Highlights
-
•
The overall prevalence of latent tuberculosis infection (LTBI) in this study was 63.36%.
-
•
The positivity rate for the tuberculin skin test was higher compared with the QuantiFERON-TB Gold Plus test.
-
•
The prevalence of LTBI was high among slaughterhouse workers (100%).
-
•
Protozoal infection was found to be significantly associated with LTBI.
Keywords: TB high-risk groups, Latent tuberculosis infection, Magnitude, Interferon-gamma release assay, Tuberculin skin test, Burkina Faso, One Health
Abstract
Objectives
To determine the prevalence and risk factors for latent tuberculosis infection (LTBI) among three high-risk groups – household contacts of TB index cases, healthcare workers and slaughterhouse workers – in Bobo-Dioulasso, Burkina Faso.
Methods
Participants were recruited to this cross-sectional study from March to July 2020 after giving informed consent. Sociodemographic, clinical and biological data were collected using a structured questionnaire. The QuantiFERON-TB Gold Plus test (QFT-Plus) and the tuberculin skin test (TST) were used for detection of LTBI. Bivariate and multivariate logistic regression analyses were performed to identify risk factors for LTBI.
Results
The prevalence of LTBI among 101 participants (age range 15–68 years) was 67.33% [95% confidence interval (CI) 57.27–76.33] and 84.16% (95% CI 75.55–90.66) based on QFT-Plus and TST results, respectively. Compared with healthcare workers and household contacts of TB index cases, the prevalence of LTBI among slaughterhouse workers was significantly higher for both QTF-Plus (96.8%; P<0.001) and TST (100%; P=0.003). Working in a slaughterhouse [adjusted odds ratio (AOR) 1.095, 95% CI 1.00–2.036], smoking (AOR 4.214, 95% CI 1.051–16.899), ≥15 years of exposure (AOR 5.617, 95% CI 1.202–32.198), having an animal at home (AOR 2.735, 95% CI 1.102–6.789) and protozoal infection (AOR 2.591, 95% CI 1.034–6.491) were significantly associated with LTBI on the QFT-Plus assay.
Conclusion
The prevalence of LTBI was high in all three groups, particularly slaughterhouse workers. The risk factors identified could form the basis of targeted intervention.
Introduction
Tuberculosis (TB) is among the top 10 causes of death in the world, despite being curable and preventable. There were 1.4 million deaths due to TB and 10 million cases of TB in 2019, and most of these cases were reported from African and Asian countries (World Health Organization, 2019). TB is caused by Mycobacterium tuberculosis complex (MTBC), and the disease is spread when the source expels bacteria into the air to a susceptible host, causing active TB disease or latent tuberculous infection (LTBI) without evidence of manifestation of symptoms of active disease (López de Goicoechea-Saiz et al., 2018).
An estimated 1.7 billion people are living with LTBI globally, and 5–10% of them are expected to develop active TB (Sadananda et al., 2020). The risk of active TB increases with occurrence of comorbidities such as human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) or occupational exposure. High prevalence of LTBI has been reported in populations at risk, such as miners, healthcare workers (HCWs) due to their professional exposure, and individuals suffering from diabetes and malnutrition in high-incidence countries (Baussano et al., 2011; Basera et al., 2017; Qader et al., 2021). Similarly, household contacts of smear-positive TB patients are at risk for LTBI because they share the same environment (Eom et al., 2018). In addition, it has been well demonstrated in developed countries that people in close contact with infected animals, such as slaughterhouse workers (SWs), can contract TB from animals (zoonotic TB) (Mbugi et al., 2017). However, the situation in low-resource countries, where disease control in cattle is poor, remains poorly documented (Torres-Gonzalez et al., 2013). Individuals infected with TB pathogens present an increased risk of progression to active disease, and constitute a potential source of infection. A significant number of cases of active TB occur in people with LTBI within a short time following primary infection, suggesting the need for LTBI intervention (Wang et al., 2020). In view of this, the World Health Organization (WHO) has set up the End TB strategy, which aims to reduce the number of TB deaths by 95% and the number of new cases by 90% in 20 years (2015–2035) (MacNeil, 2020). In order to achieve these ambitious goals, it is important to use diagnostic strategies as a central element to promote the detection and early management of cases of TB (Uplekar et al., 2015). Use of the tuberculin skin test (TST) and interferon-gamma release assay (IGRA) contitutes a valuable approach for early identification of people at risk for TB infection (Carranza et al., 2020).
In Burkina Faso, the burden of TB is high, and all segments of the population are affected. The recent WHO report estimated that there were 9500 cases of TB in Burkina Faso, including HIV co-infection, with an incidence of 48 per 100,000 population (World Health Organization, 2019). An understanding of the factors associated with acquiring and transmitting MTBC is a prerequisite for TB control in Burkina Faso. Although the 2020 WHO consolidated guidelines on TB recommend systematic testing and treatment of LTBI in populations at risk, to the authors’ knowledge, there is no data on LTBI in Burkina Faso. This study screened for LTBI among three groups at high risk for TB: household contacts of TB index cases, HCWs and SWs. The participants were recruited in urban Bobo-Dioulasso, screened for LTBI using the two WHO-recommended diagnostic tools, and factors associated with LTBI were identified.
Materials and methods
Study site
This cross-sectional study was conducted from March to July 2020 in Bobo-Dioulasso (11°10’42” N; 4°17’35” W); this city is the economical capital of Burkina Faso, and is located in the western part of the country. The study collection sites were the regional centre for TB control, the medical centre in Dafra, the medical centre in Do, and the slaughterhouse in Bobo-Dioulasso (Figure 1).
Figure 1.
Map of study area.
Study population and sample collection
The study participants were household contacts of TB index cases, HCWs and SWs who gave their informed consent. A stool sample, urine sample, 4-mL blood sample in a lithium heparin tube (for the IGRA test) and 4-mL blood sample in an ethylenediaminetetraacetic acid tube (for immuno-haematological tests) were collected from each participant. All samples were sent to Centre MURAZ for analysis. Sociodemographic, clinical and anthropometric parameters and biological data were collected.
Tests performed
QuantiFERON-TB Plus (QFT-Plus) test
To avoid the booster effect, a blood sample was collected from each participant by venipuncture into a 4-mL heparinized tube for QFT-Plus (Qiagen, Hiden, Germany), and then TST was performed.
QFT-Plus includes new antigens designed to increase the sensitivity of the test compared with the previous IGRA tool (QTF-GIT). In brief, 1 mL of blood was drawn directly into each of four separate tubes: nil control (negative control), mitogen control (positive control containing phytohemagglutinin), TB1 (containing MTBC-specific antigens ESAT-6 and CFP-10 modified for eliciting CD4+ T-cell responses) and TB2 (containing MTBC-specific antigens ESAT-6 and CFP-10 modified for eliciting CD8+ T-cell responses). After filling, the tubes were inverted slowly 10 times to coat the sides, and placed in an incubator at 37°C for 16–24 h. All tubes were centrifuged at 3000 × g for 15 min to separate the plasma, and were stored at -20°C before analysis. The QFT-Plus interferon-gamma enzyme-linked immunosorbent assay (ELISA) was performed with plasma on the EVOLIS machine (BIO-RAD, Hercules, CA, USA), an automated ELISA processor. Results were calculated using QFT-Plus Analysis Software Version 2.71.2, as described by the manufacturer (Bongomin et al., 2021; Qiagen: QuantiFERON-TB Gold Plus (QFT-Plus) ELISA... - Google Scholar, 2021 n.d.).
Results of LTBI were defined as an interferon-gamma concentration ≥0.35 IU/mL (calculated as either TB1 or TB2 antigen minus nil) according to the manufacturer's guideline. If antigen minus nil was <0.35 IU/mL or <25% of the nil value, when the mitogen value was ≥0.5 IU/mL, the result was considered negative. If (1) nil was >8 IU/mL or (2) antigen minus nil was ≥0.35 IU/mL and <25% of the nil value when nil was ≤8.0 IU/mL and the mitogen value was <0.5 IU/mL, the results were considered indeterminate (Qiagen: QuantiFERON-TB Gold Plus (QFT-Plus) ELISA... - Google Scholar, 2021 n.d.)
TST using Tubertest method
TST was performed with an intradermal 0.1-mL injection of tuberculin, equivalent to 5 IU Tubertest (Sanofi Pasteur, Paris, France), in the front side of the forearm. The diameter of the indurated area was measured 48–72 h later (Rieder et al., 2011), and was considered positive when the area was ≥5 mm, as indicated by the manufacturer.
Parasitological screening test
Each stool sample was prepared and treated using the Kato-Katz and formol ether concentration methods, as well as the direct saline/iodine method, in order to diagnose infections with intestinal parasites. In addition, urine samples were examined qualitatively to screen for Schistosoma spp. using the urinary sediment method, and by rapid point-of-care circulating cathodic antigen, as described previouly (Cisse et al., 2021).
Data management and statistical analysis
Data from questionnaires and laboratory analyses were first entered into Excel 2016 (Microsoft Corp., Redmond, WA, USA), then cleaned and exported to Stata 14 (Stata Corp., College Station, TX, USA) for analyses. Chi-squared or Fisher's exact tests were used for bivariate analyses. A univariate logistic regression was performed initially to identify potential factors associated with the occurrence of LTBI. Next, a multivariable logistic regression model was built using a stepwise backward model by including all independent variables with P<0.2 on univariate logistic regression in the model. The final model had an inclusion criterion of P<0.05 and an exclusion criterion of P>0.10. The conditions of fitness of the final model were verified. The results are presented as odds ratio (OR) and 95% confidence interval (CI). P< 0.05 was considered to indicate statistical significance.
This research involved human participants, human material and human data; all activities were performed in accordance with the Declaration of Helsinki 2018.
Results
Sociodemographic and biological characteristics of the study population
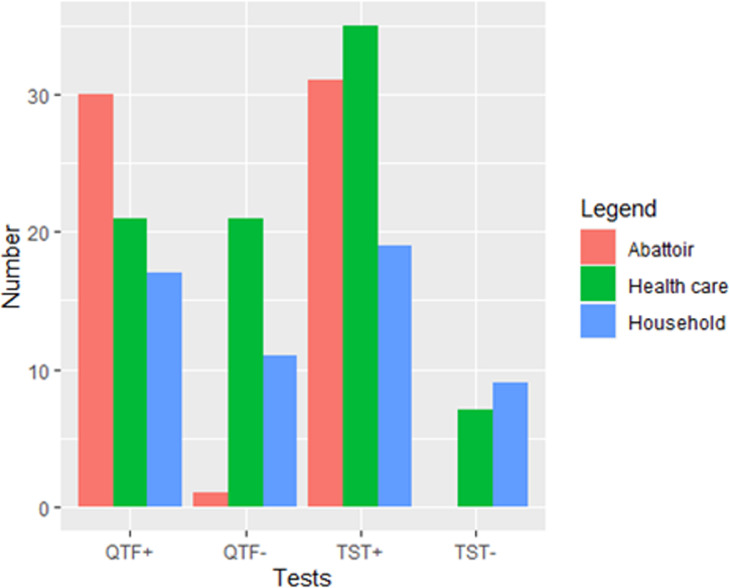
Study participants (n=103) were selected at random from slaughterhouses, healthcare facilities and households with TB index cases. Two household contacts were excluded because they had indeterminate results on the QTF-Plus assay. Baseline characteristics of the 101 subjects with reliable QTF-Plus and TST results are shown in Table 1 and Figure 2. Among these 101 subjects, 42 were HCWs, 31 were SWs and 28 were household contacts of TB index cases. The mean age was 38.52 (standard deviation 12.01) years. The sex ratio (male/female) was 1.97. Most participants (77%) had received the Bacillus Calmette-Guérin (BCG) vaccine, 52.48% of participants had a secondary education, and 61.39% were infected by intestinal protozoa.
Table 1.
Sociodemographic and biological characteristics of the study population (n=101).
| Variables | Category | n | Proportion (%) |
|---|---|---|---|
| Sex | Male | 67 | 66.34 |
| Female | 34 | 33.66 | |
| Age group (years) | 15–30 | 28 | 27.72 |
| 31–46 | 46 | 45.54 | |
| ≥47 | 27 | 26.73 | |
| Origin |
Healthcare worker | 42 | 41.58 |
| Household contact | 28 | 27.72 | |
| Slaughterhouse worker | 31 | 30.69 | |
| Body mass index (kg/m2) |
<18.5 | 4 | 3.96 |
| 18.5–24.9 | 53 | 52.48 | |
| 25–29.9 | 36 | 35.64 | |
| >30 | 8 | 7.92 | |
| Educational level |
Any | 16 | 15.84 |
| Primary | 12 | 11.88 | |
| Secondary | 53 | 52.48 | |
| University | 20 | 19.80 | |
| Marital status | Married | 73 | 72.28 |
| Single | 26 | 25.74 | |
| Other | 2 | 1.98 | |
| Smoking |
Yes | 20 | 19.80 |
| No | 81 | 80.20 | |
| Alcoholism |
Yes | 32 | 31.68 |
| No | 69 | 68.32 | |
| Diabetes |
Yes | 12 | 11.88 |
| No | 89 | 88.12 | |
| Hospitalization |
Yes | 5 | 4.95 |
| No | 96 | 95.05 | |
| Exposure (years) |
<15 | 68 | 67.33 |
| ≥15 | 33 | 32.67 | |
| BCG scar | Yes | 77 | 77.00 |
| No | 23 | 23.00 | |
| Chronic cough | Yes | 10 | 9.90 |
| No | 91 | 90.10 | |
| Presence of animal at home |
Yes | 55 | 54.46 |
| No | 46 | 45.54 | |
| Helminth infection | Yes | 15 | 14.85 |
| No | 86 | 85.15 | |
| Protozoal infection | Yes | 62 | 61.39 |
| No | 39 | 38.61 |
BCG, Bacillus Calmette-Guérin.
Figure 2.
Distribution of latent tuberculosis infection (LTBI) according to the type of participant and LTBI test.
Prevalence of LTBI based on test technique
The prevalence of LTBI among the 101 participants was found to be 67.33% (95% CI 57.27–76.33) using QFT-Plus and 84.16% (95% CI 75.55–90.66) using TST. The overall prevalence rate based on both tests was 63.36% (95% CI 52.18–71.82).
Compared with HCWs (50.0%) and household contacts of TB index cases (60.7%), the prevalence of LTBI among SWs was found to be significantly higher using QTF-Plus (96.8%; P<0.001). Based on TST results, the prevalence of LTBI among SWs (100%; P=0.003) was also higher compared with that in HCWs (83.3%) and household contacts of TB index cases (67.9%). A significant difference was observed with respect to origin of the participant and cohabitation with animals (P<0.05) for both QTF-Plus and TST. However, a positive result on QTF-Plus was significantly associated with a high frequency (61.39%) of protozoal infection in the participants (P=0.022), while TST positivity was significantly associated with gender (P<0.05), with 94.03% of cases being male (Table 2).
Table 2.
Bivariate analysis according to QuantiFERON-TB Gold Plus test (QFT-Plus) and tuberculin skin test (TST) (n=101).
| Variables | QFT-Plus–n (%) | QFT-Plus+n (%) | P-value | TST–n (%) | TST+n (%) | P-value |
|---|---|---|---|---|---|---|
| Sex | 0.194 | <0.001 | ||||
| Female | 14 (41.2) | 20 (58.8) | 12 (35.29) | 22 (64.71) | ||
| Male | 19 (28.4) | 48 (71.6) | 4 (5.97) | 63 (94.03) | ||
| Age group (years) | 0.566 | 0.095 | ||||
| 15–30 | 7 (25.0) | 21 (75.0) | 8 (28.57) | 20 (71.43) | ||
| 31–46 | 17 (37.0) | 29 (63.0) | 5 (10.87) | 41 (89.13) | ||
| ≥47 | 9 (33.3) | 18 (66.7) | 3 (11.12) | 24 (88.88) | ||
| Origin | <0.001 | 0.003 |
||||
| Healthcare worker | 21 (50.00) | 21 (50.00) | 7 (16.67) | 35 (83.33) | ||
| Household contact | 11 (39.30) | 17 (60.70) | 9 (32.14) | 19 (67.86) | ||
| Slaughterhouse worker | 1 (3.20) | 30 (96.80) | 0 (0.0) | 31 (100.00) | ||
| Body mass index (kg/m2) | 0.143 | 0.320 | ||||
| 18.5 | 3 (75.00) | 1 (25.00) | 0 (0.00) | 4 (100.00) | ||
| 18.5–24.9 | 17 (32.08) | 36 (67.92) | 7 (13.20) | 46 (86.80) | ||
| 25–29.9 | 9 (25.00) | 27 (75.00) | 6 (16.67) | 30 (83.33) | ||
| >30 | 4 (50.00) | 4 (50.00) | 3 (37.50) | 5 (62.50) | ||
| Educational level | 0.842 | 0.880 | ||||
| Any | 5 (31.20) | 11 (68.80) | 3 (18.75) | 13 (81.25) | ||
| Primary | 3 (25.00) | 9 (75.00) | 1 (8.34) | 11 (91.66) | ||
| Secondary | 17 (32.10) | 36 (67.90) | 9 (16.98) | 44 (83.02) | ||
| University | 8 (40.00) | 12 (60.00) | 3 (15.00) | 17 (85.00) | ||
| Smoking | 0.068 | 0.184 | ||||
| No | 30 (37.04) | 51 (62.96) | 1 (5.0) | 19 (95.0) | ||
| Yes | 3 (15.00) | 17 (85.00) | 15 (18.52) | 66 (81.48) | ||
| Alcoholism | 0.263 | 0.770 | ||||
| No | 25 (36.23) | 44 (63.77) | 12 (17.39) | 57 (82.61) | ||
| Yes | 8 (25.00) | 24 (75.00) | 4 (12.50) | 28 (87.50) | ||
| Diabetes | 0.199 | 0.685 | ||||
| No | 17 (30.34) | 62 (69.66) | 15 (16.85) | 74 (83.15) | ||
| Yes | 6 (50.00) | 6 (50.00) | 1 (8.33) | 11 (91.67) | ||
| Hospitalization | 0.896 | 1.000 | ||||
| No | 32 (33.33) | 64 (66.67) | 0 (0.00) | 5 (100.00) | ||
| Yes | 1 (20.00) | 4 (80.00) | 16 (16.67) | 80 (83.33) | ||
| Exposure (years) | 0.723 | 0.895 | ||||
| <15 | 11 (16.40) | 56 (83.6) | 23 (33.82) | 45 (66.18) | ||
| ≥15 | 5 (14.70) | 29 (85.3) | 10 (30.30) | 23 (69.70) | ||
| BCG scar | 0.836 | 0.847 | ||||
| No | 8 (34.78) | 15 (65.22) | 3 (13.05) | 20 (86.95) | ||
| Yes | 25 (32.47) | 52 (67.53) | 13 (16.88) | 64 (83.12) | ||
| Chronic cough | 0.218 | 0.050 | ||||
| No | 28 (30.77) | 63 (69.23) | 12 (13.19) | 79 (86.81) | ||
| Yes | 5 (50.00) | 5 (50.00) | 4 (40.00) | 6 (60.00) | ||
| Presence of animal at home | 0.011 | 0.013 | ||||
| No | 21 (45.65) | 25 (54.35) | 12 (26.09) | 34 (73.91) | ||
| Yes | 12 (21.82) | 43 (78.18) | 4 (7.27) | 51 (92.73) | ||
| Helminth infection | 0.768 | 0.702 | ||||
| No | 29 (33.72) | 57 (66.28) | 13 (15.12) | 73 (84.88) | ||
| Yes | 4 (26.67) | 11 (73.33) | 3 (20.00) | 12 (80.00) | ||
| Protozoal infection | 0.022 | 0.114 | ||||
| No | 18 (46.15) | 21 (53.85) | 9 (23.08) | 30 (76.92) | ||
| Yes | 15 (24.19) | 47 (75.81) | 7 (11.29) | 55 (88.71) |
BCG, Bacillus Calmette-Guérin.
Factors associated with LTBI among the study population
Based on QTF-Plus positivity, univariate logistic regression analysis showed that working in a slaughterhouse [crude odds ratio (COR) 30.00, 95% CI 3.739–240.651; P=0.001], having an animal at home (COR 3.009, 95% CI 1.268–7.139; P=0.012) and protozoal infection (COR 2.685, 95% CI 1.140–6.326; P=0.024) were significantly associated with LTBI. On multivariate logistic regression, working in a slaughterhouse (AOR 1.095, 95% CI 1.00–2.036), smoking (AOR 4.214, 95% CI 1.051–16.899), years of exposure (AOR 5.617, 95% CI 1.202–32.198), having an animal at home (AOR 2.735, 95% CI 1.102–6.789) and protozoal infection (AOR 2.591, 95% CI 1.034–6.491) remained significantly associated with LTBI on QFT-Plus assay. Considering the LTBI status based on TST, being male (COR 8.590, 95% CI 2.507–29.429; P=0.001) and having an animal at home (COR 4.50, 95% CI 1.339–15.119; P=0.015) were significantly associated with LTBI. However, on multivariate logistic regression, being male (AOR 10.114, 95% CI 2.744–37.270; P=0.001) and having an animal at home (AOR 5.582, 95% CI 1.484–20.995; P=0.015) were the only risk factors associated with LTBI.
Meanwhile, age, body mass index, educational level, alcoholism, diabetes, history of hospitalization and BCG scar were not associated with LTBI (Tables 3 and 4).
Table 3.
Factors associated with positive QuantiFERON-TB Gold Plus test (QTF-Plus) (n=101).
| Variables | QTF-Plus+ | COR (95% CI; P-value) | AOR (95% CI; P-value) |
|---|---|---|---|
| Sex | |||
| Female | 58.82 (20/34) | 1 | |
| Male | 71.64 (48/67) | 1.768 (0.744–4.201; 0.197) | |
| Age group (years) | |||
| 15–30 | 75.00 (21/ 28) | 1 | |
| 31–46 | 63.04 (29/46) | 0.568 (0.200–1.615; 0.289) | |
| ≥47 | 66.67 (18/27) | 0.666 (0.206–2.150; 0.497) | |
| Origin |
|||
| Healthcare worker | 83.33 (35/42) | 1 | |
| Household contact | 60.71 (17/28) | 1.545 (0.585– 4.077; 0.379) | |
| Slaughterhouse worker | 96.77 (30/31) | 30.00 (3.739–240.651; 0.001) | 1.095 (1.00–2.036; 0.023) |
| Body mass index (kg/m2) | |||
| 18.5 | 25.00 (1/4) | 1 | |
| 18.5–24.9 | 67.92 (36/53) | 0.157 (0.015–1.626; 0.121) | |
| 25–29.9 | 75.00 (27/36) | 1.416 (0.548–3.661; 0.472) | |
| ≥30 | 50.00 (4/8) | 0.472 (0.105–2.118; 0.327) | |
| Educational level |
|||
| Any | 68.75 (11/16) | 1 | |
| Primary | 75.00 (9/12) | 1.363 (0.253–7.321; 0.718) | |
| Secondary | 67.92 (36/53) | 0.962 (0.288–3.209; 0.950) | |
| University | 60.00 (12/20) | 0.681 (0.170–2.723; 0.588) | |
| Smoking |
|||
| No | 62.96 (51/81) | 1 | |
| Yes | 85.00 (17/20) | 3.333 (0.901–12.324; 0.071) | 4.214 (1.051–16.899; 0.042) |
| Alcoholism |
|||
| No | 63.77 (44/69) | 1 | |
| Yes | 75.00 (24/32) | 1.704 (0.666–4.358; 0.266) | |
| Diabetes |
|||
| No | 69.66 (62/89) | 1 | |
| Yes | 50.00 (6/12) | 0.435 (0.128–1.472; 0.181) | |
| Hospitalization |
|||
| No | 66.67 (64/96) | 1 | |
| Yes | 80.00 (4/5) | 2 (0.214–18.637; 0.543) | |
| Exposure (years) |
|||
| <15 | 65.7 (44/67) | 1 | |
| ≥15 | 70.6 (24/34) | 1.255 (0.521–3.155; 0.189) | 5.617 (1.202–32.198; 0.036) |
| BCG scar | |||
| No | 65.22 (15/23) | 1 | |
| Yes | 67.53 (52/77) | 1.109 (0.415–2.960; 0.836) | |
| Chronic cough | |||
| No | 69.23 (63/91) | 1 | |
| Yes | 50.00 (5/10) | 0.444 (0.119–1.658; 0.228) | |
| Presence of animal at home |
|||
| No | 54.35 (25/46) | 1 | |
| Yes | 78.18 (43/55) | 3.009 (1.268–7.139; 0.012) | 2.735 (1.102–6.789; 0.030) |
| Helminth infection | |||
| No | 66.28 (57/86) | 1 | |
| Yes | 73.33 (11/15) | 1.399 (0.409–4.780; 0.592) | |
| Protozoal infection | |||
| No | 53.85 (21/39) | 1 | |
| Yes | 75.81 (47/62) | 2.685 (1.140–6.326; 0.024) | 2.591 (1.034–6.491; 0.042) 0.042) |
AOR, adjusted odds ratio; BCG, Bacille Calmette-Guérin; CI, confidence interval; COR, crude odds ratio.
Table 4.
Factors associated with positive tuberculin skin test (TST).
| Variables | TST+ | COR (95% CI; P-value) | AOR (95% CI; P-value) |
|---|---|---|---|
| Sex | |||
| Female | 64.71 (22/34) | 1 | |
| Male | 94.03 (63/67) | 8.590 (2.507–29.429; 0.001) | 10.114(2.744–37.270; 0.001) |
| Age group (years) | |||
| 15–30 | 71.43 (20/28) | 1 | |
| 31–46 | 89.13 (41/46) | 3.280(0.950–11.319; 0.060) | |
| ≥47 | 88.88 (24/27) | 3.20(0.747–13.690; 0.117) | |
| Origin | |||
| Healthcare worker | 83.33 (35/42) | 1 | |
| Household contact case | 67.85 (19/28) | 0.422(0.135– 1.313; 0.136) | |
| Slaughterhouse worker | 100 (31/31) | NA | |
| Body mass index (kg/m2) | |||
| 18.5 | 100 (4/4) | 1 | |
| 18.5–24.9 | 67.92 (36/53) | Omitted | |
| 25–29.9 | 30.3 (30/36) | 0.760 (0.232–2.484; 0.651) | |
| ≥30 | 62.5 (5/8) | 0.253 (0.049–1.304; 0.101) | |
| Educational level |
|||
| Any | 81.48 (13/16) | 1 | |
| Primary | 91.66 (11/12) | 2.538(0.229–28.020; 0.447) | |
| Secondary | 83.02 (44/53) | 1.128(0.265–4.789; 0.870) | |
| University | 85.00 (17/20) | 1.307 (0.225–7.568; 0.765) | |
| Smoking |
|||
| No | 68.2 (66/81) | 1 | |
| Yes | 95.00 (19/20) | 4.318 (0.535–34.83; 0.170) | |
| Alcoholism |
|||
| No | 82.61 (57/69) | 1 | |
| Yes | 87.50 (28/32) | 1.473(0.435–4.984; 0.770) | |
| Diabetes |
|||
| No | 83.15 (74/89) | 1 | |
| Yes | 91.67 (11/12) | 2.229(0.267–18.594; 0.459) | |
| Exposure (years) |
|||
| <15 | 83.6 (56/ 67) | 1 | |
| ≥15 | 85.30 (29/34) | 1.139 (0.375–3.900; 0.824) | |
| BCG scar | |||
| No | 19.3 (20/23) | 1 | |
| Yes | 64.7 (64/77) | 0.738 (0.191– 2.854; 0.660) | |
| Chronic cough | |||
| No | 76.6 (79/91) | 1 | |
| Yes | 60 (6/10) | 0.227 (0.055–0.927; 0.039) | |
| Presence of animal at home | |||
| No | 46.3 (51/55) | 1 | |
| Yes | 38.7 (34/46) | 4.50 (1.339–15.119; 0.015) | 5.582 (1.484–20.995; 0.017) |
| Helminth infection | |||
| No | 72.4 (73/86) | 1 | |
| Yes | 80.00 (12/15) | 0.712 (0.176–2.877; 0.702) | |
| Protozoal infection | |||
| No | 76.92 (30/39) | 1 | |
| Yes | 83.33 (55/62) | 2.357 (0.797–6.963; 0.114) |
AOR, adjusted odds ratio; BCG, Bacille Calmette-Guérin; CI, confidence interval; COR, crude odds ratio.
Discussion
Prevalence of LTBI among three TB high-risk groups in Bobo-Dioulasso
To the authors’ knowledge, this is the first study on the prevalence of LTBI in three TB high-risk groups using TST and QTF-Plus in Burkina Faso, a low-income country with high incidence of TB (48/100,000 population).
The prevalence of LTBI among 101 participants was 67.33% (95% CI 57.27–76.33) using QFT-Plus and 84.16% (95% CI 75.55–90.66) using TST. The burden of LTBI in this urban city was high, with overall prevalence of 63.36% (95% CI 52.18–71.82) using both QTF-Plus and TST.
Based on IGRA, household contacts of index cases of TB in this study had relatively high prevalence of LTBI (60.7%). Similar rates have been reported in Ghana (Mensah et al., 2017) and India (Kashyap et al., 2014): 65% and 48%, respectively. In South Korea, Lee et al. (2014) reported a moderate rate of 28.6% in a similar population, while a relatively low rate (19.85%) has been estimated in Iraq (Abdulkareem et al., 2020).
Among HCWs, the prevalence of LTBI was 50% in this study. In comparison, prevalence rates of LTBI in HCWs of 47% in Iran (using QFT-GIT), 40.7% in Morocco (using QFT-GIT), 33.6% in China (using T-SPOT.TB), 26% in South Africa (using QFT-GIF) and 61.6% in **Thailand (using QFT-Plus) have been reported (Li et al., 2015; McCarthy et al., 2015; Keshavarz Valian et al., 2019; Sabri et al., 2019). These differences could be due to the incidence of TB, national income levels, and the type of IGRAs used in the studies. The rate of LTBI in SWs was significantly higher using QFT-Plus (96.8%; P<0.001). A moderately high rate of 58.5% was also found in Mexico (Torres-Gonzalez et al., 2013). This may suggest that SWs are more exposed to TB infection due to manipulating infected carcasses and using knives, which may increase the risk of transmission via inhalation of aerosols produced by infected animals or from direct contact with a wound (de la Rua-Domenech, 2006).
Based on TST results, the prevalence of LTBI in this study was 84.16% (95% CI 75.55–90.66), which was much higher compared with results from Madagascar (78.6%) (Sadananda et al., 2020) and India (55%) (Chandrasekaran et al., 2018). This could be due to the wide coverage of BCG immunization in Burkina Faso (Ouédraogo et al., 2013), and the circulation of non-tuberculous mycobacteria (NTM) (Zida et al., 2014) inducing false-positive TST results (Latorre et al., 2010). However, prevalence rates varied depending on the specific risk group and technique. For TST, prevalence was 83.3% among HCWs, 100% among SWs, and 67.9% among household contacts of TB index cases. A study conducted in 2015 reported prevalence of 60% among HCWs in Kenya (Agaya et al., 2015), 61.7% in Indonesia (Wardani et al., 2021), 79% in Ivory Coast (Kassim et al., 2000), 67% in Georgia (Mirtskhulava et al., 2008), 57.6% in Iran (Keshavarz Valian et al., 2019) and 52.1% in Morocco (Sabri et al., 2019). These inconsistent findindgs could be due to false-positive results because NTM infections could affect the TST results (Andersen et al., 2000) and TST characteristics.
For household contacts of TB index cases, the prevalence of LTBI was 67.9% on TST, which was higher compared with the results for similar populations in Taiwan (Huang et al., 2010), Iraqi Kurdistan (Abdulkareem et al., 2020) and South Korea (Lee et al., 2014): 46%, 24.05% and 38%, respectively. These differences in prevalence, although relatively moderate, could be explained by disparity in the incidence of TB in these countries (Enos et al., 2018; Chen et al., 2019; Song et al., 2019). In addition, in the context of high incidence of TB, the size of the induration of TST in a household contact could only be the corollary of exposure to a focus of mycobacteria strains from the family (MacPherson et al., 2020).
The prevalence of LTBI among SWs was 100% in this study, and a similarly high result was obtained in Mexico (76.2%) (Torres-Gonzalez et al., 2013). This suggests that SWs are highly exposed to TB pathogens.
Regardless of the population group in this study, the TST positivity rate was higher than the QTF-Plus positivity rate. This was corroborated by other studies, including a meta-analysis of 24 studies showing the same outcomes from both tests in professional groups at risk for TB (Lamberti et al., 2015).
Based on the results of both test methods (QFT-Plus/TST), the overall prevalence of LTBI in this study was 63.36% (95% CI 52.18–71.82). Similar results have been reported from Thailand (61.6%) and Georgia (55%) (Whitaker et al., 2013; Gatechompol et al., 2021). However, the prevalence found in the present study was higher compared with the prevalence in Morocco (45.2%) (Sabri et al., 2019). This could be attributed to the inclusion of different categories of participants in the two studies. Also, the discrepancies in prevalence may be linked with the tools used for LTBI diagnosis, and the disproportionate distribution of TB worldwide.
This study found that the overall prevalence of LTBI was significantly higher in men (94.03%) compared with women (P<0.05). This may be due to the fact that the global burden of TB is higher among males than females (Mumpe-Mwanja et al., 2015), and men are more susceptible to exposure to many sources of infection as well as TB due to their activities or sociocultural determinants (Neyrolles and Quintana-Murci, 2009). Also, the prevalence of LTBI in participants with an animal at home was significantly higher compared with that among those who did not have an animal at home (P<0.05). Animals provide many benefits to people, but they could carry harmful germs that can spread to people and cause zoonotic diseases, such as bovine TB (Centers for Disease Control and Prevention, 2021). In addition, the prevalence of LTBI was higher in participants with protozoal infections compared with those who were not infected with protozoal infections. Similar results were found in the USA among refugees (Board and Suzuki, 2016).
Factors associated with LTBI among the study population
Factors significantly associated with LTBI were identified using univariate and multivariate logistic regressions. These risk factors were: working in a slaughterhouse, having an animal at home, protozoal infection, smoking, being male and ≥15 years of exposure. Previous studies found that several factors are associated with positivity on both QFT-Plus and TST among people exposed to TB infection (Torres-Gonzalez et al., 2013; Lee et al., 2014; Agaya et al., 2015; McCarthy et al., 2015; Eom et al., 2018; Wardani et al., 2021).
In the logistic regression analyses (both univariate and multivariate) for QTF-Plus and/or TST, the prevalence of LTBI was approximately eight-fold higher among males than females. This corroborates results from South Africa (Ncayiyana et al., 2016) and Uganda (Mumpe-Mwanja et al., 2015). This trend has been attributed to the fact that males practice more risky activities, such as pastoral activities, butchery, mining and agriculture (Horton et al., 2016). Depending on the origin of the study population, SWs were at significantly higher risk of LTBI than HCWs and household contacts of index TB cases. Although few studies have been conducted on these three risk groups at the same time, this study supports the findings of Gompo et al. (2020) who found that SWs are at high risk due to their exposure to infected cattle. This finding highlights the need for screening of SWs at slaughterhouses where bovine TB is prevalent. It is important to note that SWs may also be exposed to TB that is not linked to M. bovis infection but linked to M. tuberculosis, as for HCWs and household contacts of TB index cases. A previous study on the prevalence of TB species in bovine carcases found that some isolated species were not M. bovis (Tarnagda et al., 2014). A relationship was found between the occurrence of LTBI and having an animal at home, with the risk of LTBI increased 10-fold compared with not having an animal at home. This could be explained by the fact that animals may constitute mycobacterial reservoirs as well as representing a source for TB infection of humans [Gompo et al., 2020]. Three of every four known infectious diseases or emerging infectious diseases in humans come from animals, due to the close connection between people and animals (Centers for Disease Control and Prevention, 2021).
In the present study, the carriage of intestinal parasites, such as protozoa, was found to increase the risk of LTBI two-fold. Other authors have recorded similar results, and provided evidence that protozoal infection was significantly associated with LTBI (Board and Suzuki, 2016; Alemu et al., 2019; Tesfaye et al., 2022). These results are likely to be due to the fact that the study population lived in settings that are co-endemic for TB and parasite infection, with poor implementation of water and hygiene sanitation. Epidemiologic studies of co-infection with TB and intestinal parasites in humans are strongly recommended to draw a roadmap for the control of both endemic diseases.
In the study population, ≥15 years of exposure increased the risk of LTBI more than five-fold. This corroborates the findings of other studies conducted elsewhere (Pai et al., 2005; Anwar et al., 2019; Sedamano et al., 2020). On multivariate logistic regression, smoking was found to be associated with LTBI. Other studies in Morocco and Nepal found that smoking was a risk factor for LTBI when assessed by QFT-GIT and/or TST (Sabri et al., 2019; Gompo et al., 2020).
This study has a few limitations. First, the results cannot be generalized to other cities in Burkina Faso. Also, other well-known risk groups, such as people living with HIV, people with chronic renal failure, and migrants, were not included. Due to difficulties with acquiring the IGRA reagents, this study was performed on a small sample (101 participants), which was not ideal to investigate the prevalence of LTBI. However, this study is considered to be of value as it is the first study to investigate LTBI in Burkina Faso.
Conclusion
To the authors’ knowledge, this is the first report of LTBI in Burkina Faso. The study found a high prevalence of LTBI among the three groups of individuals, especially SWs, and identified working in a slaughterhouse, having an animal at home, protozoal infection, smoking, being male and ≥15 years of exposure as risk factors for LTBI. These data highlight the need for targeted interventions for these risk groups to reach the end TB goals. These interventions must include pre-employment screening for SWs and HCWs, and routine screening for household contacts at high risk of TB, which will maximize opportunities to identify and treat LTBI.
Declaration of Competing Interest
None declared.
Acknowledgments
Acknowledgements
The authors wish to thank the field workers, staff at the slaughterhouses and health facilities, and TB patients and household contacts for their support.
Author contributions
DAD, GIM and SPD conceived and designed the study. DAD and KAO conducted the field study. DAD and KAO performed field data collection and performed the laboratory analysis. DAD analysed the data and wrote the manuscript. DAD, GIM, SPD, TS, KAO, LTS, ASK, MZC, HMH, AC, AMGB, KKA, RKD, PM and JZ critically revised the manuscript. All authors read and approved the final manuscript.
Funding
The field work, laboratory analyses and all other costs related to this study were funded through a PhD Fellowship to Djibougou Arthur for his PhD at the Université Nazi BONI. Afrique One-ASPIRE is funded by a consortium of donors including the African Academy of Sciences, Alliance for Accelerating Excellence in Science in Africa, the New Partnership for Africa's Development Planning and Coordinating Agency, and the Wellcome Trust (107753/A/15/Z).
Ethical approval
The protocol of the study was approved by the Ethics Committee for Health Research of Burkina Faso, known as Comité d'éthique pour la recherche en Santé (Ref. 2017-07-106/CERS). The study was conducted in accordance with the Declaration of Helsinki. Data collection authorizations were provided by the Ministry of Health and the Regional Directors of Health of Hauts-Bassins and Animal and Fisheries Resources. All participants and/or their parents/legal guardians provided written informed consent after explanation of the study procedure, risks and benefits. Participants with parasitic infections received appropriate treatment, as recommended by national parasitosis treatment guidelines.
Availability of data and materials
All data generated or analysed during this study are included in this published article
References
- Abdulkareem FN, Merza MA, Salih AM. First insight into latent tuberculosis infection among household contacts of tuberculosis patients in Duhok, Iraqi Kurdistan: using tuberculin skin test and QuantiFERON-TB Gold Plus test. Int J Infect Dis. 2020;96:97–104. doi: 10.1016/j.ijid.2020.03.067. [DOI] [PubMed] [Google Scholar]
- Agaya J, Nnadi CD, Odhiambo J, Obonyo C, Obiero V, Lipke V, et al. Tuberculosis and latent tuberculosis infection among healthcare workers in Kisumu, Kenya. Trop Med Int Health. 2015;20:1797–1804. doi: 10.1111/tmi.12601. [DOI] [PubMed] [Google Scholar]
- Alemu A, Kebede A, Dagne B, Amare M, Diriba G, Yenew B, et al. Intestinal parasites co-infection and associated factors among active pulmonary tuberculosis patients in selected health centers, Addis Ababa, Ethiopia: unmatched case control study. BMC Infect Dis. 2019;19:407. doi: 10.1186/s12879-019-4009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099–1104. doi: 10.1016/s0140-6736(00)02742-2. [DOI] [PubMed] [Google Scholar]
- Anwar MM, Ahmed DM, Elareed HR, Abdel-Latif RA-R, Sheemy MS, Kamel NM, et al. Screening for latent tuberculosis among healthcare workers in an Egyptian hospital using tuberculin skin test and QuantiFERON-TB Gold In-Tube Test. Indian J Occup Environ Med. 2019;23:106–111. doi: 10.4103/ijoem.IJOEM_184_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basera TJ, Ncayiyana J, Engel ME. Prevalence and risk factors of latent tuberculosis infection in Africa: a systematic review and meta-analysis protocol. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baussano I, Nunn P, Williams B, Pivetta E, Bugiani M, Scano F. Tuberculosis among health care workers. Emerg Infect Dis. 2011;17:488. doi: 10.3201/eid1703.100947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board AR, Suzuki S. The interrelation between intestinal parasites and latent TB infections among newly resettled refugees in Texas. Int Health. 2016;8:67–72. doi: 10.1093/inthealth/ihv033. [DOI] [PubMed] [Google Scholar]
- Bongomin F, Ssekamatte P, Nattabi G, Olum R, Ninsiima S, Kyazze AP, et al. Latent tuberculosis infection status of pregnant women in Uganda determined using QuantiFERON TB Gold-Plus. Open Forum Infect Dis. 2021;8:ofab241. doi: 10.1093/ofid/ofab241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carranza C, Pedraza-Sanchez S, de Oyarzabal-Mendez E, Torres M. Diagnosis for latent tuberculosis infection: new alternatives. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.02006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . CDC; Atlanta, GA: 2021. Zoonotic diseases.https://www.cdc.gov/onehealth/basics/zoonotic-diseases.html Available at. [Google Scholar]
- Chandrasekaran P, Mave V, Thiruvengadam K, Gupte N, Shivakumar SVBY, Hanna LE, et al. Tuberculin skin test and QuantiFERON-Gold In Tube assay for diagnosis of latent TB infection among household contacts of pulmonary TB patients in high TB burden setting. PLoS One. 2018;13 doi: 10.1371/journal.pone.0199360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-Y, Pan S-W, Shen H-S, Chuang F-Y, Feng J-Y, Su W-J. Declining trend in incidence of tuberculosis in adolescents and young adults in Taiwan. Eur Respir J. 2019;53 doi: 10.1183/13993003.01305-2018. [DOI] [PubMed] [Google Scholar]
- Cisse M, Sangare I, Djibougou AD, Tahita MC, Gnissi S, Bassinga JKW, et al. Prevalence and risk factors of Schistosoma mansoni infection among preschool-aged children from Panamasso village, Burkina Faso. Parasit Vectors. 2021;14:185. doi: 10.1186/s13071-021-04692-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rua-Domenech R. Human Mycobacterium bovis infection in the United Kingdom: incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis. 2006;86:77–109. doi: 10.1016/j.tube.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Enos M, Sitienei J, Ong'ang'o J, Mungai B, Kamene M, Wambugu J, et al. Kenya tuberculosis prevalence survey 2016: challenges and opportunities of ending TB in Kenya. PLoS One. 2018;13 doi: 10.1371/journal.pone.0209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom JS, Kim I, Kim W-Y, Jo E-J, Mok J, Kim M-H, et al. Household tuberculosis contact investigation in a tuberculosis-prevalent country: are the tuberculin skin test and interferon-gamma release assay enough in elderly contacts? Medicine. 2018;97:e9681. doi: 10.1097/MD.0000000000009681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatechompol S, Harnpariphan W, Supanan R, Suwanpimolkul G, Sophonphan J, Ubolyam S, et al. Prevalence of latent tuberculosis infection and feasibility of TB preventive therapy among Thai prisoners: a cross-sectional study. BMC Public Health. 2021;21:1206. doi: 10.1186/s12889-021-11271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompo TR, Shrestha A, Ranjit E, Gautam B, Ale K, Shrestha S, et al. Risk factors of tuberculosis in human and its association with cattle TB in Nepal: a One Health approach. One Health. 2020;10 doi: 10.1016/j.onehlt.2020.100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton KC, MacPherson P, Houben RMGJ, White RG, Corbett EL. Sex differences in tuberculosis burden and notifications in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-W, Shen G-H, Lee J-J, Yang W-T. Latent tuberculosis infection among close contacts of multidrug-resistant tuberculosis patients in central Taiwan. Int J Tuberc Lung Dis. 2010;14:1430–1435. [PubMed] [Google Scholar]
- Kashyap RS, Nayak AR, Gaherwar HM, Husain AA, Shekhawat SD, Jain RK, et al. Latent TB infection diagnosis in population exposed to TB subjects in close and poor ventilated high TB endemic zone in India. PLoS One. 2014;9:e89524. doi: 10.1371/journal.pone.0089524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassim S, Zuber P, Wiktor SZ, Diomande FV, Coulibaly IM, Coulibaly D, et al. Tuberculin skin testing to assess the occupational risk of Mycobacterium tuberculosis infection among health care workers in Abidjan, Côte d'Ivoire. Int J Tuberc Lung Dis. 2000;4:321–326. [PubMed] [Google Scholar]
- Keshavarz Valian S, Mahmoudi S, Pourakbari B, Abdolsalehi MR, Eshaghi H, Mamishi S. Screening of healthcare workers for latent tuberculosis infection in the low tuberculosis burden country: QuantiFERON-TB Gold in Tube test or tuberculin skin test? Arch Environ Occup Health. 2019;74:109–114. doi: 10.1080/19338244.2017.1394254. [DOI] [PubMed] [Google Scholar]
- Lamberti M, Uccello R, Monaco MGL, Muoio M, Feola D, Sannolo N, et al. Tuberculin skin test and QuantiFERON test agreement and influencing factors in tuberculosis screening of healthcare workers: a systematic review and meta-analysis. J Occup Med Toxicol. 2015;10:2. doi: 10.1186/s12995-015-0044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre I, De Souza-Galvão M, Ruiz-Manzano J, Lacoma A, Prat C, Altet N, et al. Evaluating the non-tuberculous mycobacteria effect in the tuberculosis infection diagnosis. Eur Respir J. 2010;35:338–342. doi: 10.1183/09031936.00196608. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lee SH, Kim YE, Cho YJ, Jeong YY, Kim HC, et al. Risk factors for latent tuberculosis infection in close contacts of active tuberculosis patients in South Korea: a prospective cohort study. BMC Infect Dis. 2014;14:566. doi: 10.1186/s12879-014-0566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-X, Chen J-X, Wang L-X, Tian L-G, Zhang Y-P, Dong S-P, et al. Prevalence and risk factors of intestinal protozoan and helminth infections among pulmonary tuberculosis patients without HIV infection in a rural county in P. R. China. Acta Trop. 2015;149:19–26. doi: 10.1016/j.actatropica.2015.05.001. [DOI] [PubMed] [Google Scholar]
- López de Goicoechea-Saiz M, Sternberg F, Portilla-Sogorb J. Prevalence and associated risk factors of latent tuberculosis infection in a Spanish prison. Rev Esp Sanid Penit. 2018;20:4–10. [PMC free article] [PubMed] [Google Scholar]
- MacNeil A. Global epidemiology of tuberculosis and progress toward meeting global targets — worldwide, 2018. MMWR Morb Mortal Wkly Rep. 2020;69:281–285. doi: 10.15585/mmwr.mm6911a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson P, Lebina L, Motsomi K, Bosch Z, Milovanovic M, Ratsela A, et al. Prevalence and risk factors for latent tuberculosis infection among household contacts of index cases in two South African provinces: analysis of baseline data from a cluster-randomised trial. PLoS One. 2020;15 doi: 10.1371/journal.pone.0230376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbugi E.V., Katale B.Z., Lupindu A.M., Keyyu J.D., Kendall S.L., Dockrell H.M., Michel A.L., Matee M.I., van Helden P.D., et al. Tuberculosis Infection: Occurrence and Risk Factors in Presumptive Tuberculosis Patients of the Serengeti Ecosystem in Tanzania. The East African health research journal. 2017;1(1):19–30. doi: 10.24248/EAHRJ-D-16-00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KM, Scott LE, Gous N, Tellie M, Venter WDF, Stevens WS, et al. High incidence of latent tuberculous infection among South African health workers: an urgent call for action. Int J Tuberc Lung Dis. 2015;19:647–653. doi: 10.5588/ijtld.14.0759. [DOI] [PubMed] [Google Scholar]
- Mensah GI, Sowah SA, Yeboah NYA, Addo KK, Jackson-Sillah D. Utility of QuantiFERON tuberculosis Gold in Tube test for detecting latent tuberculosis infection among close household contacts of confirmed tuberculosis patients in Accra, Ghana. Int J Mycobacteriol. 2017;6:27–33. doi: 10.4103/2212-5531.201891. [DOI] [PubMed] [Google Scholar]
- Mirtskhulava V, Kempker R, Shields KL, Leonard MK, Tsertsvadze T, del Rio C, et al. Prevalence and risk factors for latent tuberculosis infection among health care workers in Georgia. Int J Tuberc Lung Dis. 2008;12:513–519. [PMC free article] [PubMed] [Google Scholar]
- Mumpe-Mwanja D, Verver S, Yeka A, Etwom A, Waako J, Ssengooba W, et al. Prevalence and risk factors of latent tuberculosis among adolescents in rural Eastern Uganda. Afr Health Sci. 2015;15:851–860. doi: 10.4314/ahs.v15i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ncayiyana JR, Bassett J, West N, Westreich D, Musenge E, Emch M, et al. Prevalence of latent tuberculosis infection and predictive factors in an urban informal settlement in Johannesburg, South Africa: a cross-sectional study. BMC Infect Dis. 2016;16:661. doi: 10.1186/s12879-016-1989-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyrolles O, Quintana-Murci L. Sexual inequality in tuberculosis. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouédraogo N, Kagoné M, Sié A, Becher H, Müller O. Immunization coverage in young children: a study nested into a health and demographic surveillance system in Burkina Faso. J Trop Pediatr. 2013;59:187–194. doi: 10.1093/tropej/fms075. [DOI] [PubMed] [Google Scholar]
- Pai M, Gokhale K, Joshi R, Dogra S, Kalantri S, Mendiratta DK, et al. Mycobacterium tuberculosis infection in health care workers in rural India: comparison of a whole-blood interferon gamma assay with tuberculin skin testing. JAMA. 2005;293:2746–2755. doi: 10.1001/jama.293.22.2746. [DOI] [PubMed] [Google Scholar]
- Qader GQ, Seddiq MK, Rashidi KM, Manzoor L, Hamim A, Akhgar MH, et al. Prevalence of latent tuberculosis infection among health workers in Afghanistan: a cross-sectional study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0252307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiagen: QuantiFERON-TB Gold Plus (QFT-Plus) ELISA... - Google Scholar. 2021. n.d. Available at: https://scholar.google.com/scholar_lookup?title=QuantiFERON%C2%AE-TB+Gold+Plus+(QFT%C2%AE-Plus)+ELISA+package+insert&publication_year=2017& (Accessed 10 August 2021).
- Rieder HL, Chadha VK, Nagelkerke NJD, van Leth F, van der Werf MJ, KNCV Tuberculosis Foundation Guidelines for conducting tuberculin skin test surveys in high-prevalence countries. Int J Tuberc Lung Dis. 2011;15(1):S1–25. Suppl. [PubMed] [Google Scholar]
- Sabri A, Quistrebert J, Naji Amrani H, Abid A, Zegmout A, Abderrhamani Ghorfi I, et al. Prevalence and risk factors for latent tuberculosis infection among healthcare workers in Morocco. PLoS One. 2019;14 doi: 10.1371/journal.pone.0221081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadananda G, Knoblauch AM, Andriamiadanarivo A, Razafindrina K, Ambinintsoa I, Rabetombosoa RM, et al. Latent tuberculosis infection prevalence in rural Madagascar. Trans R Soc Trop Med Hyg. 2020;114:883–885. doi: 10.1093/trstmh/traa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedamano J, Schwalb A, Cachay R, Zamudio C, Ugarte-Gil C, Soto-Cabezas G, et al. Prevalence of positive TST among healthcare workers in high-burden TB setting in Peru. BMC Public Health. 2020;20:612. doi: 10.1186/s12889-020-08756-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-H, Huh K, Chung DR. Modern history of tuberculosis in Korea. Infect Chemother. 2019;51:414–426. doi: 10.3947/ic.2019.51.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnagda Z, Kanyala E, Zingué D, Sidibé S, Yougbaré I, Kagoné TS, et al. Prevalence of Tuberculosis spp. species in bovine carcasses in two slaughterhouses of Burkina Faso 2014. Available at: https://www.semanticscholar.org/paper/Prevalence-of-Tuberculosis-spp-.-species-in-bovine-Tarnagda-Kanyala/ea7f6df95df9e5647eb35fe84f24e44258f5464a (Accessed 19 June 2020).
- Tesfaye S, Zerfu B, Desta K. Magnitude and associated factors of intestinal parasitosis and tuberculosis among tuberculosis suspected patients attending Kuyu General Hospital, North Shewa, Oromia, Ethiopia. PLoS Negl Trop Dis. 2022;16 doi: 10.1371/journal.pntd.0010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Gonzalez P, Soberanis-Ramos O, Martinez-Gamboa A, Chavez-Mazari B, Barrios-Herrera MT, Torres-Rojas M, et al. Prevalence of latent and active tuberculosis among dairy farm workers exposed to cattle infected by Mycobacterium bovis. PLoS Negl Trop Dis. 2013;7:e2177. doi: 10.1371/journal.pntd.0002177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uplekar M, Weil D, Lonnroth K, Jaramillo E, Lienhardt C, Dias HM, et al. WHO's new end TB strategy. Lancet. 2015;385:1799–1801. doi: 10.1016/S0140-6736(15)60570-0. [DOI] [PubMed] [Google Scholar]
- Wang P-H, Lin S-Y, Lee SS-J, Lin S-W, Lee C-Y, Wei Y-F, et al. CD4 response of QuantiFERON-TB Gold Plus for positive consistency of latent tuberculosis infection in patients on dialysis. Sci Rep. 2020;10:21367. doi: 10.1038/s41598-020-78374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardani HR, Mertaniasih NM, Soedarsono S. Risk factors of latent tuberculosis infection in healthcare workers at hospitals in Jember City. Indonesia. Afr J Infect Dis. 2021;15:34–40. doi: 10.21010/ajid.v15i1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker JA, Whitaker A, J, Mirtskhulava V, Kipiani M, Harris DA, Tabagari N, et al. Prevalence and incidence of latent tuberculosis infection in Georgian healthcare workers. PLoS One. 2013;8:e58202. doi: 10.1371/journal.pone.0058202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2019. Global tuberculosis report 2019. [Google Scholar]
- World Health Organisation . World Health Organization; Geneva: 2020. WHO consolidated guidelines on tuberculosis. Module 1: prevention tuberculosis preventive treatment. Licence: CC BY-NC-SA 3.0 IGO. [PubMed] [Google Scholar]
- Zida S, Tarnagda Z, Kaboré A, Zingué D, Hien H, Sanou A, et al. Etat des lieux des mycobactérioses atypiques au Burkina Faso: résultats d'une enquête régionale. Pan Afr Med J. 2014;17:188. doi: 10.11604/pamj.2014.17.188.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article