Abstract
Risk calculators have offered a viable tool for clinicians to stratify patients at risk of prostate cancer (PCa) and to mitigate the low sensitivity and specificity of screening prostate specific antigen (PSA). While initially based on clinical and demographic data, incorporation of multiparametric magnetic resonance imaging (MRI) and the validated prostate imaging reporting and data system suspicion scoring system has standardized and improved risk stratification beyond the use of PSA and patient parameters alone. Biopsy-naïve patients with lower risk profiles for harboring clinically significant PCa are often subjected to uncomfortable, invasive, and potentially unnecessary prostate biopsy procedures. Incorporating risk calculator data into prostate MRI reports can broaden the role of radiologists, improve communication with clinicians primarily managing these patients, and help guide clinical care in directing the screening, detection, and risk stratification of PCa.
Keywords: Prostatic adenocarcinoma, Multiparametric magnetic resonance imaging, Nomograms, Risk calculators, Biopsy
Core Tip: Incorporating risk calculator data into prostate magnetic resonance imaging reports can broaden the role of radiologists, improve communication with clinicians primarily managing these patients, and help guide clinical care in directing the screening, detection, and risk stratification of prostate cancer.
INTRODUCTION
Prostate cancer (PCa) is the most common solid organ malignancy in American men and the second cause of cancer-related death in the United States[1]. Due to increased awareness, nearly 20 million men in the United States engage in screening and early detection discussions (National Comprehensive Cancer Network). Prostate specific antigen (PSA) made large-scale screening for PCa feasible, but lacked accuracy, with 15%-25% false negatives and 60% false positives[2,3]. Since PSA has proven to be an unreliable biomarker for clinically significant prostate cancer [csPCa; Grade Group (GG) ≥ 2], a large percentage of patients continue to undergo prostate biopsies with either benign or clinically indolent PCa (GG 1). Prostate biopsies are an invasive diagnostic procedure with well-established risks, such as hematuria, hematospermia, rectal bleeding, urinary tract infections, and recognized risk of sepsis[4-7]. Furthermore, potentially unnecessary biopsies and over treatment of low-risk prostate cancer has placed an undue psychological burden on patients[8].
The role of multiparametric magnetic resonance imaging (mpMRI) in prostate cancer diagnosis, surveillance, and treatment has significantly evolved and is growing in popularity as a tool to potentially avoid unnecessary biopsies in biopsy-naive patients. Controversy remains due to significant variability across patient cohorts and institutions. Risk calculators combining mpMRI with clinical variables can limit this variation and have been shown to improve predictive models[9,10]. An individualized screening algorithm using a patient’s clinical history can result in a considerable reduction in unnecessary biopsy sessions. A validated clinical risk calculator that could be incorporated into MRI reporting and aid in the decision to pursue prostate biopsies in biopsy-naive patients is needed[11]. However, such a risk calculator must be carefully validated to ensure its reliable performance and applicability to a broad population of patients undergoing prostate cancer screening when including MRI in the screening algorithm.
OVERVIEW OF RISK CALCULATORS
Historical perspective
One of the first algorithms to predict the risk of prostate cancer on prostate biopsy was the European Randomized Study for Screening of Prostate Cancer (ERSPC) risk calculator. The ERSPC has six calculators, two of which are used by patients and the remaining four used by physicians. The RC3/RC4 combined calculator uses PSA levels, digital rectal exam (DRE) exam, previous prostate biopsy history, prostate volume, and now incorporates MRI prostate imaging reporting and data system (PI-RADS) v 1.0 score to predict the detectable risk of prostate cancer on biopsy. The calculator stratifies the risk of detecting cancer to assist clinicians with the decision to pursue biopsy (https://www.prostatecancer-riskcalculator.com/). Several external validation studies have been performed for these RCs. The discriminative ability of detecting positive prostate biopsy (PBx) in biopsy-naive or previously biopsied patients using the ERSPC RC3 or RC4 was assessed, showing area under the curve (AUC) values in the range of 0.71-0.88[12-16].
Thompson et al[17] developed one of the first online individualized predictive assessments of prostate cancer before prostate biopsy extrapolated from the 5519 patients in the Prostate Cancer Prevention Trial (PCPT). It was found that PSA, family history, DRE findings, African American race, and history of a prior negative prostate biopsy provided independent predictive value to the calculation of risk of a biopsy that showed presence of cancer. The first calculator became known as the Prostate Cancer Prevention Trial Risk Calculator (PCPTRC) and has been used widely online at https://riskcalc. org/PCPTRC/. In 2012, an updated PCPTRC 2.0 was released with the added capability to provide prediction of indolent low-grade (Gleason grade < 7) vs high-grade (GG ≥ 2) PCa. Both versions of the online PCPT risk calculator were externally validated in 2014.
Independent validation and comparisons between the ERSPC and PCPTRC calculators demonstrated comparable calibration in their agreement between predicted and observed risks of prostate cancer. However, the AUC for predicting clinically significant sPCa was higher for the ERSPC risk calculator compared with the PCPTRC (0.73 vs 0.70; P = 0.043)[18]. The PCPTRC has been replaced by a more contemporary risk calculator developed by the Prostate Biopsy Collaborative Group (PBCG) that incorporates age, PSA level, DRE results, family history, race, and a history of negative biopsy along with more contemporary biopsy schemes[19]. The study demonstrates a greater inclusion of patients with diverse backgrounds and PBCG model outperformed the PCPTRC in predicting csPCa on both internal (AUC, 75.5% vs 72.3%; P < 0.0001) and external validation (AUC, 72.9% vs 69.7%; P < 0.0001). Furthermore, the PBCG model was found to be well calibrated and offered a higher net clinical benefit than the PCPT risk calculator: it led to 2.7% fewer biopsies without missing any csPCa.
Advent of imaging
Prior to 2017, mpMRI of the prostate was not commonly used in the PCa workup worldwide due to the high cost and limited availability of prostate MRI. In 2019, Alberts et al[20] published a study on the use of risk calculators and biopsy results to avoid unnecessary prostate MRI. Alberts et al[20] suggested that mpMRI of the prostate provided an opportunity to enhance the non-invasive portion of the PCa workup and introduced a nomogram integrating PI-RADS data into the ERSPC risk calculator. Alberts et al[20] demonstrated a superior nomogram compared to the ERSPC standard, achieving an AUC of 0.84, which was significantly increased compared to ERSPC calculators that did not incorporate imaging data.
As mpMRI of the prostate became more widely available and the Urology community became more aware of the potential impact of PI-RADS score on risk calculator development, prostate MRI data was more widely incorporated into PCa risk nomograms. PI-RADS data, scored on a zero to five Likert scale, is easily incorporated into nomograms due to its objective, defined numerical values. In 2019, Alberts et al[20] refined the ERSPC-RC-3/4 risk calculators, developing MRI-ERSPC-RC-3/4 by adding mpMRI examination results. The addition of MRI to the ERSPC calculators increased the discriminative ability for high-grade PCa [AUC of 0.84 (95%CI 0.81-0.88) and 0.85 (95%CI 0.81-0.89) for the MRI-ERSPC-RC3 and MRI-ERSPC-RC4, respectively][20]. Beyond the established clinical based calculators like the ERSPC and the PBCG, novel risk calculators were developed across the globe, with several large multicenter trials occurring in North America, the United Kingdom, and Australia, such as the Stanford Prostate Cancer Calculator (SPCC)[21], the PLUM cohort[22], the PCRC-MRI[23], MRI study by Chau et al[24], and the study done by van Leeuwen et al[25] PI-RADS integrated clinical calculators consistently demonstrated superior performance to calculators using clinical data alone[23-27]. Of note, due to the wide variety in study location, practice type, and timing of data collection, some of these risk calculators use data from PI-RADS v1.0 and PI-RADS 2.0. The SPCC notes that its calculator is validated for both PI-RADS v1.0 and v2.0[21].
For biopsy-naive patients, the superior performance of imaging integrated risk calculators represents a possibility to avoid invasive biopsy for low risk PCa. Trials specific to the biopsy-naive population have demonstrated promising results with high sensitivity and specificity and high net benefit. Radtke et al[27] and Chau et al[24] attained high AUC values, both in excess of 0.8, and both were trained on patient populations from the United Kingdom. The van Leeuwen et al’s risk calculator has an AUC of 0.90 and demonstrates one of the most substantial net benefits, avoiding 28.6% of biopsies at 10% risk tolerance, missing only 2.6% of PCa[25]. Additional external validation studies have demonstrated high AUC for the van Leeuwen and ERSPC based models, however both studies conclude that the use of MRI integrated risk calculators to avoid biopsy remains controversial[28,29].
DISCUSSION
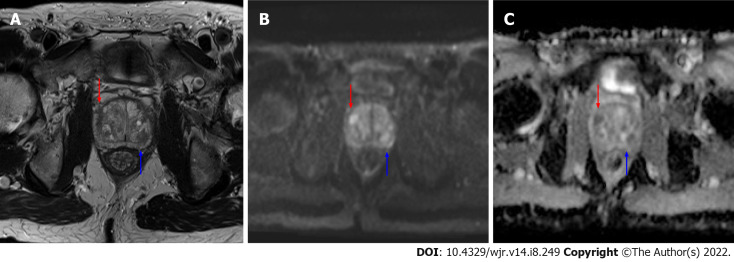
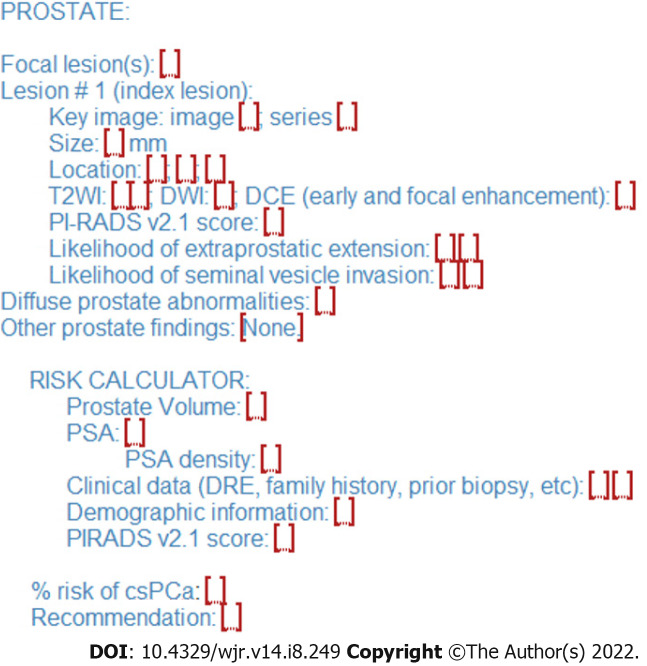
Risk calculators and nomograms provide a valuable tool in risk stratification of patients with abnormal screening PSA levels potentially allowing selection of cases to avoid biopsy in patients at low risk for harboring csPCa. Incorporation of risk calculator data into radiology reports could represent an opportunity for radiologists to add value to the patient evaluation and mitigate ambiguity of borderline results, especially PI-RADS 3 Lesions found on prostate indication MRI studies (Figures 1 and 2). In collaboration with the referring clinician, the radiologist could incorporate patient clinic and demographic information, along with the lesion PI-RADS score, calculate the percent risk of csPCa, and include this information in the final diagnostic imaging report.
Figure 1.
Axial magnetic resonance imaging images of the prostate. A: T2 weighted image; B: b1200 diffusion weighted imaging (DWI) image; C: Calculated apparent dispersion coefficient (ADC) image. A mostly encapsulated T2-hypointense transitional zone lesion is demonstrated in the left posterior central gland, measuring 10 mm (blue arrows) with focal moderate low ADC, high DWI signal, designated prostate imaging reporting and data system (PI-RADS) 3 per PI-RADS version 2.1. An additional 8 mm PI-RADS 4 Lesion of the anterior right transitional zone is present (red arrow), demonstrating non-circumscribed moderate T2 hypointensity and marked focal ADC hypointensity and DWI hyperintensity.
Figure 2.
Sample structured report for prostate lesion reporting with integrated risk calculator reporting. The calculated percent risk of clinically significant prostate cancer is included in the lesion evaluation findings with recommendations for biopsy or observation in the conclusion. csPCa: Clinically significant prostate cancer; DRE: Digital rectal exam; DWI: Diffusion weighted imaging; PI-RADS: Prostate imaging reporting and data system; PSA: Prostate specific antigen.
Three PI-RADS integrated calculators, the SPCC[21], the PLUM Prostate cancer risk calculator, and the MRI-ERSPC-R-3/4 published open access online calculators, allowing a more streamlined integration into workflow. For biopsy-naive patients, the PLUM calculator demonstrated the highest sensitivity and specificity with an AUC value of 0.87 and a net benefit of avoiding 18.1% of biopsies without missing any csPCa in biopsy-naive patients at a 15% tolerance. The MRI-ERSPC-R-3/4 calculator reported an AUC of 0.84 in its initial study from Alberts et al’s net benefit for biopsy-naive patients was not reported in the Alberts et al’s study[20], but in Petersmann et al[29], which compared the MRI-ERSPC-R-3/4 calculator to the calculator described in van Leeuwen et al[25], the MRI/ERSPC-R-3/4 nomogram avoids only 9% of biopsies in biopsy-naive patients while missing 3% at a 15% risk threshold. The SPCC trial did not report a specific AUC or net benefit for biopsy-naive patients but reported AUC values ranging from 0.78-0.83 and a net benefit of avoiding 10.3% of biopsies while missing csPCa in 0.8% of patients with a risk tolerance of 20%[21].
Additional notable nomograms have demonstrated promising results for biopsy-naive patients that outperform some of the larger and more established risk calculators. The van Leeuwen et al[25] nomogram demonstrated the highest AUC of all evaluated risk calculators and reported one of the highest net benefits, avoiding 28.6% of biopsies while missing only 2.6% of csPCa, but was developed on a smaller and more homogenous patient population (393 patients from Australia) than many of the other noted calculators. However in the external validation study by Petersmann et al[29], the van Leeuwen nomogram was demonstrated to maintain high performance, and even outperformed the ERSPC in net benefit. Petersmann et al[29] compared ERSPC and van Leeuwen risk calculator. This study showed comparable AUC values between the two studies, 0.81 for ERSPC and 0.82 for van Leeuwen, however the van Leeuwen calculator demonstrated a greater net benefit from a risk threshold of 10%-15%, avoiding 24% of biopsies while missing 6% of csPCa, compared to 14% and 5% for the MRI-ERSP-RC-3/4, respectively. Notably the ERSPC calculator had a near perfect calibration, with a calibration slope of 0.94 compared to the van Leeuwen model, 0.70. The Petersmann et al’s study population came from a hospital system in Nuremberg, Germany and likely reflected a similar demographic to the ERSPC training population, whereas the van Leeuwen study was performed in Australia[29]. The gaps in calibration between these two studies may indicate future pitfalls in generalizability, and clinicians need to be aware of the training data and population demographics when applying these calculators to their own patient population.
Novel imaging technologies such as prostate cancer directed PET imaging may further aid in refining these risk calculators, allowing for additional improvements in pre-biopsy patient risk stratification. Radiomics, a subset of clinical artificial intelligence (AI), is a promising tool on the horizon of prostate imaging and prostate cancer classification. Prostate MRI has represented a prolific area of AI research in the past decade, with algorithms demonstrating improved prostate cancer detection, classification, and upstream applications, such as deep learning reconstruction and its role in instituting abbreviated protocols. In a systematic review, Ferro et al[30] discuss 21 manuscripts related to radiomics and the detection of csPCa. These publications have demonstrated the capability of radiomics to extract salient features and develop models that predict csPCa that significantly outperform clinical models[31] and combined clinical and imaging models[32]. While these results are encouraging, the algorithms to date are often trained at a single institution and are limited by a lack of external validation and heterogeneity of the extracted radiomics features. Although further refinement and broader, multi-institution testing is needed, early successes of radiomics models suggest a promising future for AI in the evaluation, diagnosis, risk stratification, and treatment decision making in the management of csPCa.
CONCLUSION
Risk calculators have enabled physicians and patients to make a more informed decision when considering pursuit of a prostate biopsy. When evaluating biopsy-naïve patients, multiple risk calculators can be applied, each with their own strengths. The role of imaging using MRI in the diagnosis of csPCa has significantly evolved and is growing in popularity. The PI-RADS system has become a component of many currently available pre-biopsy prostate cancer risk calculators. Artificial intelligence shows promise in further advancing the role of imaging in csPCa risk assessment. Further incorporation of imaging in clinical risk calculators shows promise in aiding the decision to pursue prostate biopsies with improved confidence and patient-centric goals.
Footnotes
Conflict-of-interest statement: Rais-Bahrami S serves as a consultant to Philips/InVivo Corp, Genomic Health Inc, Blue Earth Diagnostics, Bayer Healthcare, UroViu Corp, and Intuitive Surgical.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 22, 2022
First decision: June 16, 2022
Article in press: July 25, 2022
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Andrejic-Visnjic B, Serbia; Liang L, China S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
Contributor Information
Karisma Gupta, Department of Radiology, University of Washington, Seattle, WA 98195, United States.
Jordan D Perchik, Department of Radiology, University of Alabama at Birmingham, Birmingham, AL 35233, United States.
Andrew M Fang, Department of Urology, University of Alabama at Birmingham, Birmingham, AL 35233, United States.
Kristin K Porter, Department of Radiology, University of Alabama at Birmingham, Birmingham, AL 35233, United States.
Soroush Rais-Bahrami, Department of Radiology, University of Alabama at Birmingham, Birmingham, AL 35233, United States; Department of Urology, University of Alabama at Birmingham, Birmingham, AL 35233, United States; O'Neal Comprehensive Cancer Center, University of Alabama at Birmingham, Birmingham, AL 35233, United States. soroushraisbahrami@gmail.com.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Gambert SR. Screening for prostate cancer. Int Urol Nephrol. 2001;33:249–257. doi: 10.1023/a:1015290429403. [DOI] [PubMed] [Google Scholar]
- 3.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA Jr. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 4.Halpern JA, Sedrakyan A, Dinerman B, Hsu WC, Mao J, Hu JC. Indications, Utilization and Complications Following Prostate Biopsy: New York State Analysis. J Urol. 2017;197:1020–1025. doi: 10.1016/j.juro.2016.11.081. [DOI] [PubMed] [Google Scholar]
- 5.Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830–1834. doi: 10.1016/j.juro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, Rosario DJ, Scattoni V, Lotan Y. Systematic review of complications of prostate biopsy. Eur Urol. 2013;64:876–892. doi: 10.1016/j.eururo.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 7.Ravi P, Sammon J, Meskawi M, Sun M, Karakiewicz PI, Trinh QD. Re: Complications after prostate biopsy: data from SEER-Medicare: S. Loeb, H. B. Carter, S. I. Berndt, W. Ricker and E. M. Schaeffer J Urol 2011; 186: 1830-1834. J Urol. 2012;188:677–678. doi: 10.1016/j.juro.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Loeb S, Bjurlin MA, Nicholson J, Tammela TL, Penson DF, Carter HB, Carroll P, Etzioni R. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65:1046–1055. doi: 10.1016/j.eururo.2013.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjurlin MA, Rosenkrantz AB, Sarkar S, Lepor H, Huang WC, Huang R, Venkataraman R, Taneja SS. Prediction of Prostate Cancer Risk Among Men Undergoing Combined MRI-targeted and Systematic Biopsy Using Novel Pre-biopsy Nomograms That Incorporate MRI Findings. Urology. 2018;112:112–120. doi: 10.1016/j.urology.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 10.Radtke JP, Wiesenfarth M, Kesch C, Freitag MT, Alt CD, Celik K, Distler F, Roth W, Wieczorek K, Stock C, Duensing S, Roethke MC, Teber D, Schlemmer HP, Hohenfellner M, Bonekamp D, Hadaschik BA. Combined Clinical Parameters and Multiparametric Magnetic Resonance Imaging for Advanced Risk Modeling of Prostate Cancer-Patient-tailored Risk Stratification Can Reduce Unnecessary Biopsies. Eur Urol. 2017;72:888–896. doi: 10.1016/j.eururo.2017.03.039. [DOI] [PubMed] [Google Scholar]
- 11.Fang AM, Rais-Bahrami S. Magnetic resonance imaging-based risk calculators optimize selection for prostate biopsy among biopsy-naive men. Cancer. 2022;128:25–27. doi: 10.1002/cncr.33872. [DOI] [PubMed] [Google Scholar]
- 12.Cavadas V, Osório L, Sabell F, Teves F, Branco F, Silva-Ramos M. Prostate cancer prevention trial and European randomized study of screening for prostate cancer risk calculators: a performance comparison in a contemporary screened cohort. Eur Urol. 2010;58:551–558. doi: 10.1016/j.eururo.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 13.Gayet M, Mannaerts CK, Nieboer D, Beerlage HP, Wijkstra H, Mulders PFA, Roobol MJ. Prediction of Prostate Cancer: External Validation of the ERSPC Risk Calculator in a Contemporary Dutch Clinical Cohort. Eur Urol Focus. 2018;4:228–234. doi: 10.1016/j.euf.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Trottier G, Roobol MJ, Lawrentschuk N, Boström PJ, Fernandes KA, Finelli A, Chadwick K, Evans A, van der Kwast TH, Toi A, Zlotta AR, Fleshner NE. Comparison of risk calculators from the Prostate Cancer Prevention Trial and the European Randomized Study of Screening for Prostate Cancer in a contemporary Canadian cohort. BJU Int. 2011;108:E237–E244. doi: 10.1111/j.1464-410X.2011.10207.x. [DOI] [PubMed] [Google Scholar]
- 15.van Vugt HA, Roobol MJ, Kranse R, Määttänen L, Finne P, Hugosson J, Bangma CH, Schröder FH, Steyerberg EW. Prediction of prostate cancer in unscreened men: external validation of a risk calculator. Eur J Cancer. 2011;47:903–909. doi: 10.1016/j.ejca.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Yoon DK, Park JY, Yoon S, Park MS, Moon du G, Lee JG, Schröder FH. Can the prostate risk calculator based on Western population be applied to Asian population? Prostate. 2012;72:721–729. doi: 10.1002/pros.21475. [DOI] [PubMed] [Google Scholar]
- 17.Thompson IM, Ankerst DP, Chi C, Goodman PJ, Tangen CM, Lucia MS, Feng Z, Parnes HL, Coltman CA Jr. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98:529–534. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 18.Poyet C, Nieboer D, Bhindi B, Kulkarni GS, Wiederkehr C, Wettstein MS, Largo R, Wild P, Sulser T, Hermanns T. Prostate cancer risk prediction using the novel versions of the European Randomised Study for Screening of Prostate Cancer (ERSPC) and Prostate Cancer Prevention Trial (PCPT) risk calculators: independent validation and comparison in a contemporary European cohort. BJU Int. 2016;117:401–408. doi: 10.1111/bju.13314. [DOI] [PubMed] [Google Scholar]
- 19.Ankerst DP, Straubinger J, Selig K, Guerrios L, De Hoedt A, Hernandez J, Liss MA, Leach RJ, Freedland SJ, Kattan MW, Nam R, Haese A, Montorsi F, Boorjian SA, Cooperberg MR, Poyet C, Vertosick E, Vickers AJ. A Contemporary Prostate Biopsy Risk Calculator Based on Multiple Heterogeneous Cohorts. Eur Urol. 2018;74:197–203. doi: 10.1016/j.eururo.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberts AR, Roobol MJ, Verbeek JFM, Schoots IG, Chiu PK, Osses DF, Tijsterman JD, Beerlage HP, Mannaerts CK, Schimmöller L, Albers P, Arsov C. Prediction of High-grade Prostate Cancer Following Multiparametric Magnetic Resonance Imaging: Improving the Rotterdam European Randomized Study of Screening for Prostate Cancer Risk Calculators. Eur Urol. 2019;75:310–318. doi: 10.1016/j.eururo.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 21.Wang NN, Zhou SR, Chen L, Tibshirani R, Fan RE, Ghanouni P, Thong AE, To'o KJ, Ghabili K, Nix JW, Gordetsky JB, Sprenkle P, Rais-Bahrami S, Sonn GA. The stanford prostate cancer calculator: Development and external validation of online nomograms incorporating PIRADS scores to predict clinically significant prostate cancer. Urol Oncol. 2021;39:831.e19–831.e27. doi: 10.1016/j.urolonc.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Patel HD, Koehne EL, Shea SM, Bhanji Y, Gerena M, Gorbonos A, Quek ML, Flanigan RC, Goldberg A, Gupta GN. Risk of prostate cancer for men with prior negative biopsies undergoing magnetic resonance imaging compared with biopsy-naive men: A prospective evaluation of the PLUM cohort. Cancer. 2022;128:75–84. doi: 10.1002/cncr.33875. [DOI] [PubMed] [Google Scholar]
- 23.Kinnaird A, Brisbane W, Kwan L, Priester A, Chuang R, Barsa DE, Delfin M, Sisk A, Margolis D, Felker E, Hu J, Marks LS. A prostate cancer risk calculator: Use of clinical and magnetic resonance imaging data to predict biopsy outcome in North American men. Can Urol Assoc J. 2022;16:E161–E166. doi: 10.5489/cuaj.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chau EM, Russell B, Santaolalla A, van Hemelrijck M, McCracken S, Page T, Liyanage SH, Aning J, Gnanapragasam VJ, Acher P. MRI-based nomogram for the prediction of prostate cancer diagnosis: A multi-centre validated patient-physician decision tool. J Clin Urol. 2022:E–Pub ahead of Print. [Google Scholar]
- 25.van Leeuwen PJ, Hayen A, Thompson JE, Moses D, Shnier R, Böhm M, Abuodha M, Haynes AM, Ting F, Barentsz J, Roobol M, Vass J, Rasiah K, Delprado W, Stricker PD. A multiparametric magnetic resonance imaging-based risk model to determine the risk of significant prostate cancer prior to biopsy. BJU Int. 2017;120:774–781. doi: 10.1111/bju.13814. [DOI] [PubMed] [Google Scholar]
- 26.Mehralivand S, Shih JH, Rais-Bahrami S, Oto A, Bednarova S, Nix JW, Thomas JV, Gordetsky JB, Gaur S, Harmon SA, Siddiqui MM, Merino MJ, Parnes HL, Wood BJ, Pinto PA, Choyke PL, Turkbey B. A Magnetic Resonance Imaging-Based Prediction Model for Prostate Biopsy Risk Stratification. JAMA Oncol. 2018;4:678–685. doi: 10.1001/jamaoncol.2017.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radtke JP, Giganti F, Wiesenfarth M, Stabile A, Marenco J, Orczyk C, Kasivisvanathan V, Nyarangi-Dix JN, Schütz V, Dieffenbacher S, Görtz M, Stenzinger A, Roth W, Freeman A, Punwani S, Bonekamp D, Schlemmer HP, Hohenfellner M, Emberton M, Moore CM. Prediction of significant prostate cancer in biopsy-naïve men: Validation of a novel risk model combining MRI and clinical parameters and comparison to an ERSPC risk calculator and PI-RADS. PLoS One. 2019;14:e0221350. doi: 10.1371/journal.pone.0221350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mannaerts CK, Gayet M, Verbeek JF, Engelbrecht MRW, Savci-Heijink CD, Jager GJ, Gielens MPM, van der Linden H, Beerlage HP, de Reijke TM, Wijkstra H, Roobol MJ. Prostate Cancer Risk Assessment in Biopsy-naïve Patients: The Rotterdam Prostate Cancer Risk Calculator in Multiparametric Magnetic Resonance Imaging-Transrectal Ultrasound (TRUS) Fusion Biopsy and Systematic TRUS Biopsy. Eur Urol Oncol. 2018;1:109–117. doi: 10.1016/j.euo.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Petersmann AL, Remmers S, Klein T, Manava P, Huettenbrink C, Pahernik SA, Distler FA. External validation of two MRI-based risk calculators in prostate cancer diagnosis. World J Urol. 2021;39:4109–4116. doi: 10.1007/s00345-021-03770-x. [DOI] [PubMed] [Google Scholar]
- 30.Ferro M, de Cobelli O, Vartolomei MD, Lucarelli G, Crocetto F, Barone B, Sciarra A, Del Giudice F, Muto M, Maggi M, Carrieri G, Busetto GM, Falagario U, Terracciano D, Cormio L, Musi G, Tataru OS. Prostate Cancer Radiogenomics-From Imaging to Molecular Characterization. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22189971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M, Chen T, Zhao W, Wei C, Li X, Duan S, Ji L, Lu Z, Shen J. Radiomics prediction model for the improved diagnosis of clinically significant prostate cancer on biparametric MRI. Quant Imaging Med Surg. 2020;10:368–379. doi: 10.21037/qims.2019.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deniffel D, Abraham N, Namdar K, Dong X, Salinas E, Milot L, Khalvati F, Haider MA. Using decision curve analysis to benchmark performance of a magnetic resonance imaging-based deep learning model for prostate cancer risk assessment. Eur Radiol. 2020;30:6867–6876. doi: 10.1007/s00330-020-07030-1. [DOI] [PubMed] [Google Scholar]