Abstract
Background
The rollout of GeneXpert aimed at increasing early diagnosis of tuberculosis to improve treatment outcomes and global tuberculosis targets.
Objective
This study evaluated trends in tuberculosis diagnosis and outcomes pre- and post-introduction of GeneXpert in three African countries – the Democratic Republic of the Congo (DRC), Nigeria and South Africa.
Methods
Data from 2001 to 2019 were extracted from the World Health Organization’s data repository. Descriptive analysis, paired t-tests and interrupted time series models were used.
Results
Estimated tuberculosis incidence decreased from 327/100 000 to 324/100 000 in the DRC, and from 1220/100 000 to 988/100 000 in South Africa. Incidence remained at 219/100 000 in Nigeria. The tuberculosis case notification rate did not change significantly. Increases in the new case treatment success rates were statistically significant (DRC: p = 0.0201; Nigeria: p = 0.0001; South Africa: p = 0.0017); decreases in mortality were also statistically significant (DRC: p = 0.0264; Nigeria: p = 0.0001; South Africa: p < 0.0001). Time series models showed insignificant increases in new tuberculosis cases in DRC (n = 1856, p = 0.085) and Nigeria (n = 785, p = 0.555) from 2011 to 2019; and a statistically significant decrease in South Africa (n = 15 269, p = 0.006).
Conclusion
Improvements in tuberculosis treatment outcomes were achieved, but little progress has been made in new case notification due to varied implementation and scale-up of GeneXpert across the three countries. Implementation barriers need to be addressed to achieve the required tuberculosis targets.
Keywords: tuberculosis, GeneXpert, Xpert MTB/RIF, Africa, interrupted time series
Introduction
In December 2010, the World Health Organization (WHO) approved the Xpert MTB/RIF assay for the early detection of pulmonary tuberculosis (PTB), particularly in people living with HIV and presumptive multidrug-resistant tuberculosis (MDR-TB) clients.1 Previously, sputum smear microscopy was the most used method for the diagnosis of PTB in low- and middle-income countries. Based on the type and method of microscopy, the accuracy of detection varies between 20% and 80%,2,3,4 and depends on the technologist’s reading, the quality of the sample, and the smear staining technique. Mycobacterium tuberculosis culture and drug susceptibility testing, which are the gold standard for tuberculosis diagnosis and detection of MDR-TB, are time-consuming, expensive, and requires sophisticated equipment which is not available in many facilities. Therefore, active tuberculosis disease among people living with HIV who tend to have a paucibacillary disease2 or active MDR-TB disease is detected later, resulting in poorer tuberculosis treatment outcomes; hence, the need for a more reliable and timely means of diagnosis.
GeneXpert for tuberculosis diagnosis
Although the GeneXpert platform (Cepheid, Sunnyvale, California, United States) was available as early as the 1990s, it was originally only used to detect anthrax.3 From 2006, the platform was more widely used to diagnose tuberculosis4,5 and, in 2010, received an endorsement from the WHO as a first-line tuberculosis diagnostic test among people living with HIV and those presumed to have MDR-TB.1 The Xpert MTB/RIF assay (Cepheid, Sunnyvale, California, United States) is an automated molecular (nucleic acid amplification) test for the diagnosis of tuberculosis with comparable accuracy to culture.4,6 It can also detect the M. tuberculosis complex DNA and mutations linked to rifampicin resistance (a proxy for MDR-TB) from specimens in under two hours and reduces handling of the specimen by staff.
The capability of the Xpert MTB/RIF assay to detect smear-negative tuberculosis offers an important advantage over smear microscopy, particularly for people living with HIV and children. Its ability to detect rifampicin resistance within a short period improves the prospects of timely and appropriate treatment for MDR-TB. Culture and drug susceptibility testing are, however, mandatory to complete the drug-resistance profile and to monitor treatment. The operational characteristics of the system which combines user safety, ease of use, and the potential for use as a point-of-care tool, make it an ideal system for quick diagnosis of tuberculosis. The higher cost per sample, the need for a constant power supply, and the need for regular validation are some of the challenges associated with using the system in resource-limited settings.7,8,9
The improved Xpert MTB/RIF Ultra (Cepheid, Sunnyvale, California, United States), which has enhanced performance in detecting M. tuberculosis and resistant tuberculosis, has a higher sensitivity for smear-negative tuberculosis cases resulting from HIV and tuberculosis coinfection as well as improved rifampicin resistance results. It improves case finding among smear-negative tuberculosis patients who would otherwise have been missed and potentially have transmitted tuberculosis to other people. Between 2010 and the end of 2015, 21 549 instrument modules and about 16.2 million Xpert MTB/RIF cartridges were procured by the public sector and non-governmental organisations from 122 high burden developing countries at concessionary prices.8
Country adoption of GeneXpert
The End TB strategy of 2016–2035, defined lists of countries with three types of high-burden tuberculosis infection – tuberculosis alone, MDR-TB, and tuberculosis/HIV coinfection – and identified 30 countries with a high burden of tuberculosis infection.10 Fourteen countries, eight of which are in sub-Saharan Africa, belong to all three lists. Xpert MTB/RIF rollout in the 14 countries with high burdens of tuberculosis, tuberculosis/HIV coinfection, and MDR-TB infection has been variable. Nonetheless, evidence of the impact of the introduction of Xpert MTB on tuberculosis diagnosis and detection of drug-resistance at the population level has been demonstrated.11,12,13 In Africa, the three highest burden countries are the Democratic Republic of the Congo (DRC), Nigeria and South Africa. The DRC installed their first Xpert MTB/RIF assay in 2011 but has had variable scale-up due to recurrent conflict and the Ebola epidemics. By 2016, the DRC had only 226 modules with a smear-to-Xpert ratio (defined as the market penetration of Xpert within public sector organisations) of 18 and a 6% utilisation rate.14,15 A 2019 study indicated there was an increase in tuberculosis case-finding and rifampicin resistance among diagnosed cases; however, treatment gaps had a significant impact on the treatment outcomes for drug-resistant tuberculosis patients.16 Nigeria installed the first Xpert MTB/RIF machine in 2011; by 2019, it had 1560 modules, a smear-to-Xpert ratio of 2.3, and a 27% utilisation rate.14 An early implementation assessment conducted by Mustapha et al. in 2015 reported minimal improvements in tuberculosis case-finding due to a multitude of challenges that affected accessibility;17 a follow-up assessment in 2018 found the same challenges.18 South Africa issued national guidance recommending Xpert as the first-line diagnostic test for tuberculosis in 2011–2012, because of the high HIV burden. The first Xpert MTB/RIF was installed in 2011 and by March 2019 there were 4228 installed modules, and the country had achieved a smear-to-Xpert ratio of 0 and a 40% utilisation rate.19 However, Xpert was only rolled out to the rural areas between 2013 and 2015.
Due to the variable uptake of the Xpert MTB/RIF assay since its endorsement in 2010, we set out to evaluate trends in tuberculosis incidence, case notification, and tuberculosis treatment outcomes pre- and post-introduction of GeneXpert in three high PTB-burdened African countries – the DRC, Nigeria, and South Africa. The outcomes were reported between the years 2001 and 2019.
Methods
Ethical considerations
This study used publicly available aggregated data from the World Health Organization’s website. Identifiable data were not used and there was no direct contact with individuals, hence ethical approval was not required for this study.
Study design
We used a trend design strategy20 to describe tuberculosis incidence, case notification and treatment outcome patterns before and after the introduction of GeneXpert in three selected tuberculosis high-burden countries in Africa between 2001 and 2019. The trend design uses cross-sections at two or more points in time to assess specific changes in a population over a defined period.20 We identified countries with a high burden of tuberculosis, tuberculosis/HIV coinfection, and MDR-TB that introduced Xpert within two years of WHO’s 2010 endorsement and were among the top 10 global high-burden lists based on the absolute number of reported tuberculosis cases21. Data were extracted from the updated 2020 WHO tuberculosis burden estimates22, which are annual estimates from the WHO, as well as data submitted to the WHO by different countries. This included 12 tuberculosis indicators that describe annual estimates of tuberculosis incidence, diagnosed tuberculosis cases and notifications, and tuberculosis treatment outcomes. These 12 indicators were selected based on the experience of the researchers that these were adequate to describe tuberculosis incidence, case notification, and tuberculosis treatment outcomes.
Data analysis
Data were extracted in an Excel file (Microsoft Corp., Redmond, Washington, United States) and imported into Stata 15 (Stata Statistical Software: Release 15. StataCorp LP, College Station, Texas, United States) for cleaning and analysis on 26 June 2021. The years 2001–2010 were selected to study trends before, and 2011–2019 after, rollout of GeneXpert as a routine tuberculosis diagnostic test. Different indicators that describe tuberculosis incidence, tuberculosis treatment outcome, and mortality were retained for analysis22.
Tuberculosis incidence data per 100 000 population, treatment outcomes and mortality rates for pre- and post-GeneXpert periods were summarised by medians and interquartile ranges. Additionally, these were presented in line graphs to illustrate trends in the two periods.
A paired t-test was used to compare the values of each indicator to determine if there were significant differences in the values of the different indicators between the period before and after the introduction of GeneXpert within each country.
A single group interrupted time series analysis using Newey-West standard errors assessed the impact of the introduction of GeneXpert on the number of confirmed PTB cases diagnosed from 2001 to 2019 in the three countries, using 2011 as the year the programmatic implementation of Xpert began. Missing data for diagnosed PTB cases for Nigeria in 2003 were inputted using the mean value from 2002 and 2004. Statistical significance was placed at p < 0.05 and output from the three models are presented as a summary table and charts. The variable ‘t’ represents the time since the start of the study, ‘x2011’ represents the intervention period, ‘x_t2011’ represents the interaction of ‘_x’ and ‘_t’, and ‘Treated’ represents the post-intervention linear trend. The ‘_cons’ represent the starting level of laboratory-confirmed PTB cases. A separate sensitivity model including tuberculosis/HIV coinfection rate was done for South Africa to ascertain if the initial model may have been confounded by HIV coinfection.
Results
Descriptive summary
New confirmed PTB cases, the estimated number of cases, new extrapulmonary cases, and tuberculosis notification rates were higher in the DRC and Nigeria but lower in South Africa in the period after, compared to the period before, the introduction of GeneXpert (Table 1)22.
TABLE 1.
Summary statistics of various tuberculosis indicators in three African countries 2001–2010, and 2011– 2019.
| Variable | Pre (2001–2010) |
Post (2011–2019) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Democratic Republic of the Congo (N = 10) |
Nigeria (N = 10) |
South Africa (N = 10) |
Democratic Republic of the Congo (N = 9) |
Nigeria (N = 9) |
South Africa (N = 9) |
|||||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
| New pulmonary confirmed TB cases | 65 040 | 62 192–69 477 | 42 383 | 34 402–45 721 | 133 352 | 125 864–139 136 | 76 671 | 71 526–93 555 | 54 012 | 52 811–73 660 | 132 750 | 129 840–155 473 |
| Estimated number of cases | 182 000 | 168 000–198 000 | 308 000 | 289 000–329 000 | 594 500 | 499 000–632 000 | 247 000 | 233 000–262 000 | 397 000 | 376 000–418 000 | 547 000 | 421 000–593 000 |
| Estimated incidence /100k | 327 | 327–327 | 219 | 219–219 | 1220 | 1070–1260 | 324 | 322–326 | 219 | 219–219 | 988 | 738–1110 |
| New extrapulmonary cases | 18 471 | 18 213–19 654 | 2906 | 1525–3422 | 43 193 | 37 686–48 251 | 21 579 | 20 329–24 959 | 4546 | 4227–4896 | 28 507 | 23 621–37 709 |
| Lab confirmed RR/MDR | - | - | - | - | - | - | 737 | 499–893 | 1981 | 1241–2286 | 17 360 | 13 199–19 073 |
| Lab confirmed MDR-TB | 87 | 87–87 | 21 | 21–21 | 7386 | 7386–7386 | 137 | 81–217 | 656 | 107–996 | 8981 | 8081–9956 |
| TB case notification (Numb) | 96 371 | 80 011–104 861 | 67 362 | 45 842–84 121 | 286 646 | 227 320–348 241 | 119 213 | 112 439–150 085 | 94 825 | 87 211–102 387 | 287 224 | 237 045–312 380 |
| Case notification rate 100k | 173 | 156–177 | 48 | 37–56 | 595 | 487–693 | 165 | 158–184 | 53 | 52–55 | 519 | 422–582 |
| TB case detection rate (CDR) % | 53 | 48–54 | 22 | 17–26 | 50 | 46–55 | 50 | 48–57 | 24 | 24–25 | 53 | 53–58 |
| Estimated TB/HIV % | 22 | 21–23 | 21 | 20–25 | 66 | 65–69 | 16 | 12–19 | 17 | 14–22 | 60 | 59–62 |
| Estimated mortality /100k | 84 | 83–94 | 88 | 88–93 | 395 | 371–422 | 87 | 70–89 | 85 | 81–86 | 116 | 106–137 |
| TSR new cases % | 85 | 78–86 | 79 | 76–81 | 68 | 65–70 | 89 | 88–90 | 86 | 86–87 | 78 | 77–80 |
Source: Adapted from World Health Organization (WHO). Global Tuberculosis Programme. In Global TB report [homepage on the Internet]. 2021 [cited 2021 Jun 26]. Available from: https://www.who.int/tb/country/data/download/en/
IQR, interquartile range; RR, rifampicin resistant; TSR, treatment success rate (defined as the proportion of cases registered in a particular year that successfully completed tuberculosis treatment without evidence of bacteriological failure [WHO]); CDR, case detection rate (defined as the proportion of estimated new and relapse tuberculosis cases detected in a given year under the internationally recommended tuberculosis control strategy [WHO]).
The estimated tuberculosis incidence per 100 000 population was lower in the DRC (324/100 000) and South Africa (988/100 000) post-introduction of GeneXpert, but remained the same in Nigeria (219/100 000). Data on laboratory-confirmed rifampicin-resistant/MDR-TB was not available before the introduction of GeneXpert. However, there was an increase in diagnosed MDR-TB cases across the three countries after the introduction of GeneXpert (137 for the DRC, 656 for Nigeria, and 8981 for South Africa). The tuberculosis case detection rate was comparable across the two periods in all three countries, while a decrease was observed in tuberculosis/HIV coinfection incidence post the introduction of GeneXpert (16% for the DRC, 17% for Nigeria, and 60% for South Africa). The estimated tuberculosis mortality showed a downward trend in all three countries with the largest decline in South Africa, from 395/100 000 to 116/100 000, after the introduction of GeneXpert. The treatment success rate in all three countries increased after the introduction of GeneXpert compared to the period before.
Trends in select tuberculosis indicators
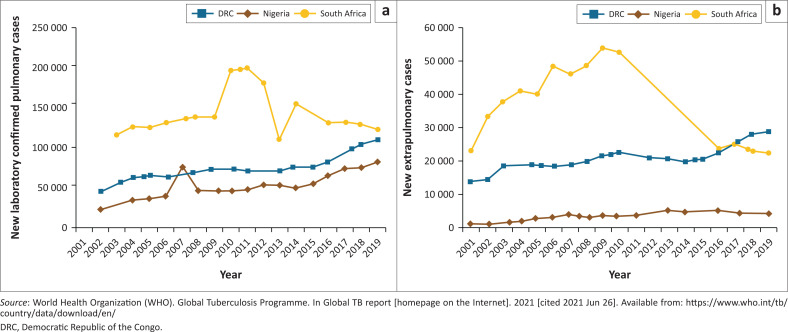
Confirmed PTB cases (Figure 1a)22 increased gradually from less than 50 000 cases in 2002 to slightly above 100 000 in the DRC and about 80 000 in Nigeria in 2019. However, in South Africa, tuberculosis cases peaked at about 200 000 in 2011, dropping to about 120 000 in 2019. New extrapulmonary cases (Figure 1b)22 followed a similar pattern to confirmed pulmonary cases but with much lower numbers; Nigeria showed a slight decline in the number of cases reported between 2017 and 2019.
FIGURE 1.
Tuberculosis cases in three countries in Africa 2001–2019 (a) New laboratory-confirmed cases 2001–2019; (b) New extrapulmonary cases 2001–2019.
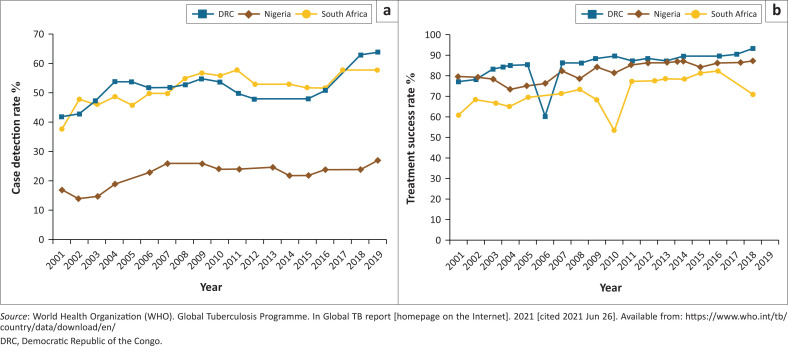
The case detection rate (Figure 2a)22 and treatment success rate (Figure 2b)22 showed a slow increase from 2001 to 2019 in all three countries. Nigeria had the lowest case detection rate with the least increase. The DRC showed a sharp decrease in treatment success rate in 2006, as did South Africa in 2010, with South Africa showing another decline after 2017. There was a marked increase in tuberculosis case detection rate in the DRC and South Africa from 2016, with a leveling off by 2019.
FIGURE 2.
Key tuberculosis indicators in three African countries 2001–2019 (a) Case detection rate (%) 2001–2019; (b) Treatment success rate (%) 2001–2019.
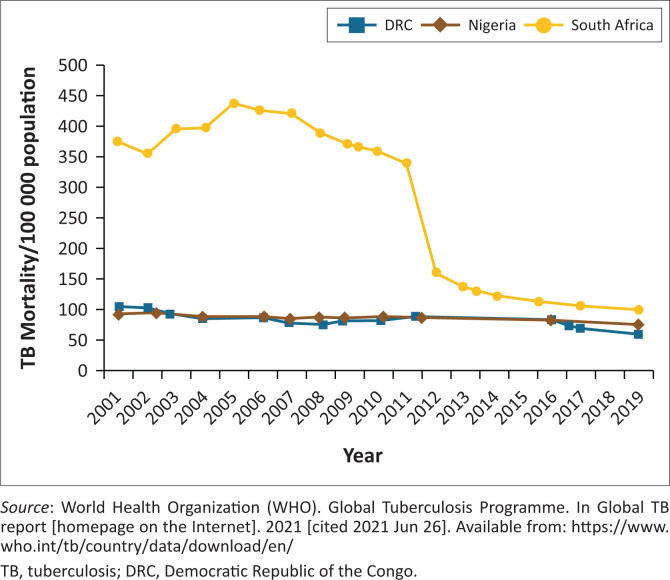
South Africa showed the most substantial decline in tuberculosis mortality per 100 000 population (Figure 3)22 from 2001 to 2019, with the steepest decline occurring between 2011 and 2012.
FIGURE 3.
Tuberculosis-related mortality in three high-burden African countries 2001–2019.
Comparison between the two periods
Data for rifampicin-resistant tuberculosis and MDR-TB were not available before the introduction of GeneXpert; hence no comparison was done. A statistically significant increase was seen in the new pulmonary confirmed tuberculosis cases and the estimated number of all cases in DRC (p = 0.0002, p < 0.0001) and Nigeria (p = 0.0011, p < 0.0001) but not in South Africa (p = 0.7413, p = 0.5081) (Table 2)22. Estimated tuberculosis incidence per 100 000 population decreased in the DRC (p = 0.0067) and South Africa (p = 0.1066), but there was no change in Nigeria. The percentage of tuberculosis/HIV incidence and mortality per 100 000 population showed a statistically significant decrease in all three countries (DRC, p = 0.0264; Nigeria and South Africa, p < 0.0001), while the percentage treatment success rate for new cases showed a statistically significant increase in the three countries (DRC, p = 0.0201; Nigeria, p < 0.0001; South Africa, p = 0.0017).
TABLE 2.
Comparison of tuberculosis indicators pre- (2001–2010) and post-introduction (2011–2019) of GeneXpert in three high tuberculosis burden countries in Africa.
| Variable | Democratic Republic of the Congo |
Nigeria |
South Africa |
|||
|---|---|---|---|---|---|---|
| Difference | p | Difference | p | Difference | p | |
| New confirmed pulmonary tuberculosis cases | 23 358 | 0.0002 | 22 335 | 0.0011 | 2125 | 0.7413 |
| Estimated number of cases | 67 667 | < 0.0001 | 92 222 | < 0.0001 | −42 889 | 0.5081 |
| Estimated incidence/100k | −3.1 | 0.0067 | - | - | −217 | 0.1066 |
| New extrapulmonary cases | 4994 | 0.0007 | 2134 | 0.0006 | −9551 | 0.1544 |
| Laboratory confirmed RR/MDR-TB† | - | - | - | |||
| Laboratory confirmed MDR-TB† | - | - | - | |||
| Tuberculosis case notification numbers | 41 726 | 0.0001 | 32 348 | 0.0001 | 6084 | 0.8818 |
| Case notification rate/100k | 7.8 | 0.2634 | 7.6 | 0.0680 | −57.1 | 0.4901 |
| Tuberculosis case detection rate % | 2.7 | 0.2305 | 3.3 | 0.0743 | 6.2 | 0.0108 |
| Estimated tuberculosis/HIV % | −4.5 | 0.0190 | −3.9 | 0.1604 | −6.6 | < 0.0001 |
| Estimated mortality/100k | −8.2 | 0.0264 | −6.0 | 0.0001 | −254 | < 0.0001 |
| TSR new cases % | 9.0 | 0.0201 | 8.4 | 0.0001 | 9.6 | 0.0017 |
Source: World Health Organization (WHO). Global Tuberculosis Programme. In Global TB report [homepage on the Internet]. 2021 [cited 2021 Jun 26]. Available from: https://www.who.int/tb/country/data/download/en/
MDR-TB, multidrug-resistant tuberculosis; TSR, treatment success rate; RR, rifampicin resistant tuberculosis
, No data to enable comparison.
Interrupted time series models
In the DRC, the initial number of laboratory-confirmed cases of PTB was 50 747, and the number of cases increased significantly every year before 2011 by 3178 (p < 0.0001) (Table 3, Supplementary Figures 1-3). In the first year of the intervention, cases of PTB decreased by 15 521 (p = 0.009), followed by a marginal increase in the yearly number of cases of diagnosed PTB (relative to the pre-intervention period) of 1856 (p = 0.085). After introducing the intervention, the post-trend estimate shows the cases of diagnosed PTB increased annually by 5034 (p ≤ 0.001). A similar trend was seen in Nigeria except for the reduction in the number of cases in the year the intervention commenced, and the increase afterwards was not statistically significant. However, the post-trend estimate showed a statistically significant increase in cases of diagnosed PTB annually by 4295 (p ≤ 0.001). South Africa showed a similar trend of a statistically significant annual increase of 7996 (p = 0.013) before 2011. There was a decline in diagnosed PTB cases by 157 in the first year of the intervention period (p = 0.994), however, this decrease was statistically significant annually after the intervention period by 15 269 cases (p = 0.006). The post-intervention linear trend also showed a significant decrease in cases of 7273 (p = 0.028).
TABLE 3.
Summary of interrupted time series models output for the three highest burden tuberculosis countries in Africa 2001–2019.
| New pulmonary confirmed cases | Democratic Republic of the Congo |
Nigeria |
South Africa |
|||
|---|---|---|---|---|---|---|
| Coefficient | p | Coefficient | p | Coefficient | p | |
| Yearly cases before 2011 | 3178 | < 0.001 | 3509 | 0.015 | 7996 | 0.013 |
| Cases in the first year of the intervention | −15 521 | 0.009 | −14 649 | 0.188 | −157 | 0.994 |
| Annual cases of PTB after 2011 relative to the period prior | 1856 | 0.085 | 785 | 0.555 | −15 269 | 0.006 |
| Baseline number of laboratory-confirmed PTB cases | 50 747 | < 0.001 | 27 173 | < 0.001 | 110 712 | < 0.001 |
| Yearly cases after the introduction of GeneXpert | 5034 | < 0.001 | 4295 | < 0.001 | −7273 | 0.028 |
PTB, pulmonary tuberculosis
The models were not influenced by seasonality or any time-varying confounder. There was no over-dispersion as our outcome variable was continuous. An autocorrelation test was done for each of the three models up to seven lags, and there was no autocorrelation in any of the models. A separate model to ascertain the impact of HIV coinfection, which could have influenced the model output in South Africa, indicated similar findings (Online Supplementary Figure 4).
Discussion
Our analysis showed that an increase in the number of diagnosed tuberculosis cases in the DRC and Nigeria was statistically significant, but the number of cases diagnosed in South Africa after the introduction of GeneXpert had decreased. Tuberculosis case notification rates and case detection rates did not show major changes. There was, however, a significant reduction in tuberculosis/HIV coinfection and mortality per 100 000 population, with an increase in treatment success rate for new cases in all three countries.
While all three countries installed their first Xpert MTB/RIF in 2011, there was variable adoption of Xpert as a first-line diagnostic test, inconsistent scale-up and inadequate utilisation of Xpert testing in each country.11,15 These factors may have considerably affected the case notification achievements of the three countries as shown in our study. In the DRC, initial evaluation post rollout indicated GeneXpert improved detection of tuberculosis cases and rifampicin resistance in patients with smear-negative sputum.9 However, later evaluation reports indicated the coverage was low, with one Xpert instrument serving 3.3 zones in the country.16 This was further complicated by recurrent stockouts and expiry of instrument cartridges as of 2018, although measures had been instituted to address these with additional staff complements and training.23
This study showed case notification and detection rates did not change considerably in Nigeria, with no difference in incidence rate between the two periods. This was corroborated by a 2019 assessment of GeneXpert performance, which reported low case findings (about 25% of estimated cases) despite the increase in Xpert modules and use of Xpert as the main diagnostic tool in 2016 (Xpert4all initiative).18 This may be because many health workers do not follow the standard protocol when a patient presents with a cough, without considering tuberculosis or ordering GeneXpert testing. In addition, there is under-utilisation of GeneXpert because of modular failures, poor power supply, inefficient sample transport mechanisms, weak data management and inadequate human resources to staff the remote test sites. However, the national programme did report an increase in tuberculosis case finding in those areas with access to Xpert.18 Additional studies on the utility of Xpert in Nigeria found that it increased tuberculosis case detection among smear-negative PTB patients.24
Implementation data from the South African National Health Laboratory services from 2011 to 2019 demonstrateed a declining trend in the number of cases detected.19 This is consistent with what we have reported. In a study to assess the impact of Xpert rollout on tuberculosis diagnostic yield and time to initiate therapy, the researchers observed marginal increases in tuberculosis case detection and time to initiate treatment, suggesting the need to address the health system to improve Xpert MTB/RIF efficiency.25 In a similar study, Xpert increased the proportion of rifampicin-resistant confirmed tuberculosis and significantly reduced the time to diagnosis, commencing therapy, and the pre-treatment mortality in patients with drug-resistant tuberculosis.26 However, on-treatment mortality and treatment outcomes did not change, reflecting a need to address treatment gaps and treatment outcomes.26
Similar to our findings, the Health Systems Trust (an organisation established to assist with the transformation of the healthcare system in South Africa) showed there has been an annual decline in tuberculosis incidence in South Africa from 2011 to 2015 and data from the University of KwaZulu-Natal suggest that the rapid drop in tuberculosis cases detected could be attributed partially to the scale-up of antiretroviral therapy that took place between 2009 and 2014.27,28,29 However, about 150 000 tuberculosis cases are missed annually in South Africa – nearly 50% of the tuberculosis burden in the country.30 Patient-side and health system-side factors may contribute to the missed cases and thus reduce the impact of Xpert on tuberculosis case detection and treatment outcomes. Major health system barriers include laboratory-running challenges such as intermittent electricity supply and limited number of Xpert cartridges, insufficient human resources, poor provider knowledge and skills to adhere to diagnostic guidelines and inadequate information management.30,31,32 On the patient side, barriers include loss to follow-up due to delayed diagnosis or family and work commitments, negative public sector care perceptions, and using private-sector care with limited financial resources.33,34
In modelling studies to determine the long-term outcome of scaling up the Xpert platform with an algorithm that used Xpert as the first-line diagnostic test, it was determined that if implemented optimally, the number of tuberculosis cases diagnosed per year would increase by 30% – 37%, while diagnosed MDR-TB cases would increase by 69% – 71%. Eighty-one percent of the cases would be diagnosed after the initial visit compared to 46% at the time.35 The data from each of the three countries, while showing a modest rise in the number of tuberculosis cases diagnosed, did not meet the expectations of the modelling studies. Despite the observed increases in the DRC and Nigeria, there are numerous health system challenges to the optimal rollout and utilisation of GeneXpert in the diagnosis of tuberculosis cases. South Africa registered a decline in the number of tuberculosis cases diagnosed. Variable scale-up, low utilisation rates and unaddressed patient-side and health-system barriers have negatively impacted the expected increase in tuberculosis case detection and rifampicin resistance detection, as well as treatment outcomes. Xpert performance in other tuberculosis high-burden countries reflects similar trends for case detection and treatment success.15,36
Limitations
This study was not without limitations. Firstly, we used data extracted from the WHO website for which we had no control in terms of how data was collected. This study did not have a mechanism for identifying data reported to the WHO that was not updated. There were missing data for some outcomes and variables but where feasible, missing data were imputed. However, the mean imputation approach used may not have yielded the optimal value, although it provided an estimate to work with. The output of our interrupted time series analysis may have been influenced by unmeasured confounders, particularly in South Africa where the rollout of the Antiretroviral Therapy Program may have impacted diagnosed tuberculosis cases. To address this, we conducted a sensitivity analysis to ascertain if HIV coinfection impacted the South African model. Noting that interrupted time series models are generally not affected by typical confounders such as age distribution or socioeconomic status, like in the modelling of individual patient data, we believe the modelling approach was appropriate.37 Nevertheless, this study has highlighted the key successes since the introduction of GeneXpert for tuberculosis diagnosis and the areas requiring improvement to be on track to end tuberculosis by 2035.
Conclusion
While the GeneXpert testing platform has been lauded as a revolutionary tool to enhance tuberculosis case finding in meeting the End TB targets,10 tuberculosis case notification remains low in the three highest tuberculosis burden countries in Africa. If low- and middle-income countries’ health systems do not address implementation challenges related to access and utilisation, the advantages of the Xpert diagnostic platform will not be realised and these targets will be missed.
Acknowledgements
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors’ contributions
V.W. designed the study, extracted, and analysed the data, and coordinated the writing of the manuscript. M.C. co-designed the study, wrote sections of the first draft, and interpreted the analysis. B.E. and C. Onwuchekwa participated in data analysis and interpretation and writing of sections of the manuscript. C. Okoro, C.C. and R.C. interpreted the analysis findings, provided input on the structure of the research, and revised the manuscript. K.O. guided the analysis, interpretation of results and presentation of the findings. All authors read and approved the final manuscript.
Sources of support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
The data that supports the findings of this research are openly available at the World Health Organization data repository (https://www.who.int/tb/country/data/download/en/).
Disclaimer
The views expressed in this manuscript are those of the authors and not those of the institutions they are affiliated with.
Footnotes
How to cite this article: Williams V, Calnan M, Edem B, et al. GeneXpert rollout in three high-burden tuberculosis countries in Africa: A review of pulmonary tuberculosis diagnosis and outcomes from 2001 to 2019. Afr J Lab Med. 2022;11(1), a1811. https://doi.org/10.4102/ajlm.v11i1.1811
Note: Additional supporting information may be found in the online version of this article as Online Supplementary Document 1.
References
- 1.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010. Sep 8;363(11):1005–1015. 10.1056/NEJMoa0907847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chamie G, Luetkemeyer A, Charlebois E, Havlir DV. Tuberculosis as part of the natural history of HIV infection in developing countries. Clin Infect Dis. 2010;50 Suppl 3:S245–S254. 10.1086/651498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ulrich M, Christensen D, Coyne S, et al. Evaluation of the Cepheid GeneXpert system for detecting Bacillus anthracis. J Appl Microbiol. 2006. May;100(5):1011–1016. 10.1111/j.1365-2672.2006.02810.x [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) . Molecular line probe assays for rapid screening of patients at risk of multidrug-resistant tuberculosis (MDR-TB). Policy statement. Geneva: WHO, 2008; p. 1–9. [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) . Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. JAMA. 2009;301(10):1014–1016. 10.1001/jama.2009.148 [DOI] [Google Scholar]
- 6.Rakotosamimanana N, Lapierre SG, Raharimanga V, et al. Performance and impact of GeneXpert MTB/RIF® and Loopamp MTBC Detection Kit® assays on tuberculosis case detection in Madagascar. BMC Infect Dis. 2019. Jun 20;19(1):1–8. 10.1186/s12879-019-4198-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khaparde S, Raizada N, Nair SA, et al. Scaling-up the Xpert MTB/RIF assay for the detection of tuberculosis and rifampicin resistance in India: An economic analysis. PLoS One. 2017. Sep 1;12(9):e0184270. 10.1371/journal.pone.0184270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albert H, Nathavitharana RR, Isaacs C, Pai M, Denkinger CM, Boehme CC. Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: What lessons have we learnt and how can we do better? Eur Respir J. 2016;48(2):516–525. 10.1183/13993003.00543-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creswell J, Codlin AJ, Andre E, et al. Results from early programmatic implementation of Xpert MTB/RIF testing in nine countries. BMC Infect Dis. 2014. Jan 2;14(1):2. 10.1186/1471-2334-14-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO) . The End TB strategy. Geneva: World Health Organization; 2016. [Google Scholar]
- 11.Schumacher SG, Denkinger CM. The impact of Xpert MTB/RIF – Do we have a final answer? Lancet Glob Health. 2019. Feb 1;7(2):e161–e162. 10.1016/S2214-109X(18)30493-5 [DOI] [PubMed] [Google Scholar]
- 12.Ershova JV, Volchenkov GV, Somova TR, et al. Impact of GeneXpert MTB/RIF® on treatment initiation and outcomes of RIF-resistant and RIF-susceptible TB patients in Vladimir TB dispensary, Russia. BMC Infect Dis. 2020. Jul 25;20(1):543. 10.1186/s12879-020-05243-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens WS, Scott L, Noble L, Gous N, Dheda K. Impact of the GeneXpert MTB/RIF technology on tuberculosis control. Microbiol Spectr. 2017. Feb 24;5(1):5–1. 10.1128/microbiolspec.TBTB2-0040-2016 [DOI] [PubMed] [Google Scholar]
- 14.Cazabon D, Pande T, Kik S, et al. Market penetration of Xpert MTB/RIF in high tuberculosis burden countries: A trend analysis from 2014–2016. Gates Open Res. 2018. Sep 18;2:35. 10.12688/gatesopenres.12842.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cazabon D, Suresh A, Oghor C, et al. Implementation of Xpert MTB/RIF in 22 high tuberculosis burden countries: Are we making progress? Eur Respir J. 2017;50(2):1700918. 10.1183/13993003.00918-2017 [DOI] [PubMed] [Google Scholar]
- 16.Bulabula ANH, Nelson JA, Musafiri EM, et al. Prevalence, predictors, and successful treatment outcomes of Xpert MTB/RIF-identified rifampicin-resistant tuberculosis in post-conflict Eastern Democratic Republic of the Congo, 2012–2017: A retrospective province-wide cohort study. Clin Infect Dis. 2019. Sep 27;69(8):1278–1287. 10.1093/cid/ciy1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mustapha G, Jumoke O, Nwadike P, et al. Assessment of Gene-Xpert MTB RIF program implementation and the challenges for enhanced tuberculosis diagnosis in Nigeria. SAARC J Tuberc Lung Dis HIV/AIDS. 2016;12(2):1–7. 10.3126/saarctb.v12i2.15948 [DOI] [Google Scholar]
- 18.Gidado M, Nwokoye N, Ogbudebe C, et al. Assessment of GeneXpert MTB/RIF performance by type and level of health-care facilities in Nigeria. Niger Med J. 2019;60(1):33. 10.4103/nmj.NMJ_12_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Department of Health, South Africa . National Xpert MTB/RIF implementation report. 2019. Pretoria: NDoH; 2019. [Google Scholar]
- 20.Babbie ER. The practice of social research. 15th ed. Belmont, CA: Wadsworth, 2020; p. 592. [Google Scholar]
- 21.World Health Organization (WHO) . WHO global lists of high burden countries for tuberculosis (TB), TB/HIV and TB (MDR/RR-TB) [homepage on the Internet]. 2021–2025. 2021. [cited 2021 Jun 26]. Available from: https://cdn.who.int/media/docs/default-source/hq-tuberculosis/who_globalhbcliststb_2021-2025_backgrounddocument.pdf
- 22.World Health Organization (WHO) . Global Tuberculosis Programme. In Global TB report [homepage on the Internet]. 2021. [cited 2021 Jun 26]. Available from: https://www.who.int/tb/country/data/download/en/
- 23.The Global Fund . Technical Evaluation Reference Group: Prospective Country Evaluations. Democratic Republic of the Congo 2019–2020 Annual Country Report [homepage on the Internet]. Geneva: The Global Fund; Jun 2020. [cited 2022 Jun 01]. Available from: https://path.azureedge.net/media/documents/DRC_PCE_Final_annual_report_2020_English.pdf [Google Scholar]
- 24.Tiamiyu AB, Iliyasu G, Dayyab FM, et al. Evaluation of GeneXpert MTB/RIF as a diagnostic tool in patients with sputum smear-negative TB in a high HIV burden region in Nigeria. Trans R Soc Trop Med Hyg. 2020. Sep 1;114(9):697–699. 10.1093/trstmh/traa019 [DOI] [PubMed] [Google Scholar]
- 25.Schmidt BM, Geldenhuys H, Tameris M, et al. Impact of Xpert MTB/RIF rollout on management of tuberculosis in a South African community. S Afr Med J. 2017. Dec 1;107(12):1078–1081. 10.7196/SAMJ.2017.v107i12.12502 [DOI] [PubMed] [Google Scholar]
- 26.Evans D, Sineke T, Schnippel K, et al. Impact of Xpert MTB/RIF and decentralized care on linkage to care and drug-resistant tuberculosis treatment outcomes in Johannesburg, South Africa. BMC Health Serv Res. 2018. Dec 17;18(1):1–12. 10.1186/s12913-018-3762-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massyn N, Day C, Ndlovu N, Padayachee T, editors. District health barometer, 2019/2020 [homepage on the Internet]. Durban: Health Systems Trust; Dec 2020. [cited 2021 Jun 27]. Available from: https://www.hst.org.za/publications/Pages/DHB2019-20.aspx [Google Scholar]
- 28.Tomita A, Smith CM, Lessells RJ, et al. Space-time clustering of recently-diagnosed tuberculosis and impact of ART scale-up: Evidence from an HIV hyper-endemic rural South African population. Sci Rep. 2019;9(1):1–9. 10.1038/s41598-019-46455-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaren ZM, Sharp A, Brouwer E, Nanoo A. The impact of anti-retroviral therapy on tuberculosis detection at the national level in South Africa. Am J Trop Med Hyg. 2018;99(6):1407. 10.4269/ajtmh.17-0530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walt van der M, Moyo S. The First National TB Prevalence Survey - South Africa 2018. Pretoria: National Department of Health; 2021. [Google Scholar]
- 31.Murdoch J, Curran R, Van Rensburg AJ, et al. Identifying contextual determinants of problems in tuberculosis care provision in South Africa: A theory-generating case study. Infect Dis Poverty. 2021;10(1):1–13. 10.1186/s40249-021-00840-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adu PA, Yassi A, Ehrlich R, Spiegel JM. Perceived health system barriers to tuberculosis control among health workers in South Africa. Ann Global Health. 2020;86(1):15. 10.5334/aogh.2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marais F, Kallon II, Dudley LD. Continuity of care for TB patients at a South African hospital: A qualitative participatory study of the experiences of hospital staff. PLoS One. 20191;14(9):e0222421. 10.1371/journal.pone.0222421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudley L, Mukinda F, Dyers R, Marais F, Sissolak D. Mind the gap! Risk factors for poor continuity of care of TB patients discharged from a hospital in the Western Cape, South Africa. PLoS One. 2018;13(1):e0190258. 10.1371/journal.pone.0190258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer-Rath G, Schnippel K, Long L, et al. The impact and cost of scaling up genexpert MTB/RIF in South Africa. PLoS One. 2012;7(5):e36966. 10.1371/journal.pone.0036966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haraka F, Kakolwa M, Schumacher SG, et al. Impact of the diagnostic test Xpert MTB/RIF on patient outcomes for tuberculosis. Cochrane Database Syst Rev. 2021;2021(5):CD012972. 10.1002/14651858.CD012972.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: A tutorial. Int J Epidemiol. 2017;46(1):348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings of this research are openly available at the World Health Organization data repository (https://www.who.int/tb/country/data/download/en/).