Abstract
Animals with altered freerunning periods are valuable in understanding properties of the circadian clock. Understanding the relationship between endogenous clock properties, entrainment, and influence of light in terms of parametric and non-parametric models can help us better understand how different populations adapt to external light cycles. Many clinical populations often show significant changes in circadian properties that in turn cause sleep and circadian problems, possibly exacerbating their underlying clinical condition. BTBR T+Itpr3tf/J (BTBR) mice are a model commonly used for the study of autism spectrum disorders (ASD). Adults and adolescents with ASD frequently exhibit profound sleep and circadian disruptions, including increased latency to sleep, insomnia, advanced and delayed sleep phase disorders, and sleep fragmentation. Here, we investigated the circadian phenotype of BTBR mice in freerunning and light-entrained conditions and found that this strain of mice showed noticeably short freerunning periods (~22.75 h). In addition, when compared to C57BL/6J controls, BTBR mice also showed higher levels of activity even though this activity was compressed into a shorter active phase. Phase delays and phase advances to light were significantly larger in BTBR mice. Despite the short freerunning period, BTBR mice exhibited normal entrainment in light-dark cycles and accelerated entrainment to both advanced and delayed light cycles. Their ability to entrain to skeleton photoperiods of 1 min suggests that this entrainment cannot be attributed to masking. Period differences were also correlated with differences in the number of vasoactive intestinal polypeptide–expressing cells in the suprachiasmatic nucleus (SCN). Overall, the BTBR model, with their unique freerunning and entrainment properties, makes an interesting model to understand the underlying circadian clock.
Keywords: autism spectrum disorders, entrainment, freerunning period, phase shift, mouse, masking, VIP, suprachiasmatic nucleus
In mammals, the suprachiasmatic nucleus (SCN) regulates daily rhythms in physiology and behavior (Antle and Silver, 2005). These rhythms persist in environments free of time cues and exhibit freerunning periods (FRPs) slightly different from the 24 h geophysical day-night cycle (Pittendrigh and Daan, 1976a). FRPs are normally distributed around a species-typical period and exhibit remarkable stability and precision in constant conditions (Pittendrigh and Daan, 1976a). These circadian rhythms arise from autoregulatory transcription-translation feedback loops (TTFL) of core clock gene expression (Mohawk and Takahashi, 2011). Alterations in various components of the TTFL can accelerate or decelerate the FRP. For instance, the clockΔ19 mutation alters the positive limb of the TTFL, leading to slower FRPs that are eventually lost in the homozygous mutant mice (Vitaterna et al., 1994). Conversely, mutation of casein kinase (CK) 1ε in hamsters leads to enhancement of the negative limb of the TTFL, shortening the FRP to ~22 h in heterozygous hamsters and ~20 h in homozygous hamsters. Some heritable sleep disorders in humans are linked to changes in the TTFL (Piggins, 2002), including mutations of the Per2 and CK1δ genes (Toh et al., 2001; Xu et al., 2005). Animals with heritable alterations in FRP, such as the duper mutation in hamsters (Monecke et al., 2011; Bittman, 2012, 2014; Manoogian et al., 2015), can also help uncover and explore factors underlying regulation of circadian rhythms. As many psychiatric conditions include alterations in sleep and circadian rhythms, the use of animal models for the study of these conditions may help uncover the underlying mechanisms leading to these changes.
While the FRP of the circadian clock is remarkably stable and precise, it is also malleable, as it can be influenced by a variety of situations. While humans maintain a stable FRP over a range of ages (Czeisler et al., 1999; Kendall et al., 2001; Duffy et al., 2015), advanced age may affect FRP in rodents, with older male hamsters and rats exhibiting a shortening of FRP (females not examined, Witting et al., 1994; Gannon, 2015) and older male mice exhibiting a lengthening of FRP (females not examined, Valentinuzzi et al., 1997). Changes in endocrine status also alter FRP. When male mice are castrated, their FRP lengthens, an effect that is reversed with testosterone treatment (Daan et al., 1975; Karatsoreos et al., 2007). While ovariectomy lengthens FRP, and estrogen treatment shortens FRP in female rats and hamsters (Morin et al., 1977; Albers, 1981), the period of female mice is unaffected by such an intervention (Iwahana et al., 2008). FRP is influenced by activity levels, with female rats and male hamsters exhibiting shorter FRPs when housed with a running wheel, with the FRP length inversely correlated with the level of activity (Yamada et al., 1988, 1990; Edgar et al., 1991; Mrosovsky, 1999). Exogenous stimuli can also influence FRP. Photic (Pittendrigh and Daan, 1976a) and non-photic zeitgebers (Weisgerber et al., 1997) can alter FRP. Housing under constant light lengthens the FRP of nocturnal mammals and shortens the FRP of diurnal birds (Aschoff, 1960). Exposure to non-24 h T-cycles alters FRP during subsequent constant darkness, with the FRP observed being intermediate between the species-typical FRP and the previously imposed T-cycle (Pittendrigh, 1960; Pittendrigh and Daan, 1976a; Boulos et al., 2002), possibly due to synaptic reorganization within the SCN (Foster et al., 2019). Different photoperiods can also influence FRP, with summer-like and winter-like photoperiods altering FRPs in subsequent constant darkness (Pittendrigh, 1964; Pittendrigh and Daan, 1976a). As many of these studies only examined males or did not specify sex, it is not clear if the described phenomena occur in the same fashion in females.
Entrainment to an imposed zeitgeber cycle requires both phase and period control. The non-parametric model of entrainment posits that the organism will achieve a phase angle of entrainment such that when they expose their PRC to the zeitgeber cycle, the net phase advances and delays that result will adjust the FRP such that τ = T (Pittendrigh and Daan, 1976b; Daan, 2000). The alternative parametric model championed by Jürgen Aschoff suggests that entrainment is achieved through continuous tonic effects of light during the day that alters the FRP by accelerating it during some parts of the day and by decelerating it during other parts of the day (Daan, 1977, 2000). While the non-parametric model is attractive due to its simplicity, heuristic value, and predictive power, some entrainment phenomena are better explained by the parametric model. For instance, animals can entrain to T-cycles that use natural dawn and dusk transitions even when the period of the T-cycle is well outside the limits of entrainment predicted based on the photic PRC (Boulos et al., 2002).
Period mutants (animals with mutations that affect the FRP) have been valuable in understanding these interactions and understanding how properties of the clock interact with each other and ultimately determine an organism’s circadian adaptation to the environment around it. In some cases, these mutations target core clock genes that are integral components of the TTFL (Ralph and Menaker, 1988; van der Horst et al., 1999; Cermakian et al., 2001; Lee et al., 2022) while in other cases the mutation affects neuropeptide signaling that likely couples the cell-autonomous oscillators (Harmar et al., 2002; Colwell et al., 2003). The circadian behavior in these animals can ultimately inform us of the reasons for a lack of alignment with external cycles or an inability to respond to time cues that results from altered light schedules, aging, or disease.
One clinical population that exhibits sleep and circadian problems are people with autism spectrum disorders (ASD, Lorsung et al., 2021), who often exhibit a long sleep latency (Richdale and Prior, 1995) characteristic of delayed sleep phase syndrome (Baker and Richdale, 2017). Animal models for the study of ASD may help uncover circadian abnormalities underlying these sleep and circadian disturbances. The inbred BTBR T+Itpr3tf/J (BTBR) mouse strain exhibits behaviors that have made it a common animal model for the study of ASD (Nadler et al., 2006; Bolivar et al., 2007; Moy et al., 2007; Meyza et al., 2013; Meyza and Blanchard, 2017). In particular, BTBR mice exhibit extremely low levels of social behavior (Bolivar et al., 2007) and have impaired reversal learning in a spatial navigation task (Moy et al., 2007). Here, we examine the freerunning and photic circadian responses of the BTBR mouse, highlighting an extremely short FRP of about 22.75 h. Despite such an extreme FRP, these mice exhibit normal entrainment to a variety of lighting conditions.
Materials Methods
Animals
In total, 62 adult BTBRT+Itpr3tf/J (BTBR; breeding and colony maintained at University of Calgary Health Sciences Animal Care Centre; originally acquired from Jackson Laboratories, USA) and 33 C57BL/6J (C57, University of Calgary Life and Environmental Science Animal Resource Centre) mice were used for these series of experiments. Due to resource and colony constraints, we were only able to examine both males and females in constant darkness, light-pulse, and neuroanatomy experiments, all other experiments examined only male mice. Animals were at least 6 weeks old and ~20 g upon arrival in the laboratory. All experiments were conducted on animals between 3 and 5 months of age and animals were age matched for all experiments. Prior to commencing the experiment, mice were group housed in polycarbonate cages, with up to 4 mice per cage. When collecting wheel running data, mice were individually housed in Nalgene Type L clear polycarbonate cages (30.3 cm long × 20.6 cm wide × 26 cm high; Nalg Nunc International, Rochester, NY), equipped with a stainless-steel running wheel (diameter of 24.2 cm). Rotation of the running wheels was monitored by magnetic switches attached to the wheels and data were collected using Clocklab (Actimetrics, Wilmette, IL, USA). Animals were maintained in a 12:12 light: dark cycle until the start of experiments, at which point they were then exposed to the light cycle appropriate for that experiment for at least 3 weeks. Animals had ad libitum access to food (Purina Lab Diet 5001) and water and were housed in temperature (21 ± 1 °C) and humidity-controlled rooms. Cages were changed approximately every 14 to 18 days and at least a week prior to experimental manipulations. All manipulations and husbandry during dark periods were performed using night-vision goggles (Model BG15, General Starlight Company, Richmond Hill, Ontario, Canada). All procedures were approved by the Life and Environmental Sciences Animal Care Committee at the University of Calgary and adhered to the policies of the Canadian Council of Animal Care for the ethical use of animals in research.
Procedures
Circadian Behavior in Constant Darkness
BTBR (male n = 8, female n = 8) and C57 (male n = 7, female n = 8) mice were used to examine circadian behavior in constant darkness (DD). Animals were allowed to freerun in DD for at least 2 weeks to achieve a stable freerunning rhythm. Data for analysis were collected for at least 3 weeks following this initial habituation period. In addition, data collected within a week following a cage or wheel change were not used for analysis in case these events altered freerunning rhythms.
Circadian Behavior in Constant Light
Male BTBR (n = 6) and C57 (n = 7) mice were used to examine circadian behavior in constant light (LL). Mice were allowed to freerun for 3 weeks in ~200 lux (measured at cage level). Behavioral assessments (period, amplitude, robustness) were made using a cosinor analysis (R. Refinetti, https://www.circadian.org/ZIP/cosinor.zip) due to the less robust locomotor rhythms observed under LL. This analysis is based on the procedure described by Nelson et al. (1979).
Phase Shifting to Brief Light Pulses
BTBR (male n = 8, female n = 8) and C57 (male n = 7, female n = 6) were first allowed to freerun in constant darkness (DD) for at least 3 weeks. They were then subjected to an early night light pulse (15 min, 40 lux) 4 h after activity onset (circadian time [CT]16; with CT12 being defined as activity onset by convention) to induce phase delays, and to a late-night light pulse 10 h after activity onset (CT22) to induce phase advances. Manipulations were counterbalanced and the early and late-night light pulse experiments were separated by 6 weeks, during which time animals were allowed to freerun in DD. After exposure to the light pulse, animals were returned to DD and allowed to freerun.
LD Entrainment
Male BTBR (n = 13) and C57 (n = 17) mice (a cohort independent from the above experiments) were used to examine circadian parameters under an LD cycle. To examine circadian parameters and entrainment to an LD cycle, all animals were exposed to 12:12 LD. Animals were allowed to stably entrain for at least 3 weeks before collecting data to be analyzed. Light intensity during LD experiments was maintained at ~200 lux (measured at cage level). LD entrainment was analyzed on at least 10 days of data.
Re-Entrainment to a Shifted LD Cycle
Male BTBR (n = 8) and C57 (n = 8) mice were tested on their ability to re-entrain to 8 h shifted light cycles. All animals were initially allowed to entrain to a 12:12 LD cycle. After 2 weeks, the LD cycle was advanced by 8 h by turning the lights on 8 h earlier on the first day of the new LD cycle (i.e., there was a 4 h period of darkness between the old and new 12 h light phases). Animals were then allowed to entrain to this new light cycle. After at least 3 weeks, the LD cycle was then delayed by 8 h by delaying the time that lights turned on by 8 h (i.e., there was a 20-h period of darkness between the old and new 12-h light phases). Again, the animals were allowed to re-entrain. Activity onsets and offsets were determined automatically by Clocklab. Animals were considered to be completely entrained to the new light cycle when their phase angle was within 20 min of their average phase angle from the 4 days prior to the LD shift. Due to the potential for the new light cycle to mask the true onset of activity in the delayed LD cycle, re-entrainment to the delayed LD cycle was also assessed by examining activity offsets. Due to the greater variability in offsets, the baseline phase angle of the offsets was averaged over the 14 days prior to shifting the LD cycle. The day of re-entrainment was defined as the first of 2 consecutive days that had an activity offset within a standard deviation of the baseline offset phase angle.
Skeleton Photoperiod
To observe the effect of light on entrainment and to further study whether masking played a role in the entrainment patterns observed in BTBR mice we subjected male BTBR (n = 8) and C57 (n = 8) mice to a skeleton photoperiod. Mice were first entrained to a 12:12 LD cycle for at least 2 weeks. Animals were then exposed to skeleton photoperiods consisting first of dawn/dusk pulses that lasted 1 h, then that lasted 10 min, and finally that lasted just 1 min before being released into DD. The dawn pulses always started at the same time as lights-on in the normal LD cycle, and the dusk pulses always ended at the same time as lights-off in the normal LD cycle. The animals were housed under each of the 3 skeleton photoperiods for at least a week before the dawn/dusk light pulses were shortened.
Tissue Collection and Immunohistochemistry
Animals (BTBR: male n = 5, female n = 5, and C57: male n = 6, female n = 4) housed in LD 12:12 were given an overdose of pentobarbital at ZT 12. When surgically anesthetized as assessed by lack of response to a toe pinch, they were perfused transcardially first with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in PBS. Brains were collected and post-fixed in 4% paraformaldehyde for 24 h and cryoprotected in 30% sucrose for a further 48 h. 35 µm sections were then collected through the anterior hypothalamus using a cryostat. Floating tissue sections were first washed in PBS containing 0.1% triton-X100 (PBSt) that included 0.3% H2O2 to inactivate endogenous peroxidase. The tissue was then washed 3 times in PBS and blocked in PBSt containing 10% normal goat serum (NGS) for 1 h. Tissue sections were then incubated in the primary antibody—a guinea pig polyclonal antibody raised against vasoactive intestinal polypeptide (VIP; Guinea Pig anti-VIP, 1:5000, Immunostar) in PBSt containing 10% NGS for 48 h at 4 °C. Tissue sections were then washed again 6 times for 10 min with PBSt before being incubated with a biotinylated secondary antibody (Goat anti-guinea pig, 1:200, Vector Labs) for 1 h. After 3 more PBSt rinses, tissue was incubated with Avidin-Biotin-Complex (Vectastain Elite ABC kit, Vector Labs). Following 3 more PBSt rinses, tissue was developed with a diaminobenzidine (DAB) reaction (0.04% DAB in Tris buffer, 60 µL of 8% NiCl, and 10 µL of 30% H2O2). The reaction was quenched in rapid PBS rinses and tissue was mounted and cover slipped. Alternate slices were then visualized with brightfield microscopy to analyze the VIP-expressing cells from the full rostrocaudal extent of the SCN (i.e., 5 section/animal) in both BTBR and C57 mice.
Microscopy Procedures
Tissue sections were visualized using brightfield microscope (Olympus BX51). The SCN were photographed though a 10× objective. Cell counting was done on the tissue itself using the 40× objective, focusing through the tissue and identifying individual cells only if the full cell body was contained within the slice. All images were collected using imaging software (ImagePro Plus 5.1.2.59; Media Cybernetics, Inc). All analysis and quantification were carried out bilaterally.
Data Analysis
Circadian period in DD was calculated using the slope of a regression line fit to daily activity onsets during a 7-day period. Phase shifts were calculated using the standard Clocklab routine as described by our group previously (Sterniczuk et al., 2008). Briefly, regression lines were fit to activity onsets for a week before the light pulse and for days 3 to 10 after the light pulse. The first 2 days following the light pulse were not analyzed to allow for transient effects (Daan and Pittendrigh, 1976). The post manipulation regression line was then extrapolated back to the day of the light pulse. The time difference between the intercepts of the 2 lines on the day following the light pulse was used as the phase shift. Due to the extremely short FRP of BTBR animals, actograms were plotted with a period closer to that of the individual (e.g., 22-23 h) so that sufficient onsets could be included both before and after the treatment. Total activity was calculated by taking the sum of daily total activity over a 10-day period using Clocklab analysis software (Actimetrics, Wilmette, IL, USA). Duration of activity (alpha), in both DD and LD, was analyzed by comparing the time difference between activity onset and activity offset as identified by Clocklab for each day. Each individual alpha was then averaged over a 10-day period. The phase angle of entrainment was determined by comparing the time of activity onset with the time of dark onset. Activity onsets preceding dark onset were positive values, while onsets occurring after dark onset were negative values. These daily phase angles of entrainment were then averaged over a 7-day period. For the re-entrainment experiments, animals were considered entrained when a stable phase angle was established—that is, activity onset coincided with lights-off with a stable phase angle that was maintained. Activity counts were exported in 1-min bins and were analyzed in 10-min bins. Activity onset was defined as the first 10 min that had more than 50 revolutions and was then followed immediately by another 10-min bin with at least 100 revolutions. Circadian parameters in DD were analyzed using 2 × 2 analyses of variance (ANOVAs) (sex × strain) for experiments that included females and using independent t-tests when only males were used. The Holm-Sidak method was used for post hoc pairwise tests to control for multiple comparisons. Statistics were conducted using Microsoft Excel or SigmaPlot 14.5 (Systat Software, Inc., San Jose, CA). All means are reported ±SD in the text and ±SEM in figures.
Results
Measures in DD
BTBR mice had significantly shorter FRPs when compared to C57 (Figure 1; F1,25 = 127.41, p < 0.0001). While male and female mice showed similar FRPs (F1,25 = 3.29; p = 0.87), there was a statistically significant interaction between strain and sex (F1,25 = 15.52, p = 0.001). BTBR male mice had shorter FRPs (22.74 ± 0.10 h) compared to both C57 males (23.8 ± 0.07 h, p < 0.0001) and BTBR females (23.14 ± 0.28 h, p < 0.0001). BTBR females had significantly shorter FRPs compared to C57 females (23.65 ± 0.17 h, p < 0.0001) and C57 males (p < 0.0001). No significant differences were found when comparing C57 males and females (p = 0.165).
Figure 1.
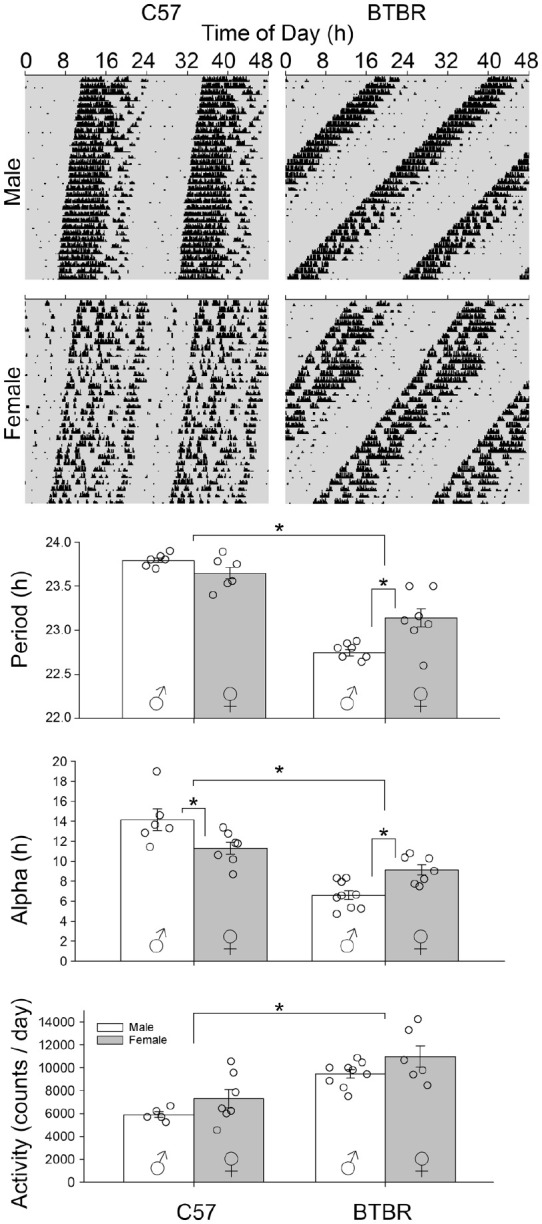
Double plotted representative actograms of male and female C57 and BTBR mice housed in DD. BTBR mice overall had shorter FRPs compared to C57 mice (p < 0.0001). BTBR male mice had significantly shorter FRPs compared to C57 male mice and BTBR female mice. BTBR female mice had significantly shorter FRPs compared to C57 male and female mice. No differences were found between C57 male and female mice. BTBR mice also had shorter alphas compared to C57 mice. BTBR male mice showed shorter alphas compared to C57 male mice and BTBR female mice and BTBR female mice showed shorter alphas compared to C57 male and female mice. BTBR mice also had higher total wheel running activity compared to C57 mice. BTBR male mice had significantly higher total wheel running activity compared to C57 male mice and BTBR female mice had significantly higher total wheel running activity compared to C57 female mice. But no differences were found when comparing BTBR male and female mice or C57 male and female mice. Abbreviations: DD = constant darkness; FRPs = freerunning periods.
*p < 0.05.
BTBR mice had a significantly shorter duration of activity (alpha) when compared to C57 mice (Figure 1, F1,25 = 61.62, p < 0.0001). In addition, there was a significant interaction between strain and sex (F1,25 = 19.18, p < 0.0001). Specifically, BTBR male mice had shorter alphas (6.6 ± 1.4 h) compared to both C57 males (14.1 ± 2.6 h, p < 0.0001) and BTBR females (9.1 ± 1.4 h, p = 0.004). BTBR females had significantly shorter alphas compared to C57 females (11.3 ± 1.6 h, p = 0.022) and C57 males had shorter alphas when compared to C57 females (p = 0.006).
BTBR mice had significantly higher total activity (10,084 ± 1767 revs/day) when compared to C57 mice (6751 ± 1760 revs/day, Figure 1, F1,25 = 32.24, p < 0.0001). There were no sex differences in total activity (F1,25 = 3.66; p = 0.67). In addition, there was no significant interaction between strain and sex on total activity (F1,25 = 0.418, p = 0.524).
Light pulses in the early subjective night (CT16) elicited large phase delays in both sexes and strains (Figure 2). BTBR mice had phase delays that were slightly, but significantly, larger than did C57 mice (F1,18 = 6.185; p = 0.023). There were no main effect of sex (F1,18 = 1.524; p = 0.233), nor was there a sex × strain interaction (F1,18 = 1.181; p = 0.292). Light pulses in the late subjective night (CT22) elicited phase advances in both sexes and strains. BTBR mice had significantly larger phase advances than did C57 mice (F1,24 = 4.582; p = 0.043), and female mice had significantly larger phase advances than did males (F1,24 = 5.626; p = 0.023). There was no sex × strain interaction (F1,24 = 1.222; p = 0.28).
Figure 2.
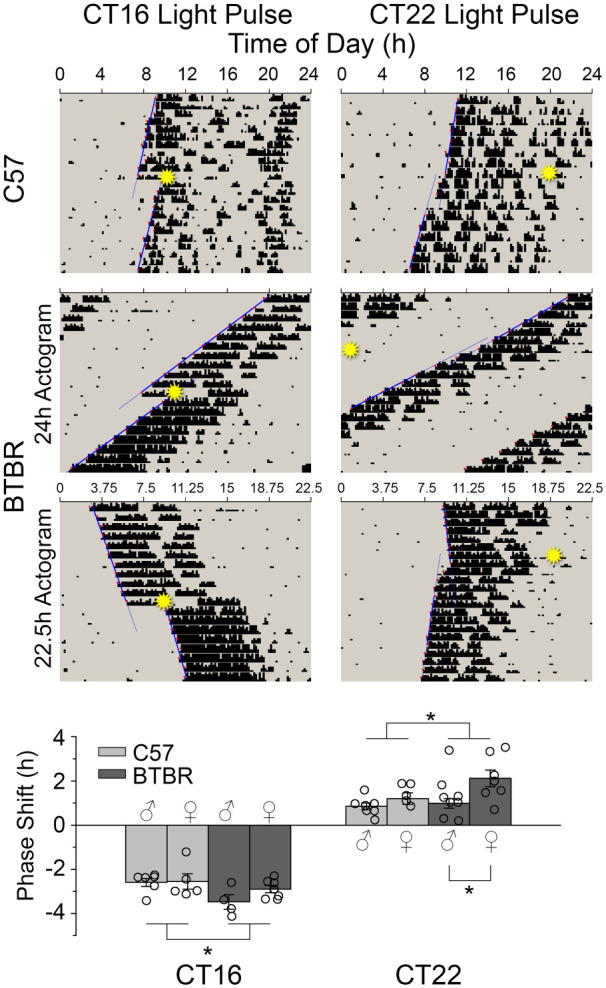
Representative actograms showing phase shift responses to early (CT16) and late (CT22) 15 min light pulses (indicated by star) in C57 and BTBR mice. Lines represent the regression lines fitted to activity onset before the light pulse and from days 3 to 10 after. The difference between the two represents the phase shift. BTBR mice had comparable phase advances but larger phase delays when compared to C57 mice.
*p < 0.05.
Measures in LL
Consistent with Aschoff’s rule, male BTBR and male C57 mice both exhibited longer periods in LL than in DD (Figure 3). While FRPs were more similar between the strains under LL than under DD, the FRPs in the BTBR mice (25.38 ± 0.17 h) were still significantly shorter (one-tailed t(11) = 2.036, p = 0.033) than in C57 mice (25.61 ± 0.22 h). There was no significant difference in the amplitude of the rhythms (t(11) = 1.306, p = 0.218). Overall, the locomotor rhythms of the BTBR mice were much less cohesive, leading to significantly less robustness of their rhythms as assessed by the cosinor analysis (BTBR: 5.4% ± 2.5%, C57: 12.4% ± 5.3%; t(11) = 2.94, p = 0.01). Only male mice were examined in LL, so sex effects were not examined.
Figure 3.
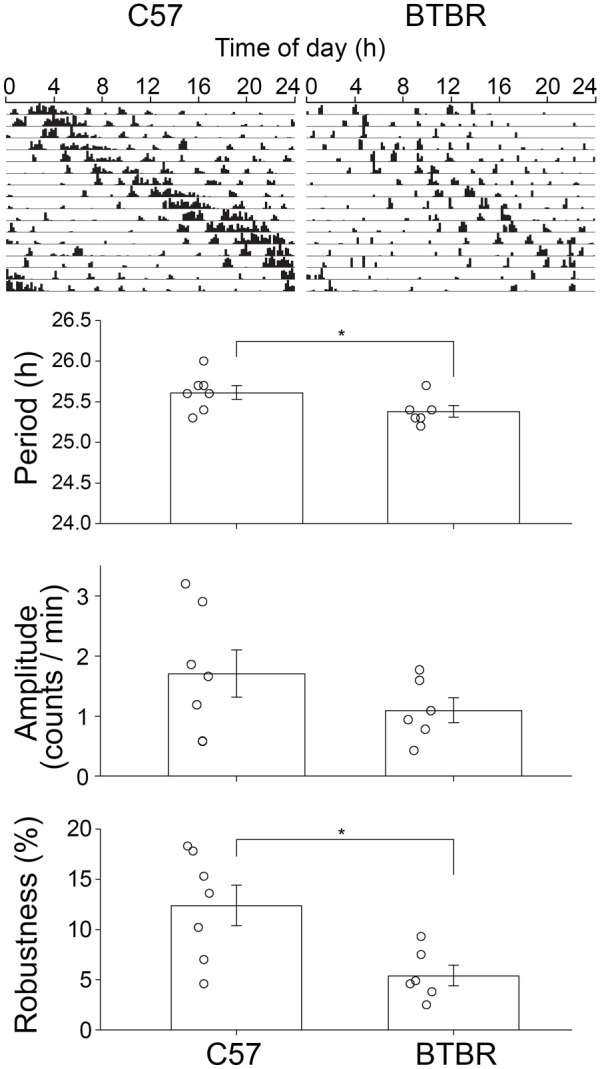
Representative actograms for BTBR and C57 in constant light. Despite the constant light induced lengthening of period, BTBR mice ran with shorter freerunning period compared to C57 mice and showed lower robustness in their activity. However, amplitude of activity was comparable between the groups.
*p < 0.05.
Measures in LD
Similar to what was observed in DD, male BTBR mice had a shorter alpha in LD (6.8 ± 0.9 h) when compared to male C57 mice (12.1 ± 0.3 h, Figure 4, t(12) = 11.79, p < 0.001). Total daily wheel running activity was significantly higher in male BTBR mice (9237 ± 1354 revs/day, t(12) = 2.784, p = 0.016) compared to male C57 mice (7232 ± 1155 revs/day). Phase angle of entrainment did not differ between the male BTBR mice (−0.1 ± 0.09 h) and male C57 mice (0.09 ± 0.09 h, t(12) = 1.524, p = 0.153). Only male mice were examined in LD, so sex effects were not examined.
Figure 4.
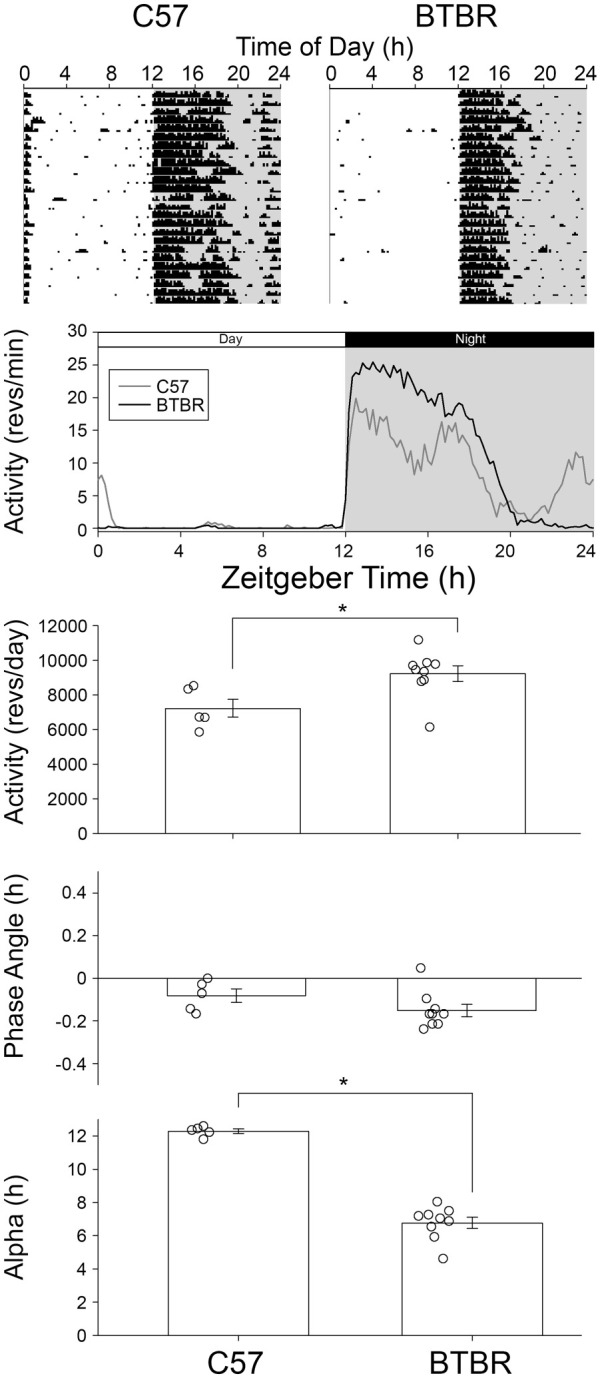
Representative actograms for BTBR and C57 in a 12:12 LD cycle. BTBR mice had significantly higher amount of total activity and a significantly shorter alpha; however, their phase angle of entrainment was comparable to that of C57 mice.
*p < 0.05.
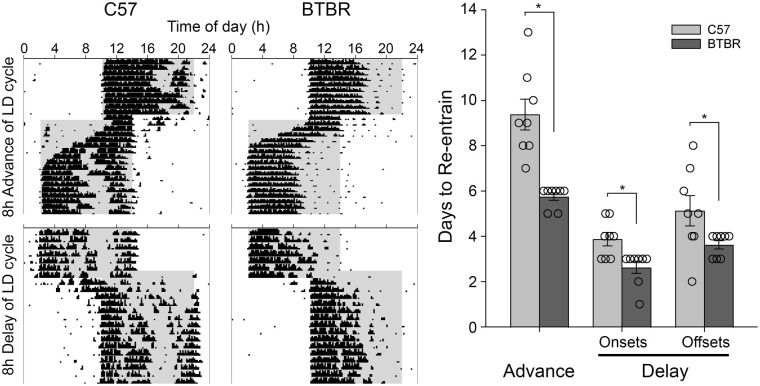
Re-entrainment to 8-h shifts in the LD cycle were examined in male BTBR and C57 mice. BTBR animals required significantly fewer days to re-entrained to an 8 h advance of their LD cycle (5.8 ± 0.5 days, Figure 5; t(14) = 5.185, p = 0.00014) than did C57 mice (9.4 ± 1.9 days). Similarly, BTBR animals required significantly fewer days to re-entrained to an 8 h delay of their LD cycle (2.6 ± 0.7 days, t(14) = 3.162, p = 0.0069) than did C57 mice (3.9 ± 0.8 days). In the re-entrainment to the delayed LD cycle, daytime activity appeared much lower in BTBR than C57 mice. As such, this quicker re-entrainment may have actually represented enhanced masking of locomotor rhythms in the BTBR mice rather than true re-entrainment. To guard against this, re-entrainment was also assessed in the phase delayed LD cycle by examining activity offsets as well. While re-entrainment took longer in both strains when assessed in this way, based on activity offsets, BTBR mice still re-entrained significantly more quickly (3.63 ± 0.5 days) than the C57 mice (5.1 ± 1.9 days, t(14) = 2.17, p = 0.048). The influence on masking was also assessed in a second test where the mice were placed into DD after 3 days on the new LD cycle, so as to reveal the true phase of the circadian clock. The activity onsets on day 3 were significantly earlier in the C57 mice than in the BTBR mice (F4,13 = 21.127, p < 0.001, Figure 6), both when actual onsets were assessed (p = 0.003), or when based on predicted onsets based on regression lines fit to onsets in DD (p < 0.001). The predicted onsets were significantly earlier than the actual onsets (F1,13 = 12.714, p = 0.003), and there was no interaction between the strain and type of onset assessed (F1,13 = 0.584, p = 0.458), indicating that some masking of activity onset was occurring in both strains. Only male mice were examined in the re-entrainment protocol, so sex effects were not examined.
Figure 5.
Representative actograms showing re-entrainment to 8 h advanced and delayed light cycles for BTBR and C57 mice. BTBR mice re-entrained significantly faster to both phase advances and phase delay shifts. This difference persisted when analyzing re-entrainment using offsets.
*p < 0.05.
Figure 6.
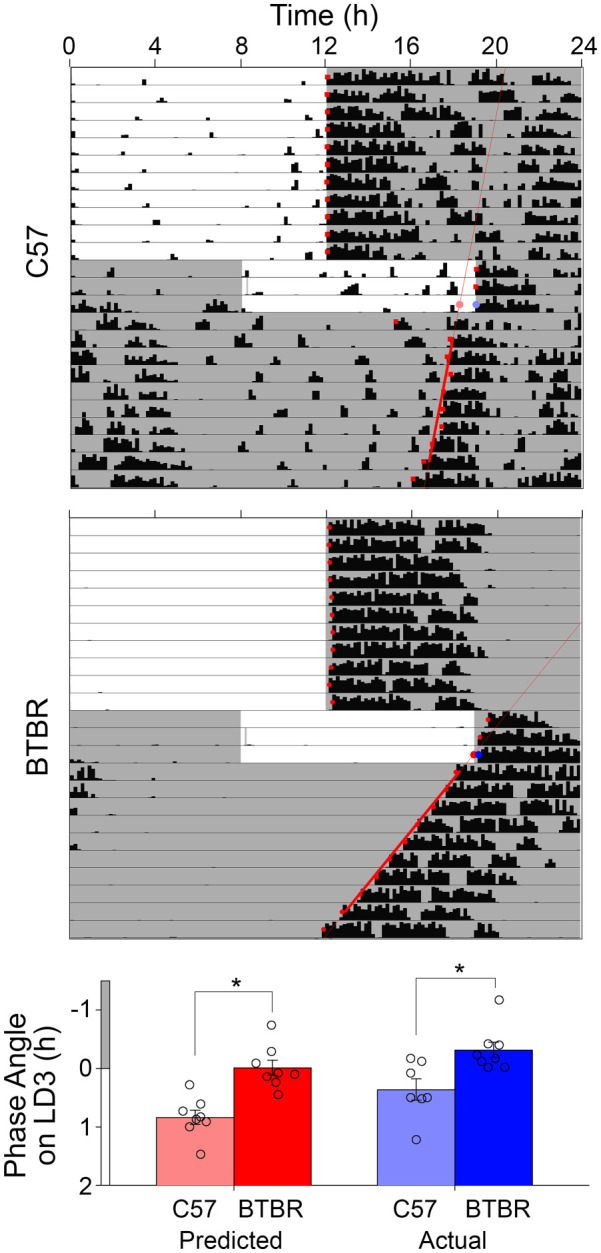
Representative actograms showing re-entrainment to 8 h advanced and delayed light cycles for C57 and BTBR mice which were subsequently placed into constant darkness to unmask the true phase of the circadian clock after 3 days of exposure to the new LD cycle. The activity onset on day 3 of the shifted LD cycle was assessed both by determining the actual onset and the predicted onset based on a regression line fit to subsequent activity onsets in constant darkness.
*p < 0.05.
Skeleton Photoperiod
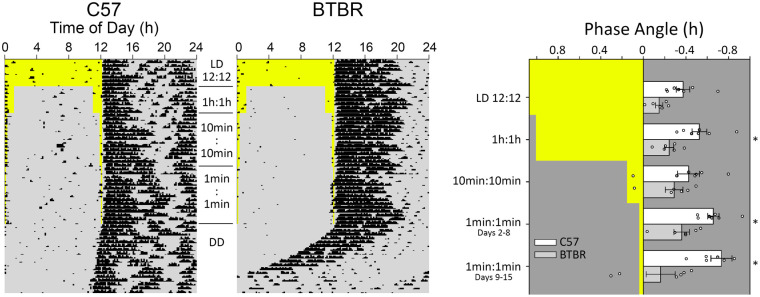
Entrainment to various skeleton photoperiods was examined in male BTBR and C57 mice. When comparing activity in skeleton photoperiods, we analyzed the phase angle of entrainment to each of the different light cycle (i.e., a normal 12:12 LD, as well as skeleton photoperiods comprised of dawn/dusk pulses of 1 h, 10 min, and 1 min duration). The phase angle of entrainment tended to become more delayed as the light portion of the skeleton photoperiod became shorter (Figure 7, main effect of light cycle, F4,44 = 3.323, p = 0.018). The BTBR mice tended to have less delayed phase angles than did the C57 mice (main effect of strain, F1,44 = 18.974, p = 0.001). Although the effect of the various skeleton photoperiods on the phase angle appeared to be different between the strains, the overall strain by light cycle interaction did not reach significance (F4,44 = 2.511, p = 0.055). However, planned comparisons between the strains revealed that the strains did not significantly differ (p = 0.058) in their phase angles under the full photoperiod, similar to what was observed with the animals contributing to the LD analysis presented in Figure 4. It was expected that given their very short FRPs, the BTBR mice would have more positive/less negative phase angles that would allow the brief phase-delaying light to fall deeper into their subjective night to produce the required larger phase delays to achieve stable entrainment. In the second week of the briefest skeleton photoperiods, 2 BTBR mice had consistent positive phase angles. Two others had activity onsets prior to the 1-min dusk light on some, but not all days, while 2 others consistently had negative phase angles. Apart from one animal, positive phase angles were never observed in the C57 mice. For that single exception, a positive phase angle was only observed with the 10-min skeleton photoperiod in a mouse that had activity onsets preceding the dusk light on only about one-third of the nights in the assessment period. Only male mice were examined in the skeleton photoperiod protocol, so sex effects were not examined.
Figure 7.
Representative actograms showing wheel running activity for C57 and BTBR mice in 12:12 LD, 1 h, 10 min, and 1 min dawn and dusk light pulses. After the final skeleton photoperiod of 1 min pulses, animals were released into DD. Overall, BTBR mice tended toward less delayed phase angles, but showed no differences in phase angles of entrainment between the different skeleton photoperiod durations. C57 mice showed differences between the 1 min and 12:12 LD, and 1 min and 10 min pulses. BTBR mice had significantly less delayed phase angles of entertainment in the 1 min and 1 h skeleton photoperiods.
*p < 0.05.
Cell Counts for VIP
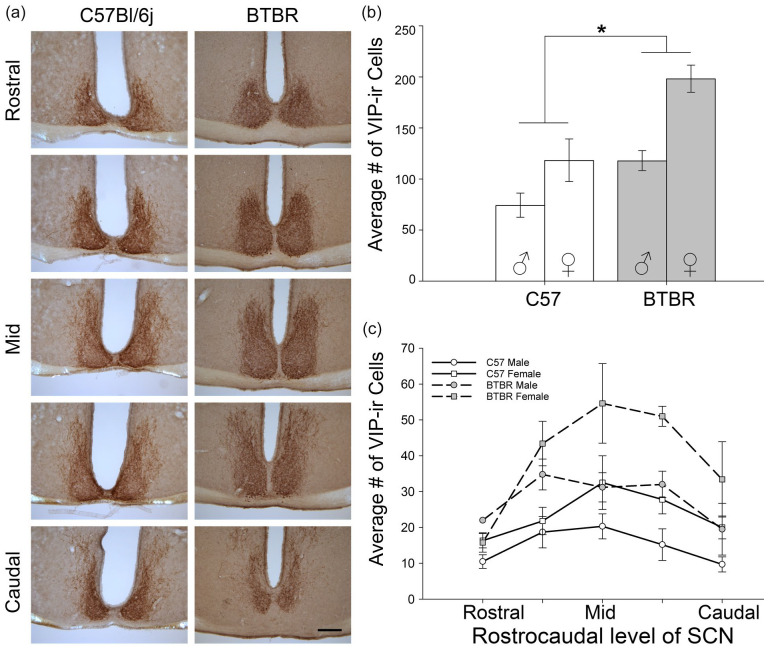
BTBR mice had a significantly higher number of VIP-expressing cells in the SCN when compared to C57 mice (main effect of strain, F1,16 = 21.453, p < 0.001, Figure 8). Females had significantly more VIP-IR cells than did males (F1,16 = 19.006, p = 0.106). There was no significant strain × sex interaction (F1,16 = 2.138, p = 0.163, however the power for the interaction test was low (Sex main effect β = 0.28, interaction β = 0.18), so this negative effect should be interpreted cautiously. In particular, planned comparisons revealed that female BTBR mice had significantly more VIP-IR cells than BTBR males (p = 0.034).
Figure 8.
(a) Representative microscopy images of VIP-expressing cells throughout the rostrocaudal extent of the SCN of female C57 and BTBR mice (male images comparable). (b) BTBR mice had a significantly higher total number of VIP-expressing cells when compared to C57. (c) Quantification of number of VIP-IR cells at each of 5 rostrocaudal levels of the SCN. Scale bar = 200 µm. Abbreviations: VIP = vasoactive intestinal polypeptide; SCN = suprachiasmatic nucleus.
p < 0.05.
Discussion
In this study, we examined the circadian behavior of BTBR mice in a variety of entrained and freerunning conditions. The BTBR strain consistently showed a short period phenotype in DD (~22.75 h) that was significantly shorter than that observed in the comparison C57 strain (~23.75 h). The FRP in BTBR mice observed here was slightly shorter than has been reported in a large screen of BTBR mice (Siepka et al., 2007) and may reflect longer time in DD for the mice examined here. While the period did lengthen under LL, it was still significantly shorter in male BTBR mice than in male C57 mice. BTBR animals exhibited phase shifts to light that were slightly, but significantly, larger than those observed in C57 mice. Male BTBR mice also re-entrained to advanced and delayed LD cycles more rapidly than did male C57 mice. Despite such a short FRP, the phase angle of entrainment observed in male BTBR mice did not differ from that observed in male C57 mice under a regular LD cycle. Phase angle differences emerged between the strains under skeleton photoperiods, with male BTBR mice on average maintaining their phase angle observed in LD, while male C57 mice became more delayed in their phase angles as the duration of light in the skeleton photoperiod decreased. BTBR mice also had higher levels of total wheel running activity when compared to C57 mice, which was consolidated within a shorter duration (alpha) each day. Differences in the underlying neuroanatomy were also noted in that the BTBR mice had significantly more VIP-immunoreactive cells in the SCN than did C57 mice.
While various genetically modified mice with altered FRPs have been generated by targeting specific clock genes or other downstream factors, the BTBR strain has among the shortest spontaneously occurring short FRP phenotype in Mus musculus (Siepka et al., 2007). While the BALB/cJ strain also has a similar short FRP (Possidente and Stephan, 1988; Shimomura et al., 2001), their circadian locomotor patterns are not as consolidated as those of the BTBR mice. Pittendrigh and Daan (1976a) reported that deer mice (Peromyscus maniculatus) exhibited periods as short as those observed in this study, but as a species their periods were highly variable, ranging from as short as 22 h to as long as 25 h. Such variability was not observed here with the BTBR mice. Spontaneous short FRP mutants have been observed in hamsters, such as the Tau mutation leading to FRPs of ~22 h in heterozygous animals and ~20 h in the homozygous mutants (Ralph and Menaker, 1988) that results from a mutation in the casine kinase 1ε gene (Lowrey et al., 2000) that accelerates the negative limb of the TTFL. Mutations in other closely related enzymes such as mCK1ε, hCK1δ, and hPer2 also accelerate degradation of PER and shorten the circadian period (Camacho et al., 2001; Akashi et al., 2002; Dey et al., 2005; Eide et al., 2005; Xu et al., 2005; Gallego and Virshup, 2007; Meng et al., 2008; Etchegaray et al., 2009; Lee et al., 2009; Walton et al., 2009). The spontaneous duper mutation observed in hamsters also shortens the circadian period (Monecke et al., 2011) and has recently been linked to a mutation in the cry1 gene (Lee et al., 2022). A similar short period mutant mouse, part-time (FRP = 21.4 h), discovered on a mutagenesis screen using BTBR mice, was also mapped to a loss of function of the cry1 gene (Siepka et al., 2007). The BTBR strain was also used in the discovery of a long period mutant mouse, Overtime (FRP = 25.8 h), mapped to a mutation of the F-box protein FBXL3 (Siepka et al., 2007) that normally degrades CRY1 proteins. These findings suggest that multiple loci can underlie a change in period and that the determination of FRP is complex (Shimomura et al., 2001; Takahashi et al., 2008). This is supported by genomic and transcriptomic studies in mice. Many candidate factors have been explored with regard to their effect on FRP. These include clock genes, as well as enzymes that regulate clock genes (Takahashi et al., 2008; Maywood et al., 2011, 2014; Hastings et al., 2019). However, findings are variable, and they differ according to the factor that is altered, suggesting a complex interplay of various factors. For instance, Clock mutant mice (termed ClockΔ19) show a long period phenotype that results from reduced transcription by the mutated clock protein (Vitaterna et al., 1994; Low-Zeddies and Takahashi, 2001). However, mice with homozygous deletion of the Clock genes show only slightly shorter FRPs (Debruyne et al., 2006). Similarly, while Cry1 null mice have a short period phenotype Cry2 null mice have a long period phenotype (Thresher et al., 1998; van der Horst et al., 1999; Vitaterna et al., 1999). High levels of variability with different types of mutations in the Per genes have also been observed (Bae et al., 2001; Cermakian et al., 2001; Zheng et al., 2001). The variability in phenotypic outcomes with different clock gene mechanisms could be attributed to (1) the part of the TTFL that is affected, (2) downstream effectors that are differentially affected due to the mutation, and (3) the presence and actions of either the clock genes themselves or clock-controlled genes and proteins in other systems. Overall, while alterations in clock genes or the SCN circuitry (see below) may contribute to the phenotypic differences we examined, given the evidence from other period mutants, clock gene variations may have a greater impact and should be explored in future studies.
Prior studies have looked at large-scale transcriptome and genome analyses in BTBR mice (Nadler et al., 2006; Jones-Davis et al., 2013). While these mice have several known polymorphisms and mutations in non-circadian-related genes, the only clock implicated gene that is known to have a polymorphism is Per3 (Jones-Davis et al., 2013). In other mouse lines, mutation of Per3 leads to only subtle changes in circadian phenotypes. For example, deletion of Per3 has no effect on circadian rhythms either in Per1/Per3 or Per2/Per3 double-mutant mice (Shearman et al., 2000; Bae et al., 2001; Maywood et al., 2014). In some cases, Per3 mutants show subtle period phenotypes in cultured cells, peripheral tissues explant and in behavior, that include both a small lengthening and shortening of the FRP (Ebisawa et al., 2001; Archer et al., 2010). Given these variable and subtle effects of Per3 on the circadian system (Shearman et al., 2000; Lee et al., 2004), the effects of these changes in Per3 in the BTBR strain will need to be further determined. However, the role of Per3 is of interest since it has been implicated in advanced sleep phase (ASPD) and delayed sleep phase (DSPD; Ebisawa et al., 2001; Archer et al., 2010) disorders. These disorders often emerge from an altered phase angle of entrainment that results from a mismatch between FRP and the 24-h day-night cycle. However, the BTBR mice examined here entrained to LD cycles and skeleton photoperiods without an altered phase angle of entrainment. While both ASPD and DSPD are associated with ASD, there is a greater incidence of DSPD (Ebisawa et al., 2001; Archer et al., 2003; Pereira et al., 2005; Jones et al., 2007; Archer et al., 2010; Lázár et al., 2012; Liberman et al., 2017, 2018; Carmassi et al., 2019). DSPD is more consistently associated with long FRPs, while ASPD is more consistent with the short FRPs such as those observed here with BTBR mice. Because of this, the BTBR mouse may not be a good model for the study of sleep and circadian problems in people with ASD. However, given the documented Per3 polymorphism in BTBR mice, and Per3’s association with circadian sleep phase disorders, BTBR mice may be a useful model for understanding the role of Per3 in these disorders.
While genetic polymorphisms could contribute to the observed phenotypes, especially with regard to the short period, another possibility would be developmental changes in neural networks underlying circadian regulation. BTBR mice exhibit a wide range of alterations in brain anatomy and function including a lack of a corpus callosum (Meyza et al., 2013), altered visual function (Cheng et al., 2017, 2020), and alterations of cortex arrangement and activity (Fenlon et al., 2015; Smith et al., 2016). The BTBR strain is known to have mutations in several genes, the most relevant to circadian rhythms being mutations in Disc1 and serotonin (5-HT)-related genes. While Disc1 knockout mice show a shorter period, this change is subtle and not as pronounced as found in the BTBR mice. Furthermore, Disc1-knockout have longer active phases than controls (Jaaro-Peled et al., 2016; Lee et al., 2021) while the BTBR mice examined here had shorter active phases. Furthermore, while BTBR mice exhibit a dysregulation in 5-HT function (Gould et al., 2011), disruption of 5-HT input to the circadian system in other models either lengthens (Mistlberger et al., 1998) or has no effect (Block and Zucker, 1976) on circadian period, suggesting that altered 5-HTergic function likely does not contribute to the phenotype observed here.
In the present study, a larger number of VIP-immunoreactive cells were noted in the SCN of BTBR mice. VIP plays a prominent role in SCN function. Altering VIP signaling can affect both freerunning and entrained rhythms. Animals lacking VIP receptors show dysrhythmia and impaired responses to light (Harmar et al., 2002; Colwell et al., 2003; Dragich et al., 2010). VIP can phase shift circadian rhythms (Piggins et al., 1995), while blocking VIP receptors impairs phase shifts to light (Chan et al., 2016). Animals with enhanced VIP receptor signaling showed more rapid re-entrainment to shifted light cycles (Shen et al., 2000; An et al., 2012). On the contrary, animals lacking VIP receptors also show dysrhythmia and impaired responses to light (Colwell et al., 2003; Dragich et al., 2010) and re-entrain to 8-h shifts in light cycles almost immediately (Harmar et al., 2002). In addition, one study reported that VIP has a dose-dependent effect on the SCN, with higher doses causing desynchrony in SCN neurons (An et al., 2012). These studies suggest that both upregulation and downregulation of VIP signaling in the SCN can adversely affect circadian behavior. It is possible that the larger population of VIP cells in the BTBR mouse SCN contributed to the observed phenotype. Further investigation is required to evaluate whether the changes in VIP cell number translate into changes in VIP signaling and to assess how VIP might contribute to the observed phenotype.
Activity levels can influence FRP. Animals housed with running wheels can engage in high amplitude exercise, and they display a shorter FRP than controls housed without running wheels (Yamada et al., 1988, 1990; Edgar et al., 1991; Mrosovsky, 1999). As BTBR mice show more activity overall, and as this activity was compressed into a shorter active phase, this high amplitude activity may have contributed to their shorter phenotype. In LL, the activity levels did not differ between the strains, and the period difference was less pronounced, although BTBR mice still have a significantly shorter FRP. In DD, the FRP of females was not as short as the BTBR males, yet their daily activity levels did not differ from that of males, suggesting that activity alone does not account for the observed FRPs. Nevertheless, examining the FRP of BTBR mice housed without wheels may uncover the contribution of high amplitude activity to the BTBR FRP.
Like male BTBR mice, female BTBR mice also have shorter FRPs, shorter alphas, and higher total activity compared to female C57 controls. However, we also found a significant interaction of sex in that the females have significantly longer FRPs and longer alphas when compared to male BTBR mice. Both male and female C57 mice in our experiment had comparable FRPs in line with previous findings that found no period difference between C57 male and female mice (Kuljis et al., 2013). Sex differences have been previously reported in other species such as the golden hamster (Davis et al., 1983; Schull et al., 1989) and Octodon degus (Labyak and Lee, 1995; Lee and Labyak, 1997); however, the male animals in these studies showed longer FRPs than females. Sex differences may be due to differences in gonadal steroids, which can influence circadian period (Morin et al., 1977; Albers, 1981; Karatsoreos et al., 2007; Royston et al., 2014). For instance, the FRP of male mice lengthens when they are castrated and then shortens with subsequent testosterone treatment (Karatsoreos et al., 2007). BTBR mice have higher testosterone levels than C57 mice (Flowers et al., 2007), which might explain their shorter FRP than BTBR females and why a sex difference was not detected in C57 mice. Some manipulations of estrogen can alter rhythm properties. In hamsters, chronic estradiol exposure shortens FRP (Morin et al., 1977), while ovariectomy lengthens FRP in rats, hamsters, and O. degus (Morin et al., 1977; Albers, 1981; Labyak and Lee, 1995). However, ovariectomy has no effect on the freerunning period in mice (Iwahana et al., 2008). Similarly, estrogen receptor lacking mice show no differences in their FRP when compared to controls (Blattner and Mahoney, 2014). This points to a species-specific difference in estrogenic regulation of circadian rhythms, especially with relevance to period. In addition, steroid hormones and androgens specifically have been implicated in the higher ASD rates observed in males (Baron-Cohen et al., 2011). Early exposure to androgens (such as testosterone) acts on the brain to produce sex differences in behavior, cognition, brain structure, and function (De Vries and Simerly, 2002; Baron-Cohen et al., 2011; Kosidou et al., 2016; Berni et al., 2018; Cherskov et al., 2018), and this has been proposed as a key mechanism in this bias. It is possible that altered hormonal signaling may be a common contributor to both the circadian and ASD-like phenotype of BTBR mice.
One interesting observation from the BTBR mice is how unremarkable most of their circadian properties are outside of their short FRP. Most other FRP models exhibit a weakening or loss of rhythmicity, altered phase angle of entrainment, or altered phase resetting responses in addition to the change in period (Thresher et al., 1998; van der Horst et al., 1999; Vitaterna et al., 1999; Dey et al., 2005; Xu et al., 2005; Meng et al., 2008; Etchegaray et al., 2009; Lee et al., 2009; Walton et al., 2009). This also includes tau and duper hamsters. In tau mutants, while rhythmicity is robust and persists in DD, animals have an unusually early phase angle of entrainment in LD (Ralph and Menaker, 1988) and have altered phase shifts to light (Shimomura and Menaker, 1994), though these effects are subtle and vary (Shimomura and Menaker, 1994; Grosse et al., 1995). Similarly, while duper and super-duper hamsters also have robust rhythms that persist in DD, they exhibit changes in entrainment and phase shifting to photic stimuli (Krug et al., 2011; Bittman, 2012, 2014). In this study, while BTBR mice had phase shifts to light that were significantly larger than were observed with C57 mice, the increase itself was rather modest (~75%) relative to the Type 0 resetting observed with the duper hamsters (Krug et al., 2011). However, as only 2 time-points were examined here, other phases might reveal larger differences in phase resetting responses.
The short FRPs and short duration of active phases observed in the BTBR mice led us to expect an advanced phase angle of entrainment, similar to what is observed with other short FRP mutants (e.g., the Tau mutant hamster; Ralph and Menaker, 1988). However, BTBR mice show precise and stable entrainment to LD cycles with activity onsets very close to dark onset. Given their short FRP, we also hypothesized that they would re-entrain to advances in the light cycle faster than C57 controls and re-entrain to delays more slowly. While they re-entrained to the advances more quickly than the C57 mice, the time taken to re-entrain was in line with their shorter FRP, which already advanced their daily onsets by ~1.5 h each day. What was surprising was the rapid re-entrainment to the delayed LD cycle, which was even quicker than re-entrainment to the advanced cycle. This is in opposition to the long-established recognition that short FRPs lead to quicker re-entrainment to advanced rather than delayed LD cycles (Wever, 1966). The rapid re-entrainment to the delayed LD cycles is remarkable, as BTBR mice already require large daily phase delays just to entrain to an LD cycle, which should limit how much extra of a delay they could accomplish to the phase delayed LD cycle. Examination of the actograms suggests that activity in the light phase might be more heavily masked in the BTBR animals, which could explain the lack of an earlier phase angle difference in LD, and the apparent more rapid re-entrainment to the delayed LD cycle. When re-entrainment was assessed by examining activity offsets that occurred in the dark phase, re-entrainment did take longer, suggesting that the assessment based on onsets may have been influenced by masking. However, re-entrainment was still quicker in the BTBR mice than the C57 mice when assessed this way. The pronounced masking observed with the delayed LD cycles prompted us to reconsider the phase angle of entrainment in LD. Other short FRP strains exhibit very early phase angles of entrainment (e.g., Ralph and Menaker, 1988), but if onsets were masked this could make it appear as if the animals had a normal phase angle of entrainment. To explore this, we used a skeleton photoperiod which could unmask earlier activity onsets. A positive phase angle of entrainment was not observed with skeleton pulses of 1 h or 10 min. It was only with the 1-min pulse that some BTBR animals started to show positive phase angles of entrainment. This, combined with the observation of the phase of the freerunning rhythm in subsequent DD, suggests that the phase angle reported here in LD is accurate and not a function of masking. The robustness of entrainment observed with skeleton pulses for 1 min was surprising. Pittendrigh and Daan (1976b) originally used 1 h pulses, as they initially doubted that 15 min pulses would be sufficient, although they later confirmed that 15 min was sufficient. Given that the non-parametric model still posits a dose effect of light duration and intensity (Pittendrigh, 1960; Nelson and Takahashi, 1991), the fact that entrainment to 1 min pulses was achieved was surprising given the large difference between FRP and the zeitgeber period. Entrainment to daily light pulses of only 1 sec has been reported (DeCoursey, 1972), although in animals (flying squirrels) with FRP periods much closer to 24 h than those of BTBR mice.
Finally, while the focus of this study was on the circadian phenotype of BTBR mice, this strain is often used as a model for the study of ASD, attention-deficit hyperactivity disorder (ADHD), and in some cases to also model anxiety and repetitive behavior (Meyza and Blanchard, 2017). While the altered rhythmicity observed here is not a good match for the sleep and circadian problems observed with ASD, the unique phenotype observed here can inform the use of these mice in other contexts. First, numerous morbidities arise when there is a large mismatch between the FRP of the animal and the period of the entraining LD cycle (e.g., Martino et al., 2008). It is possible that some of the behavioral and physiological difference exhibited by BTBR mice arise from the stress of entraining to the typical LD cycles used in vivariums. This could affect physiological parameters such as glucose tolerance, insulin resistance, digestion, and gastrointestinal health, all parameters examined in BTBR mice (Flowers et al., 2007; Newell et al., 2016). In addition, it is becoming more common to consider phase of the circadian cycle when studying behavior and physiology (Nelson et al., 2021). This can be accomplished by housing animals on a reverse LD cycle, so that they can be tested during the animal’s night phase. However, given the short active phase of BTBR mice, such testing should be confined to the first half of their dark phase, since, unlike C57 mice, BTBR mice are inactive during the latter portion of the night.
The BTBR mice exhibit several unique circadian features that make them an interesting model for understanding the underlying circadian clock. Most important among these is an extremely short FRP combined with the ability to entrain to a 24 h LD cycle with a normal phase angle of entrainment. The observation that BTBR mice could entrain to a skeleton photoperiod with just two 1-min pulses per day with only a modestly different phase angle of entrainment was surprising. This observation cannot be explained by the parametric model of entrainment, and it even presents a challenge for the non-parametric model of entrainment. Future studies in BTBR mice that generate a more detailed PRC, as well as examining phase shifts to brief 1-min light pulses, should help explain these interesting and unique circadian features. The robust activity pattern, short alpha, and short FRP of BTBR mice may also make them a useful model for exploring other circadian phenomena, such as interactions between the light- and food-entrainable oscillators (Vijaya Shankara et al., 2022).
Footnotes
Conflict of Interest Statement: The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Michael C. Antle  https://orcid.org/0000-0001-5178-4683
https://orcid.org/0000-0001-5178-4683
References
- Akashi M, Tsuchiya Y, Yoshino T, Nishida E. (2002) Control of intracellular dynamics of mammalian period proteins by casein kinase I epsilon (CKIepsilon) and CKIdelta in cultured cells. Mol Cell Biol 22:1693-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE. (1981) Gonadal hormones organize and modulate the circadian system of the rat. Am J Physiol 241:R62-R66. [DOI] [PubMed] [Google Scholar]
- An S, Tsai C, Ronecker J, Bayly A, Herzog ED. (2012) Spatiotemporal distribution of vasoactive intestinal polypeptide receptor 2 in mouse suprachiasmatic nucleus. J Comp Neurol 520:2730-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antle MC, Silver R. (2005) Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci 28:145-151. [DOI] [PubMed] [Google Scholar]
- Archer SN, Carpen JD, Gibson M, Lim GH, Johnston JD, Skene DJ, von Schantz M. (2010) Polymorphism in the PER3 promoter associates with diurnal preference and delayed sleep phase disorder. Sleep 33:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, Arendt J, von Schantz M. (2003) A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep 26:413-415. [DOI] [PubMed] [Google Scholar]
- Aschoff J. (1960) Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol 25:11-28. [DOI] [PubMed] [Google Scholar]
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. (2001) Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 30:525-536. [DOI] [PubMed] [Google Scholar]
- Baker EK, Richdale AL. (2017) Examining the behavioural sleep-wake rhythm in adults with autism spectrum disorder and no comorbid intellectual disability. J Autism Dev Disord 47:1207-1222. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, Knickmeyer R. (2011) Why are autism spectrum conditions more prevalent in males? PLoS Biol 9:e1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berni TR, Morgan CL, Berni ER, Rees DA. (2018) Polycystic ovary syndrome is associated with adverse mental health and neurodevelopmental outcomes. J Clin Endocrinol Metab 103:2116-2125. [DOI] [PubMed] [Google Scholar]
- Bittman EL. (2012) Does the precision of a biological clock depend upon its period? Effects of the duper and tau mutations in Syrian hamsters. PLoS ONE 7:e36119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittman EL. (2014) Effects of the duper mutation on responses to light: parametric and nonparametric responses, range of entrainment, and masking. J Biol Rhythms 29:97-109. [DOI] [PubMed] [Google Scholar]
- Blattner MS, Mahoney MM. (2014) Estrogen receptor 1 modulates circadian rhythms in adult female mice. Chronobiol Int 31:637-644. [DOI] [PubMed] [Google Scholar]
- Block M, Zucker I. (1976) Circadian rhythms of rat locomotor activity after lesions of the midbrain raphe nuclei. J Comp Physiol 109:235-247. [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. (2007) Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res 176:21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulos Z, Macchi MM, Terman M. (2002) Twilights widen the range of photic entrainment in hamsters. J Biol Rhythms 17:353-363. [DOI] [PubMed] [Google Scholar]
- Camacho F, Cilio M, Guo Y, Virshup DM, Patel K, Khorkova O, Styren S, Morse B, Yao Z, Keesler GA. (2001) Human casein kinase Idelta phosphorylation of human circadian clock proteins period 1 and 2. FEBS Lett 489:159-165. [DOI] [PubMed] [Google Scholar]
- Carmassi C, Palagini L, Caruso D, Masci I, Nobili L, Vita A, Dell’Osso L. (2019) Systematic review of sleep disturbances and circadian sleep desynchronization in autism spectrum disorder: toward an integrative model of a self-reinforcing loop. Front Psychiatry 10:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermakian N, Monaco L, Pando MP, Dierich A, Sassone-Corsi P. (2001) Altered behavioral rhythms and clock gene expression in mice with a targeted mutation in the Period1 gene. EMBO J 20:3967-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RK, Sterniczuk R, Enkhbold Y, Jeffers RT, Basu P, Duong B, Chow SL, Smith VM, Antle MC. (2016) Phase shifts to light are altered by antagonists to neuropeptide receptors. Neuroscience 327:115-124. [DOI] [PubMed] [Google Scholar]
- Cheng N, Khanbabaei M, Murari K, Rho JM. (2017) Disruption of visual circuit formation and refinement in a mouse model of autism. Autism Res 10:212-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N, Pagtalunan E, Abushaibah A, Naidu J, Stell WK, Rho JM, Sauvé Y. (2020) Atypical visual processing in a mouse model of autism. Sci Rep 10:12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherskov A, Pohl A, Allison C, Zhang H, Payne RA, Baron-Cohen S. (2018) Polycystic ovary syndrome and autism: a test of the prenatal sex steroid theory. Transl Psychiatry 8:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. (2003) Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol 285:R939-R949. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, et al. (1999) Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284:2177-2181. [DOI] [PubMed] [Google Scholar]
- Daan S. (1977) Tonic and phasic effects of light in the entrainment of circadian rhythms. Ann N Y Acad Sci 290:51-59. [DOI] [PubMed] [Google Scholar]
- Daan S. (2000) The Colin S. Pittendrigh Lecture. Colin Pittendrigh, Jürgen Aschoff, and the natural entrainment of circadian systems. J Biol Rhythms 15:195-207. [DOI] [PubMed] [Google Scholar]
- Daan S, Pittendrigh CS. (1976) A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curves. J Comp Physiol 106:253-266. [Google Scholar]
- Daan S, Damassa D, Pittendrigh CS, Smith ER. (1975) An effect of castration and testosterone replacement on a circadian pacemaker in mice (Mus musculus). Proc Natl Acad Sci U S A 72:3744-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FC, Darrow JM, Menaker M. (1983) Sex differences in the circadian control of hamster wheel-running activity. Am J Physiol 244:R93-R105. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Simerly RB. (2002) Anatomy, development, and function of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff DW, Arnold AP, Fahrbach SE, Etgen AM, Rubin RT, editors. Hormones, brain and behavior. San Diego (CA): Academic Press. p. 137-191. [Google Scholar]
- Debruyne JP, Noton E, Lambert CM, Maywood ES, Weaver DR, Reppert SM. (2006) A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron 50:465-477. [DOI] [PubMed] [Google Scholar]
- DeCoursey PJ. (1972) LD ratios and the entrainment of circadian activity in a nocturnal and a diurnal rodent. J Comp Physiol 78:221-235. [Google Scholar]
- Dey J, Carr AJ, Cagampang FR, Semikhodskii AS, Loudon AS, Hastings MH, Maywood ES. (2005) The tau mutation in the Syrian hamster differentially reprograms the circadian clock in the SCN and peripheral tissues. J Biol Rhythms 20:99-110. [DOI] [PubMed] [Google Scholar]
- Dragich JM, Loh DH, Wang LM, Vosko AM, Kudo T, Nakamura TJ, Odom IH, Tateyama S, Hagopian A, Waschek JA, et al. (2010) The role of the neuropeptides PACAP and VIP in the photic regulation of gene expression in the suprachiasmatic nucleus. Eur J Neurosci 31:864-875. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Zitting KM, Chinoy ED. (2015) Aging and circadian rhythms. Sleep Med Clin 10:423-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisawa T, Uchiyama M, Kajimura N, Mishima K, Kamei Y, Katoh M, Watanabe T, Sekimoto M, Shibui K, Kim K, et al. (2001) Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep 2:342-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar DM, Kilduff TS, Martin CE, Dement WC. (1991) Influence of running wheel activity on free-running sleep/wake and drinking circadian rhythms in mice. Physiol Behav 50:373-378. [DOI] [PubMed] [Google Scholar]
- Eide EJ, Kang H, Crapo S, Gallego M, Virshup DM. (2005) Casein kinase I in the mammalian circadian clock. Methods Enzymol 393:408-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchegaray JP, Machida KK, Noton E, Constance CM, Dallmann R, Di Napoli MN, DeBruyne JP, Lambert CM, Yu EA, Reppert SM, et al. (2009) Casein kinase 1 delta regulates the pace of the mammalian circadian clock. Mol Cell Biol 29:3853-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenlon LR, Liu S, Gobius I, Kurniawan ND, Murphy S, Moldrich RX, Richards LJ. (2015) Formation of functional areas in the cerebral cortex is disrupted in a mouse model of autism spectrum disorder. Neural Dev 10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers JB, Oler AT, Nadler ST, Choi Y, Schueler KL, Yandell BS, Kendziorski CM, Attie AD. (2007) Abdominal obesity in BTBR male mice is associated with peripheral but not hepatic insulin resistance. Am J Physiol Endocrinol Metab 292:E936-E945. [DOI] [PubMed] [Google Scholar]
- Foster S, Christiansen T, Antle MC. (2019) Modeling the influence of synaptic plasticity on after-effects. J Biol Rhythms 34:645-657. [DOI] [PubMed] [Google Scholar]
- Gallego M, Virshup DM. (2007) Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol 8:139-148. [DOI] [PubMed] [Google Scholar]
- Gannon RL. (2015) Circadian clock speed increases during aging in the male Syrian hamster: a large-scale study. Chronobiol Int 32:1168-1171. [DOI] [PubMed] [Google Scholar]
- Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. (2011) Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J Neurochem 116:291-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse J, Loudon AS, Hastings MH. (1995) Behavioural and cellular responses to light of the circadian system of tau mutant and wild-type Syrian hamsters. Neuroscience 65:587-597. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, et al. (2002) The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell 109:497-508. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Maywood ES, Brancaccio M. (2019) The mammalian circadian timing system and the suprachiasmatic nucleus as its pacemaker. Biology (Basel) 8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahana E, Karatsoreos I, Shibata S, Silver R. (2008) Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Horm Behav 53:422-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaro-Peled H, Altimus C, LeGates T, Cash-Padgett T, Zoubovsky S, Hikida T, Ishizuka K, Hattar S, Mongrain V, Sawa A. (2016) Abnormal wake/sleep pattern in a novel gain-of-function model of DISC1. Neurosci Res 112:63-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KH, Ellis J, von Schantz M, Skene DJ, Dijk DJ, Archer SN. (2007) Age-related change in the association between a polymorphism in the PER3 gene and preferred timing of sleep and waking activities. J Sleep Res 16:12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Davis DM, Yang M, Rider E, Osbun NC, da Gente GJ, Li J, Katz AM, Weber MD, Sen S, Crawley J, et al. (2013) Quantitative trait loci for interhemispheric commissure development and social behaviors in the BTBR T⁺ tf/J mouse model of autism. PLoS ONE 8:e61829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, Wang A, Sasanian J, Silver R. (2007) A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology 148:5487-5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall AR, Lewy AJ, Sack RL. (2001) Effects of aging on the intrinsic circadian period of totally blind humans. J Biol Rhythms 16:87-95. [DOI] [PubMed] [Google Scholar]
- Kosidou K, Dalman C, Widman L, Arver S, Lee BK, Magnusson C, Gardner RM. (2016) Maternal polycystic ovary syndrome and the risk of autism spectrum disorders in the offspring: a population-based nationwide study in Sweden. Mol Psychiatry 21:1441-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug S, McKinley Brewer J, Bois AS, Bittman EL. (2011) Effects of the duper mutation on circadian responses to light. J Biol Rhythms 26:293-304. [DOI] [PubMed] [Google Scholar]
- Kuljis DA, Loh DH, Truong D, Vosko AM, Ong ML, McClusky R, Arnold AP, Colwell CS. (2013) Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology 154:1501-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labyak SE, Lee TM. (1995) Estrus- and steroid-induced changes in circadian rhythms in a diurnal rodent, Octodon degus. Physiol Behav 58:573-585. [DOI] [PubMed] [Google Scholar]
- Lázár AS, Slak A, Lo JC, Santhi N, von Schantz M, Archer SN, Groeger JA, Dijk DJ. (2012) Sleep, diurnal preference, health, and psychological well-being: a prospective single-allelic-variation study. Chronobiol Int 29:131-146. [DOI] [PubMed] [Google Scholar]
- Lee C, Weaver DR, Reppert SM. (2004) Direct association between mouse PERIOD and CKIepsilon is critical for a functioning circadian clock. Mol Cell Biol 24:584-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Chen R, Lee Y, Yoo S, Lee C. (2009) Essential roles of CK1δ and CK1ε in the mammalian circadian clock. Proc Natl Acad Sci U S A 106:21359-21364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, Park J, Kwak Y, Park YU, Nhung TTM, Suh BK, Woo Y, Suh Y, Cho E, Cho S, et al. (2021) Disrupted-in-schizophrenia 1 enhances the quality of circadian rhythm by stabilizing BMAL1. Transl Psychiatry 11:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TM, Labyak SE. (1997) Free-running rhythms and light- and dark-pulse phase response curves for diurnal Octodon degus (Rodentia). Am J Physiol 273:R278-R286. [DOI] [PubMed] [Google Scholar]
- Lee YY, Cal-Kayitmazbatir S, Francey LJ, Bahiru MS, Hayer KE, Wu G, Zeller MJ, Roberts R, Speers J, Koshalek J, et al. (2022) duper is a null mutation of Cryptochrome 1 in Syrian hamsters. Proc Natl Acad Sci U S A 119:e2123560119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman AR, Halitjaha L, Ay A, Ingram KK. (2018) Modeling strengthens molecular link between circadian polymorphisms and major mood disorders. J Biol Rhythms 33:318-336. [DOI] [PubMed] [Google Scholar]
- Liberman AR, Kwon SB, Vu HT, Filipowicz A, Ay A, Ingram KK. (2017) Circadian clock model supports molecular link between PER3 and human anxiety. Sci Rep 7:9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsung E, Karthikeyan R, Cao R. (2021) Biological timing and neurodevelopmental disorders: a role for circadian dysfunction in autism spectrum disorders. Front Neurosci 15:642745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M, Takahashi JS. (2000) Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science 288:483-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low-Zeddies SS, Takahashi JS. (2001) Chimera analysis of the Clock mutation in mice shows that complex cellular integration determines circadian behavior. Cell 105:25-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoogian EN, Leise TL, Bittman EL. (2015) Phase resetting in duper hamsters: specificity to photic zeitgebers and circadian phase. J Biol Rhythms 30:129-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino TA, Oudit GY, Herzenberg AM, Tata N, Koletar MM, Kabir GM, Belsham DD, Backx PH, Ralph MR, Sole MJ. (2008) Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol 294:R1675-R1683. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Chesham JE, O’Brien JA, Hastings MH. (2011) A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc Natl Acad Sci U S A 108:14306-14311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Chesham JE, Smyllie NJ, Hastings MH. (2014) The Tau mutation of casein kinase 1ε sets the period of the mammalian pacemaker via regulation of Period1 or Period2 clock proteins. J Biol Rhythms 29:110-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng QJ, Logunova L, Maywood ES, Gallego M, Lebiecki J, Brown TM, Sládek M, Semikhodskii AS, Glossop NRJ, Piggins HD, et al. (2008) Setting clock speed in mammals: the CK1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron 58:78-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyza KZ, Blanchard DC. (2017) The BTBR mouse model of idiopathic autism – current view on mechanisms. Neurosci Biobehav Rev 76:99-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyza KZ, Defensor EB, Jensen AL, Corley MJ, Pearson BL, Pobbe RL, Bolivar VJ, Blanchard DC, Blanchard RJ. (2013) The BTBR T+ tf/J mouse model for autism spectrum disorders-in search of biomarkers. Behav Brain Res 251:25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE, Bossert JM, Holmes MM, Marchant EG. (1998) Serotonin and feedback effects of behavioral activity on circadian rhythms in mice. Behav Brain Res 96:93-99. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Takahashi JS. (2011) Cell autonomy and synchrony of suprachiasmatic nucleus circadian oscillators. Trends Neurosci 34:349-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monecke S, Brewer JM, Krug S, Bittman EL. (2011) Duper: a mutation that shortens hamster circadian period. J Biol Rhythms 26:283-292. [DOI] [PubMed] [Google Scholar]
- Morin LP, Fitzgerald KM, Zucker I. (1977) Estradiol shortens the period of hamster circadian rhythms. Science 196:305-307. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, et al. (2007) Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res 176:4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrosovsky N. (1999) Further experiments on the relationship between the period of circadian rhythms and locomotor activity levels in hamsters. Physiol Behav 66:797-801. [DOI] [PubMed] [Google Scholar]
- Nadler JJ, Zou F, Huang H, Moy SS, Lauder J, Crawley JN, Threadgill DW, Wright FA, Magnuson TR. (2006) Large-scale gene expression differences across brain regions and inbred strains correlate with a behavioral phenotype. Genetics 174:1229-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Takahashi JS. (1991) Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus). J Physiol 439:115-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Bumgarner JR, Walker WH, II, DeVries AC. (2021) Time-of-day as a critical biological variable. Neurosci Biobehav Rev 127:740-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W, Tong Y, Lee J, Halberg F. (1979) Methods for cosinor rhythmometry. Chronobiologia 6:305-323. [PubMed] [Google Scholar]
- Newell C, Shutt TE, Ahn Y, Hittel DS, Khan A, Rho JM, Shearer J. (2016) Tissue specific impacts of a ketogenic diet on mitochondrial dynamics in the BTBR(T+tf/j) Mouse. Front Physiol 7:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira DS, Tufik S, Louzada FM, Benedito-Silva AA, Lopez AR, Lemos NA, Korczak AL, D’Almeida V, Pedrazzoli M. (2005) Association of the length polymorphism in the human Per3 gene with the delayed sleep-phase syndrome: does latitude have an influence upon it? Sleep 28:29-32. [PubMed] [Google Scholar]
- Piggins HD. (2002) Human clock genes. Ann Med 34:394-400. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Antle MC, Rusak B. (1995) Neuropeptides phase shift the mammalian circadian pacemaker. J Neurosci 15:5612-5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS. (1960) Circadian rhythms and the circadian organization of living systems. Cold Spring Harbor Symp Quant Biol 25:159-184. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS. (1964) The entrainment of circadian oscillations by light and their role as photoperiodic clocks. Am Nat 98:261-294. [Google Scholar]
- Pittendrigh CS, Daan S. (1976. a) A functional-analysis of circadian pacemakers in nocturnal rodents. I. Stability and lability of spontaneous frequency. J Comp Physiol 106:223-252. [Google Scholar]
- Pittendrigh CS, Daan S. (1976. b) A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: pacemaker as clock. J Comp Physiol 106:291-331. [Google Scholar]
- Possidente B, Stephan FK. (1988) Circadian period in mice: analysis of genetic and maternal contributions to inbred strain differences. Behav Genet 18:109-117. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Menaker M. (1988) A mutation of the circadian system in golden hamsters. Science 241:1225-1227. [DOI] [PubMed] [Google Scholar]
- Richdale AL, Prior MR. (1995) The sleep/wake rhythm in children with autism. Eur Child Adolesc Psychiatry 4:175-186. [DOI] [PubMed] [Google Scholar]
- Royston SE, Yasui N, Kondilis AG, Lord SV, Katzenellenbogen JA, Mahoney MM. (2014) ESR1 and ESR2 differentially regulate daily and circadian activity rhythms in female mice. Endocrinology 155:2613-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schull J, Walker J, Fitzgerald K, Hiilivirta L, Ruckdeschel J, Schumacher D, Stanger D, McEachron DL. (1989) Effects of sex, thyro-parathyroidectomy, and light regime on levels and circadian rhythms of wheel-running in rats. Physiol Behav 46:341-346. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Jin X, Lee C, Reppert SM, Weaver DR. (2000) Targeted disruption of the mPer3 gene: subtle effects on circadian clock function. Mol Cell Biol 20:6269-6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Spratt C, Sheward WJ, Kallo I, West K, Morrison CF, Coen CW, Marston HM, Harmar AJ. (2000) Overexpression of the human VPAC2 receptor in the suprachiasmatic nucleus alters the circadian phenotype of mice. Proc Natl Acad Sci U S A 97:11575-11580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura K, Menaker M. (1994) Light-induced phase shifts in tau mutant hamsters. J Biol Rhythms 9:97-110. [DOI] [PubMed] [Google Scholar]
- Shimomura K, Low-Zeddies SS, King DP, Steeves TD, Whiteley A, Kushla J, Zemenides PD, Lin A, Vitaterna MH, Churchill GA, et al. (2001) Genome-wide epistatic interaction analysis reveals complex genetic determinants of circadian behavior in mice. Genome Res 11:959-980. [DOI] [PubMed] [Google Scholar]
- Siepka SM, Yoo SH, Park J, Lee C, Takahashi JS. (2007) Genetics and neurobiology of circadian clocks in mammals. Cold Spring Harb Symp Quant Biol 72:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Rho JM, Teskey GC. (2016) Ketogenic diet restores aberrant cortical motor maps and excitation-to-inhibition imbalance in the BTBR mouse model of autism spectrum disorder. Behav Brain Res 304:67-70. [DOI] [PubMed] [Google Scholar]
- Sterniczuk R, Stepkowski A, Jones M, Antle MC. (2008) Enhancement of photic shifts with the 5-HT1A mixed agonist/antagonist NAN-190: intra-suprachiasmatic nucleus pathway. Neuroscience 153:571-580. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. (2008) The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet 9:764-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thresher RJ, Vitaterna MH, Miyamoto Y, Kazantsev A, Hsu DS, Petit C, Selby CP, Dawut L, Smithies O, Takahashi JS, et al. (1998) Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science 282:1490-1494. [DOI] [PubMed] [Google Scholar]
- Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, Ptácek LJ, Fu YH. (2001) An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 291:1040-1043. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. (1997) Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol 273:R1957-R1964. [DOI] [PubMed] [Google Scholar]
- van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker AP, van Leenen D, et al. (1999) Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398:627-630. [DOI] [PubMed] [Google Scholar]
- Vijaya Shankara J, Mistlberger RE, Antle MC. (2022) Anticipation of scheduled feeding in BTBR mice reveals independence and interactions between the light- and food-entrainable circadian clocks. Front Integr Neurosci 16:896200. 10.3389/fnint.2022.896200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. (1994) Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264:719-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, Hitomi K, Thresher RJ, Ishikawa T, Miyazaki J, et al. (1999) Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci U S A 96:12114-12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KM, Fisher K, Rubitski D, Marconi M, Meng QJ, Sládek M, Adams J, Bass M, Chandrasekaran R, Butler T, et al. (2009) Selective inhibition of casein kinase 1 epsilon minimally alters circadian clock period. J Pharmacol Exp Ther 330:430-439. [DOI] [PubMed] [Google Scholar]
- Weisgerber D, Redlin U, Mrosovsky N. (1997) Lengthening of circadian period in hamsters by novelty-induced wheel running. Physiol Behav 62:759-765. [DOI] [PubMed] [Google Scholar]
- Wever R. (1966) The duration of re-entrainment of circadian rhythms after phase shifts of the Zeitgeber A theoretical investigation. J Theor Biol 13:187-201. [Google Scholar]
- Witting W, Mirmiran M, Bos NP, Swaab DF. (1994) The effect of old age on the free-running period of circadian rhythms in rat. Chronobiol Int 11:103-112. [DOI] [PubMed] [Google Scholar]
- Xu Y, Padiath QS, Shapiro RE, Jones CR, Wu SC, Saigoh N, Saigoh K, Ptácek LJ, Fu YH. (2005) Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature 434:640-644. [DOI] [PubMed] [Google Scholar]
- Yamada N, Shimoda K, Ohi K, Takahashi S, Takahashi K. (1988) Free-access to a running wheel shortens the period of free-running rhythm in blinded rats. Physiol Behav 42:87-91. [DOI] [PubMed] [Google Scholar]
- Yamada N, Shimoda K, Takahashi K, Takahashi S. (1990) Relationship between free-running period and motor activity in blinded rats. Brain Res Bull 25:115-119. [DOI] [PubMed] [Google Scholar]
- Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, et al. (2001) Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 105:683-694. [DOI] [PubMed] [Google Scholar]