Abstract
Wolbachia is a genus of obligate bacterial endosymbionts that infect a diverse range of arthropod species as well as filarial nematodes, with its single described species, Wolbachia pipientis , divided into several ‘supergroups’ based on multilocus sequence typing. Wolbachia strains in mosquitoes have been shown to inhibit the transmission of human pathogens, including Plasmodium malaria parasites and arboviruses. Despite their large host range, Wolbachia strains within the major malaria vectors of the Anopheles gambiae and Anopheles funestus complexes appear at low density, established solely on PCR-based methods. Questions have been raised as to whether this represents a true endosymbiotic relationship. However, recent definitive evidence for two distinct, high-density strains of supergroup B Wolbachia within Anopheles demeilloni and Anopheles moucheti has opened exciting possibilities to explore naturally occurring Wolbachia endosymbionts in Anopheles for biocontrol strategies to block Plasmodium transmission. Here, we utilize genomic analyses to demonstrate that both Wolbachia strains have retained all key metabolic and transport pathways despite their smaller genome size, with this reduction potentially attributable to degenerated prophage regions. Even with this reduction, we confirmed the presence of cytoplasmic incompatibility (CI) factor genes within both strains, with wAnD maintaining intact copies of these genes while the cifB gene was interrupted in wAnM, so functional analysis is required to determine whether wAnM can induce CI. Additionally, phylogenetic analysis indicates that these Wolbachia strains may have been introduced into these two Anopheles species via horizontal transmission events, rather than by ancestral acquisition and subsequent loss events in the Anopheles gambiae species complex. These are the first Wolbachia genomes, to our knowledge, that enable us to study the relationship between natural strain Plasmodium malaria parasites and their anopheline hosts.
Keywords: Anopheles, genomics, prophage, symbiosis, Wolbachia
Data Summary
Impact Statement.
Wolbachia naturally infects a wide range of arthropod species, including insect vectors of human pathogens, where they may play a role in inhibiting their replication. These bacteria have been commonly found within Aedes albopictus and Culex pipiens mosquitoes, but have been noticeably absent in the mosquito genus Anopheles, which includes all species responsible for malaria transmission. Recent PCR-based methods have suggested the potential for natural Wolbachia strains within the Anopheles gambiae species complex, which includes major malaria vector species such as Anopheles gambiae s.s., Anopheles coluzzii and Anopheles arabiensis. We recently reported the presence of stable Wolbachia strains naturally occurring within two different Anopheles species (Anopheles demeilloni and Anopheles moucheti). In this study, we update and perform comparative genomic analysis of these two Wolbachia genomes against each other and published Wolbachia strains. Updated assemblies indicate smaller genome sizes compared to other Wolbachia of insects, despite their metabolic pathway repertoire being comparable to other strains. Interestingly, prophage fragments were identified within only one of the two strains. The findings of this study will be of significant interest to researchers investigating Wolbachia as a potential malaria biocontrol strategy, giving greater insight into the evolution and diversity of this obligate intracellular endosymbiont.
Sequence data generated and used for this analysis are available in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) (BioProject number accession no. PRJNA642000). The two assembled Wolbachia genomes are available with genome accession numbers GCA_018491735.2 and GCA_018491625.2. Additional Wolbachia genomes used for comparative analysis are described in the supplementary material. Additional supplementary data files used to generate several figures can be found on FigShare (https://doi.org/10.6084/m9.figshare.19576432) [1] .
Introduction
Wolbachia has a wide host range, including insects [2] where various estimates have predicted 52–60 % of all arthropod species are naturally infected [3, 4]. Attempts to characterize the within-species diversity has resulted in the designation of Wolbachia ‘supergroups’ A through to T [5, 6], with several exceptions [7], via multilocus sequence typing of five single-copy conserved genes [8]. The relationship between Wolbachia and their hosts can range from obligate mutualism, where the endosymbiont is essential for host survival and reproduction [9, 10], to reproductive parasitism, where it manipulates the reproduction of its host to spread through the population. Currently, the best-studied phenotype (which also affects mosquito hosts) is cytoplasmic incompatibility (CI), which causes infected males to produce unviable offspring unless they mate with an infected female, while infected females have viable offspring regardless of the males’ infection status; thus, conferring a fitness advantage to Wolbachia -infected females.
Genetic studies have previously identified a pair of CI factor genes, cifA and cifB [11, 12], that have been correlated to this phenotype. These genes have often been found to co-occur as a single operon within prophage eukaryotic association modules (EAMs), and are believed to spread via horizontal transmission between Wolbachia strains due to their localization within prophage regions [13]. Despite being part of the same operon, these genes have been observed to be differentially regulated, with cifA having higher expression relative to cifB [14]. When these CI genes were first identified, they were placed into three distinct phylogenetic groups. While all three were recognized to maintain protein domains with predicted nuclease activity, the catalytic residues for these nuclease domains were predicted to be absent in one of the three groups [11], which instead contained an additional protein domain with ubiquitin-like specific protease activity [12, 15, 16]. This was later characterized as the type I group [15]. Additionally, recent research has identified genes encoding similar features in other members of the order Rickettsiales , often found associated with mobile genetic elements, such as plasmids [15, 17]. As a result, a recent study has identified up to five phylogenetic types, with one of these types being identifiable in other Rickettsia as well as Wolbachia [15].
Utilization of the CI phenotype has been explored as the basis for potential mosquito control strategies to reduce human disease transmission. The bacterium is capable of inducing CI in both natural [6, 18, 19] and artificially infected lines [20–22], and possible methods to utilize them for mosquito population control include release of males infected with Wolbachia [23], or potentially via release of genetically modified mosquitoes that carry the CI genes, but not Wolbachia [24, 25]. In addition to inducing the CI phenotype, Wolbachia has been shown to interfere with pathogen replication directly, both in those that cause disease in the insect, as well as human pathogens that utilize the insect as a vector [6, 26–28]. This has been observed to be most effective with artificial infections of Wolbachia in non-native host mosquitoes [20–22]. Recent trials have shown that Wolbachia can be used to great effect in preventing the spread of dengue virus [29, 30], while laboratory trials have indicated their potential to block Plasmodium replication in artificially infected Anopheles mosquitoes [31–33]. While there is little evidence to date of stable Wolbachia infections [31], a stable infection within Anopheles stephensi is possible [32, 33]. Infection of these mosquitoes with Wolbachia was associated with significantly reduced hatch rates however [32, 34], possibly affecting the viability of CI as a control tool in this system.
Despite Wolbachia ’s presence in a wide variety of insects, natural high-density strains within the mosquito genus Anopheles have not been conclusively proven [35, 36] until recently [37]. Previous efforts to detect this bacterium required highly sensitive PCR techniques [38–42] that amplify a select handful of Wolbachia genes. Unfortunately, this alone cannot confirm the presence of live bacteria or stable Wolbachia strains within insects. Furthermore, phylogenetic placement of these amplified Wolbachia sequences within Anopheles gambiae shows multiple strains distributed across supergroups A and B, with some strains not assigned to any supergroup [36].
We recently demonstrated high-density Wolbachia strains in two Anopheles species, Anopheles demeilloni and Anopheles moucheti [37, 43], which we observed in wild populations collected over a large geographical range in temporally distinct populations. Importantly, we further visualized these bacteria in the germline, as well as sequenced near-complete Wolbachia genomes from both host species [37]. Here, we present reassembled, circularized genomes for both strains, as well as in-depth comparative analyses of these two Wolbachia strains against each other, and in the broader context of Wolbachia supergroups A through to F, with specific focus on supergroup B. We show that, in terms of both size and predicted protein-encoding genes, both assembled genomes are at the low end of the range of Wolbachia strains found within insects whilst containing reduced or, in the case of wAnM, no prophage WO regions. Despite this, both Wolbachia genomes maintained complete pathways that are expected for the genus, such as complete haem and nucleotide biosynthetic pathways and type IV secretion systems (T4SSs). Additionally, we reconstructed the phylogenetic history using whole-genome-sequence data, which indicates that these strains may originate from independent acquisitions via horizontal transfer events, and not from an ancestral infection that has since been lost in other Anopheles mosquitoes.
Methods
Sequence data collection and genome quality assessment
Both genome assemblies of wAnD and wAnM were manually curated (i.e. gaps, indels and synteny) using the approach described by Tsai and collaborators in 2010 [44], Mummer/Nucmer software tool v4.0.0 [45], Mauve v2.4.0 [46] and Tablet v1.21.02.08 [47]. To complement the genomes of wAnD and wAnM [37], whole-genome sequences of 15 Wolbachia genomes were downloaded from the National Center for Biotechnology Information (NCBI), with these genomes spanning supergroups A through to F (full information available in Table S1, available with the online version of this article). An additional 25 Wolbachia genomes were also downloaded from the European Nucleotide Archive (ENA). These additional genomes were sequenced as part of a large-scale study [48] that looked at assembling Wolbachia genomes from a variety of existing sequencing data of various insects. All genome accession numbers used in this study, as well as a summary of their annotations used in this study, are provided in Table S1.
To confirm genome completeness, nucleotide sequences of all downloaded genomes, as well as the assembled genomes of wAnD and wAnM, were used as input into the program busco (v5.0.0) [49], with the lineage option set to ‘rickettsiales_odb10’. This program analyses genome completeness via comparison against a selection of marker genes (364 genes in total) predicted to be present in single copies based on the input genome’s lineage. Genomes that showed significantly lower completeness levels (less than 80 % completeness) were excluded from orthologue and pathway analyses. This resulted in six of the Wolbachia genomes [48] being removed from these additional analyses.
Phylogenetic, pangenome and metabolic pathway analysis
A total of 36 Wolbachia genomes were used for phylogenetic analysis of supergroup B Wolbachia specifically (genomes of wAnD and wAnM, 9 Wolbachia genomes from the NCBI and 25 from ENA). These genome sequences were used as input into the program wgsim (version 1.9) [50, 51], which ‘shreds’ the genomic template to generate genome fragments similar to sequencing reads. Base error, mutation, fraction of indels and indel extension probability were set to zero, read lengths set to 100 and a total of ten million reads simulated for each genome. These genome reads were then used to generate a single nucleotide variant (SNV) alignment via Snippy v4.6.0 [52] using the wNo genome as reference (genome accession no. GCA_000376585.1). Gubbins v3.0.0 [53] was used for removing recombinant events. Recombination-free alignment of all 34 genomes was then analysed with iq-tree v1.6.12 [54] using default parameters, with a GTR substitution model using 1000 non-parametric bootstrap replicates for branch support.
This initial tree was further validated using single-copy orthologous genes identified by OrthoFinder v2.5.1 [55] (see relevant section in Methods). All single-copy orthologous genes that were identified from each Wolbachia strain were aligned using mafft v7.455 [56], before being concatenated together using SeqKit’s concat v0.15.0 program [57], with the resultant alignment used as input into iq-tree v1.6.12 [54] using default parameters. The substitution model used was HIVw +F+R2, identified as the best-fit model by the ModelFinder program [58], and 1000 non-parametric bootstrap replicates to determine branch support.
Orthologous group detection
Orthologous group detection was performed in two separate parts – the first was to compare protein-encoding sequences amongst Wolbachia of supergroup A through to F, whilst the second was to compare protein-encoding sequences amongst Wolbachia of supergroup B specifically. For orthologue analysis amongst Wolbachia of supergroup A through to F, RefSeq protein annotations for the 15 genomes downloaded from NCBI were used, alongside RefSeq protein annotations for wAnD and wAnM. Protein sequences from these 17 genomes were used as input into the program OrthoFinder (v2.5.1) [55], using default parameters. Orthogroups that were common or unique between all 17 Wolbachia strains were subsequently plotted using the R program package UpSetR (v1.4.0) [59]. Additional querying of the data was then performed using the R program package ‘ComplexHeatmaps’ (v2.5.5) [60].
For orthologous group detection amongst a wider selection of supergroup B Wolbachia , a total of 27 Wolbachia genomes were used (6 from NCBI, 19 from ENA, alongside the assembled genomes of wAnD and wAnM) (Table S1). Annotations for all Wolbachia genomes were generated using a local installation of the NCBI PGAP (build5508 2021-07-01) [61], and were used as input into the program OrthoFinder (v2.5.1) [55] using default parameters. Orthogroups were again visualized using the R program UpSetR (v1.4.0) [59], with additional data querying performed using the R program ComplexHeatmaps (v2.5.5) [60]. Genes of interest identified within these orthogroups, e.g. those that were unique to particular genomes, were further analysed using the Pfam website’s sequence search [62, 63] and NCBI’s blastp [64]. Comparison of the identified nucleotide regions that had similarity to the Osmia lignaria gene XP_034172187.1 was performed by blastn and blastx, with visualizations performed using Easyfig [65].
Construction of metabolic pathways
The genomes of both wAnD and wAnM were submitted to the NCBI Prokaryotic Annotation Pipeline, with a GenBank flatfile being generated as a result. This flatfile was then downloaded, and used as input into BioCyc’s Pathway Tools program (v24.0) [66] and Pathologic (v24.0) [67, 68]. Pathologic is able to assign protein function and pathways to annotated genes based on name and/or automated blast hits. To address proteins with ‘ambiguous’ function within metabolic pathways, all predicted protein-encoding genes of both wAnD and wAnM were submitted to the EggNOG online server, which allows for the automated transfer of functional annotations (v2.0.1) [69]. Predicted protein-encoding genes were also submitted to the KEGG Automatic Annotation Server (KAAS, last updated 3 April 2015) [70], as a second method for functional annotation. Any proteins identified by Pathologic as having an ambiguous function were then manually cross-checked with the outputs of EggNOG and KAAS, and enzyme code numbers assigned. This process was repeated for a selection of Wolbachia genomes from supergroup B (Table S1). Once this process was completed, Pathway Tools’ Pathway Overview and Comparison options were then used to compare pathways between the different Wolbachia strains. A selection of these biosynthetic and transport pathways was then made, based on prior literature investigating their importance to the Wolbachia –host endosymbiotic relationship. Gene presence and absence within these pathways was then manually scored, and plotted out into a heatmap using R’s GGplot2 package [71].
Characterization of CI factor genes
Phylogenetic placement of the three sets of cif gene pairs from both wAnD and wAnM was made following the methods of Martinez et al. [15]. Briefly, nucleotide sequences for CI factor (cif) A and B genes for all five monophyletic types were obtained from the supplementary materials of Martinez et al. [15]. Partially sequenced cif genes were discarded, and the nucleotide sequences for cifA and cifB genes were aligned separately using the program mafft. The separate alignments were then used as input into the online GBlocks server (v0.91b) [72], with default ‘stringent’ parameters to filter out weakly conserved regions of the alignment. Once filtering was done, the separate nucleotide sequence alignments were then concatenated using SeqKit (v0.15.0) [57], and used as input for PhyML (v3.0) [73], using the GTR GAMMA substitution model of evolution and 1000 bootstrap replicates. The outputted Newick formatted tree was then annotated using the GGTree package in R (v2.2.4) [74], .
Due to concerns that the cifB gene from wAnD may be approaching pseudogenization, four uninterrupted homologues of the intact cifB gene from wAnD were identified from the generated phylogenetic tree. These were the cifB genes from wAra pair 2, wBai pair 2, wHa pair 2 and wNik pair 2. Gene sequences for these were obtained from the supplementary data of a published study [15], and used as input into the program prank (v170427) [75] for codon-based alignments using the options -codon and -translate. The resultant alignment was then manually examined for the presence of potential pseudogenization or frameshifts.
Ankyrin, prophage and insertion sequence (IS) element detection
Ankyrin domains were detected using five HMMer profiles (ID numbers PF00023.31, PF12796.8, PF13606.7, PF13637.7, PF13857.7) [63]. These profiles were generated via first downloading associated alignment files from the Pfam protein database [62] as Stockholm formatted seed files. The HMMer suite (v3.1b2) [63] was then used to build HMM (hidden Markov model) profiles from these seed files. These profiles were then compared against the protein amino acid sequences annotated from wAnD and wAnM to identify any protein-encoding genes containing an ankyrin domain. This analysis was then repeated for a selection of Wolbachia genomes to allow for direct comparisons to be made.
Prophage sequences were identified within the genomes of wAnD and wAnM using the phaster web server [76]. Assembled contig sequences of both genomes were uploaded separately to the server, checking the option to note that the input consists of multiple separate contigs. In the case of wAnD where prophage regions were detected, results were downloaded and manual curation of the identified prophage regions was performed using the Artemis genome browser [77] to identify prophage genes overlapping these regions. Additional blastx searches were performed on neighbouring genes against phage WOVitA1 sequences (GenBank nucleotide reference sequence HQ906662.1) to screen for genes that may be associated with prophage WO’s EAM.
IS element detection was performed by submission of the two completed wAnD and wAnM genomes to the ISSaga online server (v1.0) [78] and results tables obtained. Manual curation of hits identified by ISSaga was then performed, with putative IS elements first filtered for high similarity and coverage blast searches against the ISFinder database. Predicted IS elements were further compared against the assembled genome and automated annotations from PGAP, confirming that they have been annotated appropriately.
Results
Currently assembled Wolbachia genomes are small in size but supported by high completeness scores
As Wolbachia is an obligate intracellular endosymbiont, it has a highly reduced genome and can only be isolated from infected host material, posing a challenge to obtain complete, uncontaminated genome sequences. The updated, circularized genome assemblies of Wolbachia of Anopheles demeilloni (wAnD) has a total length of 1 231 247 bp, while Wolbachia of Anopheles moucheti (wAnM) has a genome length of 1 121 812 bp. This assembly showed overall even coverage throughout the genome, although there were up to three regions per genome greater than 1 kbp that showed higher than average coverage, indicating potentially collapsed repetitive regions (Fig. S1). While the currently assembled genome sizes are smaller compared to other analysed Wolbachia strains that reside within insects (particularly wAnM), they are larger than the genomes of those found in filarial nematodes, which have a maximum size of 1.08 Mbp amongst those compared in our analysis (Tables 1 and Fig. S1). RefSeq annotation of both wAnD and wAnM genomes identified 1157 and 1082 protein-encoding genes and 122 and 80 pseudogenes, respectively (Table 1). For comparison, the Wolbachia strains of Aedes albopictus (wAlbB) and Culex quinquefasciatus (wPip) maintained 1180 and 1241 protein-encoding genes, respectively.
Table 1.
Summary table of a selection of different near-complete Wolbachia genomes and their general genome properties
Note the genomes of wAnD and wAnM (highlighted in bold text) have similar genome properties compared to other Wolbachia genomes, but have a relatively lower number of protein-encoding genes when compared against Wolbachia strains of supergroups A and B. busco scores were calculated using the Rickettsiales _odb10 lineage, created on 06/03/2020 with a marker gene list total of 364.
|
Strain name |
Host organism |
Supergroup |
Size (Mb) |
G+C (mol%) |
Total genes |
No. of proteins |
No. of pseudogenes |
tRNA |
rRNA |
Other RNAs |
No. of ankyrin proteins |
busco score (out of 364) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
wAu |
Drosophila simulans |
A |
1.27 |
35.22 |
1265 |
1099 |
125 |
34 |
3 |
4 |
35 |
362 (99.5 %) |
|
wHa |
Drosophila simulans |
A |
1.30 |
35.09 |
1242 |
1110 |
91 |
34 |
3 |
4 |
36 |
362 (99.5 %) |
|
wMel |
Drosophila melanogaster |
A |
1.27 |
35.23 |
1247 |
1144 |
103 |
34 |
3 |
4 |
27 |
361 (99.2 %) |
|
wRi |
Drosophila simulans |
A |
1.45 |
35.16 |
1340 |
1245 |
95 |
35 |
3 |
4 |
33 |
360 (98.9 %) |
|
wAlbB |
Aedes albopictus |
B |
1.49 |
34.50 |
1442 |
1180 |
221 |
34 |
3 |
4 |
38 |
355 (97.5 %) |
|
wAnD |
Anopheles demeilloni |
B |
1.23 |
33.58 |
1320 |
1157 |
122 |
34 |
3 |
4 |
55 |
360 (98.9 %) |
|
wAnM |
Anopheles moucheti |
B |
1.12 |
33.59 |
1203 |
1082 |
80 |
34 |
3 |
4 |
37 |
360 (98.9 %) |
|
wMa |
Drosophila mauritiana |
B |
1.27 |
34.00 |
1196 |
1055 |
100 |
34 |
3 |
4 |
49 |
360 (98.9 %) |
|
wMau |
Drosophila mauritiana |
B |
1.27 |
34.00 |
1194 |
1054 |
99 |
34 |
3 |
4 |
49 |
361 (99.2 %) |
|
wMeg |
Chrysomya megacephala |
B |
1.38 |
33.95 |
1268 |
1116 |
111 |
34 |
3 |
4 |
52 |
363 (99.7 %) |
|
wNo |
Drosophila simulans |
B |
1.30 |
34.01 |
1208 |
1062 |
105 |
34 |
3 |
4 |
53 |
363 (99.7 %) |
|
wPip |
Culex quinquefasciatus |
B |
1.48 |
34.19 |
1385 |
1241 |
103 |
34 |
3 |
4 |
63 |
362 (99.5 %) |
|
wOo |
Onchocerca ochengi |
C |
0.96 |
32.07 |
733 |
645 |
47 |
34 |
3 |
4 |
2 |
346 (95.1 %) |
|
wOv |
Onchocerca volvulus |
C |
0.96 |
32.07 |
734 |
648 |
45 |
34 |
3 |
4 |
3 |
345 (94.8 %) |
|
wBm |
Brugia malayi |
D |
1.08 |
34.18 |
1029 |
845 |
143 |
34 |
3 |
4 |
18 |
357 (98.1 %) |
|
wFol |
Folsomia candida |
E |
1.80 |
34.35 |
1662 |
1541 |
79 |
35 |
3 |
4 |
94 |
362 (99.5 %) |
|
wCle |
Cimex lectularius |
F |
1.25 |
36.25 |
1238 |
1023 |
174 |
34 |
3 |
4 |
42 |
356 (97.8 %) |
As an obligate intracellular endosymbiont that may have multiple strains infecting the same host, assessing Wolbachia genome completeness is important to ensure contaminating reads from different strains are not incorporated, and that the assembly does not have significant gaps. Despite the smaller number of protein-encoding genes and genome size, both wAnD and wAnM were noted to contain over 98 % of essential single-copy genes as determined by the busco program [49] (Table 1), with only wAnM predicted to have one duplicated gene, indicating that both their respective hosts are infected with only a single strain of Wolbachia . These figures are in line with previously published and complete Wolbachia genomes of strains found within insects (Table 1), with examples such as the Wolbachia strains of Drosophila flies (wMel, wRi, wHa, wAu and wNo) and mosquitoes (wAlbB, wPip), all having completeness scores ranging from 97.5 to 99.5 %. Additional details on these genomes used for comparison and their associated publications are available in Table S1.
Different Anopheles species show potentially independent Wolbachia acquisition events
Whole-genome phylogenetic analysis was performed to better understand how wAnD and wAnM may have been acquired by Anopheles, utilizing the most closely related genome of Wolbachia of Drosophila simulans strain Noumea (wNo) as a reference [37]. Using a total of 36 genomes of Wolbachia strains from supergroup B, a SNV alignment of 2824 bp was generated. The midpoint-rooted tree of the SNV alignment (Fig. 1, unrooted radial tree shown in Fig. S2) placed both wAnD and wAnM within a clade that also includes wNo, and several Wolbachia strains that infect Drosophila mauritiana [48, 79, 80]. We observed a significant number of differences in this alignment between wAnD and wAnM strains, with a total of 824 SNVs between the two Anopheles-derived strains. By contrast, wAnM was shown to have a total of 408 and 417 SNVs shared between it and the Wolbachia strains of Drosophila mauritiana and wNo, respectively, suggesting that wAnM is more closely related to these strains than to wAnD. As an additional verification of results, a phylogenetic tree was also reconstructed utilizing 172 single-copy orthologues identified by the program OrthoFinder [55]. This generated a similar tree to that obtained previously, with wAnM and wAnD being members of a clade with wNo and wMa/wMau, and being distinct from known Wolbachia strains that infect mosquitoes, wAlbB and wPip. This phylogenetic tree is shown in Fig. S3.
Fig. 1.
Maximum-likelihood phylogenetic tree of whole-genome alignments of a selection of Wolbachia genomes, using 1000 bootstrap replicates. Genomes with names beginning with WOLB followed by four digits were assembled by Scholz et al. [49]. Other genomes, with the exception of wAnM and wAnD, are the results of previous sequencing efforts, with acronyms as described in Table 1. The tree is midpoint rooted. Note how wAnM and wAnD are present within a clade alongside wNo and several assembled genomes of Wolbachia from Drosophila mauritiana (green highlight). By contrast, previously sequenced Wolbachia of mosquitoes wPip/wPipMol and wAlbB are present in separate clades. Scale bar indicates relative branch lengths of the phylogenetic tree. An unrooted radial tree of this figure is included as Fig. S2.
Regardless of the method used to reconstruct the phylogenetic tree, it was observed that wAlbB and wPip, two known Wolbachia strains of mosquitoes, do not cluster together, and appear in clades separate from both wAnM and wAnD. This lack of host phylogenetic congruence can be seen throughout both generated trees (Figs 1 and S3), with Insecta host members from different orders appearing throughout. Exceptions to this observation come from Wolbachia genomes that have been sequenced from the same host, e.g. Diaphorina citri or Drosophila mauritiana. Such observations are similar to those in previous studies that predict how Wolbachia is not solely restricted to vertical transmission [81, 82] and could be an indication of independent horizontal acquisition of wAnD and wAnM in their current hosts, rather than an ancestral infection that has since been lost in other anopheline mosquitoes. Phylogenetic analysis of COII and ITS2 sequences of Anopheles demeilloni and Anopheles moucheti had previously indicated significant phylogenetic distances from both the Anopheles gambiae and Anopheles funestus complexes [37]. Furthermore, this study also provided no evidence of resident Wolbachia strains within Anopheles marshallii, a mosquito species closely related to Anopheles demeilloni and Anopheles moucheti [37].
Wolbachia core genome is conserved in wAnM and wAnD orthogroup analysis
Orthologous gene groups are important to identify in Wolbachia strains due to their wide distribution across supergroups and diverse hosts, whilst offering insights into the presence/absence of unique pathways that may be involved in host–bacterial symbiosis. For this, we compared the RefSeq annotations of wAnD and wAnM genomes against 17 Wolbachia genomes (Table 1). A total of 18 404 genes were analysed, with 96.8 % of these assigned to 1300 orthogroups, and the remainder left unassigned to any orthogroup. Across the 17 Wolbachia strains analysed, a core genome of 9031 genes distributed across 523 orthogroups was identified (i.e. 40.2 % of all identified orthogroups comprising 49.1 % of total genes analysed can be considered as part of the core genome, defined as the genes and their protein products that are present in all analysed genomes), with 501 of these orthogroups containing single-copy genes. Outside of this core genome, the number of shared orthogroups is noticeably lower (Fig. 2a), and no orthogroups were unique to Wolbachia supergroup B strains. For wAnD, 39 genes were not assigned to an orthogroup, and one species-specific orthogroup (paralogues present in only one species) was identified containing seven genes (Fig. 2a, inset). By contrast, wAnM was noted to have 27 unassigned genes, as well as three species-specific orthogroups containing a total of 64 genes. None of the protein products for these genes had identifiable protein domains. Two orthogroups containing single-copy genes were identified that were specific to both wAnD and wAnM, although again none of these had identifiable protein domains.
Fig. 2.
Overview of identified orthogroups amongst Wolbachia . (a) Graphical representation of set notation of 17 near-complete Wolbachia genomes from six of the main supergroups using UpSetR, and the protein orthologues that they encode. Each genome (one per row at the bottom half of the image) is treated as a ‘set’ containing a certain number of orthogroups (denoted by the bar graph on the bottom left of the image). The various permutations of intersections are denoted by the ball-and-stick diagram at the bottom of the image, and the size of these intersections denoted by the bar graph at the top of the image. Wolbachia genomes are colour-coded based on their supergroup organization, and the genomes of wAnD and wAnM are highlighted by an additional grey outline on their row. Note how an intersect of all 17 Wolbachia genomes was identified as containing the vast majority of orthogroups – a core proteome total of 523 orthogroups (first bar from left). All other subsequent permutations of intersects contain less than 53 orthogroups. There were no intersects that uniquely contained only supergroup B Wolbachia . The inset stacked bar chart shows the distribution of singleton (i.e. genes that do not belong to an orthogroup, dark orange/red bar segment) and strain-specific orthogroups (i.e. genes that belong to an orthogroup unique to that Wolbachia strain, light orange/red segment). (b) Graphical representation of set notation of 27 supergroup B Wolbachia genomes, using the format as described for (a). Wolbachia genomes include 6 existing published complete genomes (red highlight), and 19 recently assembled genomes from Scholz et al. [49] (blue highlight), alongside the 2 recently assembled genomes wAnM and wAnD (grey highlight). Analysis was performed on local PGAP annotations of all 27 Wolbachia genomes. Note how the intersect of all 27 Wolbachia genomes shows a core proteome of 595 orthogroups, with the second largest intersect containing 221 orthogroups shared between the Scholz et al. [49] genome assembly for Wolbachia of Dactylopius coccus and an unidentified Insecta.
Further comparisons were performed using a wider selection of Wolbachia supergroup B strains, including 6 Wolbachia genomes used in the previous analysis, as well as a further 19 draft genomes [48] with over 80 % completeness. For consistency, all genomes were annotated using a local installation of NCBI’s Prokaryotic Genome Annotation Pipeline (PGAP) [61]. In total, 32,064 genes annotated across the 27 genomes were used, of which 98.4 % were assigned to 1,669 orthogroups (Fig. 2b). A core genome (genes and their protein products that are present in all analysed genomes) was identified containing 16,957 genes distributed across 595 orthogroups (47.6 % of total genes were assigned to 36.8 % of all orthogroups). Of these 595 orthogroups, 172 contain single-copy genes. A total of 11 and eight genes were not assigned to an orthogroup for wAnD and wAnM, respectively. One species-specific orthogroup was identified in both wAnD and wAnM (containing six and 36 genes respectively). Similar to the previous comparison, one orthogroup was identified as specific to both wAnD and wAnM, containing single-copy orthologues from both genomes that did not have any identifiable protein domains.
It was interesting to see that the number of orthogroups that could be considered as part of the core genome is less than 50 % for both comparisons conducted here. We observed a total of 90 orthogroups that are not considered core due to their absence within Wolbachia strains of filarial nematodes from supergroups C and/or D specifically, whilst supergroup F strains has 30 unique orthogroups. Additionally, the genomes of Wolbachia from Drosophila mauritiana (wMa and wMau in Fig. 2a) shared 26 unique orthogroups. This observation of an extensive accessory genome has been reported in the past, even among closely related Wolbachia strains [83].
Despite smaller genomes, the Wolbachia spp. core metabolic pathways are conserved in wAnD and wAnM
Orthogroup analysis of wAnD and wAnM indicated a high degree of conservation of supergroup B metabolic capacity, and to confirm this KAAS [70] was used to assign KEGG orthology. A total of 677 and 660 protein-encoding genes were assigned a KEGG orthologue (KO) number for wAnD and wAnM, respectively. Subsequent visualization and manual annotation identified complete biosynthetic pathways that have previously been considered of interest with respect to Wolbachia –host symbiosis (Fig. 3a). This includes pathways for riboflavin, purines, pyrimidines and haem biosynthesis; and showed all pathways as present in other supergroup B isolates’ genomes. Additionally, both wAnD and wAnM also contained a suite of metabolite transport and secretion systems common to other Wolbachia strains that includes haem, zinc, iron (III), lipoproteins and phospholipids (Fig. 3a). This conservation of pathways was also observed when the analysis was focused on only Wolbachia from supergroup B strains [48] (Fig. 3b). In addition to these biosynthetic pathways, the T4SSs and Sec-secretion systems were also maintained in both Wolbachia genomes. The T4SSs are known to play roles in infection and survival for a diverse range of symbiotic and pathogenic intracellular bacteria [84, 85]. Both Wolbachia genomes contained a total of 15 T4SS-related genes, organized into two operonic regions and four individual genes spread across the genome. Wolbachia strains in the filarial nematode Brugia malayi has been predicted to utilize its T4SS to secrete protein effector molecules to avoid autophagy pathways and aid in actin cytoskeleton reformation, allowing intracellular mobility [86]. Such processes may also be conserved within Wolbachia strains residing within insects, such as wAnD and wAnM.
Fig. 3.
Heatmap representation of the presence–absence of various genes in metabolic and secretion/transport system pathways amongst a selection of Wolbachia genomes. The analysed genomes are arrayed on the x-axis, with colours of the heatmap representing the various analysed supergroups. The y-axis in turn represents different genes and metabolic pathways of interest to Wolbachia studies. Greys in the heatmap represent an absence of a gene within the respective genome. (a) Comparative illustration of 16 near-complete Wolbachia genomes. Column colours are based on Wolbachia supergroup using a similar scheme to Fig. 2, with columns representing the genomes of wAnD and wAnM highlighted with a more intense colour of the heatmap. These genomes were observed to maintain all the genes and pathways common to supergroup B Wolbachia . (b) Comparative illustration of 29 Wolbachia genomes of supergroup B. Columns are coloured based on their origin, with blue columns being genomes from the study by Scholz et al. [49], and red being genomes from existing Wolbachia , including wAnD and wAnM.
Prophage WO regions are absent, or highly degenerated, in wAnM and wAnD
Wolbachia strains found within insects are frequently infected by a bacteriophage known as phage WO [87], with prophage sequences predicted to be common in the genomes of Wolbachia strains of insects [13, 88]. These prophage regions are known to maintain an EAM [89], a group of genes that encode protein domains homologous to those found in eukaryotes. This has resulted in predictions that these genes influence host–Wolbachia interactions by mimicking and interacting with host proteins [89]. Additionally, genes that have been implicated in the mode of action for CI have typically been found localized within these prophage EAM regions [11, 12, 16, 89].
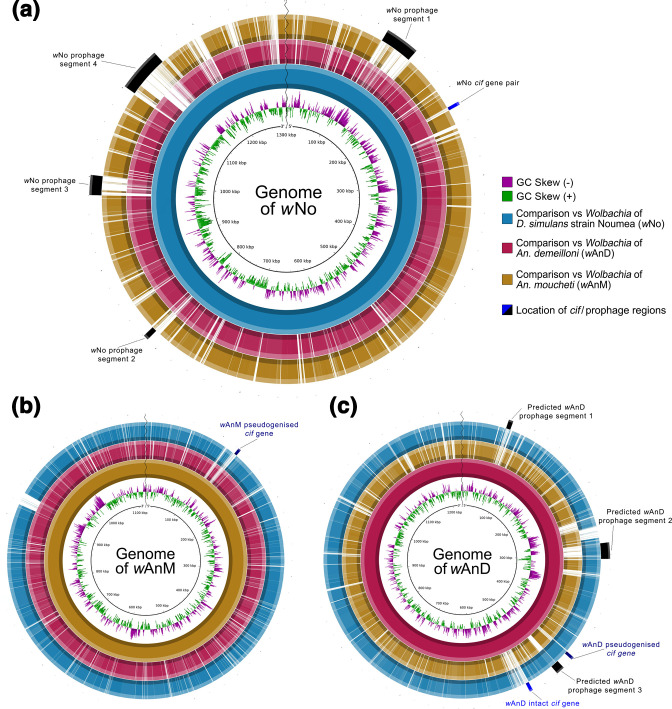
In contrast to other Wolbachia strains that reside within mosquitoes, wAnM contained no prophage fragments identifiable via the phaster web server. To confirm this, we aligned the genomes of both Wolbachia strains from the two Anopheles species to their closest relative, wNo from Drosophila simulans. The Wolbachia genome of wNo was previously observed to have four prophage-like regions [90], ranging in size from 5.7 to 47.2 kbp. Initial comparisons of the genomes showed notable gaps within the wAnM genome when compared to wNo, although the same regions appear partially present in wAnD (Fig. 4a, b). Overlaying coordinates for the four prophage regions that were known to be present in wNo [90] onto this comparison, it was observed that the gaps in alignment with wAnM were centred on these wNo prophage regions (Fig. 4a). When the original sequencing reads were mapped to the wNo genome, we observe very low read coverage on wNo prophage segments (Fig. 2), whilst these reads showed even coverage of the wAnM genome. This indicates that these prophage regions are absent in the current assembly of wAnM.
Fig. 4.
BLAST Ring Image Generator (BRIG) visualization of prophage regions in the genomes of wNo, wAnM and wAnD when compared to one another. Each individual ring represents the presence or absence of similarity for a specific genome (represented by the different colours, see the key on the right of a) against a template genome (represented by the innermost solid colour ring, and the name at the centre of the panel). Presence of similarity is defined as the query genome (the ring) having greater than 50 % similarity to the template genome. The outermost ring for each figure part contains information on predicted prophage and cif gene localizations. (a) Comparison of wAnM and wAnD against a wNo template genome (1 301 823 bp in length). Note how the black bars representing predicted prophage regions as described by Ellegaard et al. [91] overlap areas with no similarity against the wAnM genome, whilst having some similarity to the wAnD genome. Also, note how its single, intact cif gene pair is located separately from previously predicted prophage regions. (b) Comparison against a wAnM template genome (1 121 812 bp in length). Note how this genome was reported to contain no prophage regions, and its single pseudogenized cif gene pair is located in an area with no similarity to both wNo and wAnD. (c) Comparison against a wAnD template genome (1 231 247 bp in length). Predicted prophage segments 1 and 2 were predicted by the phaster web server, with segment 3 predicted by blastx searches against the prophage regions WOVitA1 and WOCauB1 through to B3, as identified by Bordentstein et al. [90]. Note how of the three predicted prophage regions, two showed similarity to the wNo genome, and one showed no similarity to either genome. Also, note how its two cif gene pairs are located separate from, but close to, predicted prophage segment 3. In addition, note how the intact cif gene pair appears within a region that shows weak to no similarity against both wAnM and wNo.
Within wAnD, analysis via the phaster web server and subsequent blastx searches of surrounding regions identified two prophage fragments of lengths 6.3 and 22.1 kbp. blastx searches also identified an additional prophage-like region of length 11.6 kbp (Fig. 4c). The total length of these prophage fragments (approx. 40 kbp) is shorter than published phage WO genomes (lengths of between 55 and 65 kbp) [89, 91]. The two prophage regions identified by phaster are predicted to encode a total of 50 genes, 16 of which were predicted to be interrupted by either stop codons or frameshifts. The prophage-like region identified after manual curation contained 13 genes, of which 7 were predicted to be interrupted. Two of these three regions contained structural phage genes that were either intact or interrupted, with examples including phage tail, baseplate, head–tail connectors and capsid proteins (Fig. S4). These observations, specifically the large number of interrupted structural phage genes identified in wAnD, indicates that this Wolbachia strain likely maintains a cryptic prophage, incapable of producing active phage particles.
CI factors are conserved in wAnM and wAnD
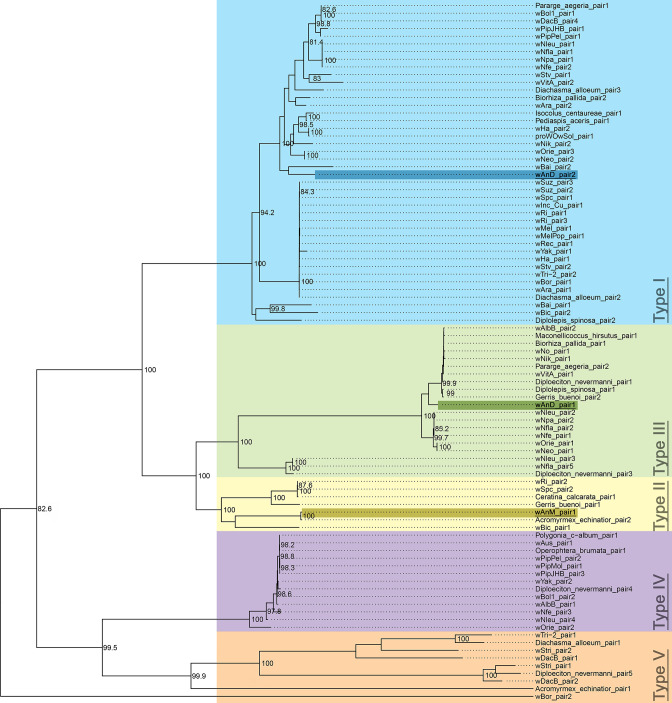
We previously reported that the genome of wAnD contains one intact pair of cif genes (JSQ73_02850, JSQ73_02855), and a second pair that showed interruptions in both genes (JSQ73_02500 through to JSQ73_02515) [37]. In turn, the genome of wAnM contains one pair of cif genes, although two internal stop codons were identified within cifB [37]. Phylogenetic analysis of the concatenated nucleotide sequences of cifA and cifB identified wAnD’s intact cif gene pair as clustering with the type I group, and its pseudogenized pair clustering with the type III group, in line with previous observations [15]. Further investigation of the intact cifB gene in wAnD via a codon-based alignment identified no evidence for potential pseudogenization events (Fig. S5). In comparison, the cif gene pair of wAnM clusters with the type II group (IYZ83_00740 through to IYZ83_00755; Fig. 5, unrooted radial tree shown in Fig. S6).
Fig. 5.
Maximum-likelihood phylogenetic tree of concatenated cif gene nucleotide alignments, built following the methods of Martinez et al. [16] with 1000 bootstrap replicates. Only bootstrap values of over 80 % are shown. The five types of concatenated cif genes are highlighted with different colours, and their corresponding types annotated. The tree is midpoint rooted. The two pairs of cif genes of wAnD were previously noted to be members of type I and type III, which is confirmed by this repeated analysis. The pair of cif genes in wAnM can be found in the well-supported type II clade. An unrooted radial tree of this figure is included as Fig. S6.
Within wAnD, the interrupted cif genes were a combined 3.6 kbp in length and were located upstream of one of the prophage regions identified by the phaster web server (Fig. 4c). Following this, the intact cif genes of wAnD combined measured 6.0 kbp in length, and were approximately 69.5 kbp downstream of the interrupted cif genes (Fig. 4c). By contrast, the interrupted cif genes of wAnM were of a combined 3.6 kbp in length (Fig. 4b). Interestingly, none of the three identified pairs of cif genes within wAnD and wAnM were located directly next to or within prophage regions, although the two within wAnD are located close to one (Fig. 4c). This is similar to wNo, whose single intact pair of cif genes was observed to be separate from predicted prophage regions (Fig. 4a). Separately, it should be noted that the cif gene pair of wAnM appears to be a unique insertion that is also not present in wAnD (Fig. 4b).
Discussion
This study provides a comprehensive analysis of two Wolbachia strains recently identified within Anopheles mosquitoes. Their high density and prevalence rates within field populations provides an opportunity to better understand Wolbachia –host interactions, as well as providing a potential tool to aid in interrupting the spread of Plasmodium parasites. One of the first observations from this study is that the Anopheles-infecting Wolbachia strains are not monophyletic with other Wolbachia strains from mosquitoes (wAlbB and wPip). Instead, both wAnD and wAnM were located within a clade that includes several Wolbachia strains found within Drosophila simulans and Drosophila mauritiana. There have been multiple studies that show horizontal transmission of Wolbachia occurs regularly [81, 82], and is even possible via a plant intermediate [92]. This potential for horizontal transmission is further emphasized by a recent survey that assembled over 1000 Wolbachia genomes from existing sequence data [48]. These genome assemblies are primarily distributed across various Wolbachia strains from supergroups A and B, whilst also generating multiple Wolbachia assemblies from the same host [48]. This study observed how closely related Wolbachia strains can be found in taxonomically unrelated hosts, as well as finding no meaningful phylogenetic clustering of different hosts and their corresponding resident Wolbachia strains. Such observations are similar with what is observed here with the whole-genome phylogeny of wAnD and wAnM, in relation to the wider supergroup B strains and their insect hosts.
When compared against these other sequenced Wolbachia strains, analysis of the wAnD and wAnM strains indicates that they maintain relatively small genome sizes for strains found within insects. This may change in the future as the short-read sequencing assembly used in this study is unable to resolve potential repetitive regions, as observed via comparison of read depths against the genomes. Despite their reduced sizes, both the wAnD and wAnM strains maintain similar metabolic and transport pathways found in other Wolbachia strains. Additionally, no biosynthetic pathways were identified that could indicate a previously unknown feature acquired in these two strains found in Anopheles mosquitoes. Known pathways of relevance for Wolbachia include haem and nucleotide biosynthetic pathways [93], as well as transport components such as the T4SS for secreting potential protein effectors [84]. The observation of smaller genome sizes could be attributed to a reduced number of mobile elements, specifically prophage regions, when compared to other Wolbachia strains that reside in mosquitoes, such as wAlbB [94] and wPip [95].
Following on from this, it is interesting to see how wAnD has degenerated, and likely cryptic, prophage regions in comparison to its closest relative wNo, whilst wAnM lacks prophage regions entirely. Prophage regions in Wolbachia genomes are known to vary significantly, with some strains maintaining duplicate prophage insertions that can encode a functional prophage [90, 95], whilst others have been found to be degenerated [90, 94, 96]. Having said this, the complete lack of prophage regions seen in wAnM has so far only been reported within Wolbachia strains that infect nematodes [97, 98]. Furthermore, these cif genes were noted to be separate from any prophage regions, contrary to previous observations and expectations for these two features to be co-localized [11, 12, 25, 89, 99]. However, this separation of cif genes is not unique to just these two Wolbachia strains in Anopheles, but is also true in the closely related Wolbachia strains wNo, wMa and wMau (the first infecting Drosophila simulans, the latter two Drosophila mauritiana), which have been shown to maintain cif genes that are distinctly separate from any prophage WO region [79, 100] (Fig. S7). It is tempting to speculate that this separation of cif genes and prophage regions may be a unique feature of this clade of Wolbachia . For comparison, the genomes of both wAlbB and wPip ( Wolbachia strains found in Aedes and Culex mosquitoes) maintain cif genes that are associated with prophage WO regions [11, 94]. In addition to this separation from prophage regions, both strains wMa and wMau were observed to have an interrupted cifB gene [79, 100], similar to what is observed in wAnM, and are both incapable of inducing CI, but capable of rescuing it, when crossed with wNo-infected mates [101, 102]. It should be noted that an interrupted cifB gene does not automatically mean an inability to induce CI, as it is known that several strains of Wolbachia that maintain intact cifA genes but interrupted cifB genes can induce a weak form of CI in their hosts [15, 103–105]. Further analysis to determine wAnM’s ability to cause CI will be necessary for further conclusions. One notable difference with the cif genes found in wAnM and wAnD is that all three of wNo, wMa and wMau's cif gene pairs are found within the type III phylogenetic group, whereas the cif gene pair identified in wAnM can be placed within type II, which is unique amongst this clade of Wolbachia. brig comparisons of the different genomes appear to indicate this cif gene pair as a unique insertion. Furthermore, whilst wAnD’s degenerated cif gene pair was noted to be a member of the type III group, its intact cif gene pair also appears unique among this group of Wolbachia as a member of type I. Like wAnM’s sole cif gene pair, this intact cif gene appears to be a unique insertion event, separate from prophage elements.
How such insertion events within both wAnD and wAnM have come to happen, and where they have come from, is currently an open question that warrants further investigation, alongside how this group of Wolbachia maintain cif gene pairs that appear separate from identifiable prophage WO regions. One possible explanation is that the recent ancestors for these strains of Wolbachia may have acquired these cif genes from a recent phage WO insertion that has very recently become degenerated [100]. Alternatively, these prophage regions could have been removed from the genome by phage excision events. Previous publications have discussed what could happen to the cif gene pairs, as well as the Wolbachia that carry them, once CI is no longer able to induce evolutionary pressure on their hosts [106–109]. For instance, a recent survey of CI genes in Wolbachia predicted how, without evolutionary pressure, these CI genes would likely degrade over time, starting with cifB, the 'toxin' component of the phenotype, followed by cifA, the 'antidote' component [15]. Alternatively, it has also been suggested that the degradation of the cif genes may be related to the absence of prophage regions [15, 16], with the former being an adaptation used by the latter to spread within Wolbachia populations. Thus, once the prophage regions are removed, it is predicted that the cif genes, and, thus, the CI phenotype, will have no evolutionary pressure to maintain themselves within Wolbachia [15, 16]. We observe this occurring to some degree in this study, with the dissociation of prophage regions from the cif genes, the interrupted type III pair observed in wAnD, and how wAnM carries interruptions in its type II cifB gene specifically. We see no evidence of such interruptions beginning to occur within the intact type I cif gene pair in wAnD. Once the phenotype these Wolbachia strains exert on their hosts can be properly elucidated, a longitudinal study on the cif genes within them is imperative. The results of such study could allow for further insights into Wolbachia biology and the evolution of the CI phenotype.
Despite the questions as to how this may have occurred, the observed similarities and differences between wAnM and its related strains wMa, wMau and wNo are intriguing, considering the high, but variable, prevalence rates of wAnM in field populations of Anopheles moucheti [37, 43]. This prevalence rate is a feature shared with wAnD [37, 43], which is more likely to be capable of inducing CI due to the presence of intact cif genes from the type I group, which wMel shares. For comparison, our previous work had shown the prevalence rates of wAnM to be between 17.5 and 75 %, which is slightly lower than wAnD prevalence rates, shown to be between 38.7 and 100 % [37]. Yet the ability for Wolbachia to persist in populations without inducing CI is known, as there are instances of Wolbachia that stably infect host populations without any overt reproductive parasitism phenotype [9, 110, 111]. Explanations for this have focused on Wolbachia providing some form of fitness benefit to their host. For instance, the Wolbachia strain wAu of supergroup A is capable of spreading through lab-based, uninfected host populations of Drosophila simulans without inducing CI [112]. This persistence of wAu could be linked to an ability to induce protection against viral infections [113], and it is tempting to speculate that Wolbachia may provide protection against pathogens of the mosquito. While such studies focus on Wolbachia of supergroup A, there has been some evidence that wMau of supergroup B may also confer a fitness benefit for their host via stimulating egg production [100, 114]. Taken together, this highlights the importance of further study regarding wAnM’s effect on their host, whether this be weak CI or some form of fitness benefit, such as benefits to reproduction or an ability to inhibit Plasmodium or viral infection.
The identification of natural Wolbachia infections in Anopheles shows promise for future control strategies of Plasmodium parasites. Whilst these strains show no pathways that are uniquely present or absent, they do exhibit unusual genomic arrangements with regards to the presence of prophage and cif genes. This has potential implications on their relationship with their respective anopheline hosts, potentially making them good candidates for transinfection into other medically relevant Anopheles species, such as Anopheles gambiae s.s. Further studies would be required to fully examine these Wolbachia strains and elucidate their predicted phenotypes of CI and pathogen blocking, both in the context of natural and artificial associations.
Supplementary Data
Funding information
E.H. and G.L.H. acknowledge support from Biotechnology and Biological Sciences Research Council (BBSRC) grant BB/V011278/1. S.T. was supported by the Wellcome SEED award 217303/Z/19/Z to E.H. L.C. was supported by National Institute of Allergy and Infectious Diseases (NIAID) grant R01-AI116811. T.W. and C.L.J. were supported by a Sir Henry Dale Wellcome Trust/Royal Society fellowship awarded to T.W. (101285) (https://wellcome.org and https://royalsociety.org). T.W. was also supported by a Royal Society challenge grant (CHG\R1\170036). G.L.H. was also supported by the BBSRC (BB/T001240/1), a Royal Society Wolfson Fellowship (RSWF\R1\180013), the NIH (R21AI138074), the Engineering and Physical Sciences Research Council (EPSRC) (EP/V043811/1), the UKRI (20197 and 85336), and the NIHR (NIHR2000907).
Author contributions
S.Q., designed methodology, conducted the investigation and formal analysis, designed visuals, and wrote the original draft. L.C., designed methodology, conducted the investigation and formal analysis, and wrote the original draft. C.L.J., conceptualized the study, conducted the investigation and resource collection, and wrote the original draft. S.T., designed methodology and software. T.W., conceptualized the study, conducted the investigation and resource collection, secured funding, and wrote the original draft. G.L.H., conceptualized and supervised the study, secured funding, and wrote the original draft. E.H., conceptualized and supervised the study, designed the methodology, and wrote the original draft. All authors have read and approved the final manuscript.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: CI, cytoplasmic incompatibility; EAM, eukaryotic association module; ENA, European Nucleotide Archive; IS, insertion sequence; KAAS, KEGG Automatic Annotation Server; NCBI, National Center for Biotechnology Information; SNV, single nucleotide variant; T4SS, type IV secretion system.
All supporting data, code and protocols have been provided within the article or through supplementary data files. Seven supplementary figures and one supplementary table are available with the online version of this article.
References
- 1.Quek S, Cerdeira C, Jeffries CL, Tomlinson S, Walker T, et al. Wolbachia endosymbionts in two Anopheles species indicates independent acquisitions and lack of prophage elements. Figshare. 2022 doi: 10.6084/m9.figshare.19576432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 3.Weinert LA, Araujo EV, Ahmed MZ, Welch JJ. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc R Soc B. 2015;282:20150249. doi: 10.1098/rspb.2015.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sazama EJ, Bosch MJ, Shouldis CS, Ouellette SP, Wesner JS. Incidence of Wolbachia in aquatic insects. Ecol Evol. 2017;7:1165–1169. doi: 10.1002/ece3.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor MJ, Bordenstein SR, Slatko B. Microbe Profile: Wolbachia: a sex selector, a viral protector and a target to treat filarial nematodes. Microbiology. 2018;164:1345–1347. doi: 10.1099/mic.0.000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur R, Shropshire JD, Cross KL, Leigh B, Mansueto AJ, et al. Living in the endosymbiotic world of Wolbachia: a centennial review. Cell Host Microbe. 2021;29:879–893. doi: 10.1016/j.chom.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldo L, Werren JH. Revisiting Wolbachia supergroup typing based on WSP: spurious lineages and discordance with MLST. Curr Microbiol. 2007;55:81–87. doi: 10.1007/s00284-007-0055-8. [DOI] [PubMed] [Google Scholar]
- 8.Baldo L, Dunning Hotopp JC, Jolley KA, Bordenstein SR, Biber SA, et al. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis . Appl Environ Microbiol. 2006;72:7098–7110. doi: 10.1128/AEM.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosokawa T, Koga R, Kikuchi Y, Meng X-Y, Fukatsu T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci. 2010;107:769–774. doi: 10.1073/pnas.0911476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikoh N, Hosokawa T, Moriyama M, Oshima K, Hattori M, et al. Evolutionary origin of insect-Wolbachia nutritional mutualism. Proc Natl Acad Sci USA. 2014;111:10257–10262. doi: 10.1073/pnas.1409284111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LePage DP, Metcalf JA, Bordenstein SR, On J, Perlmutter JI, et al. Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature. 2017;543:243–247. doi: 10.1038/nature21391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckmann JF, Ronau JA, Hochstrasser M. A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat Microbiol. 2017;2:17007. doi: 10.1038/nmicrobiol.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bordenstein SR, Wernegreen JJ. Bacteriophage flux in endosymbionts (Wolbachia): infection frequency, lateral transfer, and recombination rates. Mol Biol Evol. 2004;21:1981–1991. doi: 10.1093/molbev/msh211. [DOI] [PubMed] [Google Scholar]
- 14.Bordenstein SR, Brooks AW, Bordenstein SR, et al. Evolutionary genetics of cytoplasmic incompatibility genes cifA and cifB prophage WO Wolbachia . Genome Biol Evol. 2018;10:434–451. doi: 10.1093/gbe/evy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez J, Klasson L, Welch JJ, Jiggins FM. Life and death of selfish genes: comparative genomics reveals the dynamic evolution of cytoplasmic incompatibility. Mol Biol Evol. 2021;38:2–15. doi: 10.1093/molbev/msaa209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beckmann JF, Bonneau M, Chen H, Hochstrasser M, Poinsot D, et al. The toxin–antidote model of cytoplasmic incompatibility: genetics and evolutionary implications. Trends Genet. 2019;35:175–185. doi: 10.1016/j.tig.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillespie JJ, Driscoll TP, Verhoeve VI, Rahman MS, Macaluso KR, et al. A tangled web: origins of reproductive parasitism. Genome Biol Evol. 2018;10:2292–2309. doi: 10.1093/gbe/evy159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werren JH. Biology of Wolbachia . Annu Rev Entomol. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- 19.Serbus LR, Casper-Lindley C, Landmann F, Sullivan W. The genetics and cell biology of Wolbachia-host interactions. Annu Rev Genet. 2008;42:683–707. doi: 10.1146/annurev.genet.41.110306.130354. [DOI] [PubMed] [Google Scholar]
- 20.McMeniman CJ, Lane RV, Cass BN, Fong AWC, Sidhu M, et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti . Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 21.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 23.Crawford JE, Clarke DW, Criswell V, Desnoyer M, Cornel D, et al. Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat Biotechnol. 2020;38:482–492. doi: 10.1038/s41587-020-0471-x. [DOI] [PubMed] [Google Scholar]
- 24.Shropshire JD, On J, Layton EM, Zhou H, Bordenstein SR. One prophage WO gene rescues cytoplasmic incompatibility in Drosophila melanogaster . Proc Natl Acad Sci USA. 2018;115:4987–4991. doi: 10.1073/pnas.1800650115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shropshire JD, Bordenstein SR. Two-by-one model of cytoplasmic incompatibility: synthetic recapitulation by transgenic expression of cifA and cifB in Drosophila . PLOS Genet. 2019;15:e1008221. doi: 10.1371/journal.pgen.1008221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGraw EA, O’Neill SL. Beyond insecticides: new thinking on an ancient problem. Nat Rev Microbiol. 2013;11:181–193. doi: 10.1038/nrmicro2968. [DOI] [PubMed] [Google Scholar]
- 27.Bourtzis K, Dobson SL, Xi Z, Rasgon JL, Calvitti M, et al. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop. 2014;132:S150–S163. doi: 10.1016/j.actatropica.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Johnson KN. The impact of Wolbachia on virus infection in mosquitoes. Viruses. 2015;7:5705–5717. doi: 10.3390/v7112903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Utarini A, Indriani C, Ahmad RA, Tantowijoyo W, Arguni E, et al. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N Engl J Med. 2021;384:2177–2186. doi: 10.1056/NEJMoa2030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Indriani C, Tantowijoyo W, Rancès E, Andari B, Prabowo E, et al. Reduced dengue incidence following deployments of Wolbachia-infected Aedes aegypti in Yogyakarta, Indonesia: a quasi-experimental trial using controlled interrupted time series analysis. Gates Open Res. 2020;4:50. doi: 10.12688/gatesopenres.13122.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes GL, Koga R, Xue P, Fukatsu T, Rasgon JL. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae . PLoS Pathog. 2011;7:3–10. doi: 10.1371/journal.ppat.1002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bian G, Joshi D, Dong Y, Lu P, Zhou G, et al. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science. 2013;340:748–751. doi: 10.1126/science.1236192. [DOI] [PubMed] [Google Scholar]
- 33.Joshi D, Pan X, McFadden MJ, Bevins D, Liang X, et al. The maternally inheritable Wolbachia wAlbB induces refractoriness to Plasmodium berghei in Anopheles stephensi . Front Microbiol. 2017;8:366. doi: 10.3389/fmicb.2017.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joshi D, McFadden MJ, Bevins D, Zhang F, Xi Z. Wolbachia strain wAlbB confers both fitness costs and benefit on Anopheles stephensi . Parasit Vectors. 2014;7:336. doi: 10.1186/1756-3305-7-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker T, Moreira LA. Can Wolbachia be used to control malaria? Mem Inst Oswaldo Cruz. 2011;106:212–217. doi: 10.1590/S0074-02762011000900026. [DOI] [PubMed] [Google Scholar]
- 36.Chrostek E, Gerth M. Is Anopheles gambiae a natural host of Wolbachia? mMBio. 2019;10:e00784-19. doi: 10.1128/mBio.00784-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker T, Quek S, Jeffries CL, Bandibabone J, Dhokiya V, et al. Stable high-density and maternally inherited Wolbachia infections in Anopheles moucheti and Anopheles demeilloni mosquitoes. Curr Biol. 2021;31:2310–2320. doi: 10.1016/j.cub.2021.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baldini F, Segata N, Pompon J, Marcenac P, Shaw WR, et al. Evidence of natural Wolbachia infections in field populations of Anopheles gambiae . Nat Commun. 2014;5:3985. doi: 10.1038/ncomms4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomes FM, Hixson BL, Tyner MDW, Ramirez JL, Canepa GE, et al. Effect of naturally occurring Wolbachia in Anopheles gambiae s.l. mosquitoes from Mali on Plasmodium falciparum malaria transmission. Proc Natl Acad Sci USA. 2017;114:12566–12571. doi: 10.1073/pnas.1716181114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niang EHA, Bassene H, Makoundou P, Fenollar F, Weill M, et al. First report of natural Wolbachia infection in wild Anopheles funestus population in Senegal. Malar J. 2018;17:408. doi: 10.1186/s12936-018-2559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayala D, Akone-Ella O, Rahola N, Kengne P, Ngangue MF, et al. Natural Wolbachia infections are common in the major malaria vectors in Central Africa. Evol Appl. 2019;12:1583–1594. doi: 10.1111/eva.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong ML, Liew JWK, Wong WK, Pramasivan S, Mohamed Hassan N, et al. Natural Wolbachia infection in field-collected Anopheles and other mosquito species from Malaysia. Parasites Vectors. 2020;13:414. doi: 10.1186/s13071-020-04277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeffries CL, Lawrence GG, Golovko G, Kristan M, Orsborne J, et al. Novel Wolbachia strains in Anopheles malaria vectors from sub-Saharan Africa. Wellcome Open Res. 2018;3:113. doi: 10.12688/wellcomeopenres.14765.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai IJ, Otto TD, Berriman M. Improving draft assemblies by iterative mapping and assembly of short reads to eliminate gaps. Genome Biol. 2010;11:R41. doi: 10.1186/gb-2010-11-4-r41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marçais G, Delcher AL, Phillippy AM, Coston R, Salzberg SL, et al. MUMmer4: a fast and versatile genome alignment system. PLoS Comput Biol. 2018;14:e1005944. doi: 10.1371/journal.pcbi.1005944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darling ACE, Mau B, Blattner FR, Perna NT. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004;14:1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milne I, Stephen G, Bayer M, Cock PJA, Pritchard L, et al. Using tablet for visual exploration of second-generation sequencing data. Brief Bioinform. 2013;14:193–202. doi: 10.1093/bib/bbs012. [DOI] [PubMed] [Google Scholar]
- 48.Scholz M, Albanese D, Tuohy K, Donati C, Segata N, et al. Large scale genome reconstructions illuminate Wolbachia evolution. Nat Commun. 2020;11:5235. doi: 10.1038/s41467-020-19016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 50.Li H. Wgsim for simulating sequence reads from a reference genome. 2011. https://github.com/lh3/wgsim
- 51.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seeman T. Snippy: fast bacterial variant calling from NGS reads. 2015. https://github.com/tseemann/snippy
- 53.Croucher NJ, Page AJ, Connor TR, Delaney AJ, Keane JA, et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. 2015;43:e15. doi: 10.1093/nar/gku1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Emms DM, Kelly S. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 2015;16:157. doi: 10.1186/s13059-015-0721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen W, Le S, Li Y, Hu F, Zou Q. SeqKit: a cross-platform and ultrafast toolkit for FASTA/Q file manipulation. PLoS ONE. 2016;11:e0163962. doi: 10.1371/journal.pone.0163962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conway JR, Lex A, Gehlenborg N. UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics. 2017;33:2938–2940. doi: 10.1093/bioinformatics/btx364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32:2847–2849. doi: 10.1093/bioinformatics/btw313. [DOI] [PubMed] [Google Scholar]
- 61.Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, et al. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44:6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019;47:D427–D432. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howard Hughes Medical Institute. HMMER: biosequence analysis using profile hidden Markov models. 2013. http://hmmer.org/
- 64.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 65.Sullivan MJ, Petty NK, Beatson SA. Easyfig: a genome comparison visualizer. Bioinformatics. 2011;27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karp PD, Midford PE, Billington R, Kothari A, Krummenacker M, et al. Pathway Tools version 23.0 update: software for pathway/genome informatics and systems biology. Brief Bioinform. 2021;22:109–126. doi: 10.1093/bib/bbz104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caspi R, Billington R, Keseler IM, Kothari A, Krummenacker M, et al. The MetaCyc database of metabolic pathways and enzymes – a 2019 update. Nucleic Acids Res. 2020;48:D445–D453. doi: 10.1093/nar/gkz862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karp PD, Billington R, Caspi R, Fulcher CA, Latendresse M, et al. The BioCyc collection of microbial genomes and metabolic pathways. Brief Bioinform. 2019;20:1085–1093. doi: 10.1093/bib/bbx085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huerta-Cepas J, Forslund K, Coelho LP, Szklarczyk D, Jensen LJ, et al. Fast genome-wide functional annotation through orthology assignment by eggNOG-mapper. Mol Biol Evol. 2017;34:2115–2122. doi: 10.1093/molbev/msx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moriya Y, Itoh M, Okuda S, Yoshizawa AC, Kanehisa M. An automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007;35:W182–W185. doi: 10.1093/nar/gkm321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wickham H. ggplot2: Elegant Graphics for Data Analysis ( https://ggplot2.tidyverse.org) New York: Springer-Verlag; 2016. [Google Scholar]
- 72.Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007;56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 73.Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 74.Yu G, Smith DK, Zhu H, Guan Y, Lam TTY. Ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol. 2017;8:28–36. [Google Scholar]
- 75.Löytynoja A. Phylogeny-aware alignment with PRANK. Methods Mol Biol. 2014;1079:155–170. doi: 10.1007/978-1-62703-646-7_10. [DOI] [PubMed] [Google Scholar]
- 76.Arndt D, Grant JR, Marcu A, Sajed T, Pon A, et al. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44:W16–W21. doi: 10.1093/nar/gkw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carver T, Harris SR, Berriman M, Parkhill J, McQuillan JA. Artemis: an integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics. 2012;28:464–469. doi: 10.1093/bioinformatics/btr703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Varani AM, Siguier P, Gourbeyre E, Charneau V, Chandler M. ISsaga is an ensemble of web-based methods for high throughput identification and semi-automatic annotation of insertion sequences in prokaryotic genomes. Genome Biol. 2011;12:R30. doi: 10.1186/gb-2011-12-3-r30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baião GC, Janice J, Galinou M, Klasson L. Comparative genomics reveals factors associated with phenotypic expression of Wolbachia . Genome Biol Evol. 2021;13:evab111. doi: 10.1093/gbe/evab111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lefoulon E, Vaisman N, Frydman HM, Sun L, Voland L, et al. Large enriched fragment targeted sequencing (LEFT-SEQ) applied to capture of Wolbachia genomes. Sci Rep. 2019;9:5939. doi: 10.1038/s41598-019-42454-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O’Neill SL, Giordano R, Colbert AM, Karr TL, Robertson HM. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc Natl Acad Sci USA. 1992;89:2699–2702. doi: 10.1073/pnas.89.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Werren JH, Zhang W, Guo LR. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc Biol Sci. 1995;261:55–63. doi: 10.1098/rspb.1995.0117. [DOI] [PubMed] [Google Scholar]
- 83.Ishmael N, Hotopp JCD, Ioannidis P, Biber S, Sakamoto J, et al. Extensive genomic diversity of closely related Wolbachia strains. Microbiology. 2009;155:2211–2222. doi: 10.1099/mic.0.027581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rancès E, Voronin D, Tran-Van V, Mavingui P. Genetic and functional characterization of the type IV secretion system in Wolbachia . J Bacteriol. 2008;190:5020–5030. doi: 10.1128/JB.00377-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Christie PJ, Whitaker N, González-Rivera C. Mechanism and structure of the bacterial type IV secretion systems. Biochim Biophys Acta. 2014;1843:1578–1591. doi: 10.1016/j.bbamcr.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carpinone EM, Li Z, Mills MK, Foltz C, Brannon ER, et al. Identification of putative effectors of the type IV secretion system from the Wolbachia endosymbiont of Brugia malayi . PLoS One. 2018;13:e0204736. doi: 10.1371/journal.pone.0204736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kent BN, Bordenstein SR. Phage WO of Wolbachia: lambda of the endosymbiont world. Trends Microbiol. 2010;18:173–181. doi: 10.1016/j.tim.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gavotte L, Henri H, Stouthamer R, Charif D, Charlat S, et al. A survey of the bacteriophage WO in the endosymbiotic bacteria Wolbachia . Mol Biol Evol. 2007;24:427–435. doi: 10.1093/molbev/msl171. [DOI] [PubMed] [Google Scholar]
- 89.Bordenstein SR, Bordenstein SR. Eukaryotic association module in phage WO genomes from Wolbachia. Nat Commun. 2016;7:13155. doi: 10.1038/ncomms13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ellegaard KM, Klasson L, Näslund K, Bourtzis K, Andersson SGE. Comparative genomics of Wolbachia and the bacterial species concept. PLoS Genet. 2013;9:e1003381. doi: 10.1371/journal.pgen.1003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kupritz J, Martin J, Fischer K, Curtis KC, Fauver JR, et al. Isolation and characterization of a novel bacteriophage WO from Allonemobius socius crickets in Missouri. PLoS One. 2021;16:e0250051. doi: 10.1371/journal.pone.0250051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chrostek E, Pelz-Stelinski K, Hurst GDD, Hughes GL. Horizontal transmission of intracellular insect symbionts via plants. Front Microbiol. 2017;8:2237. doi: 10.3389/fmicb.2017.02237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gill AC, Darby AC, Makepeace BL. Iron necessity: the secret of Wolbachia’s success? PLoS Negl Trop Dis. 2014;8:e3224. doi: 10.1371/journal.pntd.0003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sinha A, Li Z, Sun L, Carlow CKS, Cordaux R. Complete genome sequence of the Wolbachia wAlbB endosymbiont of Aedes albopictus . Genome Biol Evol. 2019;11:706–720. doi: 10.1093/gbe/evz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klasson L, Walker T, Sebaihia M, Sanders MJ, Quail MA, et al. Genome evolution of Wolbachia strain wPip from the Culex pipiens group. Mol Biol Evol. 2008;25:1877–1887. doi: 10.1093/molbev/msn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miao YH, Xiao JH, Huang DW. Distribution and evolution of the bacteriophage WO and its antagonism with Wolbachia . Front Microbiol. 2020;11:595629. doi: 10.3389/fmicb.2020.595629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Foster J, Ganatra M, Kamal I, Ware J, Makarova K, et al. The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 2005;3:e121. doi: 10.1371/journal.pbio.0030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lefoulon E, Clark T, Guerrero R, Cañizales I, Cardenas-Callirgos JM, et al. Diminutive, degraded but dissimilar: Wolbachia genomes from filarial nematodes do not conform to a single paradigm. Microb Genom. 2020;6:e000487. doi: 10.1099/mgen.0.000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shropshire JD, Rosenberg R, Bordenstein SR. The impacts of cytoplasmic incompatibility factor (cifA and cifB) genetic variation on phenotypes. Genetics. 2021;217:iyaa007. doi: 10.1093/genetics/iyaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Meany MK, Conner WR, Richter SV, Bailey JA, Turelli M, et al. Loss of cytoplasmic incompatibility and minimal fecundity effects explain relatively low Wolbachia frequencies in Drosophila mauritiana . Evolution. 2019;73:1278–1295. doi: 10.1111/evo.13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bourtzis K, Dobson SL, Braig HR, O’Neill SL. Rescuing Wolbachia have been overlooked. Nature. 1998;391:852–853. doi: 10.1038/36017. [DOI] [PubMed] [Google Scholar]
- 102.Charlat S, Le Chat L, Merçot H. Characterization of non-cytoplasmic incompatibility inducing Wolbachia in two continental African populations of Drosophila simulans . Heredity. 2003;90:49–55. doi: 10.1038/sj.hdy.6800177. [DOI] [PubMed] [Google Scholar]
- 103.Shropshire JD, Leigh B, Bordenstein SR. Symbiont-mediated cytoplasmic incompatibility: what have we learned in 50 years. Elife. 2020;9:e61989. doi: 10.7554/eLife.61989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cooper BS, Ginsberg PS, Turelli M, Matute DR. Wolbachia in the Drosophila yakuba complex: pervasive frequency variation and weak cytoplasmic incompatibility, but no apparent effect on reproductive isolation. Genetics. 2017;205:333–351. doi: 10.1534/genetics.116.196238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shoemaker DD, Katju V, Jaenike J. Wolbachia and the evolution of reproductive isolation between Drosophila recens and Drosophila subquinaria . Evolution. 1999;53:1157–1164. doi: 10.1111/j.1558-5646.1999.tb04529.x. [DOI] [PubMed] [Google Scholar]
- 106.Turelli M. Evolution of incompatibility-inducing microbes and their hosts. Evolution. 1994;48:1500–1513. doi: 10.1111/j.1558-5646.1994.tb02192.x. [DOI] [PubMed] [Google Scholar]
- 107.Hurst LD, McVean GT. Clade selection, reversible evolution and the persistence of selfish elements: the evolutionary dynamics of cytoplasmic incompatibility. Proc R Soc Lond B. 1997;263:97–104. doi: 10.1098/rspb.1996.0016. [DOI] [Google Scholar]
- 108.Koehncke A, Telschow A, Werren JH, Hammerstein P. Life and death of an influential passenger: Wolbachia and the evolution of CI-modifiers by their hosts. PLoS One. 2009;4:e4425. doi: 10.1371/journal.pone.0004425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Haygood R, Turelli M. Evolution of incompatibility-inducing microbes in subdivided host populations. Evolution. 2009;63:432–447. doi: 10.1111/j.1558-5646.2008.00550.x. [DOI] [PubMed] [Google Scholar]
- 110.Dedeine F, Boulétreau M, Vavre F. Wolbachia requirement for oogenesis: occurrence within the genus Asobara (Hymenoptera, Braconidae) and evidence for intraspecific variation in A. tabida . Heredity (Edinb) 2005;95:394–400. doi: 10.1038/sj.hdy.6800739. [DOI] [PubMed] [Google Scholar]
- 111.Kriesner P, Hoffmann AA, Lee SF, Turelli M, Weeks AR. Rapid sequential spread of two Wolbachia variants in Drosophila simulans . PLoS Pathog. 2013;9:e1003607. doi: 10.1371/journal.ppat.1003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kriesner P, Hoffmann AA. Rapid spread of a Wolbachia infection that does not affect host reproduction in Drosophila simulans cage populations. Evolution. 2018;72:1475–1487. doi: 10.1111/evo.13506. [DOI] [PubMed] [Google Scholar]
- 113.Ant TH, Herd CS, Geoghegan V, Hoffmann AA, Sinkins SP, et al. The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti . PLoS Pathog. 2018;14:e1006815. doi: 10.1371/journal.ppat.1006815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fast EM, Toomey ME, Panaram K, Desjardins D, Kolaczyk ED, et al. Wolbachia enhance Drosophila stem cell proliferation and target the germline stem cell niche. Science. 2011;334:990–992. doi: 10.1126/science.1209609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.