Abstract
Stem cells are undifferentiated cells showcasing a remarkable capacity of self-replenishing and differentiating into mature cells. Their ability to proliferate connotes that a designated stem cell source is capable of generating an unrestricted number of mature cells. The ever-increasing comprehension of position, activity, and function of ocular stem cells has led to rapid progress and incessant improvement of possible procedures and therapies. A narrative review was conducted to summarize the current evidence on clinical trials and respective literature, regarding current evolution in the field of ocular regenerative medicine. We tried to ascertain the safety of experimental and clinical procedures, their effectiveness, and the ethical repercussion of their use.
Keywords: stem cells, cell-based therapies, limbal stem cells, hESC-derived retinal pigment epithelium, human induced pluripotent stem cells, olfactory mucosa mesenchymal stem cells
INTRODUCTION
According to the World Health Organization (WHO), globally, at least 2.2 billion people have vision impairment[1]. In almost half of these cases, vision impairment could have been prevented or has yet to be addressed. More than 400 million people are visually impaired due to age-related macular degeneration (AMD), glaucoma, diabetic retinopathy, Stargardt disease, and retinitis pigmentosa[1]. People suffering from these visual disorders are destined to increase dramatically in the next decades, due to population growth and aging. One of the most promising and hopeful potentials for future treatment is stem cell therapy.
Stem cells are the future of cell-based therapies due to their property to regenerate. Stem cell therapy is considered the treatment option of the 21st Century for many health conditions such as hematological disorders, Parkinson's disease, diabetes, and myocardial failure[2]. Nowadays the eye has been verified as an ideal target of stem cell-based therapies and the perfect ascertainment field of their efficacy and safety. This is due to the facility of administration and effects evaluation, using state of the art ophthalmic devices and machinery[3].
This review aims to summarize scientific evidence of the present and future prospects of stem cells for ocular therapy.
Types of Stem Cells
Stem cells, based on their origin, have been classified into embryonic stem cells (ESCs) and adult stem cells. These cells are characterized by their property of self-renewal, under appropriate conditions, and by their additional ability to differentiate into the originating tissues. The stem cells, because of their pluripotency, bear a huge potential for therapeutic use in numerous pathologies. ESCs are pluripotent, while adult stem cells are multipotent. However, the current technological advances in science have achieved to induce pluripotency to adult stem cells.
Pluripotent stem cells have the ability to evolve to any cell type[4]. These can be classified into embryonic[5], perinatal or induced pluripotent stem cells (iPSCs), based on the tissue of origin. Human induced pluripotent stem cells (hiPSCs) are derived from somatic cells exposed to reverting pattern techniques of gene expression that eventually undergo reprogramming to different specialized cell types[6]. The capability to generate hiPSCs using a patient's own cells originated a brand-new field of patient-specific therapies, always keeping in mind that undifferentiated iPSCs showcase a potential risk of tumorigenesis to the recipient.
Furthermore, stem cells can be autologous or allogeneic. When the source is a donor tissue, additional factors must be considered regardless of whether stem cells are tissue-specific or pluripotent. These include infectious agents screening, and immunosuppression due to transplant rejection threatening[7]. Another factor to be considered is the arithmetic adequacy of harvested material without affecting the functionality of donor ocular tissues. The objective is to ensure safe and functional stem cell engraftment[8], by delivering sufficient cell numbers and trying to eliminate oncogenic mutations. Moreover, it is extremely important to provide correct differentiation pathways to obtain the covetable cell type at the requested level of development.
Stem Cell Treatment in Ophthalmic Disorders
Stem cells are currently being trialed in a variety of ocular conditions and diseases. Sheets of corneal-limbal stem cells cultivated on fibrin matrices[9] are successfully employed in ocular surface impairment due to limbal cell loss[10]–[11]. The potential role and contingent success of stem cells are also currently trialed in AMD and inherited retinal diseases, being currently in phase I and II, for which safety has already been proven[12].
Ocular Surface Clinical Trials
Corneal epithelium is replenished by limbal epithelial stem cells (LESCs) located in the Vogt palisades. Any disorganization of this microenvironment can cause limbal stem cell deficiency (LSCD)[13]. This devastating condition is characterized by unstable corneal epithelium and persistent epithelial defects, leading to chronic pain and progressive visual loss. Very often this condition does not respond to penetrating keratoplasty, and in these cases, limbal stem cell transplantation is used to achieve corneal epithelial regeneration[14].
To avoid LSCD in the donor's eye, specific methods are being developed to achieve ex vivo expansion of donor's stem cells[11]. By using this approach, various substrates are utilized, such as the human amniotic membrane[15]–[16]. This substrate, unfortunately, showcases limited availability, high screening costs, and is susceptible to folding and creasing during transplantation. As an alternative fibrin matrix can be used, which exhibit high success rates at ten-year follow up[17]. Another alternative is the use of a polymer that doesn't necessitate complex procedures as a substrate[18]. Moreover, silicone hydrogel contact lenses can be used as a carrier substrate, which is highly approachable and very cost-effective[19]. Recently other substrates are also considered, such as anterior lens capsules, collagen, and synthetic polymers. These alternatives are currently under investigation and validation to determine their sustainability and effectiveness[20]. Pre-clinical models and human clinical trials are investigating the possibility of alternative tissue sources, such as allogeneic conjunctival cells, and autologous nasal and buccal mucosa. Despite the lack of specific markers for the quantification of stem cells in donor cultures, it was initially thought that corneal clarity could be restored by simply replacing lost LESCs. Nevertheless, the stem cell replacement mechanism has been recently addressed[21]. Follow-up studies, demonstrated the absence of donor's cells detection, 9mo after transplantation[22]. These results firstly indicate that long-term immunosuppression is unnecessary and secondly that the replacement theory is not the underlying mechanism. It is more likely that reconstruction of defective limbal epithelial crypts occurs by providing a microenvironment suitable for the resettlement of endogenous stem cells.
Clinical trials based on ocular surface stability and good vision indicated positive outcomes for ex vivo expansion of autologous LESCs to treat LSCD[23]. These trials showed differences in follow-up periods, some of them reporting results of more than 2y after transplantation. Considering the transparency of corneal epithelium, scarcity of conjunctivalization and vascularization, as well as best-corrected visual acuity, the overall success rate was more than 80%[24].
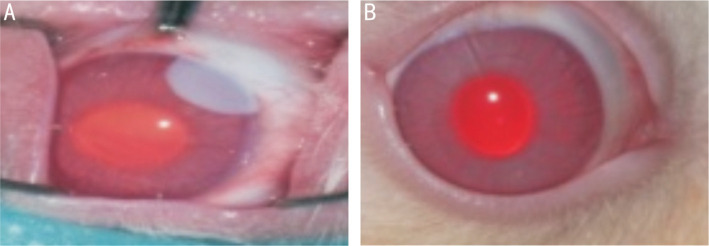
Sangwan et al[25] investigated the results of these procedures on the ocular surface of donor eyes. Following autologous limbal biopsy procedures, all donor sites showed complete healing as well as total absence of ocular surface disease and impairment, within a maximum of two weeks. In a retrospective clinical trial by Fasolo et al[26] on cases treated with autologous LESCs transplantation, indicated that 60% showed adverse events including inflammation and corneal epithelial defects. Nevertheless, these results were debatable given the relatively short duration of follow-up. On the other hand, Rama et al[17] performed clinical trials οn 113 eyes that had suffered chemical or thermal burns with resultant LSCD, and were consequently treated with stem cell transplantation via a fibrin carrier substrate. The results reported no persevering adverse effects during a decade follow-up (Figure 1). Meanwhile, using a contact lens (CL) as a carrier substrate for LSCT may be complicated by the advent of CL displacement, that necessitates the use of a supplemental CL which is a minor impediment[27].
Figure 1. Macroscopic picture of rabbit cornea after chemical (alkali) corneal injury. Photographic images were immediately after the creation of chemical burn and at 28-day post treatment.
A: Rabbit cornea is shown immediately after the creation of chemical (alkali) burn; B: 28-day follow-up: the same cornea after therapeutic use of mesenchymal stem cells (MSCs), showing excellent tissue repair.
Over the last decades several treatment options have been developed to manage patients with LSCD[10]. In 2015, Holoclar, Italy (manufactured by Holostem Terapie Avanzate S.R.L. in Italy, and originally developed by Professors Graziella Pellegrini and Michele De Luca) is an advanced therapy medicinal product (ATMP) and became the first stem cell-based treatment registered and approved by a Regulatory Body[10],[28]. Whilst not yet available in the USA, its use in National Health Service in the UK is approved and strictly regulated based upon retrospective data[29]. Holoclar is a cell-based tissue engineered therapy and uses the patient's own LESCs (autologous) from the unaffected eye to form a graft grown outside the body (ex vivo) for the treatment of LSCD. Another interesting application of cell-based treatment is the use of autologous mesenchymal stem cells (MSCs), isolated from limbal biopsies to treat cases of corneal stromal scarring thus reducing the need for corneal grafts LESCs[30].
During the past 20y, significant progress has been made in the treatment of corneal endothelium dysfunction. The global shortage of donor corneas as well as corneal graft rejection and failure remain important limiting factors to corneal transplant surgery. Consequently, novel treatments have been developed including endothelial keratoplasty techniques as well as the recently introduced promising approaches of cell-based therapies and regenerative medicine[31]–[32]. Cell-based therapies include the in vitro cultivation of human corneal endothelial cells (CECs) obtained either from the patient's own endothelium or from donor corneas. The cultured CECs can subsequently be transferred to recipients' diseased corneas. Rho-associated kinases (ROCK) inhibitors have also been used in clinical use, both as eye drops and as adjunct drugs in cell-based therapies, for the treatment of corneal endothelium dysfunction, as they enhance cell proliferation as well as the engraftment of CECs onto recipient corneas. Finally, recently introduced surgical techniques in regenerative medicine, for the treatment of corneal endothelial diseases, include descemetorrhexis without endothelial keratoplasty (DWEK)/Descemet's stripping only (DSO) and Descemet's membrane transplantation (DMT). Despite the theoretical advantages of these techniques, larger randomized comparative studies with long-term follow-up are required to establish their effectiveness.
Presbyopia and Cataract
Over a billion people worldwide, practically people aged 40y and older, are affected by presbyopia[33]. Until today, the only efficacious treatment of presbyopia consists of non-surgical refractive correction. More than 100 million people suffer from vision impairment or even blindness due to cataracts. The reduction of lens translucency caused by cataract and consecutive loss of visual clarity and sharpness can only be treated via surgical replacement of the cataractous lens, making cataract surgery the most common ophthalmic procedure worldwide[34].
The anterior lens surface is lined by a single layer of epithelial cells, which is located between the lens capsule and the lens fibers. The epithelial cells show metabolic properties that sustain the physiological health of the lens, and work as a source of stem cells. These cells are liable for lens growth throughout life. In cases of incomplete removal during cataract surgery, they often migrate to the posterior lens area causing posterior capsular bag opacification as a result of fibrotic changes they incur in. So far, the only treatment for this condition is posterior laser capsulotomy, resulting to be the second most common ophthalmic proceeding worldwide[35]. Nevertheless, these residual cells can occasionally regenerate lens fiber at various stages of transparency, around the implanted intraocular lens. They may form globular masses of epithelial and cell material known as Elschnig pearls[36]. In addition, if residual lens epithelial cells are ensnared between anterior and posterior lens capsules, subsequent proliferation and differentiation may occur generating lens-like material involving cells and fiber-like material[37]. Lin et al[38] reported a ground-breaking pediatric cataract surgery that aimed to use this postoperative complication as an alternative therapy for children aged under 24mo. This novel surgical method of cataract removal preserves endogenous lens cells, thus enabling functional crystalline lens regeneration. This ground-breaking pediatric cataract surgery is still in early stages of evaluation and requires additional study to address possible complications such as cataract reoccurrence due to underlying genetic defects and the risk of developing amblyopia, impaired vision due to inappropriate visual stimulation during the months required for lens regeneration.
Clinical trials have determined that transplantable lens stem cells provided by human embryonic stem cells (hESCs) and hiPSCs can generate lens-like organoids of various sizes and scalable clarity[39]. Several investigators have tried to define simple and practicable methods of producing lens epithelial cells using hiPSCs[40]–[41]. These cells are similar to human fetal lens epithelial cells, and, in vitro, are capable of generating light-focusing micro-lenses. Using the appropriate signaling pathways able to induce functional lens regeneration, we could pass from in vitro models to in vivo applications. The micro-lenses bear the potential to reduce the personal, social and economic consequences of cataract as they can be used to model individual or combined cataract risk factors as well as assist with in vitro drug development and toxicity assays.
Retinal Diseases
AMD is the most common cause of blindness that usually affects people aged 55y and older. Additionally, inherited retinal diseases, such as Stargardt's macular dystrophy and retinitis pigmentosa, have a noteworthy impact on the quality of life[42]. They are characterized by loss of photoreceptors, neurons, retinal pigment epithelium (RPE), and choroidal alterations. For these conditions treatment options are very limited or nonexistent, resulting in irreversible vision loss.
In AMD, RPE pathology constitutes the first clinically detectable sign, followed by photoreceptor loss and permanent impairment of visual function. In the past decades, several trials examined the conduct of the subretinal space by trying to transplant allogeneic RPE cells. Nevertheless, the results were frustrating due to the rejection mechanisms of these donor cells[43]. Currently, new clinical trials are considering the eventuality of using autologous RPE cells by accomplishing macular translocation, and RPE choroid patch graft transplantation.
Macular translocation consists of detaching and rotating the retina to reposition the macula over a healthy area of the RPE. A retrospective study by Takeuchi et al[44], on macular translocation for AMD and myopic choroidal neovascularization proved its effectiveness by demonstrating visual acuity preservation for more than 5y post-surgery.
On the other hand, RPE-choroid patch graft transplantation consists of harvesting full-thickness RPE-choroid patches from other regions of the patient's retina and transplanting them under the impaired macula[45]. Relevant clinical trials results demonstrated visual acuity preservation for more than 3y post-transplantation[46]. Unfortunately, these surgical procedures demonstrated high technical complexity and were also correlated with high risk of retinal detachment, which impeded its clinical implementation and improvement[47].
Clinical trials concerning macular translocation and RPE-choroid patch graft transplantation have established the efficacy of RPE replacement in AMD. The possibility of providing the macula with healthy RPE and fresh underlying choroid is very promising and should be taken into account in future clinical trials[48]. New techniques are being developed to manufacture RPE replacement patches in the laboratory. Sharma et al[49] developed a treatment for macular degeneration that used RPE cells derived from a patient's own healthy cells. They also designed a scaffold to support healthy RPE cells and promote their attachment inside the eye, in order to overcome the risk of retinal detachments carried by surgically harvesting autologous RPE-choroid patch grafts.
Regenerative patch technologies (RPT) is developing a stem cell-based implant technology. The CPCB-RPE1 implant consists of stem cell-derived RPE cells on an ultrathin synthetic parylene membrane creating a 6.25×3.5 mm2 patch. The implant is designed to replace the RPE and Bruch's membrane in AMD disease. The latest results from RPT's Phase 1/2a clinical trial [National Library of Medicine (NLM), NCT02590692][50] demonstrate the safety and tolerability of the implant along with the providing signals of sight-improving activity. The data positions RPT to proceed to a Phase 2b clinical trial. On the other hand, the London Project to Cure Blindness has developed an hESC-derived RPE sheet grown on a biostable polyethylene terephthalate (PET) membrane, creating a 6×3 mm2 patch. Both types of patches are delivered after a pars plana vitrectomy and a small retinotomy over the macula region[51].
Using Retinal Organoids
Retinal organoids are derived from hiPSCs constituting autologous tissues organized in 3D structures in vitro, which structurally and functionally mimic the retina[52]. In the last decade they have assisted enormously in studying several retinal diseases, that were difficult to reproduce in vitro or in rodent models. The mutation of CEP290, a primary ciliary protein, was studied in retinal organoids derived from CEP290-mutated Leber congenital amaurosis, to understand the inner mechanisms of these ciliopathies[53]–[54]. In addition retinal organoids are extremely useful in the study of several retinitis pigmentosa mutations, such as TRNT1 mutation[55], and Stargardt's disease[56]. Furthermore, retinal organoids are being used to detect the protective effects of ophthalmic supplements, such as 4-hydroxytamoxifen and diethylstilbestrol[57], and to evaluate the pharmacological effects of moxifloxacin which is retinotoxic at high doses[58]. They also emerged to be precious photoreceptor source for cell transplantation therapies, such as AMD and Stargardt's disease[59]–[60].
Human Embryonic Stem Cells, Human Induced Pluripotent Stem Cells, and Photoreceptors
Various clinical trials ascertained that transplantation of hESC-derived RPE resulted to reduced photoreceptors degeneration[21], but the safety and tolerability of this procedure had to be evaluated. Schwartz et al[12],[61] conducted two phase I/II studies involving patients with geographic atrophy in dry AMD and Stargardt disease. The 2-year results[10] were very promising with no deployment of uncontrolled proliferation, adverse reactions, or rejection. An assessment at 4y demonstrated that it is possible to safely implant hESC-RPE in order to attempt to rescue photoreceptors and visual function[61]. Patients experienced sustained improvement in visual acuity and no adverse events were noted. Indeed, there was no tumorigenicity or rejection-related inflammation. These results indicated the way to studies involving new strategies for RPE transplantation, such as sheets of cells with or without scaffolding to mimic Bruch's membrane.
Albeit hESC-RPE graft was safely tolerated, uncertainty was raised concerning the procedures with allogeneic cell grafts. Immunosuppression required in this case, caused inadmissible systemic side-effects forcing Song et al[62] to retract it. Given the unpropitious immunosuppression effects and the failure of clinical trials using cultured fetal allogeneic RPE, effectiveness on human patients is yet to be confirmed.
Ocata Therapeutics (formerly known as Advanced Cell Techonology) performed clinical trials using transplantation of hESC-derived RPE cells for Stargardt's disease and AMD[60],[63]. Since ESC-derived RPE cell transplantation is an allograft transplantation, this clinical trial was combined with immunosuppressive drug administration for 3mo. One patient developed post-surgery bacterial endophthalmitis, which was relieved after 2mo with the infusion of antibiotics and eye drops. However, the causative organism was not detected in the transplanted cells. Other reported systemic adverse events were due to the combined use of immunosuppressive drugs[64]–[65]. No abnormal proliferation or inflammatory reaction of the transplanted cells was observed. The results of transplantation revealed an increased pigmentation at the RPE cell transplantation site, which was thought to be due to the proliferation of RPE cells at the transplantation site[12].
To avoid the impediment of immunosuppression, clinical trials are directed to the use of autologous hiPSCs-derived RPE. These cells showcased their ability to restore retinal function when grafted into the sub-retinal space[66], without being mutagenic[67]. In addition, clinical trials provided evidence that the photoreceptor progenitors have the ability to ingrate into the dysfunctional murine and swine retina and partially restore visual function[68]–[69]. Nevertheless, the oncogenic likelihood of this procedure remains to be clarified as well as the necessity to be accompanied by supportive strategies of RPE and retinal neurons, to ensure their long-term graft survival.
Olfactory Mucosa Mesenchymal Stem Cells and Photoreceptors
MSCs primarily originate from the human bone marrow. Clinical studies showcased that bone mesenchymal stem cells (BMSCs) are capable to differentiate into retinal neurons under appropriate induction[70]–[71]. Nevertheless, BMSCs' quantity and ability are susceptible to multiple factors as donor's gender, age, and radiation therapy history. The application of BMSCs in clinical practice is also hindered by the intrusiveness and inconvenience of bone marrow aspiration.
However, olfactory mucosa mesenchymal stem cells (OM-MSCs) not only behave in a similar way to BMSCs with regard to pluripotent abilities, but also demonstrate convenient isolation from the nasal lamina propria as well as high proliferation rate and safety[72]. Additional advantages of OM-MSCs include the ability of autologous transplants to avoid immune rejection as well as lack of ethical, moral and societal issues and the absence of gene mutations[72].
Lu et al[73] showed that by using epidermal growth factor (EGF), taurine, and retinoic acid as inducing reagents, OM-MSCs can be induced to differentiate into photoreceptor cells in vitro as well as express a photoreceptor-specific cell marker. The application of OM-MSCs in developing neuron progenitor cells could be a new approach for novel therapies of retinal degenerative diseases.
Furthermore, Dai et al[74] studied the use of olfactory mucosal cells (OMCs) in rats showcasing elevated intraocular pressure (IOP). Their trials demonstrated that transplantation of OMCs into the optic disc region, reduced to 23% the loss of retinal ganglion cells (RGC) axons at the optic nerve head (ONH). In addition, the pattern electroretinograms (PERG) determined that the decrement of the voltage amplitudes in association with the raised IOP was significantly alleviated. Transplantation of OMCs not only is capable to diminish the loss of RGC axons induced by raised IOP in rats, but also to protect the functional activities of the axons. These findings provide a basis for future human clinical trials. Autografting OMCs from autologous nasal epithelial biopsies could treat or delay glaucoma diseases.
CONCLUSION
Stem cell therapy has become a practical reality and holds a great potential in improving the quality of life of visually impaired people. Current and continuing research increases the possibilities of stem cells applications and carries a promising future in producing novel biological therapies to treat vision loss despite the many challenges faced. Nevertheless, ethical dilemmas arise, particularly in the absence of high-quality evidence, increased marketing claims and lack of transparency regarding patient recruitment. The need to do no harm highlights the importance of rigorous clinical trial processes, appropriate regulation and post-market surveillance to avoid ineffective and unsafe products reaching the community.
International societies are committed to ensuring ethical integrity and adequate scientific standards in clinical trials and therapies concerning stem cell procedures. The International Society for Cellular Therapy (ISCT) and the International Society for Stem Cell Research (ISSCR) are global associations linking pre-clinical research to clinical trials[75]. The Association for Research in Vision and Ophthalmology (ARVO) supports the development of policies that promote stem cell research and therapies[76]. The American Academy of Ophthalmology (AAO) studies and reports the dangers of inadequate quality control of unproven stem cell interventions[77]. With the extremely rapid evolution of ocular regenerative medicine, proper regulation is demanded to avoid ineffective and unsafe products reaching the patients.
The use of treatment outcome registries is taken into account, to provide evidence on the safety and effectiveness of stem cell-based therapies. This methodology could act as a link between clinical trials and ongoing treatments already available on the market. Proper surveillance of these post-market registries by jurisdictional committees and regulators, could safeguard patients from being misguided[78].
Progress in regenerative medicine would continue to enhance welfare, while stem cell therapies would manage to improve the patient's life. In addition, patients should be protected from uncontrolled media violations and deceptive marketing assertions. Ocular regenerative medicine is currently a fast-developing area that requires independent, unbiased, and objective scientific evidence in order to ensure patient treatment decisions and outcomes are not mediated by commercial or financial interests[79].
Acknowledgments
Authors' contributions: Sotiropulos K: Project development, data management and analysis, manuscript writing and editing; Kourkoutas D: Data analysis, manuscript writing and editing; Almaliotis D: Manuscript editing; Ploumidou K: Manuscript editing; Karampatakis V: manuscript editing.
Conflicts of Interest: Sotiropulos K, None; Kourkoutas D, None; Almaliotis D, None; Ploumidou K, None; Karampatakis V, None.
REFERENCES
- 1.World Health Organization (WHO) Blindness and vision impairment. 2021. https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment.
- 2.Daley GQ, Scadden DT. Prospects for stem cell-based therapy. Cell. 2008;132(4):544–548. doi: 10.1016/j.cell.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Rubin LL. Stem cells and drug discovery: the beginning of a new era? Cell. 2008;132(4):549–552. doi: 10.1016/j.cell.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Cossu G, Birchall M, Brown T, et al. Lancet Commission: stem cells and regenerative medicine. Lancet. 2018;391(10123):883–910. doi: 10.1016/S0140-6736(17)31366-1. [DOI] [PubMed] [Google Scholar]
- 5.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Daley GQ. The promise and perils of stem cell therapeutics. Cell Stem Cell. 2012;10(6):740–749. doi: 10.1016/j.stem.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Bobba S, Di Girolamo N, Mills R, Daniell M, Chan E, Harkin DG, Cronin BG, Crawford G, McGhee C, Watson S. Nature and incidence of severe limbal stem cell deficiency in Australia and New Zealand. Clin Exp Ophthalmol. 2017;45(2):174–181. doi: 10.1111/ceo.12813. [DOI] [PubMed] [Google Scholar]
- 10.Abou-El-Enein M, Elsanhoury A, Reinke P. Overcoming challenges facing advanced therapies in the EU market. Cell Stem Cell. 2016;19(3):293–297. doi: 10.1016/j.stem.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, de Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349(9057):990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385(9967):509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 13.Tseng SCG. Concept and application of limbal stem cells. Eye (Lond) 1989;3(2):141–157. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- 14.Keivyon KR, Tseng SCG. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96(5):709–723. doi: 10.1016/s0161-6420(89)32833-8. [DOI] [PubMed] [Google Scholar]
- 15.Baylis O, Figueiredo F, Henein C, Lako M, Ahmad S. 13 years of cultured limbal epithelial cell therapy: a review of the outcomes. J Cell Biochem. 2011;112(4):993–1002. doi: 10.1002/jcb.23028. [DOI] [PubMed] [Google Scholar]
- 16.Tsai RJ, Li L, Chen J. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells(1) Am J Ophthalmol. 2000;130(4):543. doi: 10.1016/s0002-9394(00)00746-7. [DOI] [PubMed] [Google Scholar]
- 17.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363(2):147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 18.Nishida K, Yamato M, Hayashida Y, Watanabe K, Maeda N, Watanabe H, Yamamoto K, Nagai S, Kikuchi A, Tano Y, Okano T. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation. 2004;77(3):379–385. doi: 10.1097/01.TP.0000110320.45678.30. [DOI] [PubMed] [Google Scholar]
- 19.di Girolamo N, Bosch M, Zamora K, Coroneo MT, Wakefield D, Watson SL. A contact lens-based technique for expansion and transplantation of autologous epithelial progenitors for ocular surface reconstruction. Transplantation. 2009;87(10):1571–1578. doi: 10.1097/TP.0b013e3181a4bbf2. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen KN, Bobba S, Richardson A, Park M, Watson SL, Wakefield D, di Girolamo N. Native and synthetic scaffolds for limbal epithelial stem cell transplantation. Acta Biomater. 2018;65:21–35. doi: 10.1016/j.actbio.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 21.Pellegrini G, Rama P, Rocco A, Panaras A, Luca M. Concise review: hurdles in a successful example of limbal stem cell-based regenerative medicine. Stem Cells. 2014;32(1):26–34. doi: 10.1002/stem.1517. [DOI] [PubMed] [Google Scholar]
- 22.Daya SM, Watson A, Sharpe JR, Giledi O, Rowe A, Martin R, James SE. Outcomes and DNA analysis of ex vivo expanded stem cell allograft for ocular surface reconstruction. Ophthalmology. 2005;112(3):470–477. doi: 10.1016/j.ophtha.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Parihar JKS, Parihar AS, Jain VK, Kaushik J, Nath P. Allogenic cultivated limbal stem cell transplantation versus cadaveric keratolimbal allograft in ocular surface disorder: 1-year outcome. Int Ophthalmol. 2017;37(6):1323–1331. doi: 10.1007/s10792-016-0415-0. [DOI] [PubMed] [Google Scholar]
- 24.Basu SY, Sureka SP, Shanbhag SS, Kethiri AR, Singh V, Sangwan VS. Simple limbal epithelial transplantation. Ophthalmology. 2016;123(5):1000–1010. doi: 10.1016/j.ophtha.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 25.Sangwan VS, Basu SY, Vemuganti GK, Sejpal K, Subramaniam SV, Bandyopadhyay S, Krishnaiah S, Gaddipati S, Tiwari S, Balasubramanian D. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol. 2011;95(11):1525–1529. doi: 10.1136/bjophthalmol-2011-300352. [DOI] [PubMed] [Google Scholar]
- 26.Fasolo A, Pedrotti E, Passilongo M, Marchini G, Monterosso C, Zampini R, Bohm E, Birattari F, Franch A, Barbaro V, Bertolin M, Breda C, Di Iorio E, Ferrari B, Ferrari S, Meneguzzi M, Ponzin D. Safety outcomes and long-term effectiveness of ex vivo autologous cultured limbal epithelial transplantation for limbal stem cell deficiency. Br J Ophthalmol. 2017;101(5):640–649. doi: 10.1136/bjophthalmol-2015-308272. [DOI] [PubMed] [Google Scholar]
- 27.Bobba S, Chow S, Watson S, Di Girolamo N. Clinical outcomes of xeno-free expansion and transplantation of autologous ocular surface epithelial stem cells via contact lens delivery: a prospective case series. Stem Cell Res Ther. 2015;6(1):23. doi: 10.1186/s13287-015-0009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellegrini G, Ardigò D, Milazzo G, Iotti G, Guatelli P, Pelosi D, de Luca M. Navigating market authorization: the path holoclar took to become the first stem cell product approved in the European union. Stem Cells Transl Med. 2017;7(1):146–154. doi: 10.1002/sctm.17-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang A, Chen X. Market access of advanced therapy medicinal product (ATMP) In Europe: lessons learnt and key considerations for future success. Value Health. 2017;20(9):A696. [Google Scholar]
- 30.Basu SY, Hertsenberg AJ, Funderburgh ML, Burrow MK, Mann MM, Du YQ, Lathrop KL, Syed-Picard FN, Adams SM, Birk DE, Funderburgh JL. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci Transl Med. 2014;6(266):266ra172. doi: 10.1126/scitranslmed.3009644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peh GSL, Beuerman RW, Colman A, Tan DT, Mehta JS. Human corneal endothelial cell expansion for corneal endothelium transplantation: an overview. Transplantation. 2011;91(8):811–819. doi: 10.1097/TP.0b013e3182111f01. [DOI] [PubMed] [Google Scholar]
- 32.Ong HS, Ang M, Mehta J. Evolution of therapies for the corneal endothelium: past, present and future approaches. Br J Ophthalmol. 2021;105(4):454–467. doi: 10.1136/bjophthalmol-2020-316149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holden BA, Fricke TR, Ho SM, Wong R, Schlenther G, Cronjé S, Burnett A, Papas E, Naidoo KS, Frick KD. Global vision impairment due to uncorrected presbyopia. Arch Ophthalmol. 2008;126(12):1731–1739. doi: 10.1001/archopht.126.12.1731. [DOI] [PubMed] [Google Scholar]
- 34.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96(5):614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 35.Steinert RF. Nd:YAG Laser Posterior Capsulotomy. 2013. https://www.aao.org/munnerlyn-laser-surgery-center/ndyag-laser-posterior-capsulotomy-3.
- 36.Kappelhof JP, Vrensen GF. The pathology of after-cataract. A minireview. Acta Ophthalmol Suppl. 1985;1992(205):13–24. doi: 10.1111/j.1755-3768.1992.tb02176.x. [DOI] [PubMed] [Google Scholar]
- 37.Kappelhof JP, Vrensen GFJM, de Jong PTVM, Pameyer J, Willekens BLJC. The ring of Soemmerring in man: an ultrastructural study. Graefes Arch Clin Exp Ophthalmol. 1987;225(1):77–83. doi: 10.1007/BF02155809. [DOI] [PubMed] [Google Scholar]
- 38.Lin HT, Ouyang H, Zhu J, et al. Lens regeneration using endogenous stem cells with gain of visual function. Nature. 2016;531(7594):323–328. doi: 10.1038/nature17181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu QL, Qin ZW, Jin XM, Zhang LF, Chen ZJ, He JL, Ji JF, Yao K. Generation of functional lentoid bodies from human induced pluripotent stem cells derived from urinary cells. Invest Ophthalmol Vis Sci. 2017;58(1):517. doi: 10.1167/iovs.16-20504. [DOI] [PubMed] [Google Scholar]
- 40.Murphy P, Kabir MH, Srivastava T, Mason ME, Dewi CU, Lim S, Yang A, Djordjevic D, Killingsworth MC, Ho JWK, Harman DG, O'Connor MD. Light-focusing human micro-lenses generated from pluripotent stem cells model lens development and drug-induced cataract in vitro. Development. 2018;145(1):dev155838. doi: 10.1242/dev.155838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dewi CU, Mason M, Cohen-Hyams T, Killingsworth MC, Harman DG, Gnanasambandapillai V, Liyanage L, O'Connor MD. A simplified method for producing human lens epithelial cells and light-focusing micro-lenses from pluripotent stem cells. Exp Eye Res. 2021;202:108317. doi: 10.1016/j.exer.2020.108317. [DOI] [PubMed] [Google Scholar]
- 42.Jonas JB, Cheung CMG, Panda-Jonas S. Updates on the epidemiology of age-related macular degeneration. Asia Pac J Ophthalmol (Phila) 2017;6(6):493–497. doi: 10.22608/APO.2017251. [DOI] [PubMed] [Google Scholar]
- 43.da Cruz L, Chen FK, Ahmado A, Greenwood J, Coffey P. RPE transplantation and its role in retinal disease. Prog Retin Eye Res. 2007;26(6):598–635. doi: 10.1016/j.preteyeres.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi K, Kachi S, Iwata E, Ishikawa K, Terasaki H. Visual function 5 years or more after macular translocation surgery for myopic choroidal neovascularisation and age-related macular degeneration. Eye (Lond) 2012;26(1):51–60. doi: 10.1038/eye.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanga PE, Kychenthal A, Fitzke FW, Halfyard AS, Chan R, Bird AC, Aylward GW. Retinal pigment epithelium translocation after choroidal neovascular membrane removal in age-related macular degeneration. Ophthalmology. 2002;109(8):1492–1498. doi: 10.1016/s0161-6420(02)01099-0. [DOI] [PubMed] [Google Scholar]
- 46.Chen FK, Uppal GS, MacLaren RE, Coffey PJ, Rubin GS, Tufail A, Aylward GW, Da Cruz L. Long-term visual and microperimetry outcomes following autologous retinal pigment epithelium choroid graft for neovascular age-related macular degeneration. Clin Exp Ophthalmol. 2009;37(3):275–285. doi: 10.1111/j.1442-9071.2009.01915.x. [DOI] [PubMed] [Google Scholar]
- 47.Lai JC, Lapolice DJ, Stinnett SS, Meyer CH, Arieu LM, Keller MA, Toth CA. Visual outcomes following macular translocation with 360-degree peripheral retinectomy. Arch Ophthalmol. 2002;120(10):1317–1324. doi: 10.1001/archopht.120.10.1317. [DOI] [PubMed] [Google Scholar]
- 48.van Meurs JC, ter Averst E, Hofland LJ, van Hagen PM, Mooy CM, Baarsma GS, Kuijpers RW, Boks T, Stalmans P. Autologous peripheral retinal pigment epithelium translocation in patients with subfoveal neovascular membranes. Br J Ophthalmol. 2004;88(1):110–113. doi: 10.1136/bjo.88.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma R, Khristov V, Rising A, et al. Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci Transl Med. 2019;11(475):eaat5580. doi: 10.1126/scitranslmed.aat5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Regenerative Patch Technologies LLC. (2016, February 16-). Study of Subretinal Implantation of Human Embryonic Stem Cell-Derived RPE Cells in Advanced Dry AMD. (Clinicaltrials.gov Identifier NCT02590692) https://clinicaltrials.gov/ct2/show/NCT02590692.
- 51.Vitillo L, Tovell VE, Coffey P. Treatment of age-related macular degeneration with pluripotent stem cell-derived retinal pigment epithelium. Curr Eye Res. 2020;45(3):361–371. doi: 10.1080/02713683.2019.1691237. [DOI] [PubMed] [Google Scholar]
- 52.Phillips MJ, Wallace KA, Dickerson SJ, et al. Blood-derived human iPS cells generate optic vesicle-like structures with the capacity to form retinal laminae and develop synapses. Invest Ophthalmol Vis Sci. 2012;53(4):2007–2019. doi: 10.1167/iovs.11-9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimada H, Lu QL, Insinna-Kettenhofen C, Nagashima K, English MA, Semler EM, Mahgerefteh J, Cideciyan AV, Li TS, Brooks BP, Gunay-Aygun M, Jacobson SG, Cogliati T, Westlake CJ, Swaroop A. In vitro modeling using ciliopathy-patient-derived cells reveals distinct cilia dysfunctions caused by CEP290 mutations. Cell Rep. 2017;20(2):384–396. doi: 10.1016/j.celrep.2017.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rachel RA, Yamamoto EA, Dewanjee MK, May-Simera HL, Sergeev YV, Hackett AN, Pohida K, Munasinghe J, Gotoh N, Wickstead B, Fariss RN, Dong LJ, Li TS, Swaroop A. CEP290 alleles in mice disrupt tissue-specific cilia biogenesis and recapitulate features of syndromic ciliopathies. Hum Mol Genet. 2015;24(13):3775–3791. doi: 10.1093/hmg/ddv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma TP, Wiley LA, Whitmore SS, Anfinson KR, Cranston CM, Oppedal DJ, Daggett HT, Mullins RF, Tucker BA, Stone EM. Patient-specific induced pluripotent stem cells to evaluate the pathophysiology of TRNT1-associated Retinitis pigmentosa. Stem Cell Res. 2017;21:58–70. doi: 10.1016/j.scr.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 56.Khan M, Arno G, Fakin A, et al. Detailed phenotyping and therapeutic strategies for intronic ABCA4 variants in stargardt disease. Mol Ther Nucleic Acids. 2020;21:412–427. doi: 10.1016/j.omtn.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ito SI, Onishi A, Takahashi M. Chemically-induced photoreceptor degeneration and protection in mouse iPSC-derived three-dimensional retinal organoids. Stem Cell Res. 2017;24:94–101. doi: 10.1016/j.scr.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 58.Hallam D, Hilgen G, Dorgau B, et al. Human-induced pluripotent stem cells generate light responsive retinal organoids with variable and nutrient-dependent efficiency. Stem Cells. 2018;36(10):1535–1551. doi: 10.1002/stem.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Souied E, Pulido J, Staurenghi G. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;377(8):792. doi: 10.1056/NEJMc1706274. [DOI] [PubMed] [Google Scholar]
- 60.Mehat MS, Sundaram V, Ripamonti C, et al. Transplantation of human embryonic stem cell-derived retinal pigment epithelial cells in macular degeneration. Ophthalmology. 2018;125(11):1765–1775. doi: 10.1016/j.ophtha.2018.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwartz SD, Tan G, Hosseini H, Nagiel A. Subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium for the treatment of macular degeneration: an assessment at 4 years. Invest Ophthalmol Vis Sci. 2016;57(5):ORSFc1–ORSFc9. doi: 10.1167/iovs.15-18681. [DOI] [PubMed] [Google Scholar]
- 62.Song WK, Park KM, Kim HJ, Lee JH, Choi J, Chong SY, Shim SH, Del Priore LV, Lanza R. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Reports. 2015;4(5):860–872. doi: 10.1016/j.stemcr.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sugita S, Mandai M, Hirami Y, et al. HLA-matched allogeneic iPS cells-derived RPE transplantation for macular degeneration. J Clin Med. 2020;9(7):E2217. doi: 10.3390/jcm9072217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Algvere PV, Berglin L, Gouras P, Sheng YH, Kopp ED. Transplantation of RPE in age-related macular degeneration: observations in disciform lesions and dry RPE atrophy. Graefes Arch Clin Exp Ophthalmol. 1997;235(3):149–158. doi: 10.1007/BF00941722. [DOI] [PubMed] [Google Scholar]
- 65.Weisz JM, Humayun MS, de Juan E, Jr, Del Cerro M, Sunness JS, Dagnelie G, Soylu M, Rizzo L, Nussenblatt RB. Allogenic fetal retinal pigment epithelial cell transplant in a patient with geographic atrophy. Retina. 1999;19(6):540–545. doi: 10.1097/00006982-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 66.Li Y, Tsai YT, Hsu CW, Erol D, Yang J, Wu WH, Davis RJ, Egli D, Tsang SH. Long-term safety and efficacy of human-induced pluripotent stem cell (iPS) grafts in a preclinical model of retinitis pigmentosa. Mol Med. 2012;18(1):1312–1319. doi: 10.2119/molmed.2012.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kwon EM, Connelly JP, Hansen NF, Donovan FX, Winkler T, Davis BW, Alkadi H, Chandrasekharappa SC, Dunbar CE, Mullikin JC, Liu P. iPSCs and fibroblast subclones from the same fibroblast population contain comparable levels of sequence variations. Proc Natl Acad Sci U S A. 2017;114(8):1964–1969. doi: 10.1073/pnas.1616035114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bartsch U, Oriyakhel W, Kenna PF, Linke S, Richard G, Petrowitz B, Humphries P, Farrar GJ, Ader M. Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp Eye Res. 2008;86(4):691–700. doi: 10.1016/j.exer.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 69.Barnea-Cramer AO, Wang W, Lu SJ, Singh MS, Luo CM, Huo HG, McClements ME, Barnard AR, MacLaren RE, Lanza R. Function of human pluripotent stem cell-derived photoreceptor progenitors in blind mice. Sci Rep. 2016;6:29784. doi: 10.1038/srep29784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mathivanan I, Trepp C, Brunold C, Baerlocher G, Enzmann V. Retinal differentiation of human bone marrow-derived stem cells by co-culture with retinal pigment epithelium in vitro. Exp Cell Res. 2015;333(1):11–20. doi: 10.1016/j.yexcr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 71.Tracy CJ, Sanders DN, Bryan JN, Jensen CA, Castaner LJ, Kirk MD, Katz ML. Intravitreal implantation of genetically modified autologous bone marrow-derived stem cells for treating retinal disorders. Adv Exp Med Biol. 2016;854:571–577. doi: 10.1007/978-3-319-17121-0_76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hwang SH, Park SH, Choi J, Lee DC, Oh JH, Kim SW, Kim JB. Characteristics of mesenchymal stem cells originating from the bilateral inferior turbinate in humans with nasal septal deviation. PLoS One. 2014;9(6):e100219. doi: 10.1371/journal.pone.0100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu W, Duan D, Ackbarkhan Z, Lu M, Huang ML. Differentiation of human olfactory mucosa mesenchymal stem cells into photoreceptor cells in vitro. Int J Ophthalmol. 2017;10(10):1504–1509. doi: 10.18240/ijo.2017.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dai C, Xie J, Dai JM, Li DQ, Khaw PT, Yin ZQ, Huo SJ, Collins A, Raisman G, Li Y. Transplantation of cultured olfactory mucosal cells rescues optic nerve axons in a rat glaucoma model. Brain Res. 2019;1714:45–51. doi: 10.1016/j.brainres.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 75.Hyun I, Lindvall O, Ährlund-Richter L, Cattaneo E, Cavazzana-Calvo M, Cossu G, de Luca M, Fox IJ, Gerstle C, Goldstein RA, Hermerén G, High KA, Kim HO, Lee HP, Levy-Lahad E, Li LS, Lo B, Marshak DR, Daley GQ. New ISSCR guidelines underscore major principles for responsible translational stem cell research. Cell Stem Cell. 2008;3(6):607–609. doi: 10.1016/j.stem.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 76.Association for Research in Vision and Ophthalmology (ARVO) Statement on Cell and Gene Therapy Research. 2018.
- 77.Turner L, Knoepfler P. Selling stem cells in the USA: assessing the direct-to-consumer industry. Cell Stem Cell. 2016;19(2):154–157. doi: 10.1016/j.stem.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 78.Watson SL, Chan E, Daniell M, et al. Save Sight Registries Keratoconus; Tracking the outcomes of corneal cross-linking for keratoconus from routine clinical practice across Australia and New Zealand. Invest Ophthalmol Vis Sci. 2017;58(8):3523. [Google Scholar]
- 79.Bobba S, di Girolamo N, Munsie M, Chen F, Pébay A, Harkin D, Hewitt AW, O'Connor M, McLenachan S, Shadforth AMA, Watson SL. The Current state of stem cell therapy for ocular disease. Exp Eye Res. 2018;177:65–75. doi: 10.1016/j.exer.2018.07.019. [DOI] [PubMed] [Google Scholar]