Abstract
Foeniculum vulgare Mill. and Mentha piperita L. are two common medicinally important plants with a wide range of biological activities such as insecticide and antibacterial effects. In this study, the chemical composition of their essential oils was investigated using GC-MS analysis. After that, their nanoemulsions were prepared; optimum samples with droplet sizes of 74 ± 7 and 136 ± 5 nm were gelified. The viscosity of the prepared nanogels and the successful loading of the essential oil in them were investigated. The efficacy of the nanogel containing M. piperita essential oil as a repellent and antibacterial agent was more potent than the nanogel containing F. vulgare essential oil. Its completely protected time against Anopheles stephensi, the main malaria mosquito vector, was 120 ± 8 min. Moreover, the growth of Escherichia coli and Staphylococcus aureus after treatment with 5000 µg/mL of nanogel containing M. piperita essential oil was reduced by 100 and 65%, respectively. Considering natural constituents, a straightforward preparation method, and high efficacy, the nanogel containing M. piperita essential oil could be introduced for further investigation against other mosquitoes and bacterial species.
1. Introduction
Essential oils are volatile natural oils formed as secondary metabolites in aromatic plants [1]. They are extracted from different parts of plant organs, e.g., buds, flowers, leaves, stems, twigs, seeds, fruits, roots, wood, or bark [2]. They have many biological effects, such as antiseptic, antibacterial, antiviral, and fungicidal properties [3, 4]. Besides, their larvicidal activity, repellent effects, and insecticide properties have also been confirmed [5, 6]. However, they should be stabilized due to their volatility and instability [7]. Preparation of EO-based nanoformulation has been recently considered a promising approach [8]. Among the common nanoformulations such as nanoemulsions, polymeric nanoparticles, and lipid nanocarriers, nanogels have received much attention, especially in topical applications, due to proper viscosity, high loading capacity, biocompatibility, and biodegradability [9, 10].
Escherichia coli and Staphylococcus aureus are two Gram-negative and positive opportunistic pathogens that (could) cause severe and life-threatening human infections [11]. S. aureus is mainly responsible for postoperative wound infection, toxic shock syndrome, and food poisoning [12]. The E. coli is present in the human intestine and causes lower urinary tract infection, coleocystis, or septicemia [13]. These bacteria enter the body through contaminated hands and can, of course, cause skin damage. Moreover, S. aureus is an important cause of soft tissue and skin infections such as boils, impetigo, carbuncles, staphylococcal scalded skin syndrome, and cellulitis.
Furthermore, malaria, with about 241 million cases and 627,000 deaths in 2020, is still the most dreadful of mosquito-borne diseases [14]. Around 30 species of the 400 identified Anopheles mosquito species are the vectors of malaria to humans [15]. Anopheles stephensi Liston is one of the most important malaria vectors in the Middle East and South Asia [16, 17]. However, it has recently expanded to Ethiopia, Djibouti, Lakshadweep, and Sri Lanka [18]. Besides, mosquito bites can be infected by other pathogens such as bacteria. However, one recommended solution to prevent mosquito-borne disease transmission is to use repellents [19, 20]. On the other hand, resistance to industrial repellents and their adverse effects has been one of the challenges for health systems in recent years [21, 22]. For instance, DEET (N, N-diethyl-3 methylbenzamide) is one of the best known and most successful synthetic chemical repellents that, by blocking the olfactory receptors neurons, causes repellency [23, 24]. However, its usage has been questioned due to its side effects on humans such as hypotension, seizures, neurotoxic, and skin irritations [25, 26].
Foeniculum vulgare Mill. and Mentha piperita L. are two common medicinally important plants with a wide range of biological activities such as antibacterial and insecticide effects [27, 28]. For instance, the literature reported that F. vulgare EO at a concentration of 40 mg/L caused 50% mortality for the second instars larvae Culex pipiens [29]. Besides, a protection time of 0.5% M. piperita EO against An. stephensi was reported at 17 min [30].
This study was an attempt to prepare multifunctional topically administrated natural nanogels. First, two nanogels containing F. vulgare and M. piperita EOs were prepared. After that, their antibacterial activities against E. coli and S. aureus were investigated. Finally, their repellent efficacies were investigated against An. stephesni compared to DEET as a gold standard repellent.
2. Materials and Methods
2.1. Materials
F. vulgare and M. piperita EOs were bought from Tabib Daru Company (Iran) and Zardband Pharmaceuticals Company (Iran). S. aureus (ATCC 25923) and E. coli (ATCC 25922) were provided by the Pasteur Institute of Iran. Carboxymethylcellulose (CMC), Mueller–Hinton broth, Mueller–Hinton agar, and Tween 20 were bought from Merck Chemicals (Germany). DEET 40% was purchased from Reyhan Naghsh Jahan Pharmaceutical Co., Iran. It was diluted to 2.0% using distilled water as the diluent following the formulated nanogel products.
2.2. Chemical Composition of the EOs
A gas chromatography device (Agilent 6890, HP-5MS column, USA) connected to a mass spectrometer (Agilent 5973, USA) was used for chemical compositions of the EOs as described in our previous study. Besides, relative abundances were calculated by peak area normalization [31].
2.3. Preparation and Characterization of Nanoemulsion-Based Gels
A fixed amount of (2.0% v/v) each EO was mixed with different amounts of Tween 20 (2000 rpm, 3 min, room temperature) to form a homogenous mixture. Distilled water was then added dropwise up to the final volume (5000 µL) and stirred for 40 minutes. The prepared nanoemulsions were subjected to size analysis using a DLS-type apparatus (K-One Nano Ltd., Korea). Nanoemulsions with proper size characteristics, including droplet size of <200 nm and droplet size distribution (SPAN) less than 1 [32], were considered optimum samples.
An optimum nanoemulsion from each EO (No. 1 and No. 7,Table 1) was selected for gelation; CMC (3.5% w/v) was added to each and stirred (2000 rpm) overnight at room temperature to complete the gelation process. The prepared nanogels containing F. vulgare and M. piperita EO were abbreviated as FVNG and MPNG. A schematic of the described method is depicted in Figure 1. Furthermore, blank gels of each nanogel were also prepared using the same approach, only without EO.
Table 1.
Prepared nanoemulsions and their size analyses.
| No. | F. vulgare (%) | M. piperita (%) | Tween 20 (%) | Droplet size (nm) | SPANa |
|---|---|---|---|---|---|
| 1 | 2 | — | 3 | 74 | 0.97 |
| 2 | 2 | — | 4 | 45 | 3.6 |
| 3 | 2 | — | 6 | 27 | 7.4 |
| 4 | 2 | — | 8 | 12 | 5.4 |
| 5 | 2 | — | 10 | 132 | 2.7 |
| 6 | — | 2 | 3 | 76 | 5.1 |
| 7 | — | 2 | 4 | 136 | 0.96 |
| 8 | — | 2 | 6 | 7 | 1.5 |
| 9 | — | 2 | 8 | 22 | 3.2 |
| 10 | — | 2 | 10 | 395 | 1.8 |
aDroplet size distribution.
Figure 1.
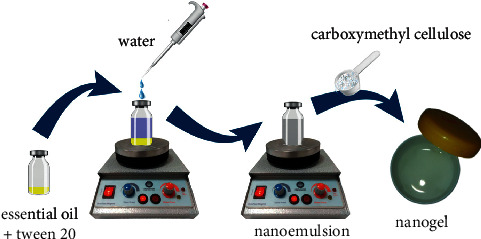
Preparation of nanoemulsion-based nanogels.
The viscosity of FVNG and MPNG at shear rates of 0.1–100 1/s was investigated using a Rheometer machine (MCR-302, Anton Paar, Austria). Moreover, ATR-FTIR analysis was used to investigate the successful loading of the EOs in the nanogels; spectra of EOs, blank gels, and nanogels (FVNG and MPNG) were recorded in a wavenumber range of 400–3900 cm−1. Without any preparation process, the samples were subjected to the spectrometer apparatus (Tensor II model, Bruker Co, Germany).
2.4. Repellent Bioassay
Susceptible mosquitoes (Bandar-e-Abbas strain) were used in the current study. They were reared and maintained at 27 ± 2 C temperature, ≥70 ± 10% relative humidity, and a 12 : 12 (light: dark) photoperiod. For repellent bioassays, 250 nonblood fed and nulliparous 5–7 days old adult female mosquitoes were kept in cages (40 × 40 × 40 cm) and not fed for 14 h before repellency tests. A 47-year-old male volunteer was employed to determine the protection time using the Arm-in-cage method with a slight modification [33]. His forearm was first washed with 70% alcohol and dried with a towel. Only the underside of the lower arm between the wrist and elbow, with an area of 8 cm × 12.5 cm (covered by fewer hairs), was exposed, and latex gloves covered the hand. The volunteer's hands were then impregnated with the samples (1g of FVNG and MPNG, 1 mL of DEET). After 5 minutes, the volunteer placed his forearm in a cage for 3 minutes. This procedure is repeated at a 30-minute interval; the test was stopped when one landing and/or probing occurred in a 3 min test.
2.5. Antibacterial Tests
For investigation of antibacterial tests of FVNG and MPNG as well as their blank gels, the ATCC100 assay was used with slight modifications [32]. Four mL of each fresh bacterial suspension (2 × 105 CFU/mL) was first filled separately in 6 cm plate dishes. Then, by adding 1.0, 0.5, and 0.25 g of each nanogel, final concentrations of EOs were fixed at 5000, 2500, and 1250 µg/mL. Antibacterial effects of blank gels were also investigated in a similar process. The treated plates were then incubated at 37°C for 24 h, and 10 µL of suspensions was cultured on a Muller–Hinton agar culture plate and incubated for 24 h. Finally, the number of grown colonies was counted and compared with the control group, and growth reduction was calculated using the following equation.
| (1) |
2.6. Statistical Analyses
All experiments were repeated three times, and the results were given as mean ± standard deviation. For the comparison of two or higher samples, the independent sample t-test and one-way ANOVA with at least a 0.05 significance level were used (STATA v11, StataCorp, USA).
3. Results
3.1. Compounds of F. vulgare and M. piperita EOs
Identified compounds in the EOs using GC-MS analysis are listed in Table 2. trans-Anethole (52.6%), limonene (11.1%), carvone (8.2%), tarragon (7.7%), and fenchone (4.5%) are five major compounds in F. vulgare EO. Besides, menthol (31.1%), menthone (22.1%), camphane (7.0%), menthofuran (6.0%), and iso-menthone (5.9%) are major compounds in M. piperita EO.
Table 2.
Identified compounds in the EOs using GC-MS analysis.
| RTa | Compound | F. vulgare | M. piperita | ||||
|---|---|---|---|---|---|---|---|
| Area | % | RIb | Area | % | RI | ||
| 7.0 | α-Pinene | 2358139618 | 1.4 | 932 | — | — | — |
| 8.3 | β-Pinene | — | — | — | 63432312 | 1.3 | 979 |
| 9.5 | α-Phellandrene | 1929797231 | 1.2 | 1027 | — | — | — |
| 11.1 | Limonene | 18097975121 | 11.1 | 1029 | — | — | — |
| 13.4 | Fenchone | 7258724167 | 4.5 | 1083 | — | — | — |
| 13.9 | 1,8-Cineole | — | — | — | 207426444 | 4.1 | 1026 |
| 15.7 | trans-Sabinene hydrate | — | — | — | 47072634 | 1.0 | 1098 |
| 18.1 | Tarragon | 12583158254 | 7.7 | 1196 | — | — | — |
| 19.6 | Fenchyl acetate | 4922665422 | 3.0 | 1218 | — | — | — |
| 20.0 | Carvone | 13304259306 | 8.2 | 1243 | — | — | — |
| 20.0 | Menthone | — | — | — | 1105246066 | 22.1 | 1152 |
| 20.3 | Iso-menthone | — | — | — | 293121099 | 5.9 | 1162 |
| 20.4 | Menthofuran | — | — | — | 301336044 | 6.0 | 1164 |
| 21.2 | Menthol | — | — | — | 1553773516 | 31.1 | 1172 |
| 23.5 | Pulegone | — | — | -- | 104297121 | 2.1 | 1273 |
| 23.8 | trans-Anethole | 85700289131 | 52.6 | 1284 | — | — | — |
| 26.2 | Camphane | — | — | — | 351577121 | 7.0 | 1131 |
| 31.5 | trans-Caryophyllene | — | — | — | 150233448 | 3.0 | 1419 |
| 34.0 | Germacrene D | — | — | — | 92534749 | 1.9 | 1481 |
| 35.6 | Dillapiole | 1547681987 | 1.0 | 1622 | — | — | — |
aRetention time (min), bretention index.
3.2. Ten Prepared Nanoemulsions and Two Nanoemulsion-Based Nanogels
Five nanoemulsions containing 2.0% F. vulgare EO were prepared (Table 2, Nos. 1–5). Sample No. 1, with proper size characteristics, i.e., droplet size of 74 ± 7 nm and SPAN of 0.97, was selected as the optimum sample for gelation. In addition, five nanoemulsions containing M. piperita EO (Table 2, Nos. 6–10) were prepared; No. 7 with 136 ± 5 nm droplet size and 0.96 SPAN was selected as the optimum nanoemulsion for gelation. DLS profiles of two selected nanoemulsions are depicted in Figure 2.
Figure 2.
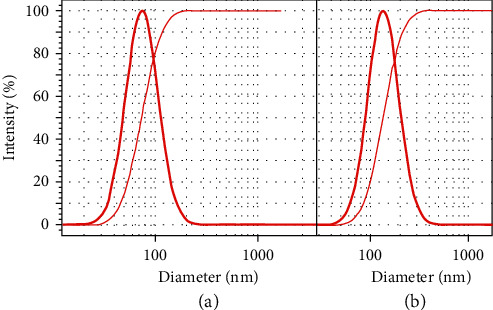
DLS profiles of optimum nanoemulsions containing EOs of (a) F. vulgare (74 ± 7 nm) and (b) M. piperita (136 ± 5 nm).
Two of the mentioned nanoemulsions were gelified using 3.5% w/v CMC. Their viscosity at different shear rates (0.1–100 1/s) was fully fitted with a well-known non-Newtonian regression, the Carreau–Yasuda model (Figure 3). In non-Newtonian fluids, viscosity decreases with an increasing shear rate [34].
Figure 3.
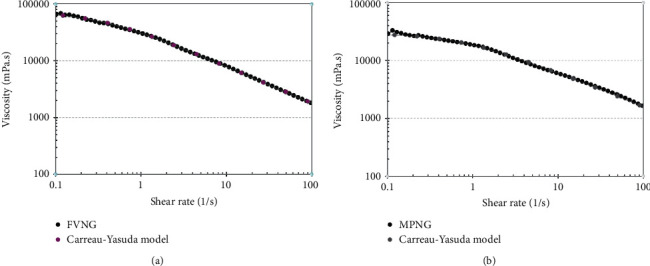
Viscosity of the nanogels containing (a) F. vulgare EO (FVNG) and (b) M. piperita EO (MPNG) at different shear rates fully fitted with the Carreau–Yasuda model.
3.3. Successful Loading of the EOs in Nanogels
In the ATR-FTIR spectrum of F. vulgare EO (Figure 4(a)), the absorption peaks at around 2834–3022 cm−1 are associated with stretching –OH and NH2 groups. The peaks observed at 1737 cm−1 and 1607 cm−1 are assigned to the stretching vibration of the –C=O group. Several characteristic peaks appeared at 1509 cm−1 (-NH is plane bend and –CN stretching), 1414, 1440, and 1243 cm−1 (-NH bending and –CN stretching), 1035–1174 cm−1 (aromatic C–H in the plane bend), and 837 cm−1 (out of the plane –NH bending). A similar observation for F. vulgare EO was reported in the literature [35]. In the spectra, blank gel (Figure 4(b)), the prominent peak at 3508–3698 cm−1 is assigned to the OH group in CMC [36]. The bands that appeared at 2922, 1581, and 1461 cm−1 are related to the stretching vibration of C-H, C=O stretching, and hydrocarbon groups (-CH2), respectively, in CMC. The peak at 1080–1252 cm−1 is attributed to the CMC's ether groups (-O- stretching). In F. vulgare, nanogel (FVNG) (Figure 4(c)) retained most of the peaks that appeared in the spectra of blank gel and F. vulgare EO confirmed the loading of the EO into the nanogel structure, although some changes in the position and intensity of peaks were identified.
Figure 4.
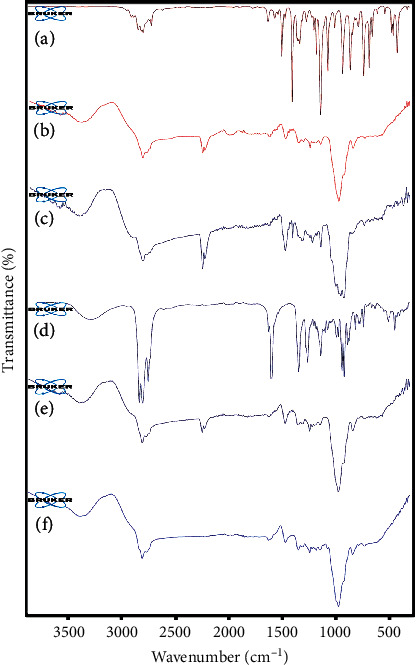
ATR-FTIR spectra of (a) F. vulgare EO, (b) blank gel, (c) nanogel containing F. vulgare EO (FVNG), (d) M. piperita EO, (e) blank gel, and (f) nanogel containing M. piperita EO (MPNG).
In the spectrum of M. piperita (Figure 4(d)), different functional groups such as alkanes, phenols, alkenes, ethers, alcohol, ester, and carboxylic acid are observed [37, 38]. The major peak at 3400 cm−1 is attributed to the hydrogen-bonded alcohol and phenols. The bands observed at 2869, 2922, 2953, 1287, 1368, and 1455 cm−1 are assigned to alkanes' C–H stretching. The absorption peak observed at 1710 cm−1 is related to C=C starching. The bands that appeared at 1044 and 1079 cm−1 are attributed to the C–O vibration of ethers, alcohol, esters, and carboxylic acids. The characteristic band around 875 cm−1 could be related to alkenes' C–H bonds. The main absorption peaks of blank gel (Figure 4(e)) of this nanogel are like previous blank gel and interpreted above. In the FTIR spectrum of M. piperita EO nanogel (Figure 4(f)), some peaks' changes in shape and intensity were identified, which correspond to the possible interaction between M. piperita EO and CMC. For instance, the absorption band at 1581 cm−1 attributed to C=O in CMC was shifted to 1579 cm−1 in the spectrum of prepared nanogel. Moreover, the intensity of the peaks observed at 2869, 2922, and 2953 cm−1 assigned to the C–H stretching of alkanes in M. piperita EO was changed in the spectrum of the final nanogel. Finally, the presence of characteristic peaks of both CMC and M. piperita EO in the FTIR spectra of MPNG indicates both components' existence in the structure of obtained nanogels.
3.4. Repellent Properties of the Nanogels
From Figure 5, MPNG, with a complete protection time of 120 ± 8 min, was significantly more potent than FVNG (70 ± 6). However, its efficacy was less than DEET, with a protection time of 140 ± 8 (P < 0.01). Besides, both blank gels with 3 min of complete protection time did not show proper efficacy.
Figure 5.
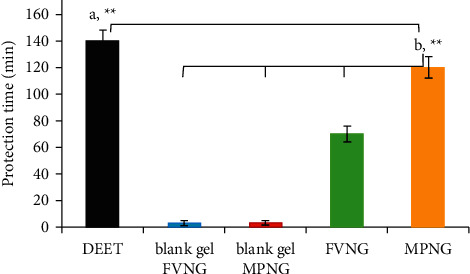
Complete protection times of nanogels containing F. vulgare and M. piperita EOs (FVNG and MPNG), blank gels (without EO), and DEET against An. stephensi. (a) Efficacy of DEET is more than MPNG (∗∗P < 0.01). (b) The efficacy of MPNG is more potent than FVNG and both blank gel (∗∗∗P < 0.001).
3.5. Antibacterial Effects of the Nanogels
The antibacterial effects of the nanogels and their blank gels against E. coli and S. aureus are depicted in Figures 6 and 7. The growth of E. coli after treatment with 1250, 2500, and 5000 µg/mL of FVNG was not significantly reduced (growth ≥ 92%). However, the growth of S. aureus after treatment with 5000 µg/mL FVNG was reduced by 30%.
Figure 6.
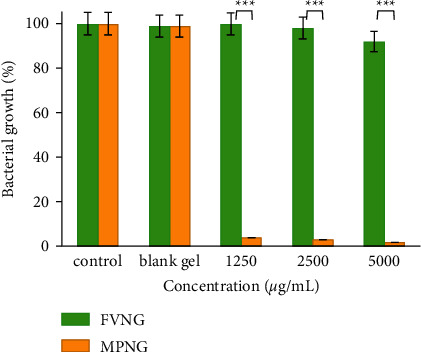
Antibacterial effects of nanogels containing F. vulgare and M. piperita EOs (FVNG and MPNG) and their blank gels (without EO) against E. coli. The efficacy of MPNG was more potent (∗∗∗P < 0.001) than FVNG.
Figure 7.
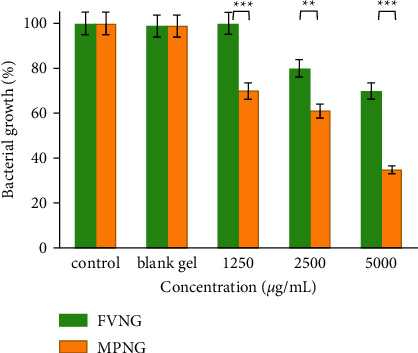
Antibacterial effects of nanogels containing F. vulgare and M. piperita EOs (FVNG and MPNG) and their blank gels (without EO) against S. aureus. The efficacy of MPNG was more potent (∗∗∗P < 0.001 and ∗∗P < 0.01) than FVNG.
Besides, after treatment with MPNG 1250, 2500, and 5000 µg/mL, the growth of E. coli was substantially reduced (≥ 96%). However, after treating S. aureus at those concentrations, bacterial growth was observed at 70, 61, and 35%. Moreover, both nanogels did not affect the growth of both bacterial types.
4. Discussion
Mosquitoes (Diptera: Culicidae) transmit malaria, dengue, yellow fever, encephalitis, filariasis, chikungunya, and Zika virus [39, 40]. Indoor residual spraying and insecticide-impregnated bed nets are core components of malaria prevention and elimination strategies, and repellents are also recommended in endemic regions [41, 42]. Repellents are substances that deter mosquitoes (or insects) from flying to, landing on, or biting human, animal skin, and surfaces [43, 44]. Due to mosquitoes' resistance to industrial repellents and their adverse effects on human health, many attempts have recently been made to develop natural nanorepellents. For instance, a solid lipid nanoparticle containing 1% Zataria multiflora EO was introduced with a 90 min protection time against An. stephensi [45]. Besides, the literature reported nanoemulsions containing 15% Eucalyptus globulus EO with 170 min protection time against a mixture of mosquitoes [46]. Moreover, nanoemulsion containing 50% M. piperita EO with 257 min protection time against An. stephensi was also reported [47].
In developing essential oil-based repellents, controlling the pungent odor of EOs is a challenge; the corresponding author of this article has observed that volunteers refuse to use pungent odor repellents. A practical solution to control the odor of colloidal nanoformulations (such as nanoemulsion or nanoparticles) is to turn them into nanogels. Besides, their topical application is also facilitated due to the increased viscosity. Therefore, in the current study, nanogels dosage form was used; interestingly, MPNG showed a 120 min repellent effect against An. stephensi.
Symbiotic and opportunistic bacteria commonly influence the skin as the body's first barrier against environmental pathogens; they can cause pain, swelling, and skin color changes [48, 49]. Moreover, some opportunistic bacteria could enter the body through open wounds, possibly leading to bloodstream infections like septicemia [50, 51]. In the current study, the growth of E. coli after treatment with 1250 µg/mL MPNG was reduced by more than 95%. On the other hand, the efficacy of MPNG against S. aureus was less than E. coli; the growth after treatment with 5000 µg/mL was reduced by 65%. Some reports with promising antibacterial effects of nonformulated EOs and nanostructures containing EOs have been found in the literature. For instance, the antibacterial activity of Juniper communis EO against standard strains of Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Streptococcus pyogenes was investigated [52]. Besides, the viability of S. aureus and E. coli after 24 h exposure to polycaprolactone nanofibers containing Mentha piperita EO was decreased to around 50% [53]. Moreover, the E. coli colonies were reduced by about 4.0 log CFU/mL after treatment with thyme EO nanoemulsion [54].
5. Conclusions
This study aimed to develop two natural nanogels using F. vulgare and M. piperita EOs as mosquito repellent and antibacterial agent prototypes. The complete protection times of the nanogels against An. stephensi were observed as 70 (±6) and 120 (±8) min. The nanogel containing F. vulgare EO showed some degree of antibacterial effects against E. coli and S. aureus. However, after treatment with nanogel of M. piperita EO 100 and 65%, their growth was reduced. Therefore, the nanogel of M. piperita EO could be considered for further investigations as mosquito repellent and antibacterial agent.
Acknowledgments
This study was supported by Fasa University of Medical Sciences (400130).
Data Availability
The data used to support this study are available from the corresponding author upon request.
Ethical Approval
This study was ethically approved by the Ethical Committee of Fasa University of Medical Sciences, IR.FUMS.REC.1400.104. Moreover, all methods in the current study were performed according to the WHO (World Health Organization) guidelines and national regulations.
Consent
Not applicable.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
ASD performed repellent assays. AA performed antibacterial tests. FK prepared nanogels. MS interpreted the ATR-FTIR spectra. GhGh contributed to the antibacterial assay and wrote the introduction. MO designed the study, analyzed the data, and drafted the MS. All authors contributed to the drafting of the manuscript and approved the final version.
References
- 1.Nazzaro F., Fratianni F., De Martino L., Coppola R., De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals . 2013;6(12):1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferraz C. A., Pastorinho M. R., Palmeira-de-Oliveira A., Sousa A. C. Ecotoxicity of plant extracts and essential oils: a review. Environmental Pollution . 2022;292 doi: 10.1016/j.envpol.2021.118319.118319 [DOI] [PubMed] [Google Scholar]
- 3.Noorpisheh Ghadimi S., Sharifi N., Osanloo M. The leishmanicidal activity of essential oils: a systematic review. Journal of Herbmed Pharmacology . 2020;9(4):300–308. doi: 10.34172/jhp.2020.38. [DOI] [Google Scholar]
- 4.Osanloo M., Ghaznavi G., Abdollahi A. Surveying the chemical composition and antibacterial activity of essential oils from selected medicinal plants against human pathogens. Iranian Journal of Microbiology . 2020;12(6):577–583. doi: 10.18502/ijm.v12i6.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nerio L. S., Olivero-Verbel J., Stashenko E. Repellent activity of essential oils: a review. Bioresource Technology . 2010;101(1):372–378. doi: 10.1016/j.biortech.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 6.Jahanian H., Kahkeshani N., Sanei-Dehkordi A., Isman M. B., Saeedi M., Khanavi M. Rosmarinus officinalis as a natural insecticide: a review. International Journal of Pest Management . 2022:1–46. doi: 10.1080/09670874.2022.2046889. [DOI] [Google Scholar]
- 7.Turek C., Stintzing F. C. Stability of essential oils: a review. Comprehensive Reviews in Food Science and Food Safety . 2013;12(1):40–53. doi: 10.1111/1541-4337.12006. [DOI] [Google Scholar]
- 8.Esmaili F., Sanei-Dehkordi A., Amoozegar F., Osanloo M. A review on the use of essential oil-based nanoformulations in control of mosquitoes. Biointerface Research in Applied Chemistry . 2021;11(5):12516–12529. [Google Scholar]
- 9.Kabanov A. V., Vinogradov S. V. Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angewandte Chemie International Edition . 2009;48(30):5418–5429. doi: 10.1002/anie.200900441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zha L., Banik B., Alexis F. Stimulus responsive nanogels for drug delivery. Soft Matter . 2011;7(13):5908–5916. doi: 10.1039/c0sm01307b. [DOI] [Google Scholar]
- 11.Lestari E. S., Severin J. A., Filius P. M. G., et al. Antimicrobial resistance among commensal isolates of Escherichia coli and Staphylococcus aureus in the Indonesian population inside and outside hospitals. European Journal of Clinical Microbiology & Infectious Diseases: Official Publication of the European Society of Clinical Microbiology . 2008;27(1):45–51. doi: 10.1007/s10096-007-0396-z. [DOI] [PubMed] [Google Scholar]
- 12.Alizadeh Behbahani B., Tabatabaei Yazdi F., Shahidi F., Mohebbi M. Antimicrobial activity of Avicennia marina extract against Staphylococcus aureus. 2nd National Congress on Medicinal Plants . 2013;2 [Google Scholar]
- 13.Jose B., Reddy L. J. Evaluation of antibacterial activity of the leaf and flower essential oils of Gliricidia sepium from south India. International Journal of Applied Pharmaceutics . 2010;2(2):20–22. [Google Scholar]
- 14.Who W. H. O. 2021. World malaria report 2021.
- 15.Who W. H. O. https://www.who.int/news-room/fact-sheets/detail/malaria Malaria fact sheet 2021 [6.2021]
- 16.Surendran S. N., Sivabalakrishnan K., Sivasingham A., et al. Anthropogenic factors driving recent range expansion of the malaria vector Anopheles stephensi. Frontiers in Public Health . 2019;7:53. doi: 10.3389/fpubh.2019.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma A., Deshmukh A., Sharma R., et al. Population genetic structure of malaria vector Anopheles stephensi using mitochondrial Cytochrome oxidase II gene in Indian populations. Indian Journal of Experimental Biology . 2014;52(10):996–1002. [PubMed] [Google Scholar]
- 18.Oshaghi M., Yaaghoobi F., Vatandoost H., Abaei M., Akbarzadeh K. Anopheles stephensi biological forms; geographical distribution and malaria transmission in malarious regions of Iran. Pakistan Journal of Biological Sciences . 2006;9(2):294–298. doi: 10.3923/pjbs.2006.294.298. [DOI] [Google Scholar]
- 19.Syafruddin D., Asih P. B. S., Rozi I. E., et al. Efficacy of a spatial repellent for control of malaria in Indonesia: a cluster-randomized controlled trial. The American Journal of Tropical Medicine and Hygiene . 2020;103(1):344–358. doi: 10.4269/ajtmh.19-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore S. J., Darling S. T., Sihuincha M., Padilla N., Devine G. J. A low-cost repellent for malaria vectors in the Americas: results of two field trials in Guatemala and Peru. Malaria Journal . 2007;6(1):101–106. doi: 10.1186/1475-2875-6-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norris E. J., Coats J. R. Current and future repellent technologies: the potential of spatial repellents and their place in mosquito-borne disease control. International Journal of Environmental Research and Public Health . 2017;14(2):124. doi: 10.3390/ijerph14020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly A. H., Koudakossi H. N. B., Moore S. J. Repellents and new “Spaces of concern” in global health. Medical Anthropology . 2017;36(5):464–478. doi: 10.1080/01459740.2017.1327957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ditzen M., Pellegrino M., Vosshall L. B. Insect odorant receptors are molecular targets of the insect repellent DEET. Science . 2008;319(5871):1838–1842. doi: 10.1126/science.1153121. [DOI] [PubMed] [Google Scholar]
- 24.Brown M., Hebert A. A. Insect repellents: an overview. Journal of the American Academy of Dermatology . 1997;36(2):243–249. doi: 10.1016/s0190-9622(97)70289-5. [DOI] [PubMed] [Google Scholar]
- 25.Swale D. R., Bloomquist J. R. Is DEET a dangerous neurotoxicant? Pest Management Science . 2019;75(8):2068–2070. doi: 10.1002/ps.5476. [DOI] [PubMed] [Google Scholar]
- 26.Rowland M., Downey G., Rab A., et al. DEET mosquito repellent provides personal protection against malaria: a household randomized trial in an Afghan refugee camp in Pakistan. Tropical Medicine and International Health . 2004;9(3):335–342. doi: 10.1111/j.1365-3156.2004.01198.x. [DOI] [PubMed] [Google Scholar]
- 27.Badgujar S. B., Patel V. V., Bandivdekar A. H. Foeniculum vulgare Mill: a review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. BioMed Research International . 2014;2014:1–32. doi: 10.1155/2014/842674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh R., Shushni M. A., Belkheir A. Antibacterial and antioxidant activities of Mentha piperita L. Arabian Journal of Chemistry . 2015;8(3):322–328. doi: 10.1016/j.arabjc.2011.01.019. [DOI] [Google Scholar]
- 29.Zoubiri S., Baaliouamer A., Seba N., Chamouni N. Chemical composition and larvicidal activity of Algerian Foeniculum vulgare seed essential oil. Arabian Journal of Chemistry . 2014;7(4):480–485. doi: 10.1016/j.arabjc.2010.11.006. [DOI] [Google Scholar]
- 30.Moemenbellah-Fard M. D., Shahriari-Namadi M., Kelidari H. R., Nejad Z. B., Ghasemi H., Osanloo M. Chemical composition and repellent activity of nine medicinal essential oils against Anopheles stephensi, the main malaria vector. International Journal of Tropical Insect Science . 2021;41(2):1325–1332. doi: 10.1007/s42690-020-00325-2. [DOI] [Google Scholar]
- 31.Ghanbariasad A., Valizadeh A., Ghadimi S. N., Fereidouni Z., Osanloo M. Nanoformulating Cinnamomum zeylanicum essential oil with an extreme effect on Leishmania tropica and Leishmania major. Journal of Drug Delivery Science and Technology . 2021;63 doi: 10.1016/j.jddst.2021.102436.102436 [DOI] [Google Scholar]
- 32.Qasemi H., Fereidouni Z., Karimi J., et al. Promising antibacterial effect of impregnated nanofiber mats with a green nanogel against clinical and standard strains of Pseudomonas aeruginosa and Staphylococcus aureus. Journal of Drug Delivery Science and Technology . 66 doi: 10.1016/j.jddst.2021.102844.102844 [DOI] [Google Scholar]
- 33.Who W. H. O. Guidelines for Efficacy Testing of Mosquito Repellents for Human Skin . Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 34.Moemenbellah-Fard M. D., Firoozian S., Shahriari-Namadi M., Zarenezhad E., Roozitalab G., Osanloo M. A natural nanogel with higher efficacy than a standard repellent against the primary malaria mosquito vector, Anopheles stephensi Liston. Chemical Papers . 2022;76(3):1767–1776. doi: 10.1007/s11696-021-02006-x. [DOI] [Google Scholar]
- 35.Kumar A., Pratap Singh P., Prakash B. Unravelling the antifungal and anti-aflatoxin B1 mechanism of chitosan nanocomposite incorporated with Foeniculum vulgare essential oil. Carbohydrate Polymers . 2020;236 doi: 10.1016/j.carbpol.2020.116050.116050 [DOI] [PubMed] [Google Scholar]
- 36.Klunklin W., Jantanasakulwong K., Phimolsiripol Y., et al. Synthesis, characterization, and application of carboxymethyl cellulose from asparagus stalk end. Polymers . 2020;13(1):p. 81. doi: 10.3390/polym13010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashrafi B., Rashidipour M., Marzban A., et al. Mentha piperita essential oils loaded in a chitosan nanogel with inhibitory effect on biofilm formation against S. mutans on the dental surface. Carbohydrate Polymers . 2019;212:142–149. doi: 10.1016/j.carbpol.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Al-Bayati F. A. Isolation and identification of antimicrobial compound from Mentha longifolia L. leaves grown wild in Iraq. Annals of Clinical Microbiology and Antimicrobials . 2009;8(1):p. 20. doi: 10.1186/1476-0711-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bowman L. R., Donegan S., McCall P. J. Is dengue vector control deficient in effectiveness or evidence?: systematic review and meta-analysis. PLoS Neglected Tropical Diseases . 2016;10(3) doi: 10.1371/journal.pntd.0004551.e0004551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franklinos L. H. V., Jones K. E., Redding D. W., Abubakar I. The effect of global change on mosquito-borne disease. The Lancet Infectious Diseases . 2019;19(9):e302–e312. doi: 10.1016/s1473-3099(19)30161-6. [DOI] [PubMed] [Google Scholar]
- 41.Soonwera M. Efficacy of essential oil from cananga odorata (lamk.) Hook.f. & thomson (annonaceae) against three mosquito species Aedes aegypti (L.), Anopheles dirus (peyton and harrison), and Culex quinquefasciatus (say) Parasitology Research . 2015;114(12):4531–4543. doi: 10.1007/s00436-015-4699-1. [DOI] [PubMed] [Google Scholar]
- 42.Neghab M., Alipour H. Tick-borne Crimean-Congo haemorrhagic fever in Fars province, southern Iran: epidemiologic characteristics and vector surveillance. Pakistan Journal of Biological Sciences . 2006;9(14):2681–2684. doi: 10.3923/pjbs.2006.2681.2684. [DOI] [Google Scholar]
- 43.Choochote W., Chaithong U., Kamsuk K., et al. Adulticidal activity against Stegomyia aegypti (Diptera: Culicidae) of three Piper spp. Revista do Instituto de Medicina Tropical de São Paulo . 2006;48(1):33–37. doi: 10.1590/s0036-46652006000100007. [DOI] [PubMed] [Google Scholar]
- 44.Blackwell A., Stuart A., Estambale B. B. The repellent and antifeedant activity of Myrica Gale oil against Aedes aegypti mosquitoes and its enhancement by the addition of salicyluric acid. The Journal of the Royal College of Physicians of Edinburgh . 2003;33(3):209–214. [Google Scholar]
- 45.Kelidari H. R., Moemenbellah-Fard M. D., Morteza-Semnani K., et al. Solid-lipid nanoparticles (SLN)s containing Zataria multiflora essential oil with no-cytotoxicity and potent repellent activity against Anopheles stephensi. Journal of Parasitic Diseases . 2021;45(1):101–108. doi: 10.1007/s12639-020-01281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Navayan A., Moghimipour E., Khodayar M. J., et al. Evaluation of the mosquito repellent activity of nano-sized microemulsion of Eucalyptus globulus essential oil against culicinae. Jundishapur Journal of Natural Pharmaceutical Products . 2017;In Press(In Press) doi: 10.5812/jjnpp.55626.e55626 [DOI] [Google Scholar]
- 47.Khoobdel M., Mohammadi R., Negahban M., Khani S. Nanoemulsified Mentha piperita and Eucalyptus globulus oils exhibit enhanced repellent activities against Anopheles stephensi. Asian Pacific Journal of Tropical Medicine . 2019;12(11):520–527. doi: 10.4103/1995-7645.271292. [DOI] [Google Scholar]
- 48.Barnes M. E., Brown M. L. A review of flavobacterium psychrophilum biology, clinical signs, and bacterial cold water disease prevention and treat. The Open Fish Science Journal . 2011;4(1):40–48. doi: 10.2174/1874401x01104010040. [DOI] [Google Scholar]
- 49.Esposito S., Ascione T., Pagliano P. Management of bacterial skin and skin structure infections with polymicrobial etiology. Expert Review of Anti-infective Therapy . 2019;17(1):17–25. doi: 10.1080/14787210.2019.1552518. [DOI] [PubMed] [Google Scholar]
- 50.Zhen X., Lundborg C. S., Sun X., Hu X., Dong H. Economic burden of antibiotic resistance in ESKAPE organisms: a systematic review. Antimicrobial Resistance and Infection Control . 2019;8(1):p. 137. doi: 10.1186/s13756-019-0590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poulakou G., Lagou S., Tsiodras S. What’s new in the epidemiology of skin and soft tissue infections in 2018? Current Opinion in Infectious Diseases . 2019;32(2):77–86. doi: 10.1097/qco.0000000000000527. [DOI] [PubMed] [Google Scholar]
- 52.Dumitrescu E., Muselin F., Dumitrescu C. S., et al. Juniper communis L. Essential oils from western Romanian carpathians: bio-structure and effective antibacterial activity. Applied Sciences . 2022;12(6) doi: 10.3390/app12062949.2949 [DOI] [Google Scholar]
- 53.Unalan I., Slavik B., Buettner A., Goldmann W. H., Frank G., Boccaccini A. R. Physical and antibacterial properties of peppermint essential oil loaded poly (ɛ-caprolactone) (PCL) electrospun fiber mats for wound healing. Frontiers in Bioengineering and Biotechnology . 2019;7 doi: 10.3389/fbioe.2019.00346.346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo M., Zhang L., He Q., et al. Synergistic antibacterial effects of ultrasound and thyme essential oils nanoemulsion against Escherichia coli O157:H7. Ultrasonics Sonochemistry . 2020;66 doi: 10.1016/j.ultsonch.2020.104988.104988 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support this study are available from the corresponding author upon request.


