Abstract
AIM
To evaluate the efficacy and safety of HLX04-O, an investigational ophthalmic formulation of HLX04 (bevacizumab biosimilar) for intravitreal injection, as a treatment for wet age-related macular degeneration (wAMD) in a phase 1/2 clinical trial (NCT04993352).
METHODS
Eligible patients with wAMD were enrolled to receive HLX04-O intravitreal injections at a dose of 1.25 mg/0.05 mL every four weeks. Efficacy and adverse events were evaluated every month during study visits.
RESULTS
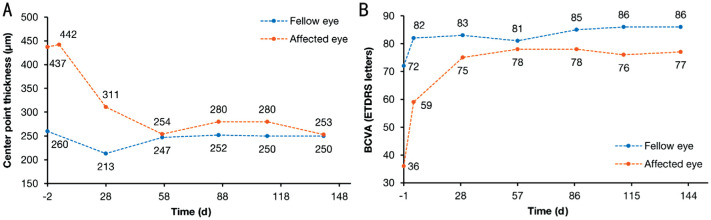
A 76-year-old male with wAMD in his left eye participated in the trial and completed six cycles of HLX04-O intravitreal injections. Changes were observed in macular center point thickness (baseline vs last study visit, 437 vs 255 µm) and best-corrected visual acuity letter score (baseline vs last study visit, 36 vs 77) of the affected eye, which indicated an improvement in wAMD over treatment. No adverse events were reported by the data cutoff date.
CONCLUSION
HLX04-O at 1.25 mg/0.05 mL every four weeks is well tolerated in this patient, demonstrating promising safety and efficacy in wAMD treatment. Large-scale studies are required to confirm the outcomes.
Keywords: wet age-related macular degeneration, anti-vascular endothelial growth factor, bevacizumab, HLX04, HLX04-O
INTRODUCTION
Age-related macular degeneration (AMD) is a major cause of irreversible visual impairment and legal blindness[1]. A Meta-analysis published in 2014 reported that the prevalence of AMD was 8.7% for people aged 45-85y, with the projected number of cases reaching 288 million by 2040[2]. Thus, AMD constitutes a major public health problem with a significant socio-economic impact. Age is the greatest risk factor for AMD, with almost all late-stage AMD cases found in people over 60y[1]. Wet AMD (wAMD), also known as neovascular or exudative AMD, is a late-stage form of the disease that accounts for only 15%-20% of AMD cases but the majority of AMD-related vision loss[3]. Choroidal neovascularization (CNV) plays a key role in the progression of wAMD[1].
The main treatment for wAMD is the intravitreal injection of an anti-vascular endothelial growth factor (VEGF) agent. Bevacizumab is the first anti-VEGF agent used to treat CNV in AMD and other ocular disorders, though it remains off-label to date. While proven effective in wAMD treatment, off-label use of intravitreal bevacizumab may increase the risk of endophthalmitis[4], and thus a formulation specifically for ophthalmic use is in need. HLX04 (Han-Bei-Tai®) is a recombinant anti-VEGF humanized monoclonal antibody developed by Shanghai Henlius Biotech, Inc. that has been approved by China National Medical Products Administration (NMPA) as a bevacizumab biosimilar for the treatment of metastatic colorectal cancer and recurrent or metastatic non-squamous non-small-cell lung cancer[5]–[6]. A new formulation of HLX04 for ophthalmic injection, currently named as HLX04-O, has been developed as a potential therapy for wAMD. By blocking VEGF, HLX04-O is expected to reduce the formation of abnormal blood vessels in the retina and inhibit the retinal edema, subretinal effusion, and visual pigment epithelial detachment (PED) caused by AMD, thereby reducing retinal thickness and improving vision[7].
A single-arm, open-label, multicenter, phase 1/2 trial is being conducted to evaluate the efficacy and safety of HLX04-O administered by intravitreal injection in patients with active wAMD (ClinicalTrials.gov identifier: NCT04993352). Here, we present a case from this phase 1/2 trial evaluating HLX04-O in wAMD treatment.
SUBJECTS AND METHODS
Ethical Approval
The study protocol and any amendments were approved by the Biomedical Research Ethics Committee of Xuzhou Central Hospital (approval number: XZXY-LY-20210528-040 and XZXY-LY-20210902-2021040). The study was conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice guidelines, and local applicable regulatory requirements. This patient provided written informed consent for publication of this case report with any accompanying images.
Patients
Eligible patients were aged ≥50y, had newly diagnosed or recurrently, active CNV lesions secondary to AMD in the affected eye, and had a best-corrected visual acuity (BCVA) letter score of 15-78 (inclusive) as assessed by the Early Treatment Diabetic Retinopathy Study (ETDRS) chart in the affected eye. Patients were excluded if they received systemic anti-VEGF therapy or intravitreal injection of any anti-VEGF drug in either eye, or other ocular use of anti-VEGF drug within three months prior to the first dose of HLX04-O.
Interventions and Assessments
HLX04-O intravitreal injections were administered at a dose of 1.25 mg/0.05 mL every four weeks. Efficacy assessments including optical coherence tomography (OCT) and BCVA letter score, as well as evaluation of adverse events, were performed every month during study visits.
Data Analysis
Rave EDC 2020.3.2 was used for collecting patient data. The EDC data were then exported into SAS v9.4 for data analysis. As this was a case report of a single patient, only descriptive data were provided in this report.
RESULTS
A 76-year-old Chinese male of Han ethnicity presented with a sudden deterioration in vision during the past week. The patient was diagnosed with wAMD and had active CNV lesions secondary to wAMD in his left eye. He did not have active intraocular, extraocular or periocular infection, a recent history (within the past month) of the aforementioned infections, or a history of idiopathic or autoimmune-associated uveitis in either eye. There was also no history of vitreous hemorrhage in the affected eye during the previous three months. The patient had not received any anti-VEGF therapy during the past three months. However, he had a history of cataract in his left eye and had undergone phacoemulsification and intraocular lens implantation in 2018. The patient provided written informed consent for inclusion in the study on July 12, 2021. Intravitreal injection of HLX04-O was administered to the affected eye with the first dose given on July 15, 2021. Six treatment cycles had been completed by December 13, 2021 (the data cutoff date).
The pre-treatment intraocular pressure (IOP) assessed by a non-contact tonometer was within the normal range in both eyes. OCT of the affected eye at screening showed a macular center point thickness of 437 µm (260 µm for the contralateral right eye, i.e., the fellow eye), apparent subretinal fluid with center subfield involvement (absent in the fellow eye), severe PED with a maximal thickness of 940 µm (absent in the fellow eye), an apparent epiretinal membrane (also present in the fellow eye), no retinal pigment epithelium rip/tear (also absent in the fellow eye), and no macular atrophy or macular hole (also absent in the fellow eye). The BCVA letter score assessed using the ETDRS chart was 36 for the affected eye and 72 for the fellow eye.
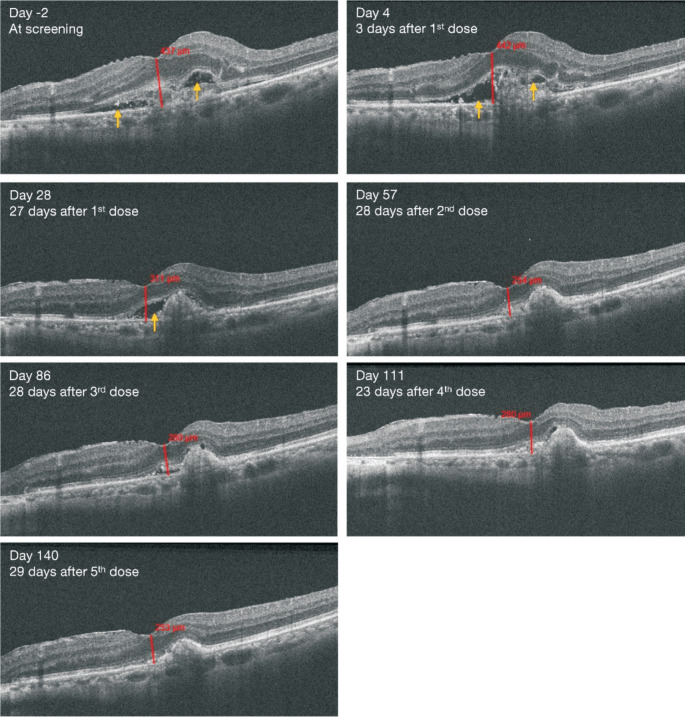
OCT showed that the center point thickness of the affected eye decreased during treatment and became similar to that of the fellow eye after the second dose (Figure 1 and Figure 2A). Subretinal effusion was also alleviated and almost disappeared after the second dose (Figure 1). Post-injection vision assessments (by counting fingers) conducted within 15min of the injections showed no significant clinical abnormalities in the affected eye. The BCVA letter score assessed using the ETDRS chart was 77 for the affected eye at the last study visit, which was getting closer to the score of 86 for the fellow eye (Figure 2B).
Figure 1. Optical coherence tomography images of the affected eye during treatment with intravitreal HLX04-O injections.
Yellow arrows indicate subretinal fluid. Numbers and line segments (in red) indicate macular center point thickness.
Figure 2. Changes during treatment with intravitreal HLX04-O injections.
A: Macular center point thickness; B: BCVA letter score. BCVA: Best-corrected visual acuity; ETDRS: Early Treatment Diabetic Retinopathy Study.
The IOP in both eyes was normal at screening, as well as at pre-injection and 30min post-injection in all study visits. The electrocardiogram and vital signs were normal. Laboratory investigations revealed normal or near-normal coagulation function (activated partial thromboplastin time, D-dimer, fibrinogen, international normalized ratio, prothrombin time, and thrombin time), glycated hemoglobin level, hepatic function, renal function, and electrolyte levels. No significant abnormalities were found on physical examination (except for the affected eye).
DISCUSSION
A 76-year-old male patient suffering from wAMD in his left eye participated in this trial and completed six cycles of intravitreal HLX04-O given at a dose of 1.25 mg/eye every four weeks. The macular center point thickness of the affected eye decreased over treatment, accompanied by BCVA improvements, and both measures became similar to those of the fellow eye at the last study visit. OCT images suggested remarkably reduced subretinal effusion in the affected eye. No adverse events were reported up to the date of the last study visit.
To achieve an effective drug concentration in the vitreous humor for wAMD treatment, intravitreal injection is still considered the optimal route of administration[8]. Current intravitreal anti-VEGF therapies for wAMD include bevacizumab (off-label), ranibizumab, aflibercept, brolucizumab, and conbercept. Ranibizumab and aflibercept have been approved by the United States Food and Drug Administration (FDA), European Medicines Agency (EMA), and NMPA, while brolucizumab has been approved by FDA and EMA, and conbercept by NMPA. Several head-to-head trials (CATT[9], GEFAL[10], IVAN[11], and MANTA[12]) and two Meta-analyses[13]–[14] demonstrated comparable efficacy (in terms of BCVA improvement) and similar safety between bevacizumab and ranibizumab in wAMD treatment; aflibercept and ranibizumab also showed similar effects, though only low-strength evidence was available. Despite promising results in clinical studies, a highly varying utilization rate of bevacizumab for wAMD (0-97%) has been observed across countries, as controversy remains over the ophthalmic off-label use[15]. Besides legal and regulatory considerations, the main concern lies with the increased risk of post-injection endophthalmitis and reduced stability arising from aliquoting and repackaging of marketed bevacizumab by clinical staff or compounding pharmacies[16]–[17]. HLX04-O, developed as a bevacizumab biosimilar specifically formulated and packaged (HLX04-O, 12.5 mg/0.5 mL or 5 mg/0.2 mL per vial; HLX04, 100 mg/4 mL per vial) for intravitreal injection, may represent an attractive therapeutic option for patients with wAMD.
The patient described in this case report had not received prior anti-VEGF therapies, which might contribute to his encouraging response to HLX04-O. Furthermore, since wAMD is a chronic disease, attention should be paid to results after a long-term follow-up to investigate whether the efficacy of HLX04-O is sustained.
In summary, the present case indicates that intravitreal HLX04-O may be an effective and safe treatment for wAMD, though this will need further confirmation in large-scale clinical investigations. Clinical trials evaluating the efficacy and safety of HLX04-O compared to those of ranibizumab are ongoing (ClinicalTrials.gov identifier: NCT04740671, NCT05003245).
Acknowledgments
We would like to express our sincere gratitude to the patient participating in the trial and his families, as well as the clinicians, study coordinators, and nurses at the study site. We thank the clinical study team (Clinical Operations: Wen-Li Duan, Hao-Yu Yu, Jing Li; Statistics: Chang Su, Jian-Cheng Cheng), Jun Zhu, and Wen-Jie Zhang from Shanghai Henlius Biotech, Inc. for their support in the study. Medical writing support was provided by Shi-Qi Zhong, PhD, and Chen Hu, PhD, from Shanghai Henlius Biotech, Inc.
Foundation: Supported by Shanghai Henlius Biotech, Inc.
Conflicts of Interest: Zhang Z, None; Wu Y, None; Lyu YL, Employee of Shanghai Henlius Biotech, Inc.; Chang MQ, None; Xu QJ, None; Liu YM, Employee of Shanghai Henlius Biotech, Inc.; Kang WY, Employee of Shanghai Henlius Biotech, Inc.; Wang QY, Employee of Shanghai Henlius Biotech, Inc.; Li CL, None.
REFERENCES
- 1.Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392(10153):1147–1159. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 2.Wong WL, Su XY, Li X, Cheung CMG, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 3.de Jong EK, Geerlings MJ, den Hollander AI. Genetics and Genomics of Eye Disease. Amsterdam: Elsevier; 2020. Age-related macular degeneration; pp. 155–180. [Google Scholar]
- 4.Khan P, Khan L, Mondal P. Cluster endophthalmitis following multiple intravitreal bevacizumab injections from a single use vial. Indian J Ophthalmol. 2016;64(9):694–696. doi: 10.4103/0301-4738.99855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henlius' 4th Biologics: Bevacizumab Biosimilar Han-Bei-Tai® Approved by National Medical Products Administration. [Accessed on December 3, 2021]. https://www.henlius.com/en/NewsDetails-3329-26.html.
- 6.Qin SK, Li J, Bai YX, Shu YQ, Li W, Yin XL, Cheng Y, Sun GP, Deng YH, Zhong HJ, Li YF, Qian XP, Zhang LM, Zhang JD, Chen KH, Kang WY, HLX04-mCRC03 Investigators Efficacy, safety, and immunogenicity of HLX04 versus reference bevacizumab in combination with XELOX or mFOLFOX6 as first-line treatment for metastatic colorectal cancer: results of a randomized, double-blind phase III study. BioDrugs. 2021;35(4):445–458. doi: 10.1007/s40259-021-00484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holz FG, Schmitz-Valckenberg S, Fleckenstein M. Recent developments in the treatment of age-related macular degeneration. J Clin Invest. 2014;124(4):1430–1438. doi: 10.1172/JCI71029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García-Quintanilla L, Luaces-Rodríguez A, Gil-Martínez M, Mondelo-García C, Maroñas O, Mangas-Sanjuan V, González-Barcia M, Zarra-Ferro I, Aguiar P, Otero-Espinar FJ, Fernández-Ferreiro A. Pharmacokinetics of intravitreal anti-VEGF drugs in age-related macular degeneration. Pharmaceutics. 2019;11(8):E365. doi: 10.3390/pharmaceutics11080365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL., 3rd Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2020;127(4S):S135–S145. doi: 10.1016/j.ophtha.2020.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Kodjikian L, Souied EH, Mimoun G, Mauget-Faÿsse M, Behar-Cohen F, Decullier E, Huot L, Aulagner G, GEFAL Study Group Ranibizumab versus bevacizumab for neovascular age-related macular degeneration: results from the GEFAL noninferiority randomized trial. Ophthalmology. 2013;120(11):2300–2309. doi: 10.1016/j.ophtha.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Chakravarthy U, Harding SP, Rogers CA, Downes S, Lotery AJ, Dakin HA, Culliford L, Scott LJ, Nash RL, Taylor J, Muldrew A, Sahni J, Wordsworth S, Raftery J, Peto T, Reeves BC. A randomised controlled trial to assess the clinical effectiveness and cost-effectiveness of alternative treatments to Inhibit VEGF in age-related choroidal neovascularisation (IVAN) Health Technol Assess. 2015;19(78):1–298. doi: 10.3310/hta19780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krebs I, Schmetterer L, Boltz A, Told R, Vécsei-Marlovits V, Egger S, Schönherr U, Haas A, Ansari-Shahrezaei S, Binder S, MANTA Research Group A randomised double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. Br J Ophthalmol. 2013;97(3):266–271. doi: 10.1136/bjophthalmol-2012-302391. [DOI] [PubMed] [Google Scholar]
- 13.Ba J, Peng RS, Xu D, Li YH, Shi H, Wang QY, Yu J. Intravitreal anti-VEGF injections for treating wet age-related macular degeneration: a systematic review and meta-analysis. Drug Des Devel Ther. 2015;9:5397–5405. doi: 10.2147/DDDT.S86269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Low A, Faridi A, Bhavsar KV, Cockerham GC, Freeman M, Fu R, Paynter R, Kondo K, Kansagara D. Comparative effectiveness and harms of intravitreal antivascular endothelial growth factor agents for three retinal conditions: a systematic review and meta-analysis. Br J Ophthalmol. 2019;103(4):442–451. doi: 10.1136/bjophthalmol-2018-312691. [DOI] [PubMed] [Google Scholar]
- 15.Bro T, Derebecka M, Jørstad ØK, Grzybowski A. Off-label use of bevacizumab for wet age-related macular degeneration in Europe. Graefes Arch Clin Exp Ophthalmol. 2020;258(3):503–511. doi: 10.1007/s00417-019-04569-8. [DOI] [PubMed] [Google Scholar]
- 16.Schargus M, Werner BP, Geerling G, Winter G. Contamination of anti-VEGF drugs for intravitreal injection: how do repackaging and newly developed syringes affect the amount of silicone oil droplets and protein aggregates? Retina. 2018;38(10):2088–2095. doi: 10.1097/IAE.0000000000001809. [DOI] [PubMed] [Google Scholar]
- 17.Vitreoretinal Society of India. Guidelines for Intravitreal Injections of Avastin (Bevacizumab) 2016. [Accessed on March 18, 2022].