Abstract
BACKGROUND
Ischemic gastritis is a clinically rare and highly fatal disease that occurs when the hemodynamics of a patient with vascular risk is disrupted. Early diagnosis and treatment are possible only with upper endoscopy after symptom appearance. We report seven cases of ischemic gastritis and its clinical features, prognosis, and indicators that may help in early detection.
CASE SUMMARY
Of the seven patients, six had vascular risk and five died within 2 wk of diagnosis. Their symptoms included hematemesis and hypotension. Although surgery is a choice for radical treatment, not all patients were tolerant. For such patients, conservative treatment was selected, but all of them died. In contrast, patients who underwent repeat endoscopy showed improved mucosal findings, suggesting that this improvement may not affect prognosis. Some ischemic changes such as wall thickening, mural emphysema, and fluid retention in the stomach were observed before diagnosis through endoscopy and computed tomography (CT). The CT scan can be effective for early detection, and improvement in circulatory failure and aggressive treatment may save the lives of patients with this disease.
CONCLUSION
The characteristic CT findings enable early detection of ischemic gastritis. Early diagnosis increases the chance of survival if early therapeutic intervention and improvement of circulatory dynamics can be achieved in this highly fatal disease.
Keywords: Celiac artery, Gastrointestinal bleeding, Ischemic gastritis, Superior mesenteric artery, Vascular risk, Case report
Core Tip: Ischemic gastritis is extremely fatal, especially in those with vascular risk. Early diagnosis and treatment is important. However, it is difficult to diagnose early unless the patient is symptomatic. Here we report seven cases of ischemic gastritis, of whom five patients died within 2 wk of diagnosis. Surgery could not be selected for our patients because of their poor general condition; instead, they were treated conservatively. Patients with improved mucosal findings on repeat endoscopy did not show increased survival. Characteristic computed tomography findings enable early detection of this disease.
INTRODUCTION
Ischemic gastritis is a rare event that occurs in the stomach where blood flow is abundant. It has a low early detection and a high mortality rate[1-9]. The risk of ischemic gastritis increases in older adults and patients with vascular risks, such as renal failure and diabetes mellitus[1]. These risks in combination with the hemodynamic disruption contribute to the high mortality rate[8]. The characteristic symptoms of this disease include abdominal pain and gastrointestinal bleeding[10]. Computed tomography (CT) reveals wall thickening, mural emphysema, and fluid retention, but rarely vascular obstruction[2,11]. Upper gastrointestinal endoscopy reveals multiple ulcers and several ischemic changes[4,6]. Surgical treatment with curative intent is sometimes necessary but is often difficult because of the patient’s poor general condition[2,3,7]. Early diagnosis and treatment of ischemic gastritis are difficult because the condition can only be diagnosed by upper gastrointestinal endoscopy when symptoms appear; therefore, early detection with other modalities is desirable. We encountered seven patients (5 men, 2 women; mean age, 75 years; age range, 53-90 years) who were diagnosed with ischemic gastritis between April 2016 and September 2021, at the Shonan Kamakura General Hospital. of this clinically rare disease and herein report its clinical features, prognosis, and indicators for early detection. The present study examined the baseline clinical and laboratory data, medical history, endoscopic and CT findings, treatment, and outcomes. Ischemic gastritis was defined as the endoscopic finding of ischemic changes, such as multiple ulcers, mucosal edema, hemorrhagic mucosa, and fractured congested mucosa[1,2,5]. The vascular risk was defined as the presence of any of the following: diabetes, dyslipidemia, hypertension, chronic kidney disease, hyperuricemia, heart failure, and any vascular disease[1-3,12].
CASE PRESENTATION
Chief complaints
The characteristics of patients with ischemic gastritis are shown in Tables 1 and 2. There were five men and two women, with a mean age of 75 years (range 53-90 years). All patients were older adults, except for one young woman (case 3), who attempted suicide by hanging. Six patients experienced shock at presentation. Only one patient's systolic blood pressure had dropped below 60 mmHg. The demographic profiles are shown in Table 1.
Table 1.
Demographic and clinical data of seven patients
|
April 2016 to September 2021: n = 7 patients
| |
| Characteristics | |
| Age | Mean age of 75 yr (range 53-90 yr) |
| Sex | Five men, two women |
| Chief complaint | Hematemesis: 7/7 (100%) |
| Shock at presentation | 6/7 (86%) |
| Past medical history | |
| Diabetes | 2/7 (28%) |
| Dyslipidemia | 2/7 (28%) |
| Hypertension | 3/7 (43%) |
| Chronic kidney disease | 3/7 (43%) |
| Hyperuricemia | 1/7 (14%) |
| Heart failure | 2/7 (28%) |
| Any vascular diseases | 3/7 (43%) |
| Smoking | History of smoking: 2/7 (28%); never smoking: 4/7 (57%) |
| Medicine | |
| Anticoagulants (warfarin) | 1/7 (14%) |
| Antiplatelet (aspirin) | 1/7 (14%) |
| Endoscopic findings | |
| Distribution | Stomach: 3/7 (43%); esophagus to duodenum: 4/7 (57%) |
| Second endoscopy | 1/7 (14%) |
| Patients undergoing CT scan before endoscopy | 7/7 (100%) On the day: 3; 2 d before: 1; 3 d before: 1; 9 d before: 1; 14 d before: 1 |
| CT findings | Wall thickening in the stomach: 4/7 (57%); mural emphysema in the stomach: 3/7 (43%) |
| Calcification at the origin of the celiac artery: 2/7 (43%); compression of the celiac artery by the median arcuate ligament: 1/7 (14%) | |
| Operation before illness onset | 4/7 (57%) Splenectomy: 1; Aortic valve replacement: 1; Ascending aorta replacement: 1; Lung cancer operation and superior vena cava repair: 1 |
| Treatment | Conservative treatment: 7/7 (100%) |
| Mechanical assistance | CHDF: 3/7 (43%) |
| Outcome | Death: 7/7 (100%) |
| Time from onset to death | |
| 1-14 d | 5/7 (71%) |
| 15-28 d | 1/7 (14%) |
| -29 d | 1/7 (14%) |
CT: Computed tomography; CHDF: Continuous hemodiafiltration.
Table 2.
Clinical characteristics of each ischemic gastritis patient
|
Case
|
Age
|
Sex
|
Symptoms
|
Shock at presentation
|
Underlying disease
|
Operation before onset
|
Anticoagulants
|
Antiplatelet
|
Treatment
|
Mechanical assistance
|
Surgery contraindications
|
Outcome
|
Time from onset to death (d)
|
Cause of death
|
| 1 | 90 | F | Hematemesis | Yes | CHF, CKD, OCI, diabetes | None | Warfarin | No | Conservative | No | Poor general condition | Death | 28 | CHF |
| 2 | 72 | M | Hematemesis | Yes | AAA, OMI | Splenectomy | No | No | Conservative | No | Poor general condition | Death | 2 | Splenic hemorrhage |
| 3 | 53 | F | Hematemesis | No | Depression | None | No | No | Conservative | No | Poor general condition | Death | 14 | Hypoxic encephalopathy |
| 4 | 84 | M | Hematemesis | Yes | AVS, CKD, CHF, thoracic aneurysm, hypertension | AVR | No | Aspirin | Conservative | CHDF | Poor general condition | Death | 12 | Multi organ failure |
| 5 | 79 | M | Hematemesis | Yes | AAA, hypertension | AAR | No | No | Conservative | CHDF | Poor general condition | Death | 2 | Aspiration pneumoniae |
| 6 | 71 | M | Hematemesis | Yes | IVF, CKD, hypertension, dyslipidemia, diabetes | None | No | No | Conservative | No | Poor general condition | Death | 2 | Myocardiac infarction |
| 7 | 77 | M | Hematemesis | Yes | OMI, dyslipidemia, lung cancer | Lung cancer operation (superior vena cava repair) | No | No | Conservative | CHDF | Poor general condition | Death | 298 | Septic shock |
CHF: Chronic heart failure; CKD: Chronic kidney disease; OCI: Old cerebral infarction; AAA: Abdominal aortic aneurysm; OMI: Old myocardiac infarction; AVS: Aortic valve stenosis; IVF: Idiopathic ventricular fibrillation; AVR: Aortic valve replacement; AAR: Ascending aorta replacement.
History of present illness
Prior to diagnosis, 7 patients were admitted to our institution for medical care for other conditions. Three patients received ventilatory support. One patient had been on warfarin, one on aspirin. The onset of hematemesis prompted urgent workup with endoscopy, leading to the diagnosis of ischemic gastritis. Four of the seven patients (57.1%) had undergone surgery within one week before onset of illness. The surgeries included splenectomy, aortic valve replacement, ascending aorta replacement, and lung cancer surgery with superior vena cava repair. In addition, two of the four patients underwent surgery on the trophic vessels of the stomach (celiac artery and superior mesenteric artery).
History of past illness
The clinical characteristics are shown in Table 2. All of the patients had at least one cardiovascular comorbidity (ranging from hypertension to congestive heart failure). Three patients had (medically controlled) arterial aneurysms, two patients had a history of myocardial infarction. Three patients had diabetes mellitus, and two had dyslipidemia. One patient had a history of cerebrovascular disease, and one had vascular surgery related to malignancy. One patient had a history of depression and suicidal attempt resulting in ischemic encephalopathy.
Personal and family history
The family history was unremarkable.
Physical examination
All patients had hematemesis and hypotension before the onset of ischemic gastritis.
Laboratory examinations
The blood tests showed no specific issue other than progressive anemia due to hematemesis and an enlarged blood urea nitrogen/ creatinine ratio.
Imaging examinations
All patients had at least a CT scan for a different investigation prior to endoscopy. The findings are presented in Table 1.
The characteristics of endoscopic and CT findings are presented in Table 3. All seven patients underwent CT scans before endoscopy, which showed wall thickening, intramural gas appearance, and peri-gastric fluid collection (Figure 1). These findings can indicate ischemic changes in the stomach layers. In four cases, these findings were observed several days before the onset of symptoms such as hematemesis. Upper gastrointestinal endoscopy revealed ischemic changes (Figure 2), which were observed only in the stomach in three patients and between the esophagus and the duodenum in the remaining four patients. In addition, vascular calcification was observed in three of the seven cases; calcification was observed at the origin of the celiac artery, and one case had compression of the celiac artery by the median arcuate ligament. Invasive treatment was difficult due to the patients’ general condition, and conservative treatment with gastric mucosal protective agents or proton pump inhibitor administration and fasting fluids was chosen in all cases.
Table 3.
Endoscopic/computed tomography findings and treatment in each ischemic gastritis patient
| Case |
Endoscopic findings
|
Endoscopic distribution | Date of CT scan from endoscopy | CT findings | |||
|
Longitudinal ulcers
|
Irregular multiple ulcers
|
Mucosal edema with redness and erosion
|
Hemorrhage
|
||||
| 1 | Yes | Yes | Yes | No | Esophagus to duodenum | On the day | Dilatation and edematous thickening of the wall of duodenum and ileum. Calcification at the origin of the celiac artery |
| 2 | Yes | Yes | Yes | Yes | Stomach | On the day | Hematoma around the spleen |
| 3 | Yes | Yes | Yes | Yes | Stomach | On the day | Fluid accumulation from the stomach to the large intestine. Compression of the celiac artery by the median arcuate ligament |
| 4 | Yes | Yes | Yes | Yes | Esophagus to duodenum | 3 d | Wall thickening and mural emphysema and fluid retention in the stomach |
| 5 | Yes | Yes | Yes | Yes | Esophagus to duodenum | 9 d | Wall thickening in the stomach |
| 6 | Yes | Yes | Yes | Yes | Esophagus to duodenum | 2 d | Wall thickening and mural emphysema and fluid retention in the stomach. Calcification at the origin of the celiac artery |
| 7 | Yes | Yes | Yes | No | Stomach | 14 d | Wall thickening and mural emphysema and fluid retention in the stomach |
CT: Computed tomography.
Figure 1.
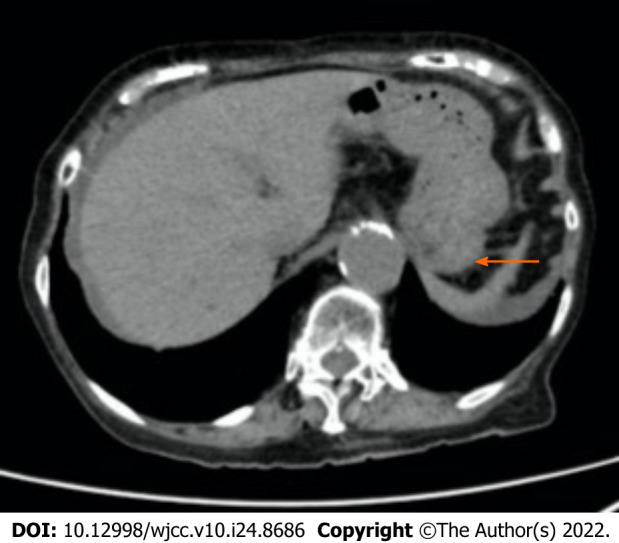
Abdominal plane computed tomography scans obtained 14 d before the onset of ischemic gastritis in case 1. Computed tomography revealed wall thickening, mural emphysema, and fluid retention in the stomach. The arrow shows the wall thickening. The arrowhead indicates the mural emphysema.
Figure 2.
Upper gastrointestinal endoscopy images performed on hospital day 2 of case 1. A: It showed longitudinal ulcers, multiple irregular ulcers, mucosal edema with redness, erosion, and hemorrhage in the stomach; B: In the duodenum, it showed longitudinal ulcers, multiple irregular ulcers, mucosal edema with redness, erosion, and hemorrhage.
Further diagnostic work-up
A notable improvement in the gastric mucosa was observed only in one patient (case 1), who underwent repeat endoscopy, (Figure 3). However, CT findings did not improve despite endoscopic mucosal improvement (Figure 4). Continuous hemodiafiltration was used in three cases to maintain circulatory dynamics; however, two patients died within 2 wk. Five of the seven patients (71.4%) died within 2 wk of onset of ischemic gastritis, and the others eventually died from their respective primary disease. The diagnosis is usually based on pathological findings; however, it is difficult to examine the histology in the presence of hematemesis and hypotension. In the single case when the patient's general condition improved and the endoscopy was repeated, the mucosal findings were improved, and the histological examination did not reveal any signs suggestive of ischemia. Postmortem autopsy could not be performed without family consent. Therefore, the diagnosis of ischemic gastritis was made based on physical examination and imaging tests.
Figure 3.
Upper gastrointestinal endoscopy images on hospital day 16 of case 1. A: It revealed improved mucosal findings in the stomach; B: It revealed improved mucosal findings in the duodenum.
Figure 4.
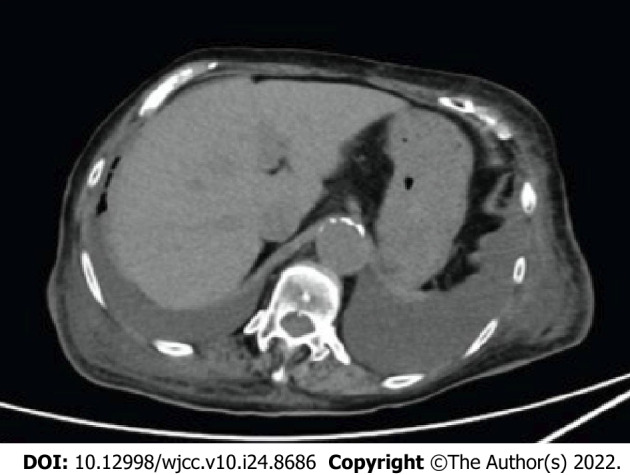
Abdominal plane computed tomography scans obtained after improvement of endoscopic findings. It revealed persistent wall thickening and mural edema and significant bilateral pleural effusion.
FINAL DIAGNOSIS
The patients were diagnosed with ischemic gastritis after endoscopy.
TREATMENT
The general condition of the patients was poor and surgical treatment was not feasible, so conservative treatment was chosen in all cases.
OUTCOME AND FOLLOW-UP
All seven patients died. However, the immediate cause of death was different in each case. The median time from the onset of symptoms of ischemic gastritis to death was 12 d (range 2-298 d).
Case 1 underwent repeat endoscopy and a notable improvement in the gastric mucosa was observed (Figure 3). However, CT findings did not improve despite endoscopic mucosal improvement (Figure 4). The patient’s abdominal symptoms gradually improved, and she was able to eat; nevertheless her circulatory dynamics were not stable, and she eventually died. Case 7, a long-term survivor, suffered vascular injury during lung cancer surgery. Postoperatively, the patient developed renal failure, and although continuous hemodiafiltration (CHDF) was introduced, his general condition did not improve, and he suffered brain death. Although he survived for a relatively long period of time, he eventually died of septic shock. All three cases, in which CHDF was introduced, underwent surgical vascular operations, and were treated for postoperative renal failure.
DISCUSSION
We reported seven cases of ischemic gastritis with a poor prognosis, five of whom died within 2 wk. Since the stomach has an abundant blood flow, it is not prone to ischemia, and not many cases of ischemic gastritis have been reported[1-9]. The gastric blood flow is provided mainly from the celiac trunk, superior mesenteric artery, and some collateral arteries. Furthermore, the stomach has an abundant submucosal vascular plexus, which is more resistant to ischemia than that of the small and large intestines[1].
The causes of ischemic gastritis can be divided into two groups: vascular and intestinal factors. Vascular factors include major arterial and venous occlusions, small vessel lesions, systemic hypoperfusion, shock, and sepsis[1,12]. Iatrogenic cessation of blood supply involves chemoembolization or surgical ligation[2,3], and polyarteritis nodosa, leukocytoclastic vasculitis, and portal hypertension have also been reported[4]. Most of our cases did not involve direct invasion by feeding vessels. The surgical vascular invasion itself may affect hemodynamics, even if the gastric feeding vessels are not directly manipulated. On the other hand, intestinal factors include increased intragastric pressure[3]. Decreased gastric motility due to aging or diabetes can cause gastric distention and increased intragastric pressure, which can lead to disease development[13,14].
In addition to recovery with conservative treatment, some cases also recover with invasive treatment including total gastrectomy[1,5,7,12] or vascular reconstruction by endovascular therapy[9]. Since most patients are in poor general condition, surgical indications need to be carefully examined. In some cases, early death occurs despite improvement in gastric mucosal findings, as in case 3[1,15,16]. Ischemic gastritis is considered as one of the phenotypes associated with impaired systemic blood flow; impaired systemic blood flow occurs in patients with systemic conditions or comorbidities that lead to ischemia of the stomach. Improvements in endoscopic findings may not be directly related to the prognosis. In contrast, CT contributed to the diagnosis of our cases. Wall thickening, mural emphysema, and fluid retention were observed in the stomach, indicating ischemic changes; these were observed on CT scan a few days before symptoms such as hematemesis appeared and persisted even after mucosal recovery on endoscopy, which was considered useful for early diagnosis and subsequent follow-up. As ischemic gastritis is associated with severe systemic arteriosclerosis, improvement of the gastric mucosal surface may not lead to an improved prognosis. In some cases, surgery or endovascular therapy saves the patient’s life, and a CT scan may be able to improve prognosis with early diagnosis and intervention[1,7,9].
CONCLUSION
Herein, we report seven cases of ischemic gastritis, which was diagnosed with upper gastrointestinal endoscopy. However, we revealed that improvement in endoscopic findings may not affect prognosis. Although characteristic CT findings enable early detection of this disease, at the stage when symptoms such as hematemesis appear, the patient's general condition is often poor, making aggressive therapeutic intervention difficult. Early diagnosis increases the chance of survival if early therapeutic intervention and improvement of circulatory dynamics can be achieved in this highly fatal disease.
ACKNOWLEDGEMENTS
The authors wish to thank Ms. Fujii M for her excellent technical and secretarial assistance.
Footnotes
Informed consent statement: Informed consent was waived by the Institutional Review Board of the hospitals.
Conflict-of-interest statement: The authors declare no conflict or competing interests.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: February 21, 2022
First decision: April 5, 2022
Article in press: July 16, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Lankarani KB, Iran; Chen CH, United States; Pan Y, China S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
Contributor Information
Kento Shionoya, Gastroenterology Medicine Center, Shonan Kamakura General Hospital, Kamakura 247-8533, Kanagawa, Japan. k_shionoya@shonankamakura.or.jp.
Akiko Sasaki, Gastroenterology Medicine Center, Shonan Kamakura General Hospital, Kamakura 247-8533, Kanagawa, Japan.
Hidekazu Moriya, Department of General Internal Medicine, Shonan Kamakura General Hospital, Kamakura 247-8533, Kanagawa, Japan.
Karen Kimura, Gastroenterology Medicine Center, Shonan Kamakura General Hospital, Kamakura 247-8533, Kanagawa, Japan.
Takashi Nishino, Gastroenterology Medicine Center, Shonan Kamakura General Hospital, Kamakura 247-8533, Kanagawa, Japan.
Jun Kubota, Gastroenterology Medicine Center, Shonan Kamakura General Hospital, Kamakura 247-8533, Kanagawa, Japan.
Chihiro Sumida, Gastroenterology Medicine Center, Shonan Kamakura General Hospital, Kamakura 247-8533, Kanagawa, Japan.
Junichi Tasaki, Gastroenterology Medicine Center, Shonan Kamakura General Hospital, Kamakura 247-8533, Kanagawa, Japan.
Chikamasa Ichita, Gastroenterology Medicine Center, Shonan Kamakura General Hospital, Kamakura 247-8533, Kanagawa, Japan.
Makomo Makazu, Gastroenterology Medicine Center, Shonan Kamakura General Hospital, Kamakura 247-8533, Kanagawa, Japan.
Sakue Masuda, Gastroenterology Medicine Center, Shonan Kamakura General Hospital, Kamakura 247-8533, Kanagawa, Japan.
Kazuya Koizumi, Gastroenterology Medicine Center, Shonan Kamakura General Hospital, Kamakura 247-8533, Kanagawa, Japan.
Jun Kawachi, Department of General Surgery, Shonan Kamakura General Hospital, Kamakura 247-8533, Kanagawa, Japan.
Toshitaka Tsukiyama, Department of Radiology, Shonan Kamakura General Hospital, Kamakura 247-8533, Japan.
Makoto Kako, Gastroenterology Medicine Center, Shonan Kamakura General Hospital, Kamakura 247-8533, Kanagawa, Japan.
References
- 1.Tomishima K, Sato S, Amano N, Murata A, Tsuzura H, Kanemitsu Y, Shimada Y, Iijima K, Genda T, Wada R, Nagahara A. A case of ischemic gastroduodenal disease in a patient who was receiving hemodialysis treatment that was managed by conservative treatment. Clin J Gastroenterol. 2018;11:386–390. doi: 10.1007/s12328-018-0865-1. [DOI] [PubMed] [Google Scholar]
- 2.Herman J, Chavalitdhamrong D, Jensen DM, Cortina G, Manuyakorn A, Jutabha R. The significance of gastric and duodenal histological ischemia reported on endoscopic biopsy. Endoscopy. 2011;43:365–368. doi: 10.1055/s-0030-1256040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trowell JE, Bell GD. Biopsy specimen appearances of ischaemic gastritis in splanchnic arterial insufficiency. J Clin Pathol. 1998;51:255–256. doi: 10.1136/jcp.51.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishino H, Takano S, Yoshitomi H, Furukawa K, Takayashiki T, Kuboki S, Suzuki D, Sakai N, Kagawa S, Nojima H, Sasaki K, Miyazaki M, Ohtsuka M. Ischemic gastropathy after distal pancreatectomy with en bloc celiac axis resection versus distal pancreatectomy for pancreatic body/tail cancer. Surg Open Sci. 2019;1:14–19. doi: 10.1016/j.sopen.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muramatsu S, Kawabe K, Yamada T, Nitta H, Ishikawa F, Yamashita S, Suwa T, Chishima J, Kusano M. A case of ischemic gastropathy. Prog Dig Endosc. 2008;72:56–57. [Google Scholar]
- 6.Tang SJ, Daram SR, Wu R, Bhaijee F. Pathogenesis, diagnosis, and management of gastric ischemia. Clin Gastroenterol Hepatol. 2014;12:246–52.e1. doi: 10.1016/j.cgh.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 7.Richieri JP, Pol B, Payan MJ. Acute necrotizing ischemic gastritis: clinical, endoscopic and histopathologic aspects. Gastrointest Endosc. 1998;48:210–212. doi: 10.1016/s0016-5107(98)70168-3. [DOI] [PubMed] [Google Scholar]
- 8.Quentin V, Dib N, Thouveny F, L'Hoste P, Croue A, Boyer J. Chronic ischemic gastritis: case report of a difficult diagnosis and review of the literature. Endoscopy. 2006;38:529–532. doi: 10.1055/s-2006-925228. [DOI] [PubMed] [Google Scholar]
- 9.Anucha J, Pinto J, Culpepper-Morgan J, Genao A, Resnick N. Ischemic Gastropathy Treated with Celiac Artery Revascularization. Cureus. 2019;11:e5949. doi: 10.7759/cureus.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakker RC, Brandjes DP, Snel P, Lawson JA, Lindeman J, Batchelor D. Malabsorption syndrome associated with ulceration of the stomach and small bowel caused by chronic intestinal ischemia in a patient with hyperhomocysteinemia. Mayo Clin Proc. 1997;72:546–550. doi: 10.4065/72.6.546. [DOI] [PubMed] [Google Scholar]
- 11.Guniganti P, Bradenham CH, Raptis C, Menias CO, Mellnick VM. CT of Gastric Emergencies. Radiographics. 2015;35:1909–1921. doi: 10.1148/rg.2015150062. [DOI] [PubMed] [Google Scholar]
- 12.Ukegawa J, Kamisago S, Takahashi S, Ishikawa J, and Takagi A. A case of ischemic duodenitis. Endosc Forum Dig Dis. 2001;17:21–27. [Google Scholar]
- 13.Wada K, Kamisaki Y, Kitano M, Kishimoto Y, Nakamoto K, Itoh T. A new gastric ulcer model induced by ischemia-reperfusion in the rat: role of leukocytes on ulceration in rat stomach. Life Sci. 1996;59:PL295–PL301. doi: 10.1016/0024-3205(96)00500-0. [DOI] [PubMed] [Google Scholar]
- 14.Varhaug JE, Svanes K, Lysen LJ, Holm P. The effect of intragastric pressure on gastric blood flow after partial devascularization of the stomach in cats. Eur Surg Res. 1980;12:415–427. doi: 10.1159/000128149. [DOI] [PubMed] [Google Scholar]
- 15.Højgaard L, Krag E. Chronic ischemic gastritis reversed after revascularization operation. Gastroenterology. 1987;92:226–228. doi: 10.1016/0016-5085(87)90864-x. [DOI] [PubMed] [Google Scholar]
- 16.de Widt-Levert LM, Nelis GF, Jörning PJ. Dyspepsia as initial symptom of splanchnic vascular insufficiency. Eur J Gastroenterol Hepatol. 1996;8:815–818. [PubMed] [Google Scholar]




