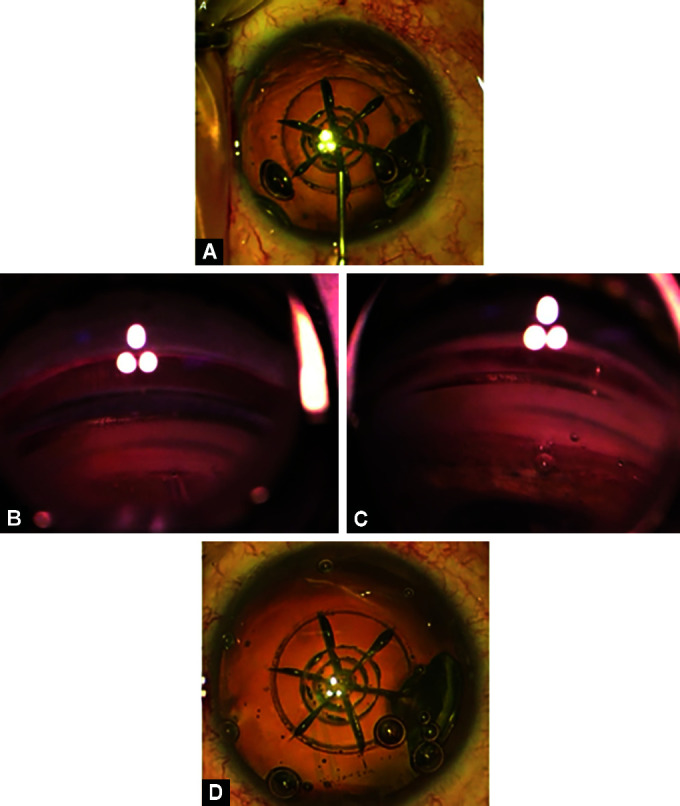
iStent inject implantation prior to phacoemulsification in FLACS case. (A) AC is deepened with OVD taking care not to disturb the open capsulotomy; (B and C) Trocar is exposed aiming at area of vascular engorgement within trabecular meshwork and istent is deployed; (D) Patient returned back to primary position with no evidence of lens injury
Patients undergoing laser-assisted cataract surgery are pretreated using the Alcon LenSx® platform with the SoftFit™ contact lens interface under topical anesthesia or peribulbar block after routine pupil dilatation. Typical laser parameters are employed with a 5.1 mm capsulotomy centered on the visual axis with a VERION guidance system and phacofragmentation performed in a sextant pattern with three concentric rings.
Patients are then transferred to the operating microscope for the remainder of the procedure.
Those undergoing manual surgery had procedures performed under topical anesthesia or peribulbar block after routine pupil dilatation.
Under microscope guidance, a 2.2 mm main wound is constructed temporally at either 0 or 180°, depending on the side of surgery. A 1 mm side port is placed at 90°. A dispersive ophthalmic viscosurgical device (OVD) is injected into the AC, taking care to deepen the angle and not disturb the open capsulotomy in FLACS cases (Fig. 2A).
The patients’ head is rotated 30° away from the surgeon, with the microscope titled 30° towards the surgeon. OVD is placed on the external nasal side of the cornea to allow coupling of a gonioprism, and the angle is visualized with the gonioprism under high magnification, with a fine focus on the trabecular meshwork.
iStent inject is introduced via the main wound into the AC with its needle extended, however, once visualized in the prism, the needle is retracted to expose the trocar. Target visualization is important at this stage, and care is taken not to cause corneal folds that can obscure the angle view. The angle is approached with the trocar, aiming at areas of vascular engorgement or hyperpigmentation, both being presumed signs of the presence of aqueous veins (Fig. 2B).
The trocar is implanted into the trabecular meshwork and, with minimal pressure and minimal dimpling, the iStent is then deployed, with two stents placed 3 clock hours apart. Care is taken to ensure the stent is adequately implanted, with its collar retained within the trabecular meshwork and its flange remaining in the AC (Fig. 2C).
The iStent introducer was removed, the patient returned to the primary position, and the microscope was adjusted so that the cataract procedure could be completed as per a normal stop and chop technique (Fig. 2D). Careful observation of the lens anatomy was undertaken, and any injury or issue was documented prior to completion of the surgery.
At the end of the procedure, a course of topical antibiotic and steroid medication is prescribed and tapered as per standard postoperative cataract care. No pharmacological miotic agents are used during the procedure, however a single dose of 250 mg acetazolamide is administered to avoid IOP spikes in the immediate postoperative period.
Results
A total number of 63 eyes underwent iStent inject implantation followed by simultaneous cataract surgery, with 37% (n = 23) undergoing femtosecond laser pretreatment. About 75% of subjects were diagnosed with open-angle glaucoma, 19% with OHT, and 6% with pseudoexfoliative disease. Table 1 summarizes demographic and clinical data of patients who underwent FLACS and conventional phacoemulsification surgery.
A total of 51% (n = 32) of all subjects were male with an average age of 71 years (range 58–93). The highest recorded untreated IOP was measured at 22.8 ± 4.3 mm Hg, with treated IOP at 19.2 ± 4.9 mm Hg, on an average of 1.4 ± 0.96 medications preoperatively. All patients were on treatment at the start of the study.
At 6 months follow-up the average IOP was 14.19 ± 1.8 mm Hg (p < 0.001) and number of medications 0.11 ± 0.3 (p < 0.001). Over 90% had an IOP at 16 mm Hg or below. This represented an average 38% reduction of IOP compared to baseline, with 88.9% (n = 57) of all subjects’ medication free (Fig. 2).
Complications identified during surgical video review are listed in Tables 1 and 2. Microhyphema (hyphema level 1) was frequently observed at the time of implantation (72%), however, there were no cases where intraoperative bleeding compromised the ability to complete the cataract surgery, there were no cases where additional washout was required at case completion (hyphema levels 2–3), nor were there any cases of significant postoperative hyphema (0%). Additionally, there were no incidents of levels 1–3 lens injury (0%) upon postoperative video review. Two stents were successfully implanted in all cases (Figs 3 and 4).
Fig. 3.
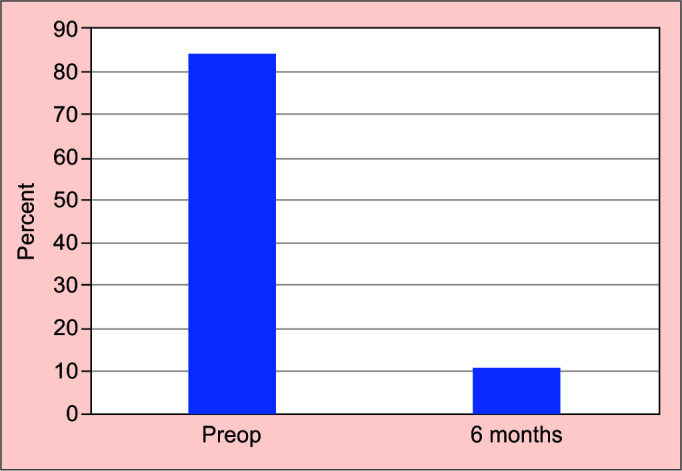
Average pre and postoperative IOP following early iStent implantation
Fig. 4.
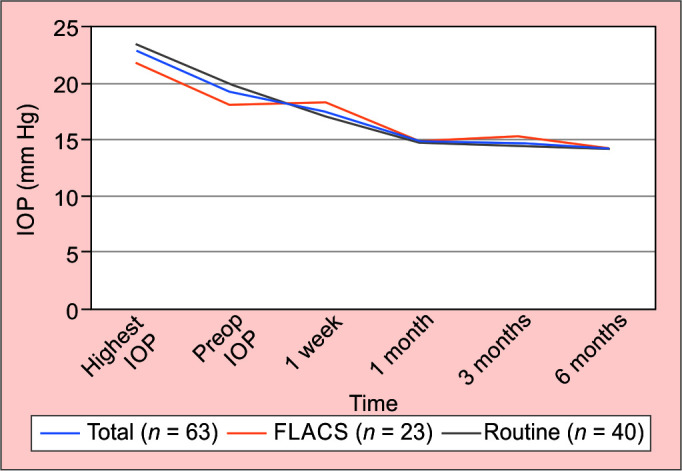
Total medication burden at 6 months following early iStent implantation
Discussion
The iStent (Glaukos, Laguna Hills, California, USA) targets the conventional aqueous outflow pathway via bypass of the trabecular meshwork (TM), the site of increased outflow resistance in primary open-angle glaucoma and OHT, allowing aqueous humor to directly drain from the AC into Schlemm's canal.
The common technique for iStent implantation, as described by Samuelson et al. involves advancing the iStent through a clear temporal corneal incision after standard phacoemulsification and intraocular lens (IOL) implantation.5 Assuming a routine and uncomplicated cataract surgery, this technique is safe, effective, and easy to perform for the non-glaucoma surgeon. This standard approach can have drawbacks: it does not guarantee corneal clarity, which may impact successful iStent implantation, and is dependent on the successful execution of the cataract operation.
Visualization of the AC angle remains the keystone of successful angle surgery, whether performing surgical goniotomy or implanting MIGS devices. Corneal and media clarity is paramount, and by moving the implantation of iStent inject to the commencement of surgery ensures iStent is implanted under optimum optical conditions as baseline corneal clarity is preserved as the impact of surgical manipulation, phacoemulsification, and IOP fluctuation associated with cataract surgery on the cornea have not occurred.
Epstein et al. recently reported on the difference in angle view before and after routine cataract surgery. They found that, on average, surgeons found no difference in their ability to clearly identify angle structures before or after surgery. However, 27.5% of responses indicated the presurgery view was either definitely better or somewhat better, which suggests there may be some cases in which preplacement of stents prior to surgery is advantageous.3
Moreover, media clarity is optimized with the use of a single ocular viscoelastic device. Some surgeons prefer the use of a dispersive OVD at the commencement of surgery to aid in corneal protection and a cohesive device at the end for IOL implantation and ease of removal. Dispersive and cohesive OVDs have different refractive indices, and the mixture of the two types in the AC can lead to visual distortion of the angle and lead to difficulty with device implantation. The use of a single OVD at surgery commencement obviates this issue.
Common postoperative complications related to iStent implantation are minor and include malposition or obstruction of the stent, with a reported incidence of 3 and 4%, respectively, reported by the iStent Study Group.5 Other studies utilizing a conventional postphacoemulsification approach to iStent implantation, have reported variable and higher rates of complications related to stent malposition: such as the 18% of cases with malpositioned stents noted by Fernandez-Barrientos et al.6 on follow-up, and the 35% of cases found by Arriola-Villalobos et al.2 in which only one of the two implanted stents was functional. Complication rates with the iStent inject have been found to be comparable to those from the previous GT 100 model.7 Further large-scale randomized studies are required to investigate the intraoperative factors leading to such complications and to iStent failure. However, visualization of the intraoperative angle anatomy remains a key factor to be considered when performing any MIGS procedure, and in this series, successful implantation occurred in all cases with two presumed functional stents in situ postoperatively. The cohort experienced a significant reduction in both IOP and medications, lending evidence to the efficacy of this technique and suggesting at least non-inferiority to expected IOP outcomes if not slightly greater reduction of medication usage to current published studies. Where failure to successfully reduce IOP occurs, this is likely due to downstream effects from the aqueous collector channels and/or the systemic circulation.
For surgeons aiming to implant two iStents, the improved injector design of the GTS 400 device, with two preloaded stents, reduces the number of surgical steps and thereby increases the chance of a successful outcome, with at least one functional stent implanted if not two. Studies have shown a larger treatment effect, and aqueous outflow facility is achieved with the concurrent implantation of two iStents as compared to one.4
Femtosecond laser-assisted cataract surgery makes many steps in routine phacoemulsification a no-touch machine-driven procedure, making results more predictable and independent of surgeon experience. Pretreatment with FLACS, in particular, offers precision and accuracy in capsulotomy construction as well as phacofragmentation. FLACS pretreatment is an advantage in the shallow AC where maneuvering space is limited and chances of endothelial damage from manual phaco and rhexis runaway are higher.8 In FLACS with the Alcon LenSx® platform, suction is used to couple the eye with the SoftFit™ interface. This process can result in vascular engorgement, which is particularly evident in the trabecular meshwork of those with a pale TM. The vascular engorgement may increase the likelihood of successful implantation due to better recognition of the angle structures, and could help identify aqueous outflow channels, enabling improved targeting for successful iStent placement.
Many surgeons fear that the potential bleeding from iStent implantation at commencement of surgery may hinder the view during subsequent surgical steps, especially manual capsulorrhexis. As noted in this and many other studies, small amounts of blood reflux through the iStent are frequently encountered after implantation and generally indicate the correct placement of the implant into Schlemm's canal.6 Irrigation and AC tamponade with a viscoelastic is employed during iStent implantation to restore visualization of the angle and the AC when the view is lost from hyphema in conventional implantation. By filling the AC with dispersive OVD at the start of an operation, hyphema is limited to the implant site. IOP is maintained due to the AC tamponade and, as a result, in this series, significant bleeding was not encountered, and all capsulotomies were completed without incident. Care must be taken, however, not to dislodge the capsulotomy created by the femtosecond laser when performing AC tamponade in FLACS cases.
Hyphema is a complication seen in the early postoperative period in conventional iStent-cataract surgery and is usually a result of reflux from the iStent, often in the setting of low early postoperative IOP. During cataract surgery, IOP is often at an elevated level, particularly during phacoemulsification, where IOP can reach in excess of 60 mm Hg.9 Early implantation enables the period of time during which surgery is performed to further tamponade the AC with elevated IOP, thereby preventing blood reflux and potentially reducing the likelihood of postoperative hyphema. In this cohort, no postoperative hyphema was encountered. Further studies with a randomized cohort would enable further clarification of this potential benefit.
It is generally accepted that when complications occur, they are often due to issues with the simultaneously performed cataract surgery rather than issues with the iStent itself.7 The iStent has a very favorable safety profile. In the event of a complicated cataract operation, iStent implantation will generally be abandoned. In the early stent placement approach, the concern is obviated as implantation has already been performed, even before capsulotomy has been undertaken.
There is concern about potential lens injury with early stent implantation. The design of the GTS 400 delivery device enables the surgeon to undertake a direct approach to the angle without sharp edges on the implement once the introducing needle has been retracted. Deployment of the iStent occurs in a linear motion, removing the sweeping approach of the previous generation of iStent. This direct-controlled approach should minimize the potential for lens injury. In this cohort, there have been no documented lens injuries. If there is inadequate access to the implant at the start of the procedure or concern about lens injury, then implantation can be delayed until after the cataract surgery is complete. It could be argued that the cohort size in this study was too small to detect lens injury, however, there was no observable injury, even capsule indentation or impression, in a reasonable number of patients. The authors expected at least some evidence of lens impaction in this group, if not a direct lens substance injury. A larger study could lend further certainty in this regard. As the lens was subsequently removed, there is no scope in this study to evaluate whether there is an increased risk of lens opacification after a stand-alone procedure.
Potential disadvantages of an early approach include the associated learning curve and inability to use miotics if that is one's preference. Further, the presence of anterior lens vault from cataract may impede the surgeon's positioning of the iStent inject introducer, although this can usually be addressed by deepening the AC with viscoelastic and was not found to be a problem in this series.
With the advancement and acceptance of MIGS as a therapeutic option for treating glaucoma, it is likely that iStent and other angle technologies will be performed as a stand-alone procedure. The early stent placement combined with cataract surgery approach enables one to attain the experience of implantation in the phakic eye in a low-risk environment.
Conclusion
In summary, iStent is becoming a mainstream treatment for mild to moderate glaucoma, especially when combined with cataract surgery. iStent implantation is a low-risk MIGS procedure that results in a meaningful reduction in IOP and reduced burden of medication. iStent implantation at the commencement of surgery appears to be a safe and effective procedure that does not detract from the expected benefits of the standard postphacoemulsification approach. By adopting an approach where iStents are implanted at the start of combined surgery, one can optimize optical clarity of the angle, through the cornea as well as the AC, especially in cases of dense cataract or potentially complicated surgery, which may lead to a greater number of successful implantations, with the attendant reduction of IOP and medication usage that follows as seen in this cohort. In addition, intra and postoperative bleeding can be minimized due to IOP control with OVD as well as intraoperative phacodynamic. In this series, there was no observed lens injury, and this technique has the advantage of enabling a surgeon to become comfortable with phakic stand-alone procedures in the low-risk environment of planned lens removal. We have observed that this is an effective and safe approach to iStent inject implantation, and the ease and success of implantation on the first attempt is increased once comfortable with the technique in both manual cataract surgery and FLACS.
Clinical Significance
iStent implantation before lens extraction does not detract from the typical results expected from the standard procedure and, in certain circumstances, increases the likelihood of successful stenting of Schlemm's canal, whilst corneal clarity is maximized, especially in complicated cases. Early implantation does not increase the likelihood of lens injury and enables surgeons to become comfortable with the implantation technique in phakic eyes, in a controlled environment, before embarking on stand-alone procedures.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Gallardo MJ, Supnet RA, Giamporcaro JE, et al. Outcomes of combined trabecular micro-bypass and phacoemulsification in a predominantly Hispanic patient population. Clin Ophthalmol. 2016;10:1931–1937. doi: 10.2147/opth.s117403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, et al. Glaukos iStent inject® trabecular micro-bypass implantation associated with cataract surgery in patients with coexisting cataract and open-angle glaucoma or ocular hypertension: a long-term study. J Ophthalmology. 2016:1056573. doi: 10.1155/2016/1056573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epstein RS, Scott AT, Young CEC, et al. Optimum time for angle visualization during ab interno glaucoma surgery: before or after phacoemulsification. J Cataract Refract Surg. 2019;45(5):615–619. doi: 10.1016/j.jcrs.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmology. 2016;10:189–206. doi: 10.2147/OPTH.S80490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuelson TW, Katz LJ, Wells JM, et al. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459–467. doi: 10.1016/j.ophtha.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Barrientos Y, Garcia-Feijoo J, Martinez-de-la-Casa JM, et al. Fluorophotometric study of the effect of the glaukos trabecular microbypass stent on aqueous humor dynamics. Invest Ophthalmol Vis Sci. 2010;51(7):3327–3332. doi: 10.1167/iovs.09-3972. [DOI] [PubMed] [Google Scholar]
- 7.Resende AF, Patel NS, Waisbourd M, et al. iStent® trabecular microbypass stent: an update. J Ophthalmology. 2016:2731856. doi: 10.1155/2016/2731856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal A, Jacob S. Current and effective advantages of femto phacoemulsification. Curr Opin Ophthalmol. 2017;28(1):49–57. doi: 10.1097/ICU.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 9.Khng C, Packer M, Fine IH, et al. Intraocular pressure during phacoemulsification. J Cataract Refract Surg. 2006;32(2):301–308. doi: 10.1016/j.jcrs.2005.08.062. [DOI] [PubMed] [Google Scholar]


