Abstract
AIM
To assess the effect of 0.01% atropine eye drops on intraocular pressure (IOP) in myopic children.
METHODS
A placebo-controlled, double-masked, randomized study. Totally 220 children aged 6 to 12y with myopia ranging from -1.00 to -6.00 D in both eyes were enrolled. Children were randomized in a 1:1 ratio to either 0.01% atropine eye drops or a placebo group using generated random numbers. All participants underwent the examination of IOP and cycloplegic refraction at baseline, 6 and 12mo. The change of IOP and the proportion of subjects with increased IOP in atropine and placebo groups were compared.
RESULTS
Of 220 children, 117 were boys (53.2%). A total of 159 (72.3%) participants completed the follow-up at the 1-year study. At baseline, the mean IOP was 15.74 mm Hg (95%CI, 15.13 to 16.34 mm Hg) for the 0.01% atropine group and 15.59 mm Hg (95%CI, 15.00 to 16.19 mm Hg) for placebo group (mean difference, 0.14 mm Hg; P=0.743) after adjusting for central corneal thickness at baseline. At one year follow-up, the mean change of IOP was 0.16 mm Hg (95%CI, -0.43 to 0.76 mm Hg) for the 0.01% atropine group and -0.11 mm Hg (95%CI, -0.71 to 0.50 mm Hg) for placebo group (mean difference, 0.27 mm Hg; P=0.525) after adjusting for central corneal thickness. The 51.4% of children have increased IOP in the 0.01% atropine group, compared with 45.9% in the placebo group (P=0.511).
CONCLUSION
The 0.01% atropine eye drops do not significantly affect the risk of elevated IOP. It is relatively safer to use in the studies that try to minimize myopia progression. However, a further long-duration study is required to be validated.
Keywords: intraocular pressure, 0.01% atropine eye drops, myopic children
INTRODUCTION
Myopia has become a severe worldwide public health issue[1]–[3]. It is predicted globally that the occurrence of myopia and high myopia is projected to a greater extent, affecting almost 5 billion and 1 billion people respectively by 2050[4]. Among urban Chinese children, the prevalence of myopia was 3.9% in grade 1 children, with 67.3% in grade 7 children[5]. Wei et al[6] conducted a cross-sectional university-based study in central China and found the prevalence of myopia was 83.2%, and 11.1% for high myopia among university students. It has been reported that high or pathologic myopia can lead to irreversible blinding conditions, such as myopic retinopathy, retinal detachment, and choroidal neovascularization[7]–[10].
Many experiments were carried out to explore the interventions of reducing myopia progression in children[11]–[13]. Atropine, a competitive antagonist of muscarinic cholinergic receptors, showed a remarkable part in the control of the progression of myopia[14]–[16]. In the atropine for the treatment of myopia 2 (ATOM 2) study in Singapore, Chia et al[17] reported the mean myopia progression over 2y was -0.30±0.60, -0.38±0.63, and -0.49±0.63 diopters (D) in the atropine 0.5%, 0.1%, and 0.01% groups, respectively. In a randomized, placebo-controlled study, Wei et al[18] showed that 0.01% atropine eye drops could reduce axial elongation and myopia progression in children without serious adverse reaction. Apart from its fruitful results, atropine was thought to have some eye complications. Previous studies have reported that myopia progression can be stopped or reversed due to installing 1% atropine eye drops but causes the risk of pupillary dilation, stinging sensation, eye pain, photophobia, and blurring.
Except for the confirmed eye complications, the potential complications were that atropine leads to elevated intraocular pressure (IOP), owing to pupil dilation. Greenstein et al[19] showed that IOP tends to rise by more than 6 mm Hg due to topical administration of anticholinergics about in 8% of normal adults. However, Mughannam et al[20] identified that no significant IOP changes by using 1% atropine in horse eye. Wu et al[21] have conducted a retrospective study on a larger scale of 621 myopic children to evaluate the effect of dosage ranging from 0.1% to 1% atropine eye drops on IOP. They found no relationship between the treatment period and increasing dose of atropine with IOP elevation. Yu et al[22] conducted a study to determine whether long-term topical atropine therapy at various concentrations induces IOP elevation and subsequent development of glaucoma. This study revealed that topical atropine eye drops do not induce ocular hypertension and effectively slow the progression of myopia. Chan et al[23] enrolled 67 eyes of 35 myopic children receiving 0.25% atropine treatment. They found no significant change in IOP, optic nerve parameters, or retinal nerve fiber layer thickness over a mean of 15.2±2.4mo of treatment.
Many studies have evaluated the effect of atropine eye drops on IOP in myopic children. At present, 0.01% atropine is increasingly used to treat myopic children[24]–[25]. However, the effect of 0.01% atropine eye drops on IOP has not been extensively evaluated in myopic children. All above studies do not include the most frequently used 0.01% atropine eye drops in clinical practice.
In this placebo-controlled, double-masked, randomized trial of one year study, we represent the findings of IOP at baseline, 6 and 12mo of school children during administration of 0.01% atropine eye drops. The main objective of this research is to assess the effect of 0.01% atropine on IOP.
SUBJECTS AND METHODS
Ethical Approval
Written informed consent was obtained from at least one parent and verbal assent from all children. This clinical trial adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Beijing Tongren Hospital, Capital Medical University. Trial registration http://www.chictr.org.cn identifier: ChiCTR-IOR-17013898.
Study Design
This is a placebo-controlled, double-masked, randomized study conducted from April 2018 to July 2019. Children aged 6-12y were recruited to receive either 0.01% atropine eye drops or a placebo in a 1:1 ratio once nightly for one year. Details of the methodology have been provided elsewhere[18].
Study Population
This study was conducted at Beijing Tongren Hospital, Beijing, China. All children are 6-12y with spherical equivalent (SE) ranging from -1.00 to -6.00 D in the right and left eyes, astigmatism of less than 1.50 D in both eyes, IOP of less than 21 mm Hg. Exclusion criteria were those with combined ocular diseases, such as strabismus, cataract, amblyopia, glaucoma, corneal scar, and ocular tumor, allergy to atropine, cyclopentolate, or excipients, current or previous use of atropine, bifocals, contact lenses, or other methods for myopia control. Poor general health children with history of cardiac or significant respiratory diseases, and long-term systemic medication use were also excluded.
Randomization and Masking
The 0.01% atropine and placebo eye drops were prepackaged in identical bottles with only the subject number and expiration date. The investigators and subjects were masked to identify the contents to make the entire process more accurate. A statistician operated the randomization independently with the schedule generated by the SAS program (SAS Institute, Cary, NC, USA). Every four children were arbitrarily randomized into the intervention or control group among eligible children.
Study Procedures
All children experienced identical standardized investigation strategies at the baseline, 6-month, and 12-month visits. Cycloplegic refraction was measured by an autorefractor (HRK7000 A, Huvitz, South Korea) at each visit after a cycloplegic procedure with 3 drops of 1% cyclopentolate at 5-minute intervals. The measurement was performed after 30min of installation of the third drop. Another drop of the same cycloplegic eye drops would be administered after the third drop if full cycloplegia had not been achieved after 30min, and the examination was performed 15min later. All 3 readings should be at most 0.25 D apart in both the spherical and cylinder components. Before cycloplegic refraction, three readings of IOP measurements were taken by a non-contact tonometer (HNT-7000, Huvitz, South Korea).
Statistical Analysis
The SE was calculated as the spherical D and half of the cylinder (sphere+0.5×cylinder). All statistical analysis was performed using SPSS V.15.0 (SPSS, Chicago, Illinois, USA) software. There was a high correlation between the right and left eyes, so the statistical analysis was performed using the right eye data. Two independent sample t-tests and an analysis of covariance were used to compare the IOP between 0.01% atropine and placebo groups. We used Chi-square tests to compare the proportion of subjects with increased IOP and categorical variables between the two groups. Age, sex, SE, axial length (AL), and IOP were calculated and analyzed. The 2-sided P-value was considered statistically significant when the values were less than 0.05.
RESULTS
A total of 220 children were recruited into the study, with equal randomization to the 0.01% atropine and the placebo groups. Of 220 children, 103 were girls (46.8%). No significant differences were found between the 0.01% atropine and the placebo groups in the baseline characteristics, including age, gender, initial AL, initial SE, parental myopia, age at myopia onset, time outdoors, and near work. A total of 159 (72.3%) participants completed the follow-up during the 1-year study, with 76 and 83 children allocated into the 0.01% atropine and placebo groups (Table 1). No significant differences were found in initial IOP between discontinued and completed subjects in the 0.01% atropine eye drops and placebo groups (P>0.05).
Table 1. Demographics and baseline characteristics of 159 participants who completed the follow-up during the 1-year study.
| Variables | Atropine group (n=76) | Placebo group (n=83) | P |
| Age at baseline (y) | 9.69±1.61 | 9.85±1.56 | 0.524 |
| Male/female | 38/38 | 43/40 | 0.820 |
| Initial SE (D) | -2.55±1.29 | -2.68±1.43 | 0.553 |
| Initial AL (mm) | 24.58±0.80 | 24.64±0.98 | 0.640 |
| IOP (mm Hg) | 15.75±2.78 | 15.64±2.58 | 0.790 |
| Central corneal thickness (mm) | 549.05±33.04 | 543.74±32.99 | 0.323 |
| Age at myopia onset (y) | 7.92±1.57 | 8.06±1.69 | 0.600 |
| Parental myopia | 0.167 | ||
| None | 5 | 11 | |
| One | 30 | 23 | |
| Both | 41 | 49 | |
| Time outdoors (h/d) | 1.28±0.59 | 1.43±0.80 | 0.200 |
| Near work (h/d) | 3.13±1.34 | 3.17±1.63 | 0.861 |
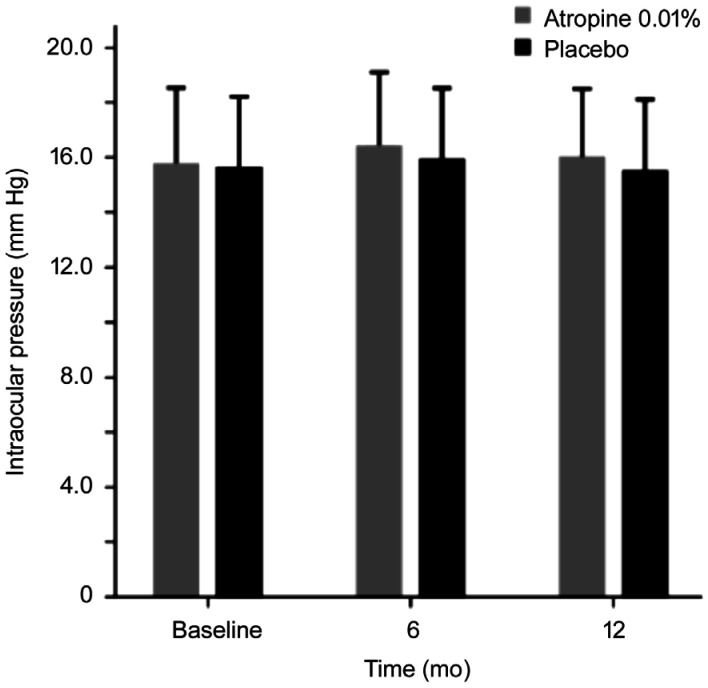
At baseline, the mean IOP was 15.74 mm Hg (95%CI, 15.13 to 16.34 mm Hg) for the 0.01% atropine group and 15.59 mm Hg (95%CI, 15.00 to 16.19 mm Hg) for placebo group (analysis of covariance, mean difference, 0.14 mm Hg; P=0.743) after adjusting for central corneal thickness at baseline. The respective mean IOP values for the 0.01% atropine eye drops and placebo groups at baseline, 6 and 12mo were shown in Figure 1. At 6mo of follow-up, the IOP ranged from 11.3 to 22.7 mm Hg in the 0.01% atropine group, and five children had increased IOP by more than 21 mm Hg. The IOP ranged from 11.1 mm Hg to 22.6 mm Hg in the placebo group, and two children had increased IOP by more than 21 mm Hg. At one year of follow-up, the IOP ranged from 11.0 to 22.0 mm Hg in the 0.01% atropine group, and one child had increased IOP by more than 21 mm Hg. The IOP ranged from 10.7 to 22.1 mm Hg in the placebo group, and two children had increased IOP by more than 21 mm Hg.
Figure 1. Mean intraocular pressure in the 0.01% atropine and placebo groups at follow-up visits.
At six months follow-up, the mean change of IOP was 0.66 mm Hg (95%CI, 0.13 to 1.18 mm Hg) for the 0.01% atropine group and 0.33 mm Hg (95%CI, -0.20 to 0.85 mm Hg) for placebo group (analysis of covariance, mean difference, 0.33 mm Hg; P=0.378) after adjusting for central corneal thickness (Table 2). At one year follow-up, the mean change of IOP was 0.16 mm Hg (95%CI, -0.43 to 0.76 mm Hg) for the 0.01% atropine group and -0.11 mm Hg (95%CI, -0.71 to 0.50 mm Hg) for placebo group (analysis of covariance, mean difference, 0.27 mm Hg; P=0.525) after adjusting for central corneal thickness (Table 2). There were no significant differences in the mean change of IOP between the two groups at 6 and 12mo.
Table 2. Mean change in intraocular pressure between 0.01% atropine eye drops and placebo groups at follow-up visits.
| Follow-ups | Atropine group (n=76) | Placebo group (n=83) | Difference | P |
| Change at 6mo | 0.66 (0.13 to 1.18) | 0.33 (-0.20 to 0.85) | 0.33 | 0.378 |
| Change at 12mo | 0.16 (-0.43 to 0.76) | -0.11 (-0.71 to 0.50) | 0.27 | 0.525 |
mm Hg (95%CI)
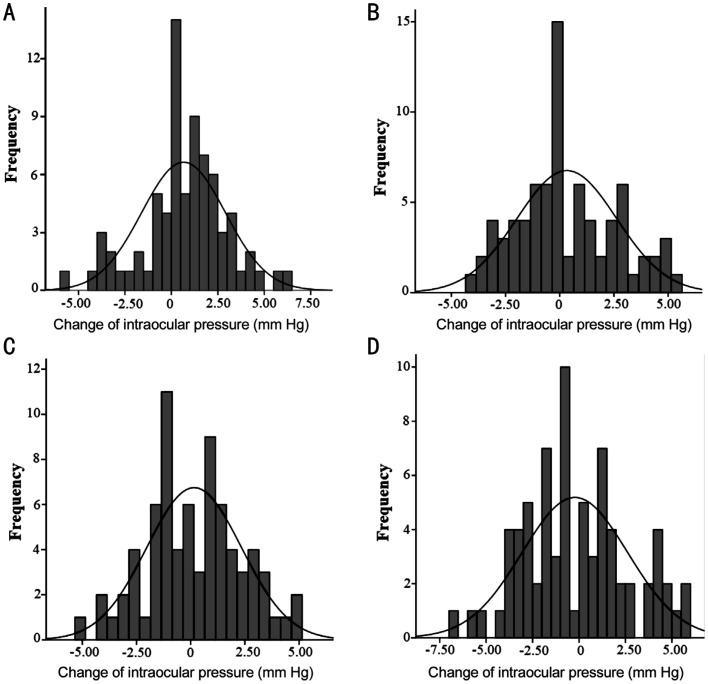
Figure 2 shows the distribution of IOP change for the 0.01% atropine and placebo groups at 6 and 12mo. At 6mo, 65.3% of children had increased IOP in the 0.01% atropine group, compared with 42.3% in the placebo group (P=0.004). At 12mo, 51.4% of children had increased IOP in the 0.01% atropine group, compared with 45.9% in the placebo group (P=0.511). In our multiple logistic regression analysis, among subjects whose IOP was increased in the 0.01% atropine group at 12mo, the increased IOP was not significantly associated with age (P=0.205), gender (P=0.407), SE (P=0.933), and AL (P=0.184).
Figure 2. Distribution of change of intraocular pressure (mm Hg) for the 0.01% atropine (A), placebo groups at 6mo (B), the 0.01% atropine (C), and placebo groups at 12mo (D).
DISCUSSION
In this randomized trial, our study showed that 0.01% atropine eye drops have no significantly elevated IOP during one year. At one year follow-up, the mean change of IOP in the 0.01% atropine group was 0.16 mm Hg (95%CI, -0.43 to 0.76 mm Hg) compared with -0.11 mm Hg (95%CI, -0.71 to 0.50 mm Hg) in the placebo group (P=0.525) after adjusting for central corneal thickness. The 51.4% of children have increased IOP in the 0.01% atropine group, compared with 45.9% in the placebo group (P=0.511). To our knowledge, the present trial was the first randomized, placebo-controlled study to present 0.01% atropine eye drops on the effect of IOP in mainland China.
Some studies had evaluated the effect of atropine eye drops on IOP in myopic children. In a retrospective study of 621 myopic children aged 10.5±2.3y to evaluate IOP for dosages ranging from 0.1% to 1% atropine eye drops, Wu et al[21] found that the cumulative dose and duration of atropine treatment are not associated with the potential risk of increased IOP. However, the change concentrations of 1%, 0.5%, 0.25%, and 0.1% atropine eye drops were used according to clinicians prescribed based on the severity of myopia for children on each visit in their study. Therefore, it is difficult to estimate the degree of influence of each concentration of atropine eye drops on IOP. Consistently, Lee et al[26] found that the respective mean changes of IOP for the 0.25% atropine, 0.125% atropine, and control groups were -1.28, 0.54, and -0.33 mm Hg at one year, showing no significant differences for three groups. However, in the study, only 32 children, 12 children, and 12 children were recruited in the 0.125 % atropine group, 0.25% atropine eye drop, and control group, respectively. In addition, both above two studies did not include the most frequently used 0.01% atropine eye drops in clinical practice. Our study found that the mean change of IOP in the 0.01% atropine group was 0.16 mm Hg (95%CI, -0.43 to 0.76 mm Hg) compared with -0.11 mm Hg (95%CI, -0.71 to 0.50 mm Hg) in the placebo group at one-year follow-up after adjusting for central corneal thickness, and no significant difference in the mean change of IOP between 0.01% atropine group and placebo group at 12mo.
In our study, we further analyzed the changes in the IOP of each subject during regular administration of 0.01% atropine eye drops. The IOP for the 0.01% atropine and placebo groups ranged from 11.0 to 22.0 and 10.7 mm Hg to 22.1 mm Hg at one-year follow-up. Only one child has increased IOP by more than 21 mm Hg in the 0.01% atropine group, and two children have increased IOP by more than 21mm Hg in the placebo group. The 51.4% of children have increased IOP in the 0.01% atropine group, compared with 45.9% in the placebo group (P=0.511) at 12mo. The present study demonstrated that a once-nightly dose of 0.01% atropine eye drops does not induce a rise in IOP in myopic children during one year.
The relationship between IOP and myopia continues to receive attention from researchers[27]–[30]. It has also been reported that moderate myopic children have significantly higher IOP than emmetropic children (P=0.022)[30]. Studies have suggested that the extension of the AL and thinning of the sclera caused by the development of myopia may lead to a decrease in eyeball compliance and an increase in IOP, thereby increasing IOP[31]. Therefore, myopic subjects with higher IOP levels may have a higher risk of glaucoma. It is shown that 0.01% of atropine eye drops did not increase IOP in myopic school-aged children.
The strength of our trial was that the first placebo-controlled, randomized study to present 0.01% atropine eye drops on the effect of IOP. It provided affirmative findings of the association between IOP and 0.01% atropine eye drops. However, there are some restrictions in our study. First, we used a non-contact tonometer instead of a Goldmann applanation tonometer as the gold standard to evaluate IOP, potentially leading to a higher reading of IOP in our study than if the IOP had been measured by Goldmann applanation tonometry[32]. However, a non-contact tonometer is clinically more tolerable for children[33]. In addition, an analysis of covariance was used to control for potential differences concerning central corneal thickness. Second, this study only evaluated the efficacy of 0.01% atropine eye drops on IOP for 1y. Thus, more years of follow-up studies should be performed.
In summary, it is concluded that 0.01% of atropine eye drops did not induce a rise in IOP in our study, and 0.01% of atropine eye drops are relatively safe to use in myopic children. In the future, further studies can conduct more details with a larger sample size and more years of follow-up to authenticate our study findings.
Acknowledgments
The authors thank all staff who contributed to this study.
Foundations: Supported by the National Natural Science Foundation of China (No.82071000); the Beijing Science Foundation for Distinguished Yong Scholars (No.JQ20029); the Capital Health Research and Development of Special (No.2020-2-1081); Supported by the Beijing Municipal Administration of Hospitals Incubating Program (No.PX2022007); the Primary Scientific Research Foundation for the Junior Researcher in Beijing Tongren Hospital, Capital Medical University (No.2020-YJJ-ZZL-011).
Conflicts of Interest: Bukhari J, None; Wei SF, None; Li SM, None; An WZ, None; Du JL, None; Liang XT, None; Gan JH, None; Tian JX, None; Bai WL, None; Cai ZN, None; Yin L, None; Wang NL, None.
REFERENCES
- 1.Baird PN, Saw SM, Lanca C, et al. Myopia. Nat Rev Dis Primers. 2020;6(1):99. doi: 10.1038/s41572-020-00231-4. [DOI] [PubMed] [Google Scholar]
- 2.Weiss RS, Park S. Recent updates on myopia control: preventing progression 1 diopter at a time. Curr Opin Ophthalmol. 2019;30(4):215–219. doi: 10.1097/ICU.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 3.Morgan IG, French AN, Ashby RS, Guo XX, Ding XH, He MG, Rose KA. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res. 2018;62:134–149. doi: 10.1016/j.preteyeres.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Li SM, Liu LR, Li SY, et al. Design, methodology and baseline data of a school-based cohort study in central China: the Anyang childhood eye study. Ophthalmic Epidemiol. 2013;20(6):348–359. doi: 10.3109/09286586.2013.842596. [DOI] [PubMed] [Google Scholar]
- 6.Wei SF, Sun YY, Li SM, Hu JP, Yang XH, Lin CX, Cao K, Du JL, Guo JY, Li H, Wang NL. Refractive errors in university students in central China: the Anyang university students eye study. Invest Ophthalmol Vis Sci. 2018;59(11):4691–4700. doi: 10.1167/iovs.18-24363. [DOI] [PubMed] [Google Scholar]
- 7.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25(5):381–391. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 8.Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol. 2014;157(1):9–25.e12. doi: 10.1016/j.ajo.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Ohno-Matsui K, Jonas JB. Posterior staphyloma in pathologic myopia. Prog Retin Eye Res. 2019;70:99–109. doi: 10.1016/j.preteyeres.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Cheong KX, Xu LQ, Ohno-Matsui K, Sabanayagam C, Saw SM, Hoang QV. An evidence-based review of the epidemiology of myopic traction maculopathy. Surv Ophthalmol. 2022 doi: 10.1016/j.survophthal.2022.03.007. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Z, Chen Y, Tan Z, Xiong R, McGuinness MB, Müller A. Interventions recommended for myopia prevention and control among children and adolescents in China: a systematic review. Br J Ophthalmol. 2021 doi: 10.1136/bjophthalmol-2021-319306. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Chen YX, Liao CM, Tan Z, He MG. Who needs myopia control? Int J Ophthalmol. 2021;14(9):1297–1301. doi: 10.18240/ijo.2021.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaiti R, Shyangbo R, Sharma IP, Dahal M. Review on current concepts of myopia and its control strategies. Int J Ophthalmol. 2021;14(4):606–615. doi: 10.18240/ijo.2021.04.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walline JJ, Lindsley K, Vedula SS, Cotter SA, Mutti DO, Twelker JD. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev. 2011(12):CD004916. doi: 10.1002/14651858.CD004916.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li SM, Wu SS, Kang MT, et al. Atropine slows myopia progression more in Asian than white children by meta-analysis. Optom Vis Sci. 2014;91(3):342–350. doi: 10.1097/OPX.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 16.Tsai HR, Chen TL, Wang JH, Huang HK, Chiu CJ. Is 0.01% atropine an effective and safe treatment for myopic children? A systemic review and meta-analysis. J Clin Med. 2021;10(17):3766. doi: 10.3390/jcm10173766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, Tan D. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (atropine for the treatment of myopia 2) Ophthalmology. 2012;119(2):347–354. doi: 10.1016/j.ophtha.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 18.Wei SF, Li SM, An WZ, Du JL, Liang XT, Sun YY, Zhang DX, Tian JX, Wang NL. Safety and efficacy of low-dose atropine eyedrops for the treatment of myopia progression in Chinese children: a randomized clinical trial. JAMA Ophthalmol. 2020;138(11):1178–1184. doi: 10.1001/jamaophthalmol.2020.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenstein SH, Abramson DH, Pitts WR., 3rd Systemic atropine and glaucoma. Bull N Y Acad Med. 1984;60(10):961–968. [PMC free article] [PubMed] [Google Scholar]
- 20.Mughannam AJ, Buyukmihci NC, Kass PH. Effect of topical atropine on intraocular pressure and pupil diameter in the normal horse eye. Vet Ophthalmol. 1999;2(4):213–215. doi: 10.1046/j.1463-5224.1999.00081.x. [DOI] [PubMed] [Google Scholar]
- 21.Wu TEJ, Yang CC, Chen HS. Does atropine use increase intraocular pressure in myopic children? Optom Vis Sci. 2012;89(2):E161–E167. doi: 10.1097/OPX.0b013e31823ac4c1. [DOI] [PubMed] [Google Scholar]
- 22.Yu TC, Wu TE, Wang YS, Cheng SF, Liou SW. A STROBE-compliant case-control study: effects of cumulative doses of topical atropine on intraocular pressure and myopia progression. Medicine. 2020;99(48):e22745. doi: 10.1097/MD.0000000000022745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan LW, Hsieh YT, Hsu WC, Cheng HC, Shen EP. Optic disc parameters of myopic children with atropine treatment. Curr Eye Res. 2017;42(12):1614–1619. doi: 10.1080/02713683.2017.1359846. [DOI] [PubMed] [Google Scholar]
- 24.Chen CW, Yao JY. Efficacy and adverse effects of atropine for myopia control in children: a meta-analysis of randomised controlled trials. J Ophthalmol. 2021;2021:4274572. doi: 10.1155/2021/4274572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brennan NA, Toubouti YM, Cheng X, Bullimore MA. Efficacy in myopia control. Prog Retin Eye Res. 2021;83:100923. doi: 10.1016/j.preteyeres.2020.100923. [DOI] [PubMed] [Google Scholar]
- 26.Lee CY, Sun CC, Lin YF, Lin KK. Effects of topical atropine on intraocular pressure and myopia progression: a prospective comparative study. BMC Ophthalmol. 2016;16:114. doi: 10.1186/s12886-016-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang PY, Chen SD, Liu YM, Lin FB, Song YH, Li TZ, Aung T, Zhang XL, Group GSHMS Lowering intraocular pressure: a potential approach for controlling high myopia progression. Invest Ophthalmol Vis Sci. 2021;62(14):17. doi: 10.1167/iovs.62.14.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pruett RC. Progressive myopia and intraocular pressure: what is the linkage? A literature review. Acta Ophthalmol. 2009;66(S185):117–127. doi: 10.1111/j.1755-3768.1988.tb02685.x. [DOI] [PubMed] [Google Scholar]
- 29.Nomura H, Ando F, Niino N, Shimokata H, Miyake Y. The relationship between intraocular pressure and refractive error adjusting for age and central corneal thickness. Oph Phys Optics. 2004;24(1):41–45. doi: 10.1046/j.1475-1313.2003.00158.x. [DOI] [PubMed] [Google Scholar]
- 30.Li SM, Iribarren R, Li H, Kang MT, Liu LR, Wei SF, Stell WK, Martin G, Wang NL. Intraocular pressure and myopia progression in Chinese children: the Anyang Childhood Eye Study. Br J Ophthalmol. 2019;103(3):349–354. doi: 10.1136/bjophthalmol-2017-311831. [DOI] [PubMed] [Google Scholar]
- 31.Schmid KL, Li RWH, Edwards MH, Lew JKF. The expandability of the eye in childhood myopia. Curr Eye Res. 2003;26(2):65–71. doi: 10.1076/ceyr.26.2.65.14513. [DOI] [PubMed] [Google Scholar]
- 32.Ito K, Tawara A, Kubota T, Harada Y. IOP measured by dynamic contour tonometry correlates with IOP measured by Goldmann applanation tonometry and non-contact tonometry in Japanese individuals. J Glaucoma. 2012;21(1):35–40. doi: 10.1097/IJG.0b013e31820275b4. [DOI] [PubMed] [Google Scholar]
- 33.Eisenberg DL, Sherman BG, McKeown CA, Schuman JS. Tonometry in adults and children. Ophthalmology. 1998;105(7):1173–1181. doi: 10.1016/S0161-6420(98)97016-6. [DOI] [PubMed] [Google Scholar]