Abstract
AIM
To compare visual quality after unilateral cataract surgery with implantation of trifocal intraocular lens (IOL) and asymmetric refractive multifocal IOL.
METHODS
The prospective nonrandom, comparative study consisted of 60 eyes of 60 patients suffering unilateral cataract surgery with implantation of two different IOLs: AT LISA tri 839MP (30 eyes; Carl Zeiss Meditec, Germany) and LS-313 MF30 (30 eyes; Oculentis GmbH, Germany). Visual acuity, refractive outcome, contrast sensitivity, defocus curves, quality of vision, and optical phenomena were evaluated at 3mo postoperatively.
RESULTS
There were no statistical differences between groups in uncorrected distance visual acuity (P=0.13) and uncorrected near visual acuity (P=0.54). In contrast, uncorrected intermediate visual acuity was better in trifocal group compared to the refractive multifocal group (P=0.02). No significant statistical between-group difference was detected in cylinder (P=0.43). Compared to trifocal group, spherical refraction and spherical equivalent in refractive multi focal group were more myopic (P<0.01). Under photopic conditions, no significant statistical differences were found between groups in contrast sensitivity at 3 and 6 cycles per degree (cpd). The refractive multifocal group performed better at 12 and 18 cpd than the trifocal group (P=0.01, P=0.034, respectively). The questionnaires of quality of vision and optical phenomena showed no differences between groups.
CONCLUSION
Trifocal IOL is superior to refractive multifocal IOL in intermediate visual acuity. Rotationally asymmetric refractive multifocal IOL is more myopic in automated refraction and significantly better for the photopic contrast sensitivity at high frequency.
Keywords: trifocal intraocular lens, rotationally asymmetric multifocal intraocular lens, visual quality, contrast sensitivity
INTRODUCTION
Presbyopia is a global problem impacting on more than a billion people[1]. The incidence of uncorrected presbyopia is up to 34% in developed countries, and it is as high as 50% of the population over the age of 50 in developing countries[2]. In 2015, there were 1.8 billion presbyopia patients worldwide, of which 826 million suffered from near vision impairment due to myopia and they had no or insufficient vision correction[3]. Now available modalities for correcting presbyopia include spectacles, contact lenses, surgical approaches, pharmaceuticals and ciliary muscle electrostimulation[2]. Scleral expansion, implantation of intraocular lens (IOL), inlays and laser refractive surgery are surgical approaches that have been carried out for years. Implantation of IOL is an effective alternative of presbyopia correction for people with cataract. According to the types of substitutes for lens, four strategies have been described: pseudophakia with monofocal IOLs, accommodative IOLs, multifocal IOLs, and extended depth of focus (EDOF) IOLs[4].
The first multifocal IOL on the market were manufactured in the late 1980s. Over the past 30y, multifocal IOLs have developed rapidly, but they are no more than diffractive, refractive or a combination of both. The previous refractive multifocal IOLs achieve multifocality by the design of annular zones that representing different refractive power and most of them are rotationally symmetric. These refractive models have many limitations, such as pupil dependence, high sensitivity for lens centration, intolerance to kappa angle, loss of contrast sensitivity, halos and glare[5]. In order to modify limitations of traditional refractive multifocal IOLs, refractive multifocal IOLs with designs of rotational asymmetry have been introduced. This type of design makes light focus on one focal point for near in a particular sector and the other sector of lens is in charge of focal point for distance. So the refractive multifocal IOLs is good at near and distance vision, with similar contrast sensitivity to monofocal IOLs[6], independence of pupil size, low sensitivity to lens decentration and less photopsia than refractive concentric multifocal IOLs[7].
The diffractive multifocal IOLs based on Huygens-Fresnel principle[5]. The physical optics shows that light has both wave and particle characteristic. Light encounters obstacles, if the size of obstacles is close to or less than the wavelength, light diffraction occurs. There are concentric diffractive steps in the central zone of optic that can produce foci. Bifocal IOLs only has a focus for far and a focus for near, not for intermediate, therefore, in some situations, the intermediate vision may not live up to expectation. With the use of computers, laptops, ipads and smart phones, good intermediate vision is becoming more and more important for patients. The first trifocal diffractive IOL was released in 2010, which provide a significant improvement in the intermediate vision.
To our knowledge, this study is the first comparison between the performance of trifocal IOLs and rotationally asymmetric refractive multifocal IOLs in basis of visions at different distances, refractive outcomes, defocus curves, contrast sensitivity, quality of vision and optical phenomena after unilateral cataract surgery.
SUBJECTS AND METHODS
Ethical Approval
All procedures in the study measured up to the ethical standards of the local Ethics Committee and conform to the Helsinki Declaration and its subsequent amendments. Each patient was invited to enter the study and signed informed written consent. Clinical Trial Registry: ChiCTR1900022519.
Patients and Study Design
This study consisted of 60 eyes of 60 patients suffering unilateral cataract surgery with implantation of IOL between July 2018 and June 2020 at Xi'an Forth Hospital, Xi'an, China. Inclusion criteria included: age-related cataract, corneal astigmatism <1.0 D, corneal higher-order aberration <0.3 µm, kappa angle <0.3 mm, alpha angle <0.3 mm. Exclusion criteria included: corneal degeneration, glaucoma, ischemic optic neuropathy, retinal and macular diseases, lens subluxation, previous corneal and intraocular surgery. The ophthalmologist recommended a specific type of IOL according to patient's occupation, habits, hobbies and demands. There were two groups: patients receiving a trifocal IOL AT LISA tri 839MP (trifocal IOL group) and a rotationally asymmetric refractive IOL LS-313 MF30 (refractive multifocal IOL group).
Surgical Technique
All surgeries in this study were phacoemulsification, operated by the same surgeon, with a Stellaris platform (Bausch & Lomb Incorporated, USA). Topical anaesthesia was used in all surgeries. The procedures included a 2.2-mm incision on the steep meridian according to the keratometry obtained in IOL Master 700 (Carl Zeiss Meditec, Germany) preoperatively, a continuous central circular capsulorhexis (approximately 5.5 mm diameter), hydrodissection, phacoemulsification, aspiration of cortex, implantation of IOL. Postoperatively, a topical treatment included a combination of antibiotics and steroids (levofloxacin hydrochloride 0.5%, 4 times a day for 2wk, tobramycin 0.3% and dexamethasone 0.1%, 4 times a day for 2wk).
Intraocular Lens
AT LISA tri 839MP is a preloaded aspheric diffractive multifocal IOL, made of hydrophilic acrylate (refractive index 1.46) with a water content of 25% and covered with a hydrophobic surface. Its overall diameter is 11.0 mm, with single-piece design and 0 degree haptic angulation. It has a 6.0-mm biconvex optic. The central 4.34 mm diameter is trifocal zone and the peripheral 4.34 to 6 mm is bifocal zone. In addition to a far focus, IOL provides an addition power of +3.33 D for near focus and +1.66 D addition power for intermediate focus in optic. The light distribution is asymmetric and the distance, intermediate and near focus are 50%, 20% and 30% respectively. Spherical aberration of IOL was -0.18 µm. It can correct the positive corneal spherical aberration.
Lentis Mplus LS-313 MF30 is a rotationally asymmetric segmental refractive multifocal IOL, with a posterior aspheric distance vision zone and anterior +3.00 D sector-shaped near vision zone. It is a single-piece biconvex hydrophilic IOL with hydrophobic surface. Its overall length is 11.0 mm, too. It has a 6.0 mm optic with a 360° sharp posterior optic edge reducing the incidence of posterior capsular opacity. A flat haptic strengthens stability.
Sample Size
The data of visual acuity in previous studies are used as parameters in sample-size calculation. A study by Bilbao-Calabuig et al[8] shows monocular uncorrected near visual acuity (UNVA) at 3mo postoperatively was 0.07±0.10 logMAR in AT LISA tri839 MP IOL. The monocular UNVA of LS-313 MF30 IOL was 0.16±0.14 logMAR in another study[9]. Assuming that a significance level is 0.05 and a test power is 0.8, the sample size is calculated with the above parameters (PASS for windows, version15.0). Thirty eyes were required in each group.
Examination Protocol
All patients had a comprehensive preoperative ophthalmological examination containing visual acuity, intraocular pressure, slit-lamp, fundoscopy, corneal topography, endothelial cell count analysis and biometry with IOL Master 700. Uncorrected and corrected visual acuity at 5 m, 80 and 40 cm were all tested by decimal chart under photopic condition. Then the visual acuity was converted to logarithm of the minimum angle of resolution (logMAR) for analysis.
A large sample study reported that Barrett universal II formula had the highest accuracy for eyes with axial length >25.0 mm, followed by Olsen and Haigis formula[10]. In 2018, Barrett universal II and Olsen were unavailable in our hospital. Due to overcorrection of hyperopic outcomes, the Wang-Koch adjustment for eyes with axial length >25.0 mm resulted in myopic errors, especially for Haigis formula[10]. The modified Wang-Koch adjustment for longer eyes performed better only for Holladay 1[11] but not for SRK/T[12]. For the above reasons, we chose Haigis formula without adjustment to calculate the IOL power and the target refraction was closest to emmetropia.
Follow up examinations were carried out at 1d, 1 and 3mo postoperatively. Three months after surgery, additional measurements were performed including refraction with the OPD Scan III (Nidek Co. Ltd., Japan), contrast sensitivity with RM800 Contrast Sensitivity unit (Raymon Photoelectricity Tech.Co., China), defocus testing, Catquest-9SF questionnaire and questionnaire about optical phenomena. Monocular contrast sensitivity was evaluated with and without glare under photopic condition. Defocus testing was conducted monocularly, under photopic conditions, by adding lens from -4.0 D to +1.0 D in gradient of 0.5 D over distance-corrected refraction.
The Catquest-9SF questionnaire was used to evaluate visual function and satisfaction after cataract surgery since 2008[13]. It consists of 9 items. Items A and B are global assessments about difficulty in daily life and vision satisfaction. Item C1-C7 are concerned with specific daily-life activities. There are 4 response options for each item: 4 =“very great difficulty or very dissatisfied”, 3 =“great difficulty or fairly dissatisfied”, 2 =“some difficulty or fairly satisfied” and 1=“no difficulty or very satisfied”. Additional option “Cannot decide” is considered as missing data in analysis. Halo and glare symptoms were evaluated with a questionnaire. The response to the questionnaire was classified into: none, slight, moderate, and severe.
Statistical Analysis
SPSS software 23.0 for windows was used for statistical analysis. The normal distribution of all data was tested by Shapiro-Wilk test. When the data were normally distributed, the Student's t-test was used to analyze the comparison between groups. When the data didn't conform to the normal distribution, the Mann-Whitney U test was used. The Fisher exact test was used to assess sex and questionnaire about halo and glare between groups. Differences were considered statistically significant with a P value less than 0.05.
RESULTS
This study comprised 30 cases (30 eyes) in trifocal group and 30 cases (30 eyes) in refractive multifocal group. Patient demographics and preoperative clinical data in both groups are shown in Table 1. There were no significant statistical differences in age, gender, axial length, corneal astigmatism, IOL power, target refraction, preoperative visual acuity, spherical dioptre, refractive cylinder and sphere equivalent between the two groups. All patients completed follow-up. None of the eyes were excluded from this study because of intraoperative or postoperative complications.
Table 1. Patient demographics and preoperative clinical data.
| Parameter | Trifocal IOL |
Refractive multifocal IOL |
P | ||
| Mean±SD | Range | Mean±SD | Range | ||
| Age (y) | 57.33±7.97 | 48-77 | 57.20±9.07 | 43-84 | 0.91 |
| Sex (M/F) | 23/7 | 17/13 | 0.17 | ||
| Axial length (mm) | 24.44±1.82 | 22.24 to 29.94 | 24.44±1.31 | 22.61 to 27.65 | 0.63 |
| Corneal astigmatism (D) | -0.51±0.27 | -0.97 to 0 | -0.56±0.23 | -0.93 to -0.15 | 0.39 |
| IOL power (D) | 18.63±4.48 | 5 to 24.5 | 18.63±3.65 | 9.5 to 22.5 | 0.51 |
| Target refraction (D) | -0.09±0.13 | -0.43 to 0.26 | -0.17±0.20 | -0.50 to 0.41 | 0.10 |
| UDVA, logMAR | 0.72±0.34 | 0.40 to 1.60 | 0.85±0.41 | 0.30 to 2.00 | 0.16 |
| CDVA, logMAR | 0.62±0.28 | 0.30 to 1.60 | 0.74±0.32 | 0.30 to 1.50 | 0.12 |
| UIVA, logMAR | 0.62±0.25 | 0.30 to 1.20 | 0.69±0.33 | 0.30 to 2.00 | 0.49 |
| DCIVA, logMAR | 0.54±0.20 | 0.30 to 1.00 | 0.63±0.34 | 0.30 to 2.00 | 0.31 |
| UNVA, logMAR | 0.66±0.24 | 0.30 to 1.30 | 0.73±0.25 | 0.30 to 1.10 | 0.27 |
| DCNVA, logMAR | 0.60±0.19 | 0.3 to 1.0 | 0.69±0.25 | 0.30 to 1.10 | 0.22 |
| Sphere (D) | -1.70±3.97 | -14.75 to 2.75 | -2.26±3.36 | -10.75 to 2.25 | 0.19 |
| Cylinder (D) | -0.73±0.49 | -2.5 to 0.00 | -0.66±0.48 | -2.00 to 0.00 | 0.48 |
| Spherical equivalent (D) | -2.06±3.99 | -15.13 to 2.25 | -2.59±3.39 | -11.00 to 1.63 | 0.24 |
SD: Standard deviation; UDVA: Uncorrected distance visual acuity; CDVA: Corrected distance visual acuity; UIVA: Uncorrected intermediate visual acuity; DCIVA: Distance-corrected intermediate visual acuity; UNVA: Uncorrected near visual acuity; DCNVA: Distance-corrected near visual acuity.
Visual Acuity and Refractive Outcomes
Table 2 shows the monocular visual acuity and refractive outcome at 3mo postoperatively. There were no significant statistical differences between groups in uncorrected distance visual acuity (P=0.13) and UNVA (P=0.54). By contrast, uncorrected intermediate visual acuity (UIVA) in trifocal group was better than that in refractive multifocal group (P=0.02). Compared with preoperative visual acuity, patients in both groups had significantly improvements in uncorrected visual acuity at all distances.
Table 2. Monocular visual acuity and refractive outcome at 3mo postoperatively.
| Parameter | Trifocal IOL |
Refractive multifocal IOL |
P | ||
| Mean±SD | Range | Mean±SD | Range | ||
| UDVA, logMAR | 0.00±0.08 | -0.1 to 0.1 | 0.04±0.07 | -0.1 to 0.1 | 0.13 |
| UIVA, logMAR | 0.06±0.07 | -0.1 to 0.2 | 0.11±0.08 | 0.00 to 0.30 | 0.02a |
| UNVA, logMAR | 0.11±0.10 | -0.1 to 0.3 | 0.13±0.10 | 0.00 to 0.40 | 0.54 |
| Sphere (D) | -0.16±0.34 | -0.75 to 0.5 | -0.98±0.52 | -1.5 to 0.25 | <0.01a |
| Cylinder (D) | -0.44±0.22 | -0.75 to 0.00 | -0.45±0.21 | -0.75 to 0.00 | 0.85 |
| Spherical equivalent (D) | -0.38±0.36 | -1.13 to 0.38 | -1.21±0.48 | -1.88 to 0 | <0.01a |
aStatistically significant (P<0.05).
OPD Scan III provided the objective refraction in the center of cornea and in 4 mm of cornea. The data in center of cornea were analyzed in this study. At follow-up of 3mo after surgery, no statistically significant between-group difference was founded in refractive cylinder (P=0.43). Compared to trifocal group, spherical refraction and spherical equivalent in refractive multifocal group were more myopic (P<0.01).
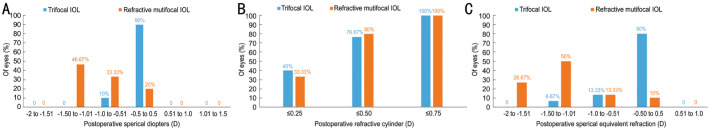
Figure 1 shows the distribution of monocular refractive outcomes at 3mo postoperatively. Monocular spherical dioptre was within ±0.5 D in 27 eyes (90%) and ±1.0 D in 30 eyes (100%) in trifocal group. In the case of refractive multifocal group, only 6 eyes (20%) were within ±0.5 D, other 24 eyes (80%) ranged from -1.5 D to 0.51 D (Figure 1A). Postoperative monocular refractive cylinder of all eyes in both groups was less than or equal to 0.75 D. The 23 eyes (76.67%) and 24 eyes (80%) were less than or equal to 0.50 D, 12 eyes (40%) and 10 eyes (33.33%) were less than or equal to 0.25 D, respectively in trifocal group and refractive multifocal group (Figure 1B). The distribution of monocular spherical equivalent was similar to that of spherical dioptre. Twenty-four eyes (80%) were within ±0.5 D in trifocal group. Only 3 eyes (10%) were within ±0.5 D in refractive multifocal group, other 27 eyes (90%) were in the range from -1.5 D to -0.51 D (Figure 1C).
Figure 1. Distribution of monocular refractive outcomes for trifocal and refractive multifocal groups at 3mo postoperatively.
A: Spherical dioptre; B: Refractive cylinder; C: Spherical equivalent refraction.
Defocus Curves
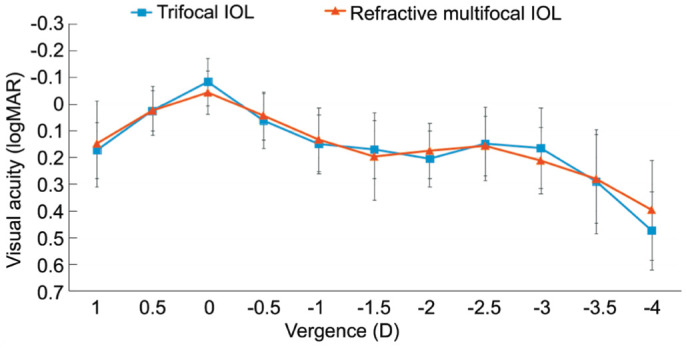
Monocular defocus curves of both groups at 3mo postoperatively are shown in Figure 2. There were no significant statistical differences at any vergence between the trifocal group and the refractive multifocal group. The curves of both groups were almost identical and had two peaks. The first peak of visual acuity representing the best corrected distance visual acuity was obtained at 0.00 D. The second peak of visual acuity representing the best corrected near visual acuity at 40 cm was observed at -2.50 D. Due to the +1.66 D addition power for intermediate focus, the defocus curve of trifocal IOL is smoother than that of refractive multifocal IOL in the range of 0-2.5 D, with vision of 0.2 logMAR or better.
Figure 2. Monocular defocus curves at 3mo postoperatively.
Contrast Sensitivity
Monocular contrast sensitivity under photopic conditions for different IOLs 3mo postoperatively are shown in Figure 3. As Figure 3A displays, contrast sensitivity without glare in both groups were only slightly lower than the normal 50-70 aged cohort at 6 cycles per degree (cpd). Normal data come from Pomerance and Evans[14].
Figure 3. The 3-month postoperative monocular contrast sensitivity under photopic conditions.
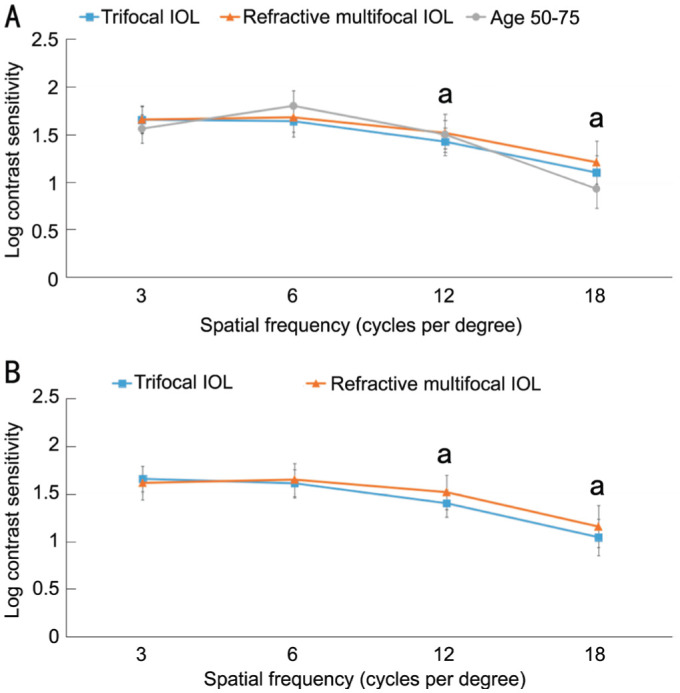
A: Without glare; B: With glare. aStatistically significant (P<0.05).
For the refractive multifocal group, the photopic contrast sensitivity without glare was better at 12 and 18 cpd (P=0.017; P=0.043), but there was no statistically significant difference at 3 and 6 cpd. As Figure 3B displays under the bright light condition with glare, there was no statistically significant difference in contrast sensitivity with glare under photopic conditions between the groups at 3 and 6 cpd. Compared with the trifocal group, the refractive multifocal group performed better at 12 and 18 cpd (P=0.01, P=0.034).
Catquest-9SF Questionnaire
There were no statistically significant differences in results for the Catquest-9SF questionnaire between groups in Figure 4 (item A, P=0.74; item B, P=0.66; item C1, P=0.26; item C2, P=0.72; item C3, P=0.37; item C4, P=0.17; item C5, P=0.32; item C6, P=0.28; item C7, P=0.62).
Figure 4. Results for the Catquest-9SF questionnaire.
A: Results for item A; B: Results for item B; C: Results for item C1-C7.
Halo and Glare Symptoms
The 66.7% and 60% of patients reported halo symptom in trifocal group and refractive multifocal group, respectively (Table 3). The differences were not statistically significant between groups (P=0.65). The 53.3% and 46.7% of patients reported glare symptom and the differences was also not statistically significant between groups (P=0.73).
Table 3. The 3-month postoperative monocular halo and glare symptoms.
| Parameters | Trifocal IOL | Refractive multifocal IOL | P |
| Halo | 0.65 | ||
| None | 10 (33.3) | 12 (40.0) | |
| Slight | 12 (40.0) | 8 (26.7) | |
| Moderate | 6 (20.0) | 6 (20.0) | |
| Severe | 2 (6.7) | 4 (13.3) | |
| Glare | 0.73 | ||
| None | 14 (46.7) | 16 (53.3) | |
| Slight | 6 (20.0) | 8 (26.7) | |
| Moderate | 7 (23.3) | 4 (13.3) | |
| Severe | 3 (10.0) | 2 (6.7) |
n (%)
DISCUSSION
As far as we know, this study is the first clinical prospective study comparing trifocal IOL with rotationally asymmetric refractive multifocal IOL. AT Lisa tri 839MP IOL and Lentis Mplus LS-313 MF30 IOL offered satisfactory distance, intermediate, and near visual outcomes after surgery. The former was a little better than the latter in intermediate visual acuity. However, this difference was not reflected in defocus curves. Trifocal diffractive IOL provide significantly better intermediate vision over bifocal diffractive IOL[15]–[19], apodized diffractive IOL[17], and symfony IOL[20]. From the above studies and our study, we can speculate that due to the design of focus for intermediate distance, trifocal IOLs perform better at intermediate distance visual acuity than other IOLs, including bifocal diffractive IOLs, apodized diffractive IOLs, rotationally asymmetric refractive multifocal IOLs and extended depth of field IOLs. A lot of researches[20]–[23] demonstrated the near vision in trifocal group was also better than that in extended range of focus group. In a comparative analysis, Mojzis et al[16] showed better monocular UNVA and distance-corrected near visual acuity in the trifocal group than that in the bifocal group. In our study, Lentis Mplus LS-313 MF30 offered the same level of near visual acuity as that of AT Lisa tri 839 MP, owing to the refractive design and an +3.00 D add power in near vision zone on optic. Different from trifocal IOLs, rotationally asymmetric refractive multifocal IOLs provide different near addition powers. Lentis Mplus MF15 IOL with the +1.5 D power addition provided good intermediate visual acuity with high quality of vision[24].
All postoperative refraction were carried out with OPD Scan III. Refractive cylinders in both groups were roughly the same, but the spherical dioptre in refractive multifocal group was more myopic than that in trifocal group. Since the calculation of spherical equivalent is based on spherical dioptre, a similar result was found in spherical equivalent. Why is there a difference about spherical dioptre evaluated by auto refractometer between trifocal IOL and refractive multifocal IOL? van der Linden et al[25] reported that after implantation of rotationally asymmetric multifocal IOL, the spherical dioptre with automated refraction was +0.98 D more myopic than subjective manifest refraction. Compared to subjective manifest refraction, the spherical equivalent with automated refraction was +1.11 D more myopic. Albarrán-Diego et al[26] found a similar result that the difference between automated refraction and subjective manifest refraction was about 1.25 D in rotationally asymmetric refractive multifocal IOL. We can infer from the above studies that spherical refraction measuring with auto refractometer may cause mean error of +1.0 D approximately in rotationally asymmetric multifocal IOL. In our study, the difference of mean spherical dioptre between trifocal IOL and refractive multifocal IOL was +0.83±0.60 D. The reason for pseudomyopia with auto refractometer in the sector-shaped addition multifocal IOLs may lie in its geometrical asymmetry. Two different refractive surfaces in these IOLs yield two foci in pupil area. Therefore, for the emmetropia implanted with refractive multifocal IOL, auto refractometer detects two refractions of 0.00 D and -2.5 D and results in an average of about -1.25 D[27]. In addition to refractive measuring inaccuracy, other differences caused by geometrical asymmetry is worthy of further study.
The defocus curves in our study were largely similar to that investigated by previous studies[9],[20],[24],[27]. The curves of AT Lisa tri 839 MP IOL (+3.33 D addition) and Lentis Mplus LS-313 MF30 IOL (+3.0 D addition) are almost identical. Both IOLs have near power addition about +3.0 D, and the second peaks of visual acuity representing the best corrected near visual acuity were all observed at -2.50 D. For the defocus levels of -1.00, -1.50, and -2.0 D, the visual acuity of the trifocal group was significantly better than that of the bifocal group[16]–[17]. However, in our study, there was no statistically significant difference in defocus levels of -1.00, - 1.50, and -2.0 D between trifocal group and refractive multifocal group. We speculate a seamless transition zone between distance vision zone and near vision zone in refractive multifocal IOL may be the reason for better performance of intermediate distance visual acuity in defocus curve than bifocal IOL.
In the past, the multifocal IOLs are considered to give rise to the loss of contrast sensitivity. In our study, we found that the monocular photopic contrast sensitivity without glare was similar to that of normal people aged 50-75y[14], with a little decline at 6 cpd. Pedrotti et al[24], Alió et al[27] and Muñoz et al[28] found no difference in photopic contrast sensitivity between rotationally asymmetric multifocal IOL and monofocal IOL. Alio et al[29] also found for the asymmetric multifocal IOL with spatial frequencies of 12 and 18 cpd, its photopic contrast sensitivity was significantly better than that of diffractive bifocal IOL. The photopic contrast sensitivity with and without glare in asymmetrical multifocal group was significantly superior to trifocal group for the spatial frequencies of 12 and 18 cpd in our study. A seamless transition in refractive multifocal IOL which makes light loss only about 8% and avoids influence of diffraction may contributes to better contrast sensitivity.
In addition to the clinical report outcome such as visual acuity and contrast sensitivity, the patient report outcome is also an important evaluation of quality of vision after cataract surgery[30]. The short-form cataract questionnaire (Catquest-9SF) has been translated to many languages and validated in many countries including China[31]. Patients in both groups had high satisfaction with postoperative vision and little difficulty int daily life. Differences of difficulty with specific daily-life activities (item C1-C7) were not statistically significant between the trifocal group and the refractive multifocal group. Rementería-Capelo et al[32] evaluated patients' subjective perception of vision using the Catquest-9SF and found the outcomes with no differences between trifocal IOLs and toric trifocal IOLs.
In our study, 66.7% of patients reported halo, and 53.3% of patients reported glare in trifocal group. The percentages in the study by Mencucci et al[22] were 70% and 50%, and in the study by Hayashi et al[18] were 65.6% and 43.8%. Obviously, the incidence of halo was higher than that of glare. We found that there was no significant differences in optical phenomena between trifocal IOL and refractive multifocal IOL. Previous study has also demonstrated that the halo and glare of trifocal IOLs is not significantly different from that of symfony IOLs[22]. Kim et al[33] reported that unilateral implantation of trifocal IOL in patients who had implantation of monofocal IOL previously led to a low rate of severe glare or halo.
This study has some limitations. First, it was a prospective nonrandom comparative study. The IOLs were not assigned randomly. Second, the data in this study were monocular. In addition, the follow-up time is short. In future study, patients with bilateral cataract surgery will be included and the follow-up time will be longer.
In conclusion, trifocal IOL was superior to refractive multifocal IOL in intermediate visual acuity. Rotationally asymmetric refractive multifocal IOL was more myopic in automated refraction and significantly better for the photopic contrast sensitivity at high frequency.
Acknowledgments
Authors' contributions: Hui N contributed guarantor of integrity of entire study and wrote the manuscript; Chu MF contributed to data analysis; Li Y contributed to literature research and manuscript preparation; Wang CY performed surgeries in the study; Yu L and Ma B contributed to patients' enrollment and follow up.
Foundations: Supported by Key Research and Development Projects of Shaanxi Province (No.2017SF-246); Science and Technology Planning Project of Xi'an (No.2017116SF/YX010; No.201805097YX5SF31).
Conflicts of Interest: Hui N, None; Chu MF, None; Li Y, None; Wang CY, None; Yu L, None; Ma B, None.
REFERENCES
- 1.Holden BA, Fricke TR, Ho SM, Wong R, Schlenther G, Cronjé S, Burnett A, Papas E, Naidoo KS, Frick KD. Global vision impairment due to uncorrected presbyopia. Arch Ophthalmol. 2008;126(12):1731–1739. doi: 10.1001/archopht.126.12.1731. [DOI] [PubMed] [Google Scholar]
- 2.Wolffsohn JS, Davies LN. Presbyopia: effectiveness of correction strategies. Prog Retin Eye Res. 2019;68:124–143. doi: 10.1016/j.preteyeres.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Fricke TR, Tahhan N, Resnikoff S, Papas E, Burnett A, Ho SM, Naduvilath T, Naidoo KS. Global prevalence of presbyopia and vision impairment from uncorrected presbyopia. Ophthalmology. 2018;125(10):1492–1499. doi: 10.1016/j.ophtha.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Bilbao-Calabuig R, Gónzalez-López F, Llovet-Rausell A, Ortega-Usobiaga J, Tejerina Fernández V, Llovet-Osuna F. Lens-based surgical correction of presbyopia. Where are we in 2020? Arch Soc Esp Oftalmol (Engl Ed) 2021;96(2):74–88. doi: 10.1016/j.oftal.2020.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Alió JL, Pikkel J. Multifocal intraocular lenses: neuroadaptation. Essentials in Ophthalmology. Cham: Springer International Publishing; 2019. pp. 53–60. [Google Scholar]
- 6.Moore JE, McNeely RN, Pazo EE, Moore TCB. Rotationally asymmetric multifocal intraocular lenses: preoperative considerations and postoperative outcomes. Curr Opin Ophthalmol. 2017;28(1):9–15. doi: 10.1097/ICU.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 7.Venter JA, Barclay D, Pelouskova M, Bull CEL. Initial experience with a new refractive rotationally asymmetric multifocal intraocular lens. J Refract Surg. 2014;30(11):770–776. doi: 10.3928/1081597X-20141021-09. [DOI] [PubMed] [Google Scholar]
- 8.Bilbao-Calabuig R, Llovet-Rausell A, Ortega-Usobiaga J, et al. Visual outcomes following bilateral lmplantation of two diffractive trifocal intraocular lenses in 10 084 eyes. Am J Ophthalmol. 2017;179:55–66. doi: 10.1016/j.ajo.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Kohnen T, Hemkeppler E, Herzog M, Schönbrunn S, DeLorenzo N, Petermann K, Böhm M. Visual outcomes after implantation of a segmental refractive multifocal intraocular lens following cataract surgery. Am J Ophthalmol. 2018;191:156–165. doi: 10.1016/j.ajo.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125(2):169–178. doi: 10.1016/j.ophtha.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Popovic M, Schlenker MB, Campos-Möller X, Pereira A, Ahmed IIK. Wang-Koch formula for optimization of intraocular lens power calculation: evaluation at a Canadian center. J Cataract Refract Surg. 2018;44(1):17–22. doi: 10.1016/j.jcrs.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 12.Cheng HH, Liu LP, Sun A, Wu MX. Accuracy of modified axial length adjustment for intraocular lens power calculation in Chinese axial myopic eyes. Curr Eye Res. 2020;45(7):827–833. doi: 10.1080/02713683.2019.1698053. [DOI] [PubMed] [Google Scholar]
- 13.Lundström M, Kugelberg M, Montan P, Nilsson I, Zetterberg M, Pesudovs K, Behndig A. Catquest-9SF functioning over a decade - a study from the Swedish National Cataract Register. Eye Vis (Lond) 2020;7(1):56. doi: 10.1186/s40662-020-00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pomerance GN, Evans DW. Test-retest reliability of the CSV-1000 contrast test and its relationship to glaucoma therapy. Invest Ophthalmol Vis Sci. 1994;35(9):3357–3361. [PubMed] [Google Scholar]
- 15.Kim M, Kim JH, Lim TH, Cho BJ. Comparison of reading speed after bilateral bifocal and trifocal intraocular lens implantation. Korean J Ophthalmol. 2018;32(2):77–82. doi: 10.3341/kjo.2017.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mojzis P, Kukuckova L, Majerova K, Ziak P, Piñero DP. Postoperative visual performance with a bifocal and trifocal diffractive intraocular lens during a 1-year follow-up. Int J Ophthalmol. 2017;10(10):1528–1533. doi: 10.18240/ijo.2017.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaymak H, Breyer D, Alió JL, Cochener B. Visual performance with bifocal and trifocal diffractive intraocular lenses: a prospective three-armed randomized multicenter clinical trial. J Refract Surg. 2017;33(10):655–662. doi: 10.3928/1081597X-20170504-04. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi K, Sato T, Igarashi C, Yoshida M. Comparison of visual outcomes between bilateral trifocal intraocular lenses and combined bifocal intraocular lenses with different near addition. Jpn J Ophthalmol. 2019;63(6):429–436. doi: 10.1007/s10384-019-00693-4. [DOI] [PubMed] [Google Scholar]
- 19.Jin SS, Friedman DS, Cao K, et al. Comparison of postoperative visual performance between bifocal and trifocal intraocular Lens based on randomized controlled trails: a meta-analysis. BMC Ophthalmol. 2019;19(1):78. doi: 10.1186/s12886-019-1078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lubiński W, Podborączyńska-Jodko K, Kirkiewicz M, Mularczyk M, Post M. Comparison of visual outcomes after implantation of AtLisa tri 839 MP and Symfony intraocular lenses. Int Ophthalmol. 2020;40(10):2553–2562. doi: 10.1007/s10792-020-01435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rementería-Capelo LA, García-Pérez JL, Gros-Otero J, Carrillo V, Pérez-Lanzac J, Contreras I. Real-world evaluation of visual results and patient satisfaction for extended range of focus intraocular lenses compared to trifocal lenses. Int Ophthalmol. 2021;41(1):163–172. doi: 10.1007/s10792-020-01563-6. [DOI] [PubMed] [Google Scholar]
- 22.Mencucci R, Favuzza E, Caporossi O, Savastano A, Rizzo S. Comparative analysis of visual outcomes, reading skills, contrast sensitivity, and patient satisfaction with two models of trifocal diffractive intraocular lenses and an extended range of vision intraocular lens. Graefes Arch Clin Exp Ophthalmol. 2018;256(10):1913–1922. doi: 10.1007/s00417-018-4052-3. [DOI] [PubMed] [Google Scholar]
- 23.Monaco G, Gari M, di Censo F, Poscia A, Ruggi G, Scialdone A. Visual performance after bilateral implantation of 2 new presbyopia-correcting intraocular lenses: Trifocal versus extended range of vision. J Cataract Refract Surg. 2017;43(6):737–747. doi: 10.1016/j.jcrs.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 24.Pedrotti E, Mastropasqua R, Bonetto J, Demasi C, Aiello F, Nucci C, Mariotti C, Marchini G. Quality of vision, patient satisfaction and long-term visual function after bilateral implantation of a low addition multifocal intraocular lens. Int Ophthalmol. 2018;38(4):1709–1716. doi: 10.1007/s10792-017-0652-x. [DOI] [PubMed] [Google Scholar]
- 25.van der Linden JW, Vrijman V, Al-Saady R, van der Meulen IJ, Mourits MP, Lapid-Gortzak R. Autorefraction versus subjective refraction in a radially asymmetric multifocal intraocular lens. Acta Ophthalmol. 2014;92(8):764–768. doi: 10.1111/aos.12410. [DOI] [PubMed] [Google Scholar]
- 26.Albarrán-Diego C, Muñoz G, Rohrweck S, García-Lázaro S, Albero JR. Validity of automated refraction after segmented refractive multifocal intraocular lens implantation. Int J Ophthalmol. 2017;10(11):1728–1733. doi: 10.18240/ijo.2017.11.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alió JL, Piñero DP, Plaza-Puche AB, Chan MJR. Visual outcomes and optical performance of a monofocal intraocular lens and a new-generation multifocal intraocular lens. J Cataract Refract Surg. 2011;37(2):241–250. doi: 10.1016/j.jcrs.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 28.Muñoz G, Albarrán-Diego C, Ferrer-Blasco T, Sakla HF, García-Lázaro S. Visual function after bilateral implantation of a new zonal refractive aspheric multifocal intraocular lens. J Cataract Refract Surg. 2011;37(11):2043–2052. doi: 10.1016/j.jcrs.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 29.Alio JL, Plaza-Puche AB, Javaloy J, Ayala MJ, Moreno LJ, Piñero DP. Comparison of a new refractive multifocal intraocular lens with an inferior segmental near add and a diffractive multifocal intraocular lens. Ophthalmology. 2012;119(3):555–563. doi: 10.1016/j.ophtha.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 30.Kabanovski A, Hatch W, Chaudhary V, El-Defrawy S, Reid R, Ahmed IIK, Schlenker MB. Validation and application of Catquest-9SF in various populations: a systematic review. Surv Ophthalmol. 2020;65(3):348–360. doi: 10.1016/j.survophthal.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Xu ZQ, Wu S, Li WZ, Dou Y, Wu Q. The Chinese Catquest-9SF: validation and application in community screenings. BMC Ophthalmol. 2018;18(1):77. doi: 10.1186/s12886-018-0743-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rementería-Capelo LA, Contreras I, García-Pérez JL, Blázquez V, Ruiz-Alcocer J. Visual quality and patient satisfaction with a trifocal intraocular lens and its new toric version. J Cataract Refract Surg. 2019;45(11):1584–1590. doi: 10.1016/j.jcrs.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Kim JY, Lee YH, Won HJ, Jeong H, Park JH, Kim MJ, Tchah H. Diffractive multifocal intraocular lens implantation in patients with monofocal intraocular lens in the contralateral eye. Int J Ophthalmol. 2020;13(5):737–743. doi: 10.18240/ijo.2020.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]