Abstract
Objective
To study the prognostic role of right ventricular systolic pressure (RVSP) in patients with heart failure (HF).
Background
Although RVSP is a readily available echocardiographic parameter, it is often underused. Its prognostic role in patients with heart failure is not well established compared with pulmonary artery pressure measured by right heart catheterization.
Methods
This single-center retrospective cohort study included patients with acute heart failure hospitalization admitted to the hospital from January 2005 to December 2018. The primary predictor was right ventricular systolic pressure (RVSP) obtained from bedside transthoracic echocardiography at admission. We divided RVSP into two groups, RVSP <40 mm Hg (reference group) and RVSP ≥40 mm Hg. Primary outcome was all-cause mortality. Secondary outcomes were all-cause readmission and cardiac readmission. We conducted propensity-score matching and applied cox-proportional hazard model to compute hazard ratio (HR) with 95% confidence interval (CI).
Results
Out of 972 HF patients, 534 patients had RVSP <40 mm Hg and 438 patients had RVSP ≥40 mm Hg. Patients with RVSP ≥40 mm Hg compared with RVSP <40 mm Hg were associated with higher rates of death [HR: 1.60, 95% CI: 1.22–2.09, P-value = 0.001], all-cause readmissions [HR: 1.37, 95% CI: 1.09–1.73, P-value = 0.008] and cardiac readmissions [HR: 1.41, 95% CI: 1.07–1.85, P-value = 0.014].
Conclusion
Higher RVSP (≥40 mm Hg) in HF patients was associated with higher rates of death, all-cause readmissions, and cardiac readmissions. RVSP can be considered as a prognostic marker for mortality and readmission.
Keywords: Heart failure, Right ventricular systolic pressure, Death, Readmission
Abbreviations
- CI
Confidence interval
- HR
Hazard ratio
- HFrEF
Heart failure with reduced ejection fraction
- HFpEF
Heart failure with preserved ejection fraction
- LVEF
Left ventricular ejection fraction
- PSM
Propensity-score matched
- RVSP
Right ventricular systolic pressure
1. Introduction
With the aging population, the prevalence of heart failure (HF) is increasing in the United States and around the world. A recent report suggesting an estimated prevalence of 6.2 million American adults ≥20 years of age with HF between 2013 and 2016, compared with an estimated 5.7 million between 2009 and 2012.1 Presence of pulmonary hypertension (PH) in patients with heart failure, a consequence of increased left atrial pressure either secondary to enhanced stiffness of the left ventricular wall or systolic dysfunction, has been associated with worse clinical outcomes and increased mortality.2 Increasing prevalence of PH coupled with resulting right ventricular dysfunction have been identified as a significant modifier of natural disease prognosis in HG.3 It is estimated that nearly 50% of patients with HF have PH present on their echocardiogram.2,4 Therefore, the accurate diagnosis of PH requires invasive right heart catheterization. However, the prognostic value of right ventricular systolic pressure (RVSP) in HF patients measured using transthoracic echocardiography as an alternative method to assess PH is not well established.
The present study aimed at studying the prognostic role of RVSP in patients with heart failure and subgroups of heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF).
2. Method
2.1. Study population
This was a single-center retrospective cohort study of confirmed cases of acute heart failure hospitalization. Patients admitted to the hospital from January 2005 to December 2018 and meeting the inclusion criteria were included in the study. The institutional review board approved this study and permitted a waiver of informed consent from the study participants. Acute heart failure was defined as acute onset or change in signs and/or symptoms of heart failure needing urgent hospitalization and intravenous treatment.5 The exclusion criteria were age <18 years, acute coronary syndrome at the time of presentation, primarily right-sided heart failure, unavailable echocardiography during the hospital stay, presence of an aortic or mitral valve abnormality, and discharge within 24 h of admission. For patients with previous heart failure, the first admission to the hospital was considered an index event.
2.2. Procedure
A team of resident physicians reviewed and collected demographic, clinical, laboratory, medication information, and outcomes from the electronic medical records. The definitions of all extracted data and outcomes were recorded separately and checked by two authors. All the data extraction was done manually, which was verified by a second physician. Any disparity was resolved by consulting the primary investigator. Patient confidentiality was protected by allocating data storage to a locked, password-protected computer.
2.3. Primary predictor and outcomes
Our primary predictor was right ventricular systolic pressure (RVSP) obtained from bedside echocardiography at admission. We divided RVSP into two groups, RVSP <40 mm Hg (reference group) and RVSP ≥40 mm Hg. Our primary outcome was all-cause mortality. Secondary outcomes were all-cause readmission, cardiac readmission, and readmission or mortality at 6 months. All outcomes were time-to-event type. They were defined as the time from discharge date to the date of occurrence of events. The clinical outcomes were determined by electronic medical records from all the hospitals of the organization. Death was determined from EMR, telephone call, or social security death index master file by two-point identifier name and date of birth. Cardiac readmission was defined as readmission due to heart failure, myocardial infarction, or atrial fibrillation. Further, we performed a subgroup analysis by ejection fraction (EF). Heart failure with reduced ejection fraction (HFrEF) was defined as EF< 40%, and heart failure with preserved ejection fraction (HFpEF) was defined as EF ≥ 40%.
2.4. Statistical analysis
Baseline characteristics of both groups were expressed using descriptive statistics. The continuous variables were demonstrated as median with interquartile range (IQR). Categorical variables were extrapolated in frequency and proportion. The Mann-Whitney-Wilcoxon tests were applied to compare continuous variables. Fisher's exact test or Pearson's chi2 tests were implemented to compare categorical variables. For death, censoring was applied at last objective evidence of survival available. For readmission, censoring was applied at six months. We built a multivariable cox-proportional hazard model (Supplemental Text 1 and Text 2) to determine the hazard ratio (HR) and 95% confidence interval (CI) for the RVSP ≥40 mmHg group compared with the group with RVSP <40 mm Hg. We also utilized a propensity score with 1:1 near neighbor matching without replacement using caliper 0.1 to keep the standardized difference of the baseline characteristics between the two groups <10%. In the propensity- score-matched (PSM) cohort, we executed univariate cox-proportional hazard analysis to determine the HR with 95% CI. We applied the test for proportionality assumption based on Schoenfeld Residuals to check the violation of proportional assumption. Missing data were not imputed. In the PSM cohort, the Kaplan–Meier curves were used to demonstrate time-to-event outcomes in each group; the comparison between the two groups was calculated with the log-rank test. A P-value less than 0.05 was considered statistically significant.
2.5. Unmeasured bias analysis
To evaluate the robustness of our findings, we conducted an ‘E-value’ analysis to determine the validity of the study. E-value identifies the minimum strength of association that unmeasured confounders may need to have with both treatment and outcome, conditional on measured covariates, to fully explain the observed association. This estimates what the relative risk may have to be for any unmeasured confounder to overcome the observed association of study intervention with study outcomes. All statistical analyses were performed using STATA version 16.1 (StataCorp LLC).
3. Results
The present analysis included 972 HF patients, of which 534 patients had RVSP <40 mmHg and 438 patients had RVSP ≥40 mmHg. Out of 972 patients, 621 (64%) patients had HFrEF, and 351 (36%) patients had HFpEF.
3.1. Baseline characteristics [Table 1]
Table 1.
Baseline demographics and clinical characteristics of patients stratified by RVSP.
| No PH (RVSP <40 mm Hg) (N = 534, 54.94%) | PH (RVSP ≥40 mm Hg) (N = 438, 45.06%) | P-value | |
|---|---|---|---|
| Demographic | |||
| Age (years), mean (SD) | 63.37 (14.30) | 65.76 (13.99) | 0.007 |
| Male, n (%) | 342 (64.04) | 252 (57.53) | 0.038 |
| Black, n (%) | 206 (38.58) | 182 (41.55) | 0.346 |
| Hispanic, n (%) | 310 (58.05) | 248 (56.62) | 0.653 |
| Comorbidities | |||
| Hypertension, n (%) | 459 (85.96) | 374 (85.39) | 0.802 |
| Diabetes, n (%) | 271 (50.75) | 231 (52.74) | 0.537 |
| Coronary artery disease, n (%) | 173 (32.46) | 104 (23.85) | 0.003 |
| Stroke, n (%) | 52 (9.74) | 33 (7.53) | 0.226 |
| Cancer, n (%) | 50 (9.36) | 35 (7.99) | 0.451 |
| Atrial fibrillation, n (%) | 118 (22.10) | 123 (28.08) | 0.032 |
| Obesity, n (%) | 270 (50.56) | 172 (39.27) | <0.001 |
| Chronic lung disease, n (%) | 88 (16.48) | 94 (21.46) | 0.048 |
| Parameters on admission | |||
| MAP (mm Hg), mean (SD) | 104.57 (20.06) | 101.74 (18.88) | 0.031 |
| Pro-BNP (pg/ml), median (IQR) | 3789 (1454–11354) | 6996 (3197–17666) | <0.001 |
| Hb (g/dL), median (IQR) | 12.25 (10.6–13.6) | 11.65 (10.3–13.1) | <0.001 |
| GFR (ml/min/m2), median (IQR) | 64.87 (38.20–89.46) | 56.70 (35.43–80.21) | 0.003 |
| Sodium (mEq/L), median (IQR) | 139 (137–142) | 139 (137–141) | 0.006 |
| Echocardiographic features | |||
| EF >40%, n (%) | 197 (36.89) | 153 (35.16) | 0.576 |
| LAD, median (IQR) | 4.2 (4–4.7) | 4.4 (4.1–5) | <0.001 |
| LVIDD, median (IQR) | 5.5 (4.9–6) | 5.5 (5–6.1) | 0.29 |
| Discharge Medications | |||
| Loop diuretics, n (%) | 400 (75.05) | 371 (84.70) | <0.001 |
| Beta blocker, n (%) | 434 (81.27) | 345 (78.77) | 0.33 |
| ACE inhibitors/ARB, n (%) | 395 (73.97) | 324 (73.97) | 0.99 |
| Calcium channel blocker, n (%) | 139 (26.03) | 123 (28.08) | 0.473 |
| Spironolactone, n (%) | 85 (15.92) | 100 (22.83) | 0.006 |
| Digoxin, n (%) | 129 (24.16) | 133 (30.37) | 0.030 |
| Aspirin/Clopidogrel, n (%) | 442 (82.77) | 325 (74.20) | 0.001 |
| Statin, n (%) | 364 (68.16) | 269 (61.42) | 0.028 |
| Outcomes | |||
| Death (n, %) | 142 (26.59) | 170 (38.81) | <0.001 |
| All-cause readmission (n, %) | 194 (36.33) | 195 (44.52) | 0.010 |
| Cardiac readmission (n, %) | 140 (26.22) | 146 (33.33) | 0.015 |
| Death or readmission (n, %) | 273 (51.12) | 265 (60.50) | 0.003 |
| Length of stay (median, IQR) | 4 (2–6) | 4 (2–8) | 0.010 |
Abbreviations.
RVSP – right ventricular systolic pressure, PH – pulmonary hypertension, IQR – interquartile range, SD – standard deviation, CAD – coronary artery disease, MAP – mean arterial pressure, BMI – body mass index, pro-BNP – pro-brain natriuretic peptide, Hb – hemoglobin, GFR – glomerular filtration rate, EF – ejection fraction, LAD – left atrial diameter, LVIDD – left ventricular internal diastolic diameter, ACE - angiotensin-converting enzyme, ARB – angiotensin receptor blocker.
Patients with RVSP ≥40 mm Hg had a higher mean age [65.76 ± 13.99 vs. 63.37 ± 14.30, P-value = 0.007], but the difference was of little clinical significance. RVSP ≥40 mmHg had lower frequency of males compared with RVSP <40 mmHg (64.04% vs. 57.33%, P-value = 0.038). RVSP ≥40 mmHg had lower percentage of coronary artery disease (23.85% vs. 32.46%, P-value = 0.003) and obesity (39.27% vs. 50.56%, P-value <0.001) compared with RVSP <40 mmHg. However, RVSP ≥40 mmHg had a higher percentage of atrial fibrillation (28.08% vs. 22.10%, P-value = 0.032) and chronic lung disease (21.46% vs. 16.48%, P-value = 0.048) compared with RVSP <40 mmHg. RVSP ≥40 mmHg had lower mean arterial pressure, hemoglobin, glomerular filtration rate, higher pro-BNP, and higher left atrial diameter compared with patients with RVSP <40 mm Hg. RVSP ≥40 mmHg had more patients on loop diuretic (84.70% vs. 75.05%, P-value <0.001), spironolactone (22.83% vs. 15.92%, P-value = 0.006), and digoxin prescription (129 (24.16) vs. 133 (30.37), P-value = 0.030). However, patients with RVSP <40 mmHg compared with RVSP ≥40 mmHg had a higher percentage of aspirin/clopidogrel (82.77% vs. 74.20%, P-value = 0.001) and statin prescription (68.16% vs. 61.42%, P-value = 0.028). After propensity-score matching, 345 patients with HF were each included in RVSP <40 mmHg and RVSP ≥40 mmHg cohorts [Table 2]. Supplemental Table 1 presents baseline characteristics by subgroup of HFrEF and HFpEF.
Table 2.
Baseline demographics and clinical characteristics of patients stratified by RVSP after propensity-score matching.
| No PH (RVSP <40 mm Hg) (N = 345, 50%) | PH (RVSP ≥40 mm Hg) (N = 345, 50%) | P-value | |
|---|---|---|---|
| Demographic | |||
| Age (years), median (IQR) | 64 (54–75) | 65 (55–75) | 0.63 |
| Male, n (%) | 202 (58.55) | 203 (58.84) | 0.938 |
| Black, n (%) | 137 (39.71) | 146 (42.32) | 0.486 |
| Hispanic, n (%) | 199 (57.68) | 195 (56.52) | 0.758 |
| Comorbidities | |||
| Hypertension, n (%) | 293 (84.93) | 299 (86.67) | 0.513 |
| Diabetes, n (%) | 183 (53.04) | 182 (52.75) | 0.939 |
| Coronary artery disease, n (%) | 115 (33.43) | 86 (25.07) | 0.016 |
| Stroke, n (%) | 26 (7.54) | 32 (9.28) | 0.410 |
| Cancer, n (%) | 33 (9.57) | 29 (8.41) | 0.594 |
| Atrial fibrillation, n (%) | 88 (25.51) | 85 (24.64) | 0.792 |
| Obesity, n (%) | 150 (43.48) | 155 (44.93) | 0.702 |
| Chronic lung disease, n (%) | 70 (20.29) | 68 (19.71) | 0.849 |
| Parameters on admission | |||
| MAP (mm Hg), median (IQR) | 101 (90–115) | 100 (90–115) | 0.995 |
| Pro-BNP (pg/ml), median (IQR) | 5264 (1855–12189) | 6614 (3133–14000) | 0.033 |
| Hb (g/dL), median (IQR) | 12 (10.5–13.4) | 11.6 (10.3–13.1) | 0.157 |
| GFR (ml/min/m2), median (IQR) | 63.25 (36.25–89.73) | 57.58 (35.78–80.15) | 0.053 |
| Sodium (mEq/L), median (IQR) | 139 (137–142) | 139 (136–141) | 0.781 |
| Echocardiographic features | |||
| EF >40%, n (%) | 127 (36.81) | 129 (37.39) | 0.875 |
| LAD, median (IQR) | 4.2 (4–4.7) | 4.4 (4.1–5) | <0.001 |
| LVIDD, median (IQR) | 5.4 (4.8–6) | 5.5 (5–6.1) | 0.061 |
| Discharge Medications | |||
| Loop Diuretics, n (%) | 284 (82.32) | 282 (81.74) | 0.843 |
| Beta blocker, n (%) | 268 (77.68) | 271 (78.55) | 0.782 |
| ACE inhibitors/ARB, n (%) | 251 (72.75) | 254 (73.62) | 0.797 |
| CCB, n (%) | 97 (28.12) | 97 (28.12) | 1.00 |
| Spironolactone, n (%) | 67 (19.42) | 58 (16.81) | 0.374 |
| Digoxin, n (%) | 91 (26.38) | 88 (25.51) | 0.794 |
| Aspirin/clopidogrel, n (%) | 279 (80.87) | 260 (75.36) | 0.080 |
| Statin, n (%) | 228 (66.09) | 232 (67.25) | 0.747 |
| Anticoagulants, n (%) | 61 (17.68) | 67 (19.42) | 0.557 |
| Outcomes | |||
| Death (n, %) | 94 (27.25) | 128 (37.10) | 0.006 |
| All-cause readmission (n, %) | 134 (38.84) | 162 (46.96) | 0.031 |
| Cardiac readmission (n, %) | 93 (26.96) | 122 (35.36) | 0.017 |
| Death or readmission (n, %) | 162 (50.31) | 239 (64.95) | <0.001 |
| Length of stay (median, IQR) | 4 (3–6) | 4 (3–7) | 0.87 |
Abbreviations.
RVSP – right ventricular systolic pressure, PH – pulmonary hypertension, IQR – interquartile range, SD – standard deviation, CAD – coronary artery disease, MAP – mean arterial pressure, BMI – body mass index, pro-BNP – pro brain natriuretic peptide, Hb – hemoglobin, GFR – glomerular filtration rate, EF – ejection fraction, LAD – left atrial diameter, LVIDD – left ventricular internal diastolic diameter, ACE - angiotensin-converting enzyme, ARB – angiotensin receptor blocker.
3.2. Outcome in heart failure cohort [Table 3]
Table 3.
Primary and secondary outcome stratified by Right Ventricular Systolic Pressure by various Statistical Methods.
| Univariate Model | Multivariable Model | PSM Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Death | 1.79 | 1.43–2.24 | <0.001 | 1.66 | 1.31–2.10 | <0.001 | 1.60 | 1.22–2.09 | 0.001 | |
| All-cause readmission | 30-Days | 1.62 | 1.18–2.23 | 0.003 | 1.50 | 1.07–2.12 | 0.019 | 1.53 | 1.07–2.19 | 0.021 |
| 6-months | 1.39 | 1.14–1.70 | 0.001 | 1.28 | 1.04–1.59 | 0.022 | 1.37 | 1.09–1.73 | 0.008 | |
| Cardiac readmission | 30-Days | 1.47 | 1.00–2.15 | 0.052 | 1.35 | 0.89–2.04 | 0.154 | 1.35 | 0.87–2.09 | 0.184 |
| 6-months | 1.37 | 1.08–1.73 | 0.009 | 1.30 | 1.01–1.67 | 0.040 | 1.41 | 1.07–1.85 | 0.014 | |
| Death or readmission | 30-Days | 1.74 | 1.28–2.37 | <0.001 | 1.64 | 1.19–2.28 | 0.003 | 1.60 | 1.13–2.27 | 0.008 |
| 6-months | 1.48 | 1.22–1.80 | <0.001 | 1.35 | 1.10–1.66 | 0.004 | 1.44 | 1.15–1.80 | 0.002 | |
Abbreviation: PSM – propensity-score matched, HR – hazard ratio, CI – confidence interval.
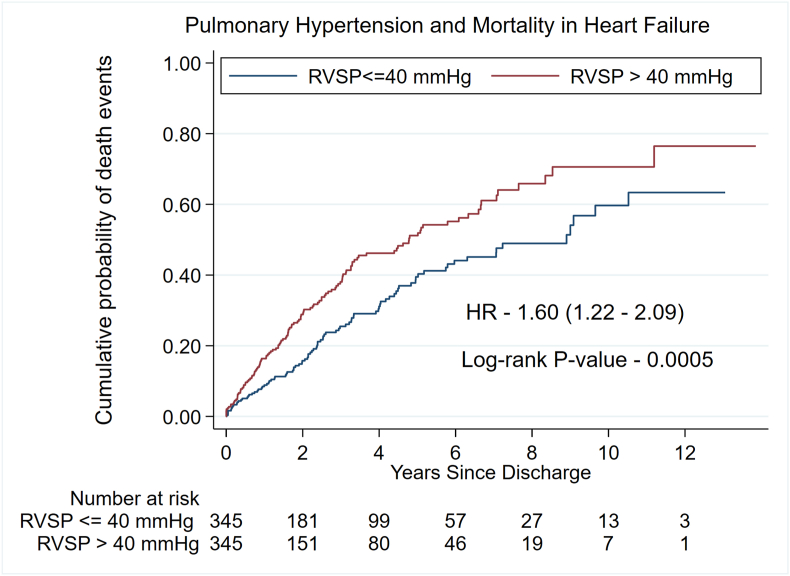
A RVSP ≥40 mm Hg compared with RVSP <40 mmHg was associated with higher rates of death [PSM cohort; HR: 1.60, 95% CI: 1.22–2.09, P-value = 0.001] [Fig. 1]. Further, RVSP ≥40 mmHg compared with RVSP <40 mmHg was associated with higher rates of all-cause readmissions [PSM cohort; HR: 1.37, 95% CI: 1.09–1.73, P-value = 0.008] [Fig. 2], cardiac readmissions [PSM cohort; HR: 1.41, 95% CI: 1.07–1.85, P-value = 0.014] [Fig. 3], and combined death/readmissions [PSM cohort; HR: 1.44, 95%CI: 1.15–1.80, P-value = 0.002] [Fig. 4] at 6-month follow-up.
Fig. 1.
Kaplan–Meier graph of mortality by Right Ventricular Systolic Pressure.
Fig. 2.
Kaplan–Meier graph of all-cause readmission by Right Ventricular Systolic Pressure.
Fig. 3.
Kaplan–Meier graph of cardiac readmission by Right Ventricular Systolic Pressure.
Fig. 4.
Kaplan–Meier graph of death/readmission by Right Ventricular Systolic Pressure.
3.3. Outcomes in subgroups of HFrEF and HFpEF [Table 4]
Table 4.
Primary and secondary outcome in subgroups of HFrEF and HFpEF by propensity-sscore matching method.
| HFrEF (N = 434) | HFpEF (N = 256) | ||||||
|---|---|---|---|---|---|---|---|
| Outcomes | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Death | 1.57 | 1.12–2.21 | 0.009 | 1.68 | 1.09–2.59 | 0.019 | |
| All-cause readmission | 30-Days | 1.56 | 0.97–2.50 | 0.064 | 1.47 | 0.84–2.57 | 0.175 |
| 6-months | 1.22 | 0.90–1.64 | 0.203 | 1.63 | 1.13–2.35 | 0.009 | |
| Cardiac readmission | 30-Days | 1.36 | 0.78–2.36 | 0.280 | 1.33 | 0.65–2.74 | 0.440 |
| 6-months | 1.21 | 0.86–1.71 | 0.269 | 1.81 | 1.14–2.86 | 0.012 | |
| Death or readmission | 30-Days | 1.72 | 1.10–2.71 | 0.018 | 1.41 | 0.82–2.43 | 0.211 |
| 6-months | 1.32 | 1.01–1.76 | 0.046 | 1.61 | 1.12–2.31 | 0.010 | |
Abbreviation: PSM – propensity-score matched, HR – hazard ratio, CI – confidence interval.
In both HFrEF and HFpEF, patients with RVSP ≥40 mmHg compared with RVSP <40 mmHg had higher rates of death and a composite of death or readmission. In addition, in patients with HFpEF, RVSP ≥40 mmHg compared with RVSP <40 mmHg was associated with higher rates of all-cause readmission and cardiac readmission at follow-up. However, in patients with HFrEF, RVSP ≥40 mmHg compared with RVSP <40 mmHg was associated with similar rates of all-cause readmission and cardiac readmission at follow-up.
3.4. Unmeasured bias analysis
In the ‘E-value’ analysis, the observed HR of 1.60 for mortality, 1.37 for all-cause readmission, 1.41 for cardiac readmission, and 1.44 for death/readmission could be explained by an unmeasured confounder that was associated with both the treatment and the outcome by HR of 2.58-fold, 2.08-fold, 2.17-fold, and 2.24-fold each, respectively, above the measured confounders, but weaker confounding could not do so. This signifies that the point estimate required by the unmeasured confounder to explain the observed effect is high.
4. Discussion
In this retrospective cohort study, RVSP ≥40 mmHg had a higher hazard of death compared with RVSP <40 mmHg. Further, RVSP ≥40 mmHg was associated with higher rates of all-cause readmission, cardiac readmission, and death/readmission at 6-month follow-up compared with RVSP <40 mmHg. Likewise, in the subgroups of HFpEF and HFrEF, RVSP ≥40 mm Hg was associated with higher rates of death and composite of death or readmission compared with RVSP <40 mmHg. However, the rates of all-cause readmission and cardiac readmission were higher in RVSP ≥40 mmHg population only in the HFpEF subgroup but similar in the HFrEF subgroup compared with RVSP <40 mmHg.
At baseline, there was no clinically significant difference in the mean age between the two cohorts. Patients with RVSP ≥40 mmHg had a lower percentage of coronary artery disease and obesity, whilst having a higher percentage of atrial fibrillation and chronic lung disease compared with RVSP <40 mmHg, which is expected and observed in previous studies.6,7 RVSP ≥40 mmHg was also associated with a lower mean of mean atrial pressure, median hemoglobin, glomerular filtration rate, and sodium, whilst a higher median of pro-BNP, which combined signifies severe disease. In addition, a higher percentage of chronic lung disease in patients with RVSP ≥40 mmHg is likely explained by the established role of chronic lung disease in the development of PH, and hence raised RVSP.7 Further, patients with RVSP ≥40 mmHg compared with RVSP <40 mmHg had a lower percentage of statin, and aspirin/clopidogrel prescription explained by the lower percentage of coronary artery disease in patients with RVSP ≥40 mmHg.
Based on our literature review, we realized that RVSP is an underrated echocardiographic parameter with regard to its prognostic value. It is also well-known that cardiac hemodynamic parameters are highly interdependent. For example, there is a strong correlation between pulmonary artery systolic pressure (PASP) and pulmonary capillary wedge pressure (PCWP), as demonstrated by Dranzer et al.8 In conditions without right ventricular outflow obstruction (e.g., absence of pulmonary stenosis, RV outlet obstruction or teratology of Fallot), RVSP is equal to PASP.9, 10, 11 An increase in RVSP corresponds to an increase in PASP, and possible simultaneous worsening of other hemodynamic parameters like PCWP and left ventricular function. Many centers lack the facilities for a right heart catheterization, and immediate right heart catheterization for a HF hospitalization is not routinely done. This is because the use of right heart catheterization showed a lack of benefit, and an increased infection rate in heart failure patients.12 In such cases, we believe that RVSP can be used as a non-invasive alternative to right heart catheterization to determine the basic right heart hemodynamics, including PASP.
Further, similar to our results, previous studies have demonstrated that PASP (and thereby PH) is independently associated with increased mortality.13, 14, 15, 16, 17, 18 The reason, as explained in these studies, showed that PH, when induced by monocrotaline in rats, is associated with reversible and irreversible damage to the epithelium of lung parenchyma by increasing basic fibroblast growth factors (bFGF),19 laminin (LM), and fibronectin (FN) in the basement membrane of lungs and tenascin synthesis.20,21 These changes lead to increased type IV collagen in the airway, vascular and gas exchange region of lungs, and an increase in right ventricular mass, thereby compromising lung reserve, lung functional capacity, oxygenation, and reducing right ventricular function, increasing RVSP. This, in turn, increases the burden on the already compromised and compensated heart failure pathophysiology. Further, these studies have shown that the molecular changes precede a few days before increasing pulmonary vasculature pressure. As a result, when RVSP rises, the molecular changes at the gas exchange membrane have already occurred. Thus, an increased RVSP points to a damaged lung epithelial membrane. The above studies also showed that elevated PH increases right ventricular mass. This increased right ventricular mass competes with the left ventricle in the shared pericardial space.22 This leads to a constellation of increased left ventricular filling pressure and reduced left ventricular end-diastolic volume, thereby compromising the left ventricular function. Also, left ventricular dysfunction, regardless of the pathology of the left side heart disease, leads to an increased left-sided filling pressure triggering a sequel of PH followed by pulmonary vasoconstriction, endothelial dysfunction, and remodeling of small pulmonary resistance vessels, which ultimately results in right-sided ventricular dysfunction and hence poor prognosis.23
PH can occur either from intrinsic lung or heart dysfunction. As a result, RVSP can act as an indirect marker for either lung or left heart function. Elevated RVSP acts as a surrogate giving an idea of the left heart and lung function irrespective of the underlying primary cause. Poor outcomes for patients with higher RVSP act as a testimony to the above statement. Based on the above explanation, it is evidenced that RVSP gives an insight into the pulmonary vasculature, hence might act as an excellent prognostic indicator in heart failure patients. The idea is not to deter patients from right heart catheterization as it gives other invaluable information, but to identify a bedside, non-invasive method, which is a part of routine practice, and without subjecting patients to additional invasive tests during heart failure hospitalization. We also believe that this strategy when coupled with guideline-directed medical therapy (GDMT) is expected to improve the outcomes in HF patients.
A novel finding of our study is the higher rates of all-cause and cardiac readmission with RVSP ≥40 mm Hg compared with RVSP <40 mm Hg in patients with HFpEF, while no effects on all-cause or cardiac readmissions in patients with HFrEF. There have been several studies done showing poor outcomes in patients with HFpEF and PH.4 There has been difference noted in the underlying causes and cardiac remodeling leading to PH from HFrEF and HFpEF. HFrEF has well established guideline-directed medical therapy, leading to less readmissions; however, ambiguous medical management for HFpEF may lead to higher readmissions as seen in this study.24 Further studies are required to validate this disparity.
Our study has several limitations, which must be considered before generalizing its results. First, ours is a single-center, retrospective study, fraught with inherent unmeasured biases that might exist despite robust adjustments. However, the size of the study population, the consistency of results with prior studies, application of propensity-score match analysis, and ‘E-value’ analysis reassure validity of conclusions, which may be more generalizable. The outcomes were reported after multivariate cox-regression and propensity-score matching, utilizing 25 variables for robust adjustment and matching. Though the non-invasive nature of echocardiographic measurement of RVSP has its benefits, it is not without limitations. The possibility of RVSP measurement may not be possible in all patients due to the lack of a tricuspid regurgitation jet and lack of a transthoracic echocardiographic window, reducing its universal applicability. The right atrial pressure is assumed as a constant number in most cases, which may influence the measured RVSP, especially when RVSP is low.25 The interpreters' variability in RVSP measurement were not accounted for in the present analysis. The accuracy of measurement of tricuspid jet is based on the skills of the technician. The technique of determining right ventricular function was not fixed and varied from technician preferences over the years. A combination of TAPSE (tricuspid annular plane systolic excursion), annular velocity, 2D strain imaging, and fractional area change were used in some, while in others only some of the techniques were used. In this study, as all transthoracic echocardiographic readings are read and verified by two personnel, conflicting results from interpersonal variability are subject to minimum errors. Further, the same technicians performed transthoracic echocardiography for all patients in both groups leading to a lower probability of intergroup errors in reporting.
5. Conclusion
In conclusion, RVSP ≥40 mmHg compared with RVSP <40 mmHg in patients with HF was associated with higher rates of death, all-cause readmissions, and cardiac readmissions. Further, in the HFpEF subgroup, RVSP ≥40 mmHg compared with RVSP <40 mmHg was associated with higher rates of death, all-cause readmissions, and cardiac readmissions, while in the HFrEF subgroup, RVSP ≥40 mmHg compared with RVSP <40 mmHg was associated with higher rates of death, but similar rates of all-cause and cardiac readmissions. RVSP can be considered a prognostic marker for mortality and readmission.
Funding
makeadent.org Ram and Sanjita Kalra Aavishqaar Fund.
Declaration of competing interest
The authors reported no potential conflicts of interest, relationships with pharmaceutical companies, biomedical device manufacturers, or other corporations whose products or services are related to the subject matter of the article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ihj.2022.03.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Virani S.S., Alonso A., Benjamin E.J., et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020:E139–E596. doi: 10.1161/CIR.0000000000000757. Published online. [DOI] [PubMed] [Google Scholar]
- 2.Rao S.D., Adusumalli S., Mazurek J.A. Pulmonary hypertension in heart failure patients. Card Fail Rev. 2020;6 doi: 10.15420/cfr.2019.09. e05-e05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin J.T., Semigran M.J. Heart failure and pulmonary hypertension. Heart Fail Clin. 2010;6(2):215–222. doi: 10.1016/j.hfc.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guazzi M. Pulmonary hypertension in heart failure preserved ejection fraction: prevalence, pathophysiology, and clinical perspectives. Circ Heart Fail. 2014;7(2):367–377. doi: 10.1161/CIRCHEARTFAILURE.113.000823. [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P., Voors A.A., Anker S.D., et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. 2016. [DOI] [PubMed] [Google Scholar]
- 6.Rottlaender D., Motloch L.J., Schmidt D., et al. Clinical impact of atrial fibrillation in patients with pulmonary hypertension. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033902. e33902-e33902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaouat A., Naeije R., Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J. 2008;32(5):1371. doi: 10.1183/09031936.00015608. LP - 1385. [DOI] [PubMed] [Google Scholar]
- 8.Drazner M.H., Hamilton M.A., Fonarow G., Creaser J., Flavell C., Stevenson L.W. Relationship between right and left-sided filling pressures in 1000 patients with advanced heart failure. J Hear lung Transplant Off Publ Int Soc Hear Transplant. 1999;18(11):1126–1132. doi: 10.1016/s1053-2498(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 9.Yock P.G., Popp R.L. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70(4):657–662. doi: 10.1161/01.CIR.70.4.657. [DOI] [PubMed] [Google Scholar]
- 10.Berger M., Haimowitz A., Van Tosh A., Berdoff R.L., Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985;6(2):359–365. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- 11.Currie P.J., Seward J.B., Chan K.L., et al. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6(4):750–756. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- 12.Binanay C., Califf R.M., Hasselblad V., et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294(13):1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 13.Shalaby A., Voigt A., El-Saed A., Saba S. Usefulness of pulmonary artery pressure by echocardiography to predict outcome in patients receiving cardiac resynchronization therapy heart failure. Am J Cardiol. 2008;101(2):238–241. doi: 10.1016/j.amjcard.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 14.Kjaergaard J., Akkan D., Iversen K.K., et al. Prognostic importance of pulmonary hypertension in patients with heart failure. Am J Cardiol. 2007;99(8):1146–1150. doi: 10.1016/j.amjcard.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 15.Lam C.S.P., Roger V.L., Rodeheffer R.J., Borlaug B.A., Enders F.T., Redfield M.M. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53(13):1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damy T., Goode K.M., Kallvikbacka-Bennett A., et al. Determinants and prognostic value of pulmonary arterial pressure in patients with chronic heart failure. Eur Heart J. 2010;31(18):2280–2290. doi: 10.1093/eurheartj/ehq245. [DOI] [PubMed] [Google Scholar]
- 17.Szwejkowski B.R., Elder D.H.J., Shearer F., et al. Pulmonary hypertension predicts all-cause mortality in patients with heart failure: a retrospective cohort study. Eur J Heart Fail. 2012;14(2):162–167. doi: 10.1093/eurjhf/hfr159. [DOI] [PubMed] [Google Scholar]
- 18.Bursi F., McNallan S.M., Redfield M.M., et al. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59(3):222–231. doi: 10.1016/j.jacc.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arcot S.S., Fagerland J.A., Lipke D.W., Gillespie M.N., Olson J.W. Basic fibroblast growth factor alterations during development of monocrotaline-induced pulmonary hypertension in rats. Growth Factors. 1995;12(2):121–130. doi: 10.3109/08977199509028958. [DOI] [PubMed] [Google Scholar]
- 20.Lipke D.W., Arcot S.S., Gillespie M.N., Olson J.W. Temporal alterations in specific basement membrane components in lungs from monocrotaline-treated rats. Am J Respir Cell Mol Biol. 1993;9(4):418–428. doi: 10.1165/ajrcmb/9.4.418. [DOI] [PubMed] [Google Scholar]
- 21.Lipke D.L., Aziz S.M., Fagerland J.A., Majesky M., Arcot S.S. Tenascin synthesis, deposition, and isoforms in monocrotaline-induced pulmonary hypertensive rat lungs. Am J Physiol Cell Mol Physiol. 1996;271(2):L208–L215. doi: 10.1152/ajplung.1996.271.2.L208. [DOI] [PubMed] [Google Scholar]
- 22.Janicki J.S., Weber K.T. The pericardium and ventricular interaction, distensibility, and function. Am J Physiol Cell Physiol. 1980;238(4):H494–H503. doi: 10.1152/ajpheart.1980.238.4.H494. [DOI] [PubMed] [Google Scholar]
- 23.Guazzi M., Labate V. Pulmonary hypertension in heart failure patients: pathophysiology and prognostic implications. Curr Heart Fail Rep. 2016;13(6):281–294. doi: 10.1007/s11897-016-0306-8. [DOI] [PubMed] [Google Scholar]
- 24.Guazzi M., Ghio S., Adir Y. Pulmonary hypertension in HFpEF and HFrEF: JACC review topic of the week. J Am Coll Cardiol. 2020;76(9):1102–1111. doi: 10.1016/j.jacc.2020.06.069. [DOI] [PubMed] [Google Scholar]
- 25.Kiatchoosakun S., Wongvipaporn C., Nanagara R., Hoit B.D. Right ventricular systolic pressure assessed by echocardiography: a predictive factor of mortality in patients with scleroderma. Clin Cardiol. 2011;34(8):488–493. doi: 10.1002/clc.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.