Abstract
AIM
To compare the efficacy and safety of intravitreal aflibercept with dexamethasone implant in the treatment of macular edema (ME) associated with diabetic retinopathy (DR) or retinal vein occlusion (RVO).
METHODS
A comprehensive search of studies comparing dexamethasone and aflibercept in patients with ME was conducted at PubMed, Embase, and Cochrane Central Register of Controlled Trials from the beginning of library to April 16, 2021. Extracting the data including best-corrected visual acuity (BCVA), central retinal thickness (CRT), number of injections and serious adverse events (SAEs) from the final qualified articles. RevMan 5.3 software was used for Meta-analysis of the included studies.
RESULTS
Totally 7 studies with 369 eyes were included. The causes of ME in the final screening study included RVO and DR. Compared with the aflibercept treatment group, the BCVA of the dexamethasone implant treatment group showed no significant difference in the follow-up for 3mo [mean difference (MD): -0.05, 95% confidence interval (CI): -0.11, 0.02; P=0.17] and 12mo (MD: -0.01, 95%CI: -0.38, 0.37; P=0.98), but it was slightly worse than the aflibercept group at 6mo (MD: 0.12, 95%CI: 0.03, 0.21; P=0.008). In terms of CRT reduction, there was no significant difference between the two groups at 3mo (MD: -28.14, 95%CI: -79.95, 23.67; P=0.29), 6mo (MD: 27.67, 95%CI: -84.89, 140.24; P=0.63), and 12mo (MD: -59.00, 95%CI: -127.37, 9.37; P=0.09). However, dexamethasone implant had fewer injections, but more adverse events such as elevated intraocular pressure (IOP) and cataract.
CONCLUSION
Intravitreal injection of aflibercept and dexamethasone implant can both effectively increase BCVA and reduce CRT. Compared with aflibercept, dexamethasone implant is not inferior in improving vision and reducing CRT in the initial treatment period (3mo) and long-term treatment period (12mo). Besides, it has fewer injections and more likely to cause elevated IOP and cataract.
Keywords: Meta-analysis, macular edema, dexamethasone, aflibercept, best-corrected visual acuity, central retinal thickness
INTRODUCTION
Diabetic retinopathy (DR) and retinal vein occlusion (RVO) are the two most common retinal vascular diseases[1]–[2]. DR is a common cause of moderate and severe visual impairment in working-age population. At present, there are 92.6 million DR patients worldwide, of which approximately 20.6 million suffer from diabetic macular edema (DME), and nearly 28.4 million suffer from visual impairment[3]–[5]. Its main clinical manifestations include retinal microaneurysm, spot or patchy hemorrhage, cotton wool spots, macular edema (ME), etc. RVO is the second most common retinal vascular disease. It is estimated that there are 5.2 to 16 cases per 1000 patients with RVO and there are nearly 16 million cases worldwide. Vascular dilation and tortuosity, retinal hemorrhages as well as cystoid macular edema (CME) are the characteristics of RVO[6]–[7]. When the retinal vascular changes only affect the peripheral retina, it may not have a significant impact on vision. Once the macular area involved, there will be a significant decrease in vision. ME is a common serious complication of both DR and RVO. Thus, seeking therapeutic strategy for ME have attracted great concern of ophthalmologists and retinal specialists.
Two of the most important pathogenic mechanism of ME are the increased release of vascular endothelial growth factor (VEGF) and the production of pro-inflammatory cytokines[8]–[10]. Therefore, anti-VEGF and anti-inflammation are the main treatment regimens for ME[11]–[12]. Anti-VEGF agents such as aflibercept have been proved to effectively prevent vision loss and is currently recognized as a preferred treatment for ME[13]. Aflibercept is a recombinant fusion protein consisting of the extracellular domain of human VEGF receptor-1 and 2 fused to the Fc fragment of human IgG1. Previous studies have shown that aflibercept has a significantly greater binding affinity for VEGF than bevacizumab or ranibizumab, and it may last longer in the eye[14]–[15]. However, the effects of intraocular anti-VEGF agents can only be sustained for a short period with a single administration. Inflammation plays a pivotal role in the pathophysiology of ME. Thus, corticosteroids have been clinically used in the treatment of ME for years. The sustained dexamethasone intravitreal implant (DEX implant; Ozurdex), a biodegradable device, was first approved by the Food and Drug Administration in 2009 for the treatment of ME with RVO. Its unique dosage form can overcome the ocular administration barrier and prolong the action time of dexamethasone in the eye[11],[16].
In previous studies, both the aflibercept and dexamethasone implant have been shown to be effective in the treatment of ME[17]–[24]. They can slow the progression of vision loss in most patients and alleviate ME. However, there have been few systematic reviews or Meta-analyses comparing the clinical efficacy and safety between the aflibercept and dexamethasone implant. Thus, in this context, we conducted this Meta-analysis and systematic review to evaluate the efficacy and safety of aflibercept and dexamethasone for patients with RVO or DR associated ME, including best-corrected visual acuity (BCVA), central retinal thickness (CRT) and other indicators such as number of injections and serious adverse events (SAEs).
MATERIALS AND METHODS
Search Strategy
In this Meta-analysis, Embase, PubMed and Cochrane Central Register of Controlled Trials were respectively used for comprehensive retrieval to screen the articles consistent with the research topic. The searched keywords are including: “aflibercept,” “dexamethasone,” “retinal vein occlusion,” “diabetic macular edema” and its relevant synonyms. All these processes were performed simultaneously and separately by two researchers.
Inclusion and Exclusion Criteria
We screened the article in accordance with the following criteria. 1) The causes of ME include DR and RVO; 2) The final articles should be controlled trial design comparing the efficacy and safety of intravitreal aflibercept with dexamethasone implant in the treatment of ME; 3) There are useful data to be extracted in the final articles including BCVA and CRT; 4) The final selected documents are not time-limited but must be in English; 5) The final article must have 3mo or more follow-up period.
Article Selection and Data Extraction
The titles, abstracts, and full texts are screened by two researchers independently using the above selection criteria. The differences of opinions were resolved through discussion. This includes randomized controlled trials (RCTs), real-world prospective and retrospective clinical studies. The following data should be extracted and organized from the final articles: the name of first author, year of publication, the type of study design, key characteristics of subjects (such as: number of research subjects, age, sex, and number of eyes in the study) as well as data of research results (such as: BCVA, CRT, mean number of intravitreal injections, SAEs).
Quality Assessment
Quality evaluation of RCTs were performed using Revised Cochrane risk of bias tool for individually randomized, parallel group trials (RoB2.0). Methodological Index for Non-randomized Studies (MINORS) were used to perform quality assessment for non-RCTs. Quality of cohort studies were assessed with Newcastle-Ottawa scale (NOS).
Statistical Analysis
Meta-analysis was performed using RevMan5.3 software. Enumeration data (SAEs) were described by relative risk (RR) and 95% confidence interval (CI), while measurement data including BCVA, CRT, mean number of intravitreal injections were described by mean difference (MD) and 95%CI. I2 test was used for heterogeneity test. If P≥0.1 and I2≤50%, a fixed-effect model would be used for Meta-analysis. P<0.1 and I2>50% indicated statistical heterogeneity among references. In this case, the source of the heterogeneity needs to be analyzed and sensitivity analysis should be performed to detect stability. If there was no clinical heterogeneity, random effects model would be used for Meta-analysis. If there was large heterogeneity and the source of heterogeneity could not be known, descriptive analysis would be used. P<0.05 was considered statistically significant.
RESULTS
Study Selection
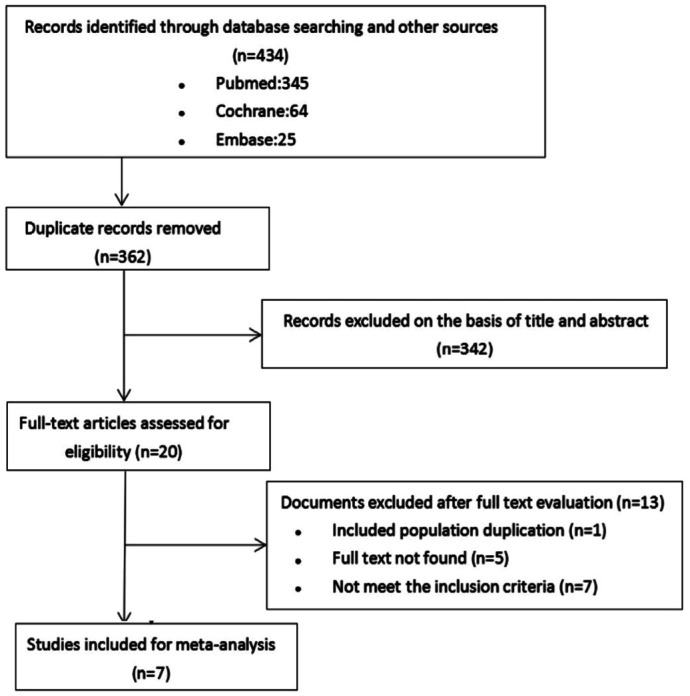
The flow chart of the selection process is shown in Figure 1. In the literature search, 434 studies were identified in PubMed, Cochrane, and Embase. After checking for duplications, 362 studies were left. Of these studies, 20 articles that were relevant to the study topic remained for full-text review. Finally, after full-text review of these 20 articles, 7 studies met inclusion criteria.
Figure 1. Flow chart of studies meeting inclusion and exclusion criteria from literature review.
Final Included Literature
After screening, 1 RCT, 1 non-RCT, and 5 retrospective cohort studies were finally selected. Table 1 showed the basic characteristics of the 7 included studies. Sample sizes ranged from 22 to 98 eyes. The mean age of patients ranged from 56.4 to 70.6y. The dose of Dexamethasone was 0.7 mg in the Dexamethasone implant group of the included studies. The dose of Aflibercept was 2mg in the Aflibercept group of the included studies. Moreover, the duration of follow-up varied from 3 to 12mo among these studies. According to their study designs, different methodological quality evaluation methods were used. We used the RoB2.0 tool for the RCT, MINORS for the non-RCT and NOS for the five retrospective cohort studies.
Table 1. Study characteristics of the included studies.
| Study | Design | Disease | Gender (M/F) |
Age (y) |
Eyes (n) |
Intervention |
Follow-up (mo) | NOS | ||||
| IDI | IVA | IDI | IVA | IDI | IVA | IDI | IVA | |||||
| Aksoy 2020[17] | Retrospective cohort study | DR | 18/19 | 18/16 | 61.3±11.3 | 59.3±10.3 | 37 | 34 | 0.7 mg at baseline | 3 injections/mo (2 mg) | 6 | 6 |
| Bolukbasi 2019[18] | Retrospective cohort study | DR | 9/16 | 13/19 | 65.1±13.2 | 56.4±13.5 | 25 | 32 | 0.7 mg at baseline | 3 injections/mo (2 mg) | 3 | 5 |
| Comet 2021[19] | Non-RCT | DR | 12/9 | 11/9 | 66.3±7.8 | 69.6±9.2 | 21 | 20 | 0.7 mg +PRN | 3 injections/mo (2 mg)+ PRN | 12 | N/A |
| Hanhart 2017[20] | Retrospective cohort study | RVO | 6/4 | 6/6 | 63.60±7.12 | 62.08±8.87 | 10 | 12 | NA | NA | 12 | 7 |
| Kaldirim2018[21] | Retrospective cohort study | RVO | 12/8 | 13/7 | 70.6±3.9 | 70.45±3.9 | 20 | 20 | 0.7 mg at baseline | 3 injections/mo (2 mg)+ PRN | 6 | 6 |
| Ozsaygili 2020[22] | RCT | DR | 15/14 | 20/13 | 64.8±7.9 | 66.4±2.0 | 48 | 50 | 0.7 mg +PRN | 3 injections/mo (2 mg)+ PRN | 12 | N/A |
| Yucel 2019[23] | Retrospective cohort study | RVO | NA | NA | 65.4±2.3 | 66.2±3.2 | 24 | 16 | PRN (0.7 mg) | PRN (2 mg) | 6 | 6 |
M: Male; F: Female; RCT: Randomized controlled trial; DR: Diabetic retinopathy; RVO: Retinal vein occlusion; IDI: Intravitreal dexamethasone implant; IVA: Intravitreal aflibercept; NA: Not applicable; NOS: Newcastle-Ottawa scale; PRN: pro re nata.
Changes in Best-Corrected Visual Acuity
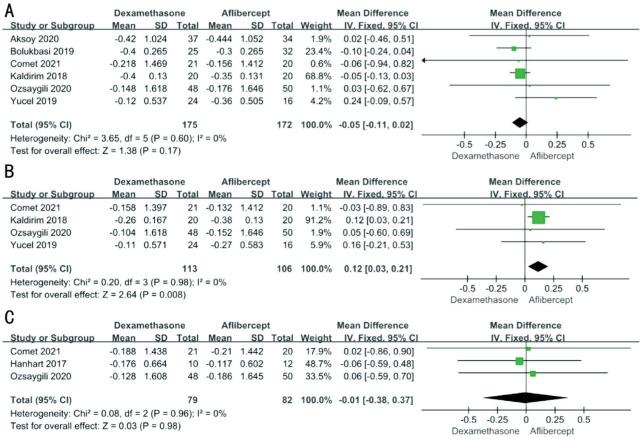
The change of BCVA is the most important index to observe the therapeutic efficacy. Different studies recorded BCVA in a different way. Among them, logarithm of minimum angle of resolution (logMAR) visual chart was used in 3 studies, Early Treatment Diabetic Retinopathy Study (ETDRS) letters was used in 2 studies, and Snellen visual chart was used in 2 studies. For the purpose of statistical analysis, all visual acuity data were converted into logMAR. To extrapolate all available data, 6 studies (n=347) were used for the analysis of 3-month outcomes, 4 studies (n=219) were used for the analysis of 6-month outcomes and 3 studies (n=161) were included for 12-month analysis. After testing the heterogeneity, we all used the fixed effects model (3mo: P=0.60, I2=0; 6mo: P=0.98, I2=0; 12mo: P=0.96, I2=0). The pooled results demonstrated no significant difference in BCVA gain between aflibercept and dexamethasone in either 3mo (MD -0.05; 95%CI -0.11, 0.02; P=0.17) or 12mo (MD -0.01; 95%CI -0.38, 0.37; P=0.98). But there was a significant difference of BCVA gain at 6mo (MD 0.12; 95%CI 0.03, 0.21; P=0.008) between two groups with original data showing slightly worse of dexamethasone than aflibercept for BCVA improvement (Figure 2).
Figure 2. Differences in BCVA (logMAR) changes between aflibercept and dexamethasone implant treatment at 3mo (A), 6mo (B), and 12mo (C).
BCVA: Best-corrected visual acuity; logMAR: Logarithm of minimum angle of resolution; SD: Standard deviation; CI: Confidence interval.
Changes in Central Retinal Thickness
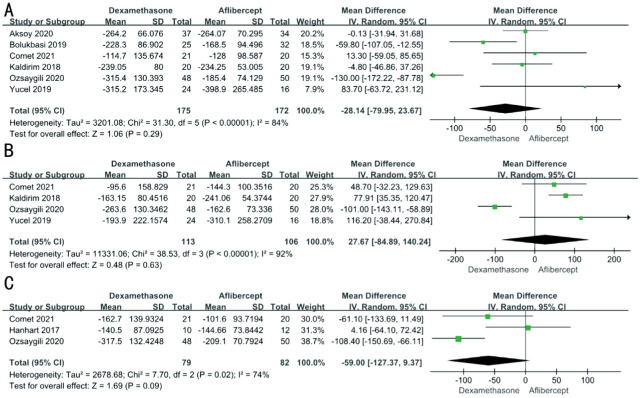
After heterogeneity test, we found there was great heterogeneity among these studies, so the random effects model was used (3mo: P<0.01 I2=84%; 6mo: P<0.01 I2=92%; 12mo: P=0.02 I2=74%). After comparison between aflibercept and dexamethasone, the two medications had no significant differences in reducing CRT overall (3mo: MD -28.14; 95%CI -79.95, 23.67; P=0.29; 6mo: MD 27.67; 95%CI -84.89, 140.24; P=0.63; 12mo: MD -59.00; 95%CI -127.37, 9.37; P=0.09; Figure 3).
Figure 3. Differences in CRT changes between aflibercept and dexamethasone treatment at 3mo (A), 6mo (B), and 12mo (C).
CRT: Central retinal thickness; SD: Standard deviation; CI: Confidence interval.
Serious Adverse Events
Elevation of intraocular pressure
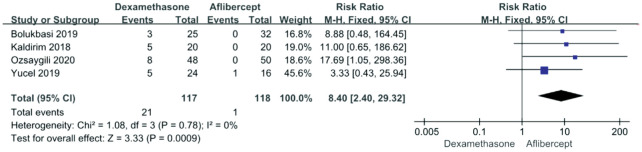
Four included articles reported adverse events related to intraocular pressure (IOP). The pooled results demonstrated a significant difference between aflibercept and dexamethasone treatment (RR 8.40; 95%CI 2.40, 29.32; P<0.0009) without heterogeneity (P=0.78, I2=0). It showed that the dexamethasone group was more prone to have elevated IOP than the aflibercept group (Figure 4).
Figure 4. Forest plot showing the elevation of intraocular pressure.
Cataract
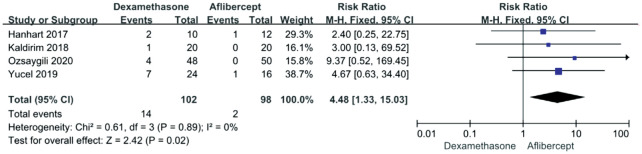
Four of the included studies reported the occurrence of cataracts after treatments. We used the fixed effects model because the heterogeneity was not detected between studies (P=0.89, I2=0). Figure 5 showed that there was a significant difference in the incidence of cataract between the dexamethasone and the aflibercept (RR 4.48; 95%CI 1.33, 15.03; P=0.02). Moreover, the Aflibercept group had fewer cataracts than the dexamethasone group.
Figure 5. Forest plot showing the adverse events: cataract.
Mean Number of Intravitreal Injections
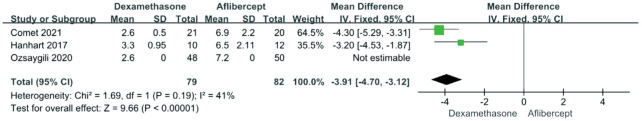
Three included articles reported the number of injections in 12mo. The heterogeneity was detected between studies (P=0.19; I2=41%). Analysis using a fixed effects model noted that fewer injections were given in the dexamethasone group than in the aflibercept group (RR -3.91; 95%CI -4.70, -3.12; P<0.00001; Figure 6).
Figure 6. Forest plot showing the mean number of intravitreal injections.
Heterogeneity, Sensitivity Analysis, and Publication Bias
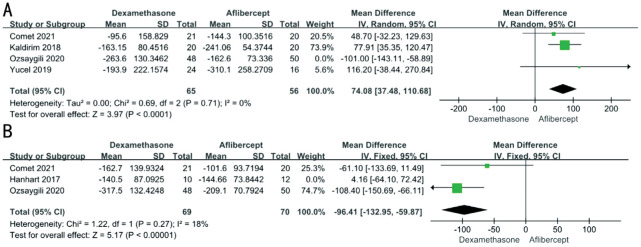
We had found the heterogeneity in the part of CRT and number of injections. A leave-one-out sensitivity analysis showed that none of the single studies had a significant effect on overall effect in the BCVA. As for the CRT, we found that after excluding the Ozsaygili and Duru[22] in 6mo and the Hanhart and Rozenman[20] in 12mo, the heterogeneity was significantly reduced. After excluding them, CRT reduction between dexamethasone group and aflibercept group was significantly different (6mo: MD 74.08; 95%CI 37.48, 110.68; P<0.0001; 12mo: MD -96.39; 95%CI -132.93, -59.85; P<0.00001; Figure 7). Combined analysis of original data, we concluded that the CRT reduction of dexamethasone group was slightly worse than aflibercept group in 6mo but slightly better than aflibercept group in 12mo. Funnel plots and Egger's test were not used, because there were less than ten studies for each comparison.
Figure 7. Differences in central retinal thickness changes between aflibercept and dexamethasone treatment at 6mo (A) and 12mo (B).
DISCUSSION
DR and RVO are the top two causes of vision impairment among all the retinal vascular diseases. Currently, there are available treatments for ME caused by RVO and DR[25], and the most common strategies are intravitreal anti-VEGF agents and steroid. Intravitreal injection of anti-VEGF drug is the preferred treatment for ME secondary to RVO and DR[26]. The basic principle is that elevated VEGF levels can disrupt the blood-retina barrier (BRB), causing retinal vascular leakage, and ME. VEGF tyrosine kinase receptors are quite potent in mitosis and permeability for retinal vasculature[11]. Aflibercept, a recombinant fusion protein, competitively inhibits the binding and activation of VEGF to its receptor and significantly reduces vascular permeability[27]. Past studies have shown that it can effectively relieve ME and improve vision during long-term follow-up[28]. However, patients who treated by aflibercept may need at least seven injections and monthly follow-ups in the first year[29]. Frequently intravitreous injections may increase the patient's economic burden, cause psychological pressure and high risks of surgical complications such as endophthalmitis. Intravitreal dexamethasone sustained release system has become a substitute of anti-VEGF agents in ME management due to its advantages of efficacy, release duration, and tolerance. Its main mechanism is to inhibit the release of various inflammatory factors and stabilize the BRB. It has been shown to indirectly inhibit VEGF for alleviation of blood vessel leakage, thereby promoting the resolution of ME[30]–[33]. On the other hand, dexamethasone implant have potential side effects, including increased IOP and cataracts[34]. In clinical practice, dexamethasone implant is often used as a complementary therapy to obtain better efficacy and reduce the number of drug injection.
To date, there have been no systematic studies comparing the efficacy and safety of aflibercept and dexamethasone implant in treating ME caused by DR or RVO. Therefore, we conducted the current Meta-analysis and systematic review to evaluate the efficacy and safety of these two treatments on ME, including changes in BCVA, CRT and so on. A total of seven clinical controlled studies were collected, including 347 patients (172 in the aflibercept group and 175 in the dexamethasone group). Our Meta-analysis results indicated that both dexamethasone implant and aflibercept can achieve significant functional and anatomical improvements for ME secondary to DR or RVO. The BCVA results showed that although the visual acuity improvement of the dexamethasone implant was slightly worse than that of the aflibercept group at 6mo of follow-up, there was no significant difference between the two groups at 3 or 12mo of follow-up. Differences in half-life and administration interval between the two strategies may account for the results. The half-life of aflibercept is short, and monthly follow-up administration is required. The half-life of dexamethasone plant was nearly about 4mo, and at the 6mo follow-up, the effect of the first dose was weak and the accumulative effect of the second dose was still not fully achieved.
The results of CRT showed that in terms of anatomic improvement, compared with intravitreal injection of aflibercept, dexamethasone implant was not significantly inferior to aflibercept. The statistical results told us that compared with the intravitreal injection of aflibercept, the dexamethasone implant can effectively reduce the number of injections. Thus, dexamethasone demonstrated its unique advantages including less repeated injection and subsequent better patient compliance. Although dexamethasone is not a prime anti-VEGF drug compared with aflibercept, as a hormone, it can indirectly decrease VEGF expression, stabilize white blood cells, and reduce relief of inflammatory cytokines. Studies have shown that dexamethasone is slightly superior to aflibercept, in both anatomy and function during the first three months of administration. It indicated that in clinical practice, dexamethasone implant may be the first choice for patients with injection anxiety, heavy economic burden, or poor compliance.
Statistical results of adverse reactions told us that dexamethasone has similar drawbacks as other steroids. For example, it may increase IOP to some extent and accelerate the progress of cataract. In this Meta-analysis, 25% (5 patients) of the Kaldırım and Yazgan[21] dexamethasone group and 12% (3 patients) of the Bolukbasi et al[18] dexamethasone group used antiglaucoma drugs regularly to control their IOP, and the Hanhart and Rozenman[20] dexamethasone group had elevated IOP without medication control. There was no significant change of IOP in Comet et al[19] and Aksoy et al[17] dexamethasone groups. Therefore, we can conclude that dexamethasone implant was more likely to cause elevated IOP in patients than aflibercept, but they were manageable.
We found no significant heterogeneity in the part of BCVA, but large heterogeneity in the CRT. Therefore, sensitivity analysis and subgroup analysis were performed to analyze the source of heterogeneity in the CRT. We found that Ozsaygili and Duru[22] in 6mo and the Hanhart and Rozenman[20] in 12mo may be main sources of the heterogeneity of CRT. Moreover, the Meta-analysis indicated that the CRT reduction of dexamethasone group was slightly worse than aflibercept group in 6mo but slightly better than aflibercept group in 12mo. Therefore, dexamethasone may be more effective than aflibercept from the long-term effect in CRT reduction. We speculate that the reasons of heterogeneity may include differences in research design types and research sample size. In terms of differences in research design types, Ozsaygili and Duru[22] is an RCT, but the remaining studies are all case-control studies in 6mo. In terms of the difference in sample size, Hanhart and Rozenman[20] has a small number of cases and relatively low credibility in the literature. Thus, this study has some shortcomings. First, it is only based on publicly available information. Second, the included literature is mostly retrospective cohort studies, which cannot be completely randomized.
To sum up, the results of this Meta-analysis and systemic review showed that the effect of dexamethasone on BCVA and CRT is not worse than that of aflibercept in the initial treatment period within 3mo and long-term treatment period after 12mo. Besides, dexamethasone can significantly reduce the number of operations, relieve the economic burden, and reduce frequent follow-up pressure of patients.
In addition, several studies in recent years have also provided new evidence on this topic, which may be helpful to us. In terms of the preferred treatment option for patients with DME, Meduri et al[35] demonstrated that naïve DME patients treated with dexamethasone implant show a better functional response in patients with the integrity of the ellipsoid zone (EZ) and absence of vitreomacular alterations. Ceravolo et al[36] reported that DME patients with serous detachment of neuro-epithelium (SDN) and a high number of hyper-reflective spots (HRS) showed a better response to intravitreal steroids than anti-VEGF treatment. The reason may be that DME associated with SDN and a high number of HRS describes a specific inflammatory pattern. Therefore, these patients showed a better response to dexamethasone implant than to anti-VEGF treatment.
It has been proven that the appropriate number and timing of intravitreal injections can determine long-term vision outcomes in patients with retinal disease[37]–[39]. Conversely, delayed treatment often result in serious and irreversible vision impairment[40]–[42]. During the coronavirus disease 2019 (COVID-19) pandemic outbreak, the number of vitreous injections had decreased significantly in many countries. Billioti et al[43] reported that in France the number of intravitreal injections decreased by 47.1% during the first 5 weeks of lockdown. In Italy, the reduction in the number of intravitreal injections ranged from 60% to 91.7%[44]–[45]. Consequently, vision and anatomical outcomes in patients with retinal disease are negatively affected in the short term[46]–[47]. Furthermore, Scorcia et al[48] suggested that dexamethasone implant greatly improved anatomic and functional outcomes in patients who were unable to receive an appropriate anti-VEGF therapeutic regime during the epidemic.
In conclusion, although anti-VEGF therapy is still the preferred treatment for ME, in an era of widespread global novel coronavirus epidemic, we need to comprehensively consider the risk-benefit of patients with retinal diseases while maintaining COVID-19 infection control broadly. Therefore, combined with the conclusions of this Meta-analysis and systematic review, we conclude that compared with aflibercept, dexamethasone implant is expected to be the primary choice for patients with ME caused by different causes, especially in the following specific populations: 1) patients with pseudophakic eye; 2) patients with low risk of IOP elevation; 3) patients with cataract who need surgery; 4) patients who do not respond to anti-VEGF therapy; 5) patients who are unable or unwilling to return for regular examinations; 6) naïve DME patients with integrity of the EZ integrity and absence of vitreomacular alterations; 7) DME patients with SDN and a high number of HRS; 8) patients with a recent history of major cardiovascular events; 9) pregnant woman. At the same time, regular follow-up and timely supplementary administration should be paid attention to when using dexamethasone treatment.
Acknowledgments
Foundations: Supported by Key Research and Development Program of Shaanxi Province (No.2021SF-155); Natural Science Foundation of Jiangxi Province (No.20192BAB205049); National Innovation and Entrepreneurship Training Program for College Students (No.X202110698092); Clinical Research Grant from First Affiliated Hospital of Xi'an Jiaotong University (No.20ZD02).
Conflicts of Interest: Qiu XY, None; Hu XF, None; Qin YZ, None; Ma JX, None; Liu QP, None; Qin L, None; Li JM, None.
REFERENCES
- 1.Ferris FL, 3rd, Patz A. Macular edema. A complication of diabetic retinopathy. Surv Ophthalmol. 1984;28 Suppl:452–461. doi: 10.1016/0039-6257(84)90227-3. [DOI] [PubMed] [Google Scholar]
- 2.Patz A, Schatz H, Berkow JW, Gittelsohn AM, Ticho U. Macular edema—an overlooked complication of diabetic retinopathy. Trans Am Acad Ophthalmol Otolaryngol. 1973;77(1):OP34–OP42. [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin epidemiologic study of diabetic retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102(1):7–16. doi: 10.1016/s0161-6420(95)31052-4. [DOI] [PubMed] [Google Scholar]
- 4.Ting DSW, Cheung GCM, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44(4):260–277. doi: 10.1111/ceo.12696. [DOI] [PubMed] [Google Scholar]
- 5.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, Kowalski JW, Nguyen H, Wong TY, International Eye Disease Consortium The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117(2):313–319.e1. doi: 10.1016/j.ophtha.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa JV, Moura-Coelho N, Abreu AC, Neves P, Ornelas M, Furtado MJ. Macular edema secondary to retinal vein occlusion in a real-life setting: a multicenter, nationwide, 3-year follow-up study. Graefes Arch Clin Exp Ophthalmol. 2021;259(2):343–350. doi: 10.1007/s00417-020-04932-0. [DOI] [PubMed] [Google Scholar]
- 8.Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes. 1998;47(12):1953–1959. doi: 10.2337/diabetes.47.12.1953. [DOI] [PubMed] [Google Scholar]
- 9.Hillier RJ, Ojaimi E, Wong DT, et al. Aqueous humor cytokine levels as biomarkers of disease severity in diabetic macular edema. Retina. 2017;37(4):761–769. doi: 10.1097/IAE.0000000000001210. [DOI] [PubMed] [Google Scholar]
- 10.Mastropasqua R, D'Aloisio R, Di Nicola M, Di Martino G, Lamolinara A, Di Antonio L, Tognetto D, Toto L. Relationship between aqueous humor cytokine level changes and retinal vascular changes after intravitreal aflibercept for diabetic macular edema. Sci Rep. 2018;8(1):16548. doi: 10.1038/s41598-018-35036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatziralli I, Nicholson L, Sivaprasad S, Hykin P. Intravitreal steroid and anti-vascular endothelial growth agents for the management of retinal vein occlusion: evidence from randomized trials. Expert Opin Biol Ther. 2015;15(12):1685–1697. doi: 10.1517/14712598.2015.1086744. [DOI] [PubMed] [Google Scholar]
- 12.Amoaku WM, Ghanchi F, Bailey C, et al. Diabetic retinopathy and diabetic macular oedema pathways and management: UK Consensus Working Group. Eye(Lond) 2020;34(1):1–51. doi: 10.1038/s41433-020-0961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sırakaya E, Küçük B, Ağadayı A. Aflibercept treatment for macular edema following branch retinal vein occlusion: age-based responses. Ophthalmologica. 2020;243(2):94–101. doi: 10.1159/000502042. [DOI] [PubMed] [Google Scholar]
- 14.Behl T, Kotwani A. Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacol Res. 2015;99:137–148. doi: 10.1016/j.phrs.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Salimi A, Vila N, Modabber M, Kapusta M. One-year outcomes of Aflibercept for refractory diabetic macular edema in Bevacizumab nonresponders. Indian J Ophthalmol. 2021;69(2):360–367. doi: 10.4103/ijo.IJO_459_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karti O, Saatci AO. Place of intravitreal dexamethasone implant in the treatment armamentarium of diabetic macular edema. World J Diabetes. 2021;12(8):1220–1232. doi: 10.4239/wjd.v12.i8.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aksoy M, Yilmaz G, Vardarli I, Akkoyun I. Choroidal thickness after dexamethasone implant or aflibercept in patients with diabetic macular edema persistent to ranibizumab. J Ocul Pharmacol Ther. 2020;36(8):629–635. doi: 10.1089/jop.2020.0004. [DOI] [PubMed] [Google Scholar]
- 18.Bolukbasi S, Cakir A, Erden B, Karaca G. Comparison of the short-term effect of aflibercept and dexamethasone implant on serous retinal detachment in the treatment of naive diabetic macular edema. Cutan Ocul Toxicol. 2019;38(4):401–405. doi: 10.1080/15569527.2019.1657884. [DOI] [PubMed] [Google Scholar]
- 19.Comet A, Gascon P, Ramtohul P, Donnadieu B, Denis D, Matonti F. INVICTUS: Intravitreal anti-VEGF and dexamethasone implant comparison for the treatment of diabetic macular edema: a 12 months follow-up study. Eur J Ophthalmol. 2021;31(2):754–758. doi: 10.1177/1120672120930603. [DOI] [PubMed] [Google Scholar]
- 20.Hanhart J, Rozenman Y. Comparison of intravitreal ranibizumab, aflibercept, and dexamethasone implant after bevacizumab failure in macular edema secondary to retinal vascular occlusions. Ophthalmologica. 2017;238(1-2):110–118. doi: 10.1159/000473864. [DOI] [PubMed] [Google Scholar]
- 21.Kaldırım HE, Yazgan S. A comparison of three different intravitreal treatment modalities of macular edema due to branch retinal vein occlusion. Int Ophthalmol. 2018;38(4):1549–1558. doi: 10.1007/s10792-017-0618-z. [DOI] [PubMed] [Google Scholar]
- 22.Ozsaygili C, Duru N. Comparison of intravitreal dexamethasone implant and aflibercept in patients with treatment-naive diabetic macular edema with serous retinal detachment. Retina. 2020;40(6):1044–1052. doi: 10.1097/IAE.0000000000002537. [DOI] [PubMed] [Google Scholar]
- 23.Yucel OE, Birinci H, Sullu Y. The short-term efficacy of intravitreal ranibizumab, aflibercept and dexamethasone implant in the treatment of macular edema due to non-ischemic central retinal vein occlusion. Int Ophthalmol. 2019;39(4):891–901. doi: 10.1007/s10792-018-0890-6. [DOI] [PubMed] [Google Scholar]
- 24.Comet A, Gascon P, Sauvan L, Donnadieu B, Matonti F. INVICTUS: intravitreal anti-VEGF and dexamethasone implant comparison for the treatment of diabetic macular edema: a 6 months follow-up Study. Acta Ophthalmol. 2019;97(6):e937–e938. doi: 10.1111/aos.14057. [DOI] [PubMed] [Google Scholar]
- 25.Haller JA, Bandello F, Belfort R, Jr, Blumenkranz MS, Gillies M, Heier J, Loewenstein A, Yoon YH, Jiao J, Li XY, Whitcup SM. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion. Ophthalmology. 2011;118(12):2453–2460. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Diabetic Retinopathy Clinical Research Network. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193–1203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 28.Rudge JS, Thurston G, Davis S, Papadopoulos N, Gale N, Wiegand SJ, Yancopoulos GD. VEGF trap as a novel antiangiogenic treatment currently in clinical trials for cancer and eye diseases, and VelociGene-based discovery of the next generation of angiogenesis targets. Cold Spring Harb Symp Quant Biol. 2005;70:411–418. doi: 10.1101/sqb.2005.70.052. [DOI] [PubMed] [Google Scholar]
- 29.Campochiaro PA, Clark WL, Boyer DS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion. Ophthalmology. 2015;122(3):538–544. doi: 10.1016/j.ophtha.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 30.Rezar-Dreindl S, Eibenberger K, Pollreisz A, et al. Effect of intravitreal dexamethasone implant on intra-ocular cytokines and chemokines in eyes with retinal vein occlusion. Acta Ophthalmol. 2017;95(2):e119–e127. doi: 10.1111/aos.13152. [DOI] [PubMed] [Google Scholar]
- 31.McAllister IL, Vijayasekaran S, Chen SD, Yu DY. Effect of triamcinolone acetonide on vascular endothelial growth factor and occludin levels in branch retinal vein occlusion. Am J Ophthalmol. 2009;147(5):838–846. 846.e1–2. doi: 10.1016/j.ajo.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 32.van der Wijk AE, Canning P, van Heijningen RP, Vogels IMC, van Noorden CJF, Klaassen I, Schlingemann RO. Glucocorticoids exert differential effects on the endothelium in an in vitro model of the blood-retinal barrier. Acta Ophthalmol. 2019;97(2):214–224. doi: 10.1111/aos.13909. [DOI] [PubMed] [Google Scholar]
- 33.Edelman JL, Lutz D, Castro MR. Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res. 2005;80(2):249–258. doi: 10.1016/j.exer.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Yoon YH, Kim JW, Lee JY, et al. Dexamethasone intravitreal implant for early treatment and retreatment of macular edema related to branch retinal vein occlusion: the multicenter COBALT study. Ophthalmologica. 2018;240(2):81–89. doi: 10.1159/000487547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meduri A, Oliverio GW, Trombetta L, Giordano M, Inferrera L, Trombetta CJ. Optical coherence tomography predictors of favorable functional response in Naïve diabetic macular edema eyes treated with dexamethasone implants as a first-line agent. J Ophthalmol. 2021;2021:6639418. doi: 10.1155/2021/6639418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ceravolo I, Oliverio GW, Alibrandi A, Bhatti A, Trombetta L, Rejdak R, Toro MD, Trombetta CJ. The application of structural retinal biomarkers to evaluate the effect of intravitreal ranibizumab and dexamethasone intravitreal implant on treatment of diabetic macular edema. Diagnostics (Basel) 2020;10(6):413. doi: 10.3390/diagnostics10060413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korobelnik JF, Loewenstein A, Eldem B, et al. Guidance for anti-VEGF intravitreal injections during the COVID-19 pandemic. Graefes Arch Clin Exp Ophthalmol. 2020;258(6):1149–1156. doi: 10.1007/s00417-020-04703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen SY, Mimoun G, Oubraham H, Zourdani A, Malbrel C, Queré S, Schneider V, LUMIERE Study Group Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the LUMIERE study. Retina. 2013;33(3):474–481. doi: 10.1097/IAE.0b013e31827b6324. [DOI] [PubMed] [Google Scholar]
- 39.Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99(2):220–226. doi: 10.1136/bjophthalmol-2014-305327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toro MD, Brézin AP, Burdon M, et al. Early impact of COVID-19 outbreak on eye care: insights from EUROCOVCAT group. Eur J Ophthalmol. 2021;31(1):5–9. doi: 10.1177/1120672120960339. [DOI] [PubMed] [Google Scholar]
- 41.Tognetto D, Brézin AP, Cummings AB, et al. Rethinking elective cataract surgery diagnostics, assessments, and tools after the COVID-19 pandemic experience and beyond: insights from the EUROCOVCAT group. Diagnostics (Basel) 2020;10(12):1035. doi: 10.3390/diagnostics10121035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elfalah M, AlRyalat SA, Toro MD, et al. Delayed intravitreal anti-VEGF therapy for patients during the COVID-19 lockdown: an ethical endeavor. Clin Ophthalmol. 2021;15:661–669. doi: 10.2147/OPTH.S289068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Billioti de Gage S, Drouin J, Desplas D, Cuenot F, Dray-Spira R, Weill A, Zureik M. Intravitreal anti-vascular endothelial growth factor use in France during the coronavirus disease 2019 pandemic. JAMA Ophthalmol. 2021;139(2):240–242. doi: 10.1001/jamaophthalmol.2020.5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carnevali A, Giannaccare G, Gatti V, Scuteri G, Randazzo G, Scorcia V. Intravitreal injections during COVID-19 outbreak: real-world experience from an Italian tertiary referral center. Eur J Ophthalmol. 2021;31(1):10–12. doi: 10.1177/1120672120962032. [DOI] [PubMed] [Google Scholar]
- 45.Viola F, Milella P, Pozzo Giuffrida F, Ganci S, Invernizzi A. Impact of coronavirus disease pandemic on intravitreal injections treatment for macular diseases: report from a referral hospital in Milan. Retina. 2021;41(4):701–705. doi: 10.1097/IAE.0000000000002941. [DOI] [PubMed] [Google Scholar]
- 46.Naravane AV, Mundae R, Zhou Y, et al. Short term visual and structural outcomes of anti-vascular endothelial growth factor (anti-VEGF) treatment delay during the first COVID-19 wave: a pilot study. PLoS One. 2021;16(2):e0247161. doi: 10.1371/journal.pone.0247161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sindal MD, Chhabra K, Khanna V. Profile of patients receiving intravitreal anti-vascular endothelial growth factor injections during COVID-19-related lockdown. Indian J Ophthalmol. 2021;69(3):730–733. doi: 10.4103/ijo.IJO_2807_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scorcia V, Giannaccare G, Gatti V, et al. Intravitreal dexamethasone implant in patients who did not complete anti-VEGF loading dose during the COVID-19 pandemic: a retrospective observational study. Ophthalmol Ther. 2021;10(4):1015–1024. doi: 10.1007/s40123-021-00395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]