Abstract
Purpose
To report the efficacy of the XEN45 implant in advanced to end-stage glaucoma patients, after a 6 months follow-up.
Methods
Retrospective, noncomparative electronic health record audit of patients who had undergone an XEN45 procedure. The main outcome measures were intraocular pressure (IOP) reduction and the number of antihypertensive medications. Secondary outcome measures were the rates of early postoperative complications. Complete and qualified success; failure and hypotony were defined according to the World Glaucoma Association guidelines (Shaarawy TM et al.). Needling rates and short-term complications were assessed and a subgroup analysis was performed.
Results
A total of 39 eyes with advanced to end stage-glaucoma were included. Twenty eyes (51%) had undergone combined cataract surgery and 19 (49%), the XEN45 procedure alone. Mean IOP decreased from 19.67 ± 7.87 mm Hg to 13.18 ± 6.09 mm Hg; the number of medications decreased from a median use of 4 (IQR 2–5) to 0 (IQR 0–1). Complete success was achieved in 24 (61.5%) of the eyes, qualified success in 10 (25.6%), and failure in five (12.82%). Needling was required in 15 (38.46%) of the eyes at 6 months. Choroidal detachment occurred in eight (20.51%) eyes, numerical hypotony (IOP ≤ 5 mm Hg) at day 1 was noted in seven (17.95%) eyes with a full resolution by 2 weeks.
Conclusion
In this short-term follow-up, we have seen that XEN45 is a viable, effective, and safe procedure utilized in advanced to end-stage glaucoma patients. Treating cases of significant hypotony using AC reformation with sulfur hexafluoride (SF6) is a safe and effective procedure.
How to cite this article
Hindi I, Berkowitz E, Waizer I, et al. Efficacy of the XEN45 Implant in Advanced to End-stage Glaucoma Patients. J Curr Glaucoma Pract 2022;16(2):84-90.
Keywords: Advanced glaucoma, End-stage glaucoma, Hypotony, Needling, XEN45
Introduction
Glaucoma is the leading cause worldwide of irreversible blindness. It is estimated that globally 76 million people were treated for glaucoma during 2020.1 This disease is characterized by a progressive optic neuropathy highly associated with elevated intraocular pressure (IOP). Many risk factors have been identified. Of these, IOP reduction is the only one that can be modified, thereby, preventing further progression of the disease.2,3
Minimally invasive glaucoma surgery (MIGS) is defined as any glaucoma procedure avoiding conjunctival dissection performed via an ab interno incision.4 MIGS are a safe, effective, and simple solution for patients with mild and moderate glaucoma.5 This type of surgery minimizes tissue damage, thus, diminishing the disruption of the normal anatomy and physiology.4 Reduction in the IOP may be similar but is often inferior to traditional filtering surgeries. According to Manasses et al., after MIGS, the IOP reduction should be at least 20%, with a better safety profile and a shorter surgical time.6
The XEN45 implant (Allergan, Irvine, California, USA) is a 6 mm cross-linked porcine-derived collagen tube with a lumen diameter of 45 μm.7 The implant is inserted through a 27-gauge needle preloaded with the implant via an ab interno incision, thus allowing the aqueous fluid to drain into the subconjunctival space. Subconjunctival filtration has the advantage of bypassing physiological barriers. Whereas the use of the subconjunctival space has been the mainstay of glaucoma procedures, it may also induce subconjunctival fibrosis, leading to blockage and requiring subsequent bleb needling and revisions. This may necessitate close follow-up and increased postoperative (PO) management compared with other MIGS procedures.8 The XEN45 implant has been described as a treatment option for mild to moderate glaucoma patients. De Gregorio et al. investigated the XEN45 implant in a prospective nonrandomized control trial and observed a success rate of 80.4% at 12 months.9 Pérez-Torregrosa et al. performed phacoemulsification procedures in conjunction with the XEN45 implant in 30 eyes with mild to moderate glaucoma, reporting a decrease in the IOP of 29.34% at 12 months.10 Tan et al. reported a complete success of 87.0% and qualified success of 92.0%.8
Due to subconjunctival scarring, a large number of patients require needling. As well, early hypotony has been shown to differ between studies, ranging from 22.1–46.2%.11–16 There is scarce evidence as to the efficacy of the XEN45 implant in advanced to end-stage glaucoma patients. According to the World Glaucoma Association's Guidelines on Design and Reporting of Glaucoma Surgical Trials, the target pressure for advanced-stage glaucoma can be defined in two ways: IOP ≤ 15mm Hg and ≥ 40% IOP reduction, or IOP ≤ 12 mm Hg.17 Laborda-Guirao et al's recent study of patients afflicted with advanced primary open-angle glaucoma (POAG), defined success as an IOP reduction of at least 20% and an IOP value ≤ 18 mm Hg without treatment (complete) or treatment with hypotensive medication (qualified). The authors reported a mean IOP reduction of 27.4% (23.3–31.5%), complete success of 36.3% and 28.7% as qualified. No differences in the success rate of combined and XEN45 standalone procedures were observed.18 It is known that XEN45 has a good safety and efficacy profile in mild to moderate stages, however, there are limited data regarding its efficacy and safety in advanced to end-stage glaucoma patients. Our aim was to report our clinical experience with the efficacy and safety of a XEN45 implant in patients with advanced to end-stage glaucoma.
Materials and Methods
This is a retrospective, consecutive, noncomparative interventional study of advanced glaucoma patients who underwent standalone XEN45 implantation or implantation in combination with cataract surgery from June 2018 until December 2019. This single-center study included 39 eyes of 37 patients. Inclusion criteria included glaucoma patients after XEN45 implantation, afflicted with advanced-stage disease. Advanced glaucoma was defined as any of the following: MD greater than −12 dB. More than 50% of the points (37) are depressed below the 5% level or more than 20 points are depressed below the 1% level on the pattern deviation. At least one point in the central 5 degrees has a sensitivity of 0 dB. Points within the central 5 degrees with sensitivity <15 dB in both hemifields),as defined by Hodapp, Parrish, and Anderson.19 Exclusion criteria included loss of follow-up in one-time point (1 week, 1 month, 3 months) until the end of the study (6 months postsurgery). Patients underwent a complete preoperative examination encompassing visual acuity (measured by the Snellen chart), a slit-lamp examination, IOP (measured by the Goldmann applanation tonometer), and the DRI OCT Triton (DRI Optical Coherence Tomography Triton, Topcon, Canada) measuring the retinal nerve fiber layer (RNFL), ganglion cell plus inner plexiform layer thickness (GCL+), ganglion cell complex thickness (GCL++), and macular. The Humphrey visual field (VF) measured (Carl Zeiss Meditec Dublin, CA) 24.2 and 10.2. Informed consent was obtained from all patients after an explanation of the surgical procedure and potential associated risks. This study adhered to the tenets of the Declaration of Helsinki and was approved by the center's committee. Using the World Glaucoma Association guidelines (2009) complete success was defined as ≤15 mm Hg IOP and ≥40% IOP reduction, without additional glaucoma medications. Qualified success was defined as an IOP reduction of ≥40% and an IOP ≤ 15 mm Hg with medication. Failure was defined as an IOP level measured above the upper limit ≥15 mm Hg) or below the lower limit (≤6 mm Hg) during two consecutive visits. Numerical hypotony was defined as IOP ≤ 5 mm Hg without clinical signs of hypotony and clinical hypotony was defined as eyes with clinical signs of hypotony irrespective of IOP.18
All operations were performed by a single glaucoma specialist (BLINDED). A standard dose of 0.2 mg/mL of mitomycin-C (MMC) combined with 0.2 cc of air was injected into the subconjunctival space in order to dissect the conjunctiva from the tenon at 12 o'clock, 6–8 mm behind the surgical limbus. In the cases of combined surgery, cataract extraction by phacoemulsification, and intraocular lens (IOL) implantation were performed before the XEN45 implantation procedure. A corneal incision was made in the inferotemporal area. Miochol-E (Bausch and Lomb, Surrey, UK) was injected to constrict the pupil and HanitaVisco 1.8% (Hanita Lenses Investments, Hanita, Israel) ophthalmic viscosurgical device (OVD) to deepen the AC. Subsequently, the XEN45 was implanted ab interno using a pre-loaded injector, superior to the trabecular meshwork via a scleral tunnel into the subconjunctival space. The ideal placement was 3 mm of the exposed implant into the subconjunctival space, 1 mm into the AC, and 2 mm tunneled through the sclera. A mirrored gonioscopic lens was used to verify the correct implant placement. It was critical for the surgeon to ensure that the implant was inserted correctly, with subsequent bleb formation noted at the end of the surgery. Upon completion of the procedure, HanitaVisco 1.8% OVD was left in the AC. All patients received intracameral antibiotics.
All topical and systemic glaucoma medications were ceased on the day of the surgery. Patients were treated with a topical fluoroquinolone for a week and prednisolone 1%, five times a day for 2 months before tapering down (depending on the level of inflammation and fibrosis). All patients were examined on the first PO day, 1 week PO, 1 month PO, 3 months PO, and 6 months PO. At 6 months, evaluations with VF and OCT were performed.
In the event of a shallow anterior chamber, patients were also treated with cycloplegic drops. Medical treatment or further intervention was offered as deemed necessary by the treating physician. When AC reformation was required, it was performed by filling at least a third of the AC with a bubble of sulfur hexafluoride (SF6) diluted to 50%. All needling procedures were performed during the first 3 months of follow-up. After the needling procedure, the patients were treated with topical fluoroquinolone for a week and prednisolone 1%, five times a day. The primary outcome measures were IOP reduction and the number of antihypertensive medications taken at each time point during the PO follow-up. Secondary outcome measures were PO complications, VF 24.2 mean deviation (MD), and RNFL thickness.
Statistics
All continuous parameters are reported as mean and standard deviation. Noncontinuous parameters are reported as the median and interquartile range (IQR). An unpaired t-test compared continuous data and the χ2 test compared nonparametric data.
Results
A total of 39 eyes of 37 patients with advanced to end-stage glaucoma participated in the study. Demographic and baseline characteristics are presented in Table 1. The cohort was stratified into subgroups based on their basic characteristics (glaucoma and surgery type) for the assessment of XEN45 efficacy. Preoperative visual acuity was 0.48 ± 0.36 LogMAR and 0.37 ± 0.38 LogMAR at the 6-month follow-up. Baseline MD (VF 24.2) was 19.37 ± 7.54 dB and RNFL total thickness was 57.43 ± 17.31 μm. Upon completion of the study, the MD and RNFL total thicknesses were −17.57 ± 11.01 dB and 56.79 ± 13.83 μm, respectively. The mean baseline IOP was 19.67 ± 7.87 mm Hg. At the 6-month follow-up, the IOP dropped to 13.18 ± 6.09 mm Hg which was statistically significant (p < 0.0001) (Fig. 1). At each time point during the follow-up, the IOP decreased (Fig. 2). No statistically significant differences were found in any of the measured IOP time points between the XEN45 alone vs the combined procedure.
Table 1.
Demographic and medical characteristics
| Total: 39 eyes of 37 patients | |
|---|---|
| Age (years) | |
| Mean ± SD | 69.4 ± 10.8 |
| Range | 43–92 |
| Sex, n (%) | |
| Female | 8 (22.0) |
| Male | 29 (78.0) |
| Study eye, n (%) | |
| Right | 21 (53.9) |
| Left | 18 (46.2) |
| Glaucoma type, n (%) | |
| Pseudoexfoliative | 22 (56.4) |
| POAG | 11 (28.2) |
| Pigmentary | 2 (5.1) |
| SIG | 2 (5.1) |
| NVG | 1 (2.6) |
| NTG | 1 (2.6) |
| Previous procedures, n (%) | |
| SLT | 10 (25.6) |
| Express | 4 (10.3) |
| Trabeculectomy | 3 (7.7) |
| Micropulse | 1 (2.6) |
| IOP at baseline | |
| Mean ± SD | 19.7 ± 7.9 |
| Range | 13–50 |
| VF 24.2 MD | |
| Mean ± SD | -19.4 ± 7.5 |
| Range | 7.2–32.6 |
| RNFL total thickness | |
| Mean ± SD | 57.4 ± 17.3 |
| Range | 37–79 |
| Medications at baseline | |
| Mean ± SD | 4.0 ± 1.0 |
| None, n (%) | 0 (0.0) |
| One, n (%) | 0 (0.0) |
| Two, n (%) | 2 (5.1) |
| Three, n (%) | 8 (20.5) |
| Four, n (%) | 16 (41.0) |
| Five or more, n (%) | 13 (33.3) |
| Procedure, n (%) | |
| XEN45 stent and cataract surgery | 20 (51.3) |
| XEN45 stent as standalone | 19 (48.7) |
| Phakic, n (% of 19) | 13 (68.4) |
SD, standard deviation; POAG, primary open-angle glaucoma; SIG, Steroid-induced glaucoma; NVG, neovascular glaucoma; NTG, normal tension glaucoma; SLT, selective laser trabeculotomy; IOP, intraocular pressure; VF, visual field; MD, mean deviation; RNFL, retinal nerve fiber layer
Fig. 1.
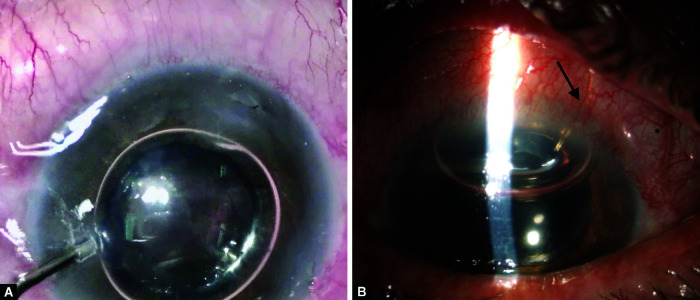
A patient with over-filtration, chemosis 360 degrees, and shallow anterior chamber. The pupil is pharmacologically dilated
Figs 2A and B.
(A) Demonstrates an intraoperative injection of SF6 into the anterior chamber (B) Postoperative day 2 of the same patient, showing deepening of the anterior chamber, arrow marks XEN45, asterisk the subconjunctival gas
At baseline, 37 eyes (95%, n = 39) were treated with at least three antihypertensive medications, median use of 4 (IQR 2–5) antihypertensive medications. After the 6-month follow-up, the median was 0 (IQR 0–1). Similarly, this drop in the number of medications was statistically significant (p < 0.0001) with an IOP reduction of 32.94% and an 89.78% reduction in the use of medications (Fig. 3). At the 6-month follow-up, complete success was achieved in 24 (61.5%) of the eyes, qualified success in 10 (25.6%), and failure in five (12.82%). Four out of the five eyes that failed, underwent a combined procedure after 6 months (80%). Multivariate analysis did not indicate any potential risk factors including surgery type that were associated with surgical success or failure. Upon completion of the study, in 33 eyes (84.62%) the IOP was <15 mm Hg and in 20 (51.28%) <12 mm Hg (Table 2).
Fig. 3.
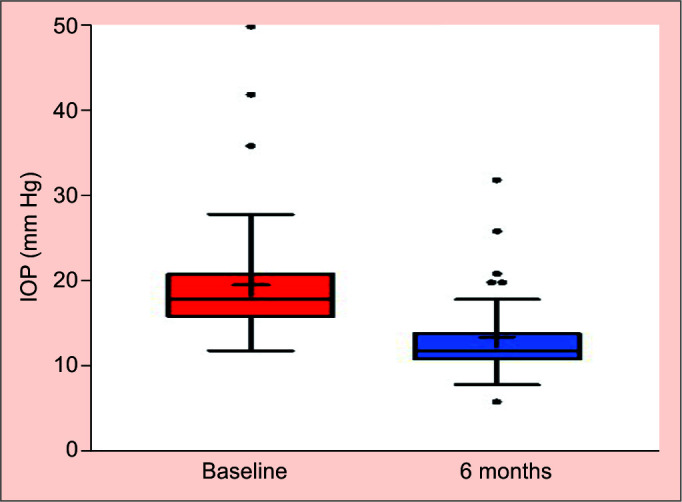
IOP at baseline and 6 months postsurgery (Tukey test)
Table 2.
IOP rates 6 months after the surgery
| Patients with IOP ≤15 mm Hg at 6 months follow-up, n (%) | |||
|---|---|---|---|
| With medications | Without medications | Total | |
| Total | 8/33 (24.2) | 25/33 (75.8) | 33/39 (84.6) |
| One | 7 | − | − |
| Two | 1 | − | − |
| Patients with IOP ≤12 mm Hg at 6 months follow-up, n (%) | |||
| With medications | Without medications | Total | |
| Total | 4/20 (20.0) | 16/20 (80.0) | 20/39 (51.3) |
| One | 3 | − | − |
| Two | 1 | − | − |
IOP, intraocular pressure
Fifteen (38.46%) eyes developed subconjunctival fibrosis with signs of decreasing bleb function, thus, requiring needling. Needling was performed at the slit lamp using 5-Fluorouracil (5-FU); 10 eyes (66.67%) required a single needling procedure, and five eyes (33.33%) underwent two procedures. Improvement of the IOP and bleb function was observed in 86.67% of the eyes at 14 days postneedling. There was no difference in the needling rates in eyes that had undergone only XEN45 vs the combined procedure (Figs 4 and 5). Multivariate analysis found no correlation between the needling rate and patients’ demographic characteristics.
Fig. 4.
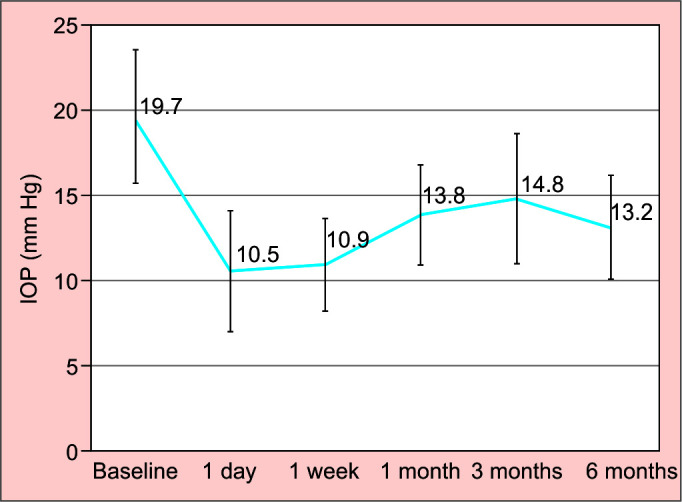
IOP measurement during the study (line graph)
Fig. 5.
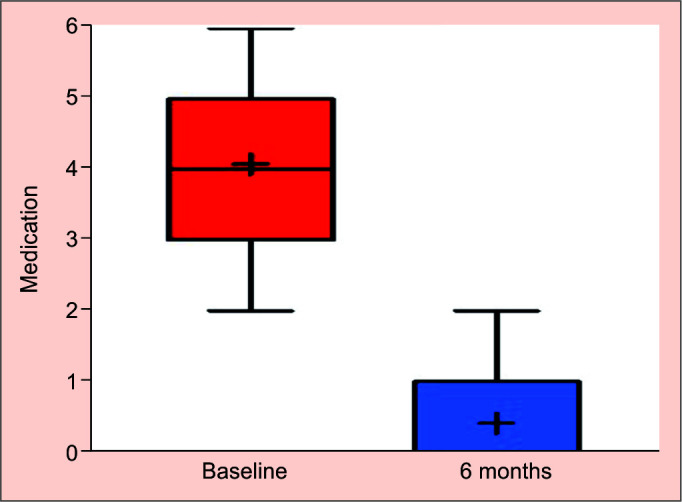
Medication at baseline and 6 months postsurgery (Tukey test)
Adverse Events
During the follow-up period, no cases of endophthalmitis or significant vision loss were observed (Fig. 1). Seventeen (43.59%) eyes presented with numerical hypotony and eight eyes (20.51%) with clinical hypotony and choroidal detachment during the first week (mean IOP of 4.3). Three out of eight patients were treated conservatively with prednisolone acetate 1% and atropine sulfate 1% eye drops; five were treated surgically with the reformation of the AC using SF6 50% (Figs 2A and B). One out of the five had undergone an AC reformation with HanitaVisco 1.8% and a subsequent SF6 reformation. There was one case of XEN45 exchange due to iris obstruction that was considered a failure; other adverse events can be seen in Table 3. PO corneal edema in patients who had undergone a combined procedure spontaneously resolved.
Table 3.
Adverse events and hypotony rate
| Patients, n (%) | ||
|---|---|---|
| Numerical hypotonya | 17 | (43.6) |
| Choroidal detachmentb | 8 | (20.5) |
| IOP spikesc | 3 | (7.7) |
| Malignant glaucoma | 1 | (2.6) |
| High IOP after OVD | 1 | (2.6) |
| Hypotony maculopathy | 1 | (2.6) |
| Subluxated IOL, TASS, XEN45 exchange | 1 | (2.6) |
| Hyphema | 1 | (2.6) |
| Flat ACd | 1 | (2.6) |
| Aqueous misdirection | 1 | (2.6) |
| Cystoid macular edema | 1 | (2.6) |
| Endophthalmitis | 0 | (0) |
| Vision loss | 0 | (0) |
| Disk swelling | 0 | (0) |
a Transient numerical hypotony (IOP < 6 mm Hg), during the first week after surgery. Mean IOP was 4.3 mm Hg;
b 5 eyes (62.5%) surgical reformation with SF6, 1 eye (12.5%) surgical reformation with OVD, 3 eyes (37.5%) medical treatment;
c Pressure spikes above 30 mm Hg during 3 months follow-up;
d Spontaneous resolution; IOP, intraocular pressure; OVD, ophthalmic viscosurgical device; TASS, toxic anterior segment syndrome; AC, anterior chamber
Discussion
We present a retrospective study (historical cohort), utilizing short-term data, reporting on advanced to end-stage glaucoma patients who had undergone an alkylating agent (MMC) augmented XEN45 implantation in a university-affiliated hospital in Israel. Baseline characteristics reveal a cohort mainly comprised of patients with pseudoexfoliative glaucoma (PXF) and POAG of Caucasian ethnicity, matching the ethnic mix of the area. Overall, our data showed a 32.94% reduction in mean IOP at the 6-month time point. Since our patients were afflicted with advanced to end-stage glaucoma, the relevant success criteria for this stage were employed: 61.50% of the eyes demonstrated complete success and 25.61% demonstrated qualified success. Over half (51.28%) of our eyes achieved an IOP of equal to or less than 12 mm Hg. No significant difference was found in the IOP between patients who had undergone only XEN45 (12.60 mm Hg) and patients who had undergone a combined procedure (13.26 mm Hg) p = 0.6824.
Several studies have reported on the short-term efficacy of XEN45, albeit, there is insufficient information with regard to advanced glaucoma patients. A large study performed by Widder et al.20 showed a reduction in IOP from a mean of 24.3–16.8 mm Hg (31% reduction in IOP); Heidinger et al.11 reported a 22.7% reduction in IOP and Smith et al.21 a reduction of 33% at 12 months. Sheybani et al.22 described a 36.4% reduction and Tan et al.8 a 41.8% reduction, as well as a significant reduction in glaucoma medications. Our patients achieved a mean IOP reduction of 32.94% at the completion of the follow-up. In the tube versus trabeculectomy (TVT) study23 which included eyes with advanced glaucoma, the mean IOP of the tube arm dropped from 25.1 ± 5.3 mm Hg to 13.5 ± 4.2 mm Hg (46.2% reduction) and in the trabeculectomy, arm dropped from 25.6 ± 5.3 mm Hg to 12.8 ± 5.9 mm Hg (50.0% reduction) at 6 months follow-up. A similar drop was achieved in other studies comparing the Ahmed valve to a trabeculectomy.24 Whereas, in our study, the drop in IOP was lower, from 19.67 ± 7.87 mm Hg to 13.18 ± 6.09 mm Hg at 6 months (32.94% of reduction). While trabeculectomy and tube shunts may offer a more significant reduction in IOP, XEN45 offers a less invasive, shorter procedure with a favorable safety profile.
Although it was not originally planned in the study protocol, we found that 56.41% were afflicted with PXF glaucoma since we have many eastern European patients. Little is known about the efficacy and safety of XEN45 in patients with advanced to end-stage PXF glaucoma. A recent study by Dar et al. found that implantation succeeded in these patients, however, the decrease in IOP was lower compared to non-PXF glaucoma patients.25 Their inclusion criteria for severity was MD <-6dB, thus, not only advanced patients were included, in our study, we included only patients with MD <-12dB.
Numerical hypotony was observed in 43.59% during the first PO week compared to Kalina et al.26 who observed 21.3% and 20.5% reported by Tan et al.8 On our first PO day, numerical hypotony was seen in 17.95% of patients. Our practice is to leave the AC filled with OVD at the completion of surgery. We used an OVD with an osmolarity of 200–400 mOsM and viscosity of 30,000–60,000 cps. PO pressure spikes and high IOP remained low even when the entire AC was filled with OVD. Our percentage of PO hypotony is similar to the values described in the literature. In cases where AC reformation was required, our preferred method was using SF6% 50%. No additional reformation after using this method was required in our cohort.
Needling was required by 38.46% of patients at the 3 months follow-up and was considered part of the PO management and not an adverse event.26 Hengerer et al.27reported a rate of 27.7%, Galal et al.,12 30.7%, Grover et al.,28 32.3%, and Mansouri et al.,13 37%. Our rate was lower than reported by Fea et al.,29 50%, Sheybani et al.,7 47%, and Schlenker et al.,30 43.2%.
No significant adverse events were seen in our cohort. One potential benefit of a minimally invasive procedure is the favorable visual outcome as well as a rapid improvement in vision. Our results demonstrated decreased visual acuity during the immediate PO period in the combined group, secondary to corneal edema, which improved at the 1-month time point and remained constant throughout the follow-up period.
Limitations
The study has several limitations. It is a short-term retrospective study with relatively small cohort size. Since the patients are mainly advanced to end-stage glaucoma patients a relatively small number of patients can be expected. Because no needling took place after 3 months, it is uncertain whether this procedure will be beneficial at later stages. It is hard to compare pre and post-operative pressures without an effective “washout” period in order to examine the “real” IOP change, but since these patients were with advanced glaucoma, medication washout would have increased their risk of additional loss of vision.
Conclusion
In this short-term study, we conclude that XEN45 is a viable, effective, and safe procedure after 6 months of follow-up in patients with advanced to end-stage glaucoma, as a standalone procedure or combined with cataract surgery. It is a favorable option due to its minimally invasive nature and safety profile. In select cases, it should be considered as one of the treatment options for advanced glaucoma patients. Patients must be advised regarding the failure rate as well and the possible need for bleb revisions and PO topical treatment.
No difference was found between treatment with XEN45 alone vs a combined procedure. Studies have shown that a XEN45 implant works via subconjunctival drainage, therefore, offering the possibility of achieving IOPs similar to trabeculectomy, but with fewer side effects. Our study confirmed that XEN45 is a viable, effective, and safe procedure after 6 months of follow-up in patients with advanced to end-stage glaucoma. It is a beneficial treatment of PXF glaucoma, and, therefore, should be considered as one of the treatment options for advanced glaucoma. However, further investigations with a larger number of patients is essential. Leaving OVD in the AC does not reduce the rates of early hypotony. Treating clinical hypotony with AC reformation and SF6 is a safe and effective procedure.
Acknowledgments
The authors thank Mrs. Phyllis Curchack Kornspan for her editorial services.
Footnotes
Source of support: Nil
Conflict of interest: None
Orcid
Isaac Hindi https://orcid.org/0000-0002-4843-695X
Eran Berkowitz https://orcid.org/0000-0001-6817-7513
References
- 1.Tham Y-C, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Kidd MN, O'Connor M. Progression of field loss after trabeculectomy: a five-year follow-up. Br J Ophthalmol. 1985;69(11):827–831. doi: 10.1136/bjo.69.11.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 4.Saheb H, Ahmed IIK. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23(2):96–104. doi: 10.1097/ICU.0b013e32834ff1e7. [DOI] [PubMed] [Google Scholar]
- 5.Ansari E. An update on implants for minimally invasive glaucoma surgery (MIGS). Ophthalmol Ther. 2017;6(2):233–241. doi: 10.1007/s40123-017-0098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manasses DT, Au L. The new era of glaucoma micro-stent surgery. Ophthalmol Ther. 2016;5(2):135–146. doi: 10.1007/s40123-016-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheybani A, Reitsamer H, Ahmed IIK. Fluid dynamics of a novel micro-fistula implant for the surgical treatment of glaucoma. Invest Ophthalmol Vis Sci. 2015;56(8):4789–4795. doi: 10.1167/iovs.15-16625. [DOI] [PubMed] [Google Scholar]
- 8.Tan SZ, Walkden A, Au L. One-year result of XEN45 implant for glaucoma: efficacy, safety, and postoperative management. Eye (Lond) 2018;32(2):324–332. doi: 10.1038/eye.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregorio AD, Pedrotti E, Russo L, et al. Minimally invasive combined glaucoma and cataract surgery: clinical results of the smallest ab interno gel stent. Int Ophthalmol. 2018;38(3):1129–1134. doi: 10.1007/s10792-017-0571-x. [DOI] [PubMed] [Google Scholar]
- 10.Pérez-Torregrosa VT, Olate-Pérez Á, Cerdà-Ibáñez M, et al. Combined phacoemulsification and XEN45 surgery from a temporal approach and 2 incisions. Arch Soc Esp Oftalmol. 2016;91(9):415–421. doi: 10.1016/j.oftal.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Heidinger A, Schwab C, Lindner E, et al. A retrospective study of 199 xen45 stent implantations from 2014 to 2016. J Glaucoma. 2019;28(1):75–79. doi: 10.1097/IJG.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 12.Galal A, Bilgic A, Eltanamly R, et al. XEN glaucoma implant with Mitomycin C 1-year follow-up: result and complications. J Ophthalmol. 2017;2017:5457246. doi: 10.1155/2017/5457246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mansouri K, Guidotti J, Rao HL, et al. Prospective evaluation of standalone XEN gel implant and combined phacoemulsification-xen gel implant surgery: 1-year results. J Glaucoma. 2018;27(2):140–147. doi: 10.1097/IJG.0000000000000858. [DOI] [PubMed] [Google Scholar]
- 14.Karimi A, Lindfield D, Turnbull A, et al. A multi-centre interventional case series of 259 ab-interno Xen gel implants for glaucoma, with and without combined cataract surgery. Eye (Lond) 2019;33(3):469–477. doi: 10.1038/s41433-018-0243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reitsamer H, Sng C, Vera V, et al. Two-year results of a multicenter study of the ab interno gelatin implant in medically uncontrolled primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2019;257(5):983–996. doi: 10.1007/s00417-019-04251-z. [DOI] [PubMed] [Google Scholar]
- 16.Fea AM, Bron AM, Economou MA, et al. European study of the efficacy of a cross-linked gel stent for the treatment of glaucoma. J Cataract Refract Surg. 2020;46(3):441–450. doi: 10.1097/j.jcrs.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 17.Shaarawy TM, Sherwood MB, Grehn F. Guidelines on design and reporting of glaucoma surgical trials. World Glaucoma Association. 2009:15–24. [Google Scholar]
- 18.Laborda-Guirao T, Cubero-Parra JM, Hidalgo-Torres A. Efficacy and safety of XEN 45 gel stent alone or in combination with phacoemulsification in advanced open angle glaucoma patients: 1-year retrospective study. Int J Ophthalmol. 2020;13:1250–1256. doi: 10.18240/ijo.2020.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodapp E, Parrish RK, II, Anderson DR. St. Louis: CV Mosby; 1993. Clinical Decisions in Glaucoma. [Google Scholar]
- 20.Widder RA, Dietlein TS, Dinslage S, et al. The XEN45 gel stent as a minimally invasive procedure in glaucoma surgery: success rates, risk profile, and rates of re-surgery after 261 surgeries. Graefes Arch Clin Exp Ophthalmol. 2018;256(4):765–771. doi: 10.1007/s00417-018-3899-7. [DOI] [PubMed] [Google Scholar]
- 21.Smith M, Charles R, Abdel-Hay A, et al. 1-year outcomes of the Xen45 glaucoma implant. Eye (Lond) 2019;33(5):761–766. doi: 10.1038/s41433-018-0310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheybani A, Dick HB, Ahmed IIK. Early clinical results of a novel ab interno gel stent for the surgical treatment of open-angle glaucoma. J Glaucoma. 2016;25(7):e691–e696. doi: 10.1097/IJG.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 23.Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the tube versus trabeculectomy study after one year of follow-up. Am J Ophthalmol. 2007;143(1):9–22. doi: 10.1016/j.ajo.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 24.Wilson MR, Mendis U, Smith SD, et al. Ahmed glaucoma valve implant vs trabeculectomy in the surgical treatment of glaucoma: a randomized clinical trial. Am J Ophthalmol. 2000;130(3):267–273. doi: 10.1016/s0002-9394(00)00473-6. [DOI] [PubMed] [Google Scholar]
- 25.Dar N, Sharon T, Hecht I, et al. Efficacy and safety of the ab interno gelatin stent in severe pseudoexfoliation glaucoma compared to non-pseudoexfoliation glaucoma at 6 months. Eur J Ophthalmol. 2020;30(5):1028–1033. doi: 10.1177/1120672119848277. [DOI] [PubMed] [Google Scholar]
- 26.Kalina AG, Kalina PH, Brown MM. XEN® gel stent in medically refractory open-angle glaucoma: results and observations after one year of use in the United States. Ophthalmol Ther. 2019;8(3):435–446. doi: 10.1007/s40123-019-0192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hengerer FH, Kohnen T, Mueller M, et al. Ab interno gel implant for the treatment of glaucoma patients with or without prior glaucoma surgery: 1-year results. J Glaucoma. 2017;26(12):1130–1136. doi: 10.1097/IJG.0000000000000803. [DOI] [PubMed] [Google Scholar]
- 28.Grover DS, Flynn WJ, Bashford KP, et al. Performance and safety of a new ab interno gelatin stent in refractory glaucoma at 12 months. Am J Ophthalmol. 2017;183:25–36. doi: 10.1016/j.ajo.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 29.Fea AM, Spinetta R, Cannizzo PML, et al. Evaluation of bleb morphology and reduction in IOP and glaucoma medication following implantation of a novel gel stent. J Ophthalmol. 2017;2017:9364910. doi: 10.1155/2017/9364910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlenker MB, Gulamhusein H, Conrad-Hengerer I, et al. Efficacy, safety, and risk factors for failure of standalone ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmology. 2017;124(11):1579–1588. doi: 10.1016/j.ophtha.2017.05.004. [DOI] [PubMed] [Google Scholar]