Abstract
Tuberculous uveitis (TBU) comprises a broad clinical spectrum of ocular manifestations, making its diagnosis challenging. Ophthalmologists usually require evidence from investigations to confirm or support a clinical diagnosis of TBU. Since direct isolation of the causative organism from ocular specimens has limitations owing to the small volume of the ocular specimens, resultant test positivities are low in yield. Immunodiagnostic tests, including the tuberculin skin test and interferon-gamma release assays (IGRAs), can help support a clinical diagnosis of TBU. Unlike the tuberculin skin test, IGRAs are in vitro tests that require a single visit and are not affected by prior Bacillus Calmette-Guerin vaccination. Currently, available IGRAs consist of different techniques and interpretation methods. Moreover, newer generations have been developed to improve the sensitivity and ability to detect active tuberculosis. This narrative review collates salient practice points as a reference for general ophthalmologists, such as evidence for the utilization of IGRAs in patients with suspected TBU, and summarizes basic knowledge and details of clinical applications of these tests in a clinical setting.
Keywords: ocular tuberculosis, tuberculous uveitis, interferon-gamma release assays, review
INTRODUCTION
Tuberculosis (TB) is an infection caused by Mycobacterium tuberculosis (Mtb). The global TB report 2020 revealed that a quarter of the world's population was infected with Mtb, and the incidence rate for developing the disease was 30 000 people per day[1]. It was reportedly one of the top ten causes of death, responsible for 1.4 million fatalities worldwide in 2019[1]. Currently, active TB and latent tuberculosis infection (LTBI) are believed to constitute a comprehensive spectrum rather than being considered as separate stages of the infection. World Health Organization guidelines define LTBI as a state of persistent immune response stimulated by Mtb antigens without evidence of clinical manifestations of active TB[2]. LTBI has several stages that affect individuals who could be asymptomatic; they may experience a controlled infection with nonreplicating but viable organisms. LTBI is therefore considered an important reservoir of TB infection that can subsequently develop into an active disease[3].
Besides lungs, TB can affect multiple organs throughout the body, including the eyes. Ocular TB is an extrapulmonary infection that may involve any part of the ocular tissue and may occur without a history of pulmonary TB. Tuberculous uveitis (TBU) is one of the most common clinical presentations of ocular TB. The prevalence of TBU varies according to geography. While endemic areas, including India and Saudi Arabia reported a high prevalence of 5.6%-26.2%, non-endemic countries, such as the United States, Europe, and Japan, showed a lower prevalence of 0.2%-7%[4]–[5].
Considering the immune-related pathogenesis of TBU, immunological tests can be useful for its diagnosis. The tuberculin skin test (TST) and interferon-gamma release assays (IGRAs) are well-known immunological tests. Poor specificity of the TST for diagnosing TB in those who had been vaccinated with Bacillus Calmette-Guerin (BCG) and in residents of endemic regions, led to the subsequent development of IGRAs that enabled immunodiagnosis of LTBI[6]. These assays performed along with or without the TST, are now increasingly used to diagnose TBU.
In this narrative review, we provide a brief description of the clinical characteristics of TBU. We further comparatively review the principles of IGRAs, including diagnostic accuracy, advantages and disadvantages, interpretation, and clinical application in adults with suspected TBU.
Clinical Characteristics of Tuberculous Uveitis
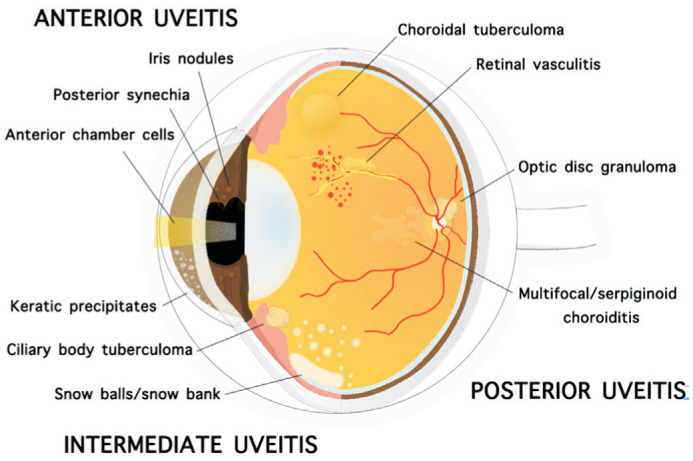
TBU has a wide spectrum of clinical presentations that may vary from anterior uveitis to intermediate uveitis, posterior uveitis, and panuveitis. It may present as unilateral or bilateral, granulomatous or non-granulomatous inflammation involving the eye. The clinical findings of TBU include iris nodules, ciliary body tuberculoma, organizing exudates in the pars plana/peripheral uvea, choroidal tubercle/tuberculoma, subretinal abscess, serpiginoid choroiditis, retinitis, retinal vasculitis, neuroretinitis, optic neuropathy, endophthalmitis, and panophthalmitis[7].
Gupta et al[8] suggested that the presence of cellular infiltrate either in the anterior chamber or in the vitreous along with broad posterior synechiae, retinal perivasculitis with or without discrete choroiditis/scars, multifocal serpiginoid choroiditis, choroidal granuloma (single or multifocal), optic disc granuloma, and optic neuropathy were ophthalmic signs consistent with a diagnosis of ocular TB. Affected patients in endemic areas often present with broad-based posterior synechiae, retinal vasculitis, choroiditis, and serpiginoid choroiditis findings that are highly specific to TBU[9]. Studies from nonendemic areas demonstrated occlusive retinal vasculitis and serpiginoid choroiditis as more common clinical presentations of TBU[10]. Figure 1 illustrates clinical characteristics of TBU.
Figure 1. Clinical findings of tuberculous uveitis include keratic precipitates, anterior chamber cells, posterior synechia, iris nodules, ciliary body tuberculoma, snowballs/snow bank, retinal vasculitis, multifocal/serpiginoid choroiditis, choroidal tuberculoma (single or multifocal), optic disc granuloma.
Current Immunodiagnostic Tests for Tuberculous Uveitis
Generally, Mtb may not be isolated from the ocular tissue in all TBU patients. Ocular TB may be classified as such, based on an affected patient's clinical findings meeting diagnostic criteria or a clinical definition[4],[8]. Figueira et al[11] proposed that investigations for TB should be performed in any of the following situations: 1) Uveitis of unknown etiology, either recurrent or unresponsive to conventional therapy; 2) Ocular findings highly suggestive of ocular TB; 3) Before initiating immunosuppressive therapy, particularly that with biologic agents.
It should be noted that most patients with ocular TB have no history of pulmonary or other systemic TB infections. An absence of pulmonary TB; therefore, does not exclude the possibility of ocular TB. Up to 60% of patients with extrapulmonary TB do not have evidence of pulmonary disease, and chest X-rays are normal in those with LTBI[4],[12].
A definitive diagnosis of TBU requires isolation of Mtb from intraocular tissue specimens obtained through invasive procedures such as aqueous paracentesis, vitreous aspiration, or retinal biopsy[11]. Mtb culture remains the gold standard for a definitive diagnosis of ocular TB. Other methods include detection of acid-fast bacilli on smear examination, amplification of Mtb nucleic acids, and histopathological examination of ocular tissues[6],[13]. However, there are limitations in identifying the organism in the eye. Usually, only a limited amount of ocular tissue sample can be extracted, and associated procedural complications may potentially damage vision. The Collaborative Ocular Tuberculosis Study showed that patients with presumed TBU demonstrated low positive yield of Mtb from intraocular fluid samples on polymerase chain reaction[14]. Given the limitations of ocular sampling and associated low positivity yield, TBU was rarely diagnosed using this method. This may contribute to delays in diagnosis and treatment, resulting in poor visual outcomes. Consequently, a finding of uveitis with intraocular features characteristic of TBU in conjunction with positive results of indirect tests, is considered sufficient evidence for a diagnosis.
Two indirect investigations, including the TST and IGRAs, evaluate the intensity of the host immunological reaction to TB antigens, which may manifest as a T lymphocyte-mediated immune response or as a delayed hypersensitivity reaction. The TST is an in vivo test performed using a purified protein derivative consisting of >200 protein precipitates derived from a heat-inactivated Mtb. The diagnostic feature of a positive TST is the development of skin induration, interpreted within 48-72h after an intradermal injection of the purified protein derivative. The American Thoracic Society and Centers for Disease Control and Prevention have provided guidelines for the interpretation of positive TST findings, as shown in Table 1[15].
Table 1. Positive tuberculin skin testing interpretation according to risk groups.
| Risk groups | Induration |
| HIV infection | ≥5 mm |
| History of recent contact with an active TB patient | |
| Fibrotic changes in chest X-ray suggestive of TB | |
| Transplanted and immunocompromised patients (including patients under treatment at least 1mo with ≥15 mg/d of prednisone or equivalent)a | |
| Immigrants coming from high-endemic areas (migration in the last 5y) | ≥10 mm |
| Intravenous drugs users | |
| Residents and employees of prisons, hospitals and so onb | |
| Microbiology laboratories staff | |
| Individuals with pathological risk conditions (Silicosis, diabetes, chronic renal failure, hematological disorders and neoplasms, malnutrition, gastrectomy or jejunoileal bypass) | |
| Children under 4 years old and/or young adults in contact with adults at risk | |
| Individuals without TB risk factors | ≥15 mm |
HIV: Human immunodeficiency virus; TB: Tuberculosis. aThe risk of tuberculosis in patients treated with corticosteroids increases with higher doses and longer durations; bFor persons who are otherwise at low risk and are tested at the start of employment, a reaction of ≥15 mm induration is considered positive. Adapted from the Center for Disease Control and Prevention. Screening for tuberculosis and tuberculosis infection in high-risk populations recommended by the Advisory Council for the Elimination of Tuberculosis.
Notably, a technique that helped identify different mycobacterial antigens, including early secretory antigenic target-6 (ESAT-6) and culture filtrate protein-10 (CFP-10), was developed into a test for blood-based in vitro evaluation of the immune response to TB. In the absence of cross-reactivity due to prior BCG vaccination, measurement of interferon-gamma (IFN-γ) produced by T lymphocytes in response to these antigens, forms the basis of IGRAs performed for the diagnosis of TB[16].
Principles of Interferon-Gamma Release Assays and Interpretation
IGRAs are in vitro immunodiagnostic tests that use antigens to stimulate specific T lymphocyte responses to Mtb. Once specific T-cells are stimulated, they activate an immune response by releasing cytokines, including IFN-γ. IFN-γ is a crucial cytokine of the CD4 T helper 1 (Th1) subset and is responsible for the delayed hypersensitivity immune response. In vitro T-cell responses can be evaluated by estimating either the number of IFN-γ producing T-cells or by measuring IFN-γ production using an enzyme-linked immune absorbent spot assay (ELISpot) and an enzyme-linked immunosorbent assay (ELISA), respectively[16]. The United States Food and Drug Administration has approved commercial IGRAs as indirect and adjunct tests for identifying TB, to be performed in conjunction with risk assessment, radiography and other medical and diagnostic evaluations.
Enzyme-linked immune absorbent spot assay technique
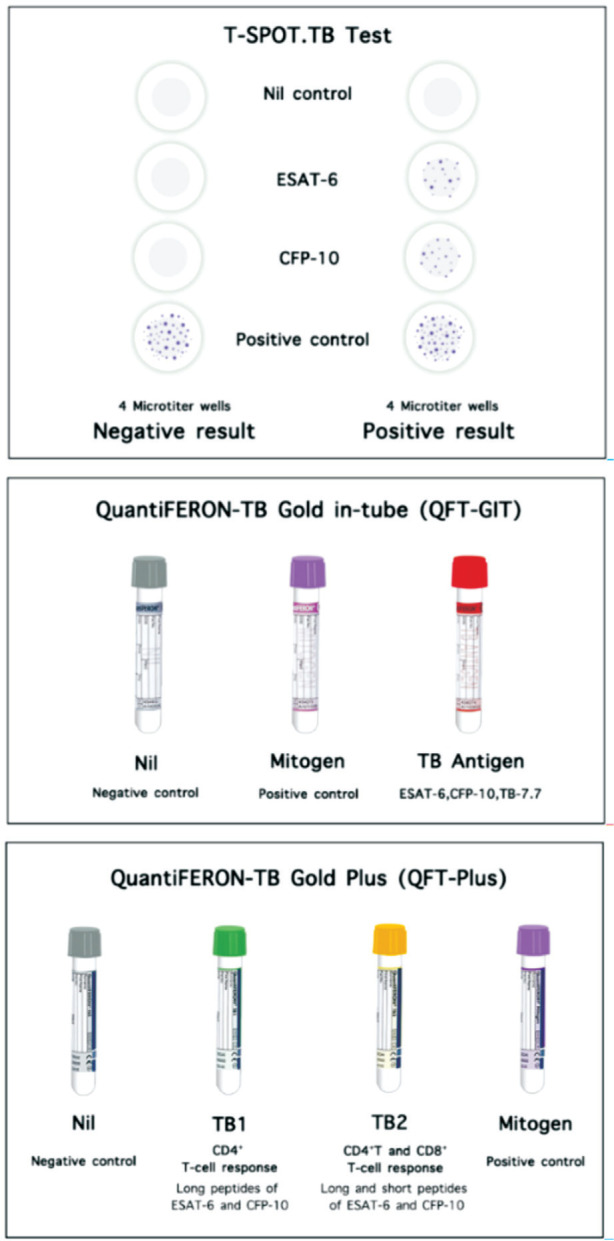
The ELISpot technique was used for the T-SPOT. TB test (Oxford Immunotec, Abingdon, United Kingdom) facilitates the enumeration of peripheral blood mononuclear cells that produce IFN-γ after stimulation with Mtb-specific antigens[17]. Peripheral blood mononuclear cells are added into 4 separated 2-microtiter wells coated with monoclonal antibodies to IFN-γ. The first two wells are each filled with either of the two different antigens, ESAT-6 and CFP-10, which are peptide antigens located in the region of difference-1 (RD-1) of the Mtb genome but are not found in the BCG vaccine strains and most strains of non-tuberculous mycobacteria, except for M. kansasii, M. marinum, M. szulgai, and M. flavescens. Likewise, there is a report of cross-reactivity between antigens of M. tuberculosis and M. leprae[18].
Both ESAT-6 and CFP-10 have multiple epitopes for T lymphocytes and can, therefore, generate an intense reaction. The two remaining wells act as controls for internal test validation. The positive control well contains phytohemagglutinin (mitogen) for stimulating nonspecific T-cell response, whereas no antigen is added in the negative control (nil) well. The production of IFN-γ is then detected via a sandwich capture technique by conjugation with secondary antibodies revealing a “spot”. These spots, considered “footprints” of effector T-cells producing IFN-γ, are then enumerated[3],[19].
An interpretation of T-SPOT. TB test is based on the enumeration of spot-forming units. A test result is considered positive if a spot count induced by either antigen ≥6 spots after subtracting the spots from the negative control well. A negative result is indicated if ≤5 spots are induced by both antigens after subtracting the spots from the negative control well. An “indeterminate” result refers to >10 spots in the negative control well, or when the positive control well shows <20 spots unless either of the antigen well shows a positive result. This suggests that another sample should be collected and repeatedly tested. If the spot counts from the antigen wells minus the negative control well are equal to or near the cut-off value (5, 6, or 7 spots), these results may be considered as “borderline” or “equivocal”, leading to less reliable results and warranting a repeat test with another sample. Therefore, in the United States, different cut-off values for positive and negative results are indicated by ≥8 spot counts and ≤4 spot counts, respectively[3],[20].
Enzyme-linked immunosorbent assay technique
The ELISA technique has been commercially developed through many generations, which include QuantiFERON (QFT), QuantiFERON-TB Gold (QFT-G), QuantiFERON-TB Gold in-tube (QFT-GIT), and QuantiFERON-TB Gold Plus (QFT-Plus). Initially, purified protein derivative was used as the antigen in the QFT test. Then, TB-specific antigens, ESAT-6 and CFP-10, were representatively used in subsequent versions named QFT-G. The third-generation test, QFT-GIT, includes three tubes, the TB antigen-coated tube, the positive control (mitogen) tube, and the negative (nil) control tube. TB antigen-coated tube contains ESAT-6, CFP-10, and an additional peptide antigen TB7.7[3],[19].
CD4 T lymphocytes were believed to play a significant role in the immune response to TB until recent studies provided evidence to support the additive role played by CD8 T lymphocytes[21]. RD-1-specific CD8 T lymphocytes are also more frequently detected in recent or active TB infections than in LTBI. These findings led to the development of the fourth generation of IGRAs, QFT-Plus. This test package contains two tubes with TB-specific antigens, TB1 and TB2. The TB1 tube containing long peptides derived from ESAT-6 and CFP-10, was designed to induce a specific CD4 T-cell response. The TB2 tube also contains the same long peptides as TB1 along with shorter peptides that can stimulate the CD8 T-cell response. The ability to elicit both CD4 and CD8 T-cell responses is believed to improve the sensitivity of this generation of IGRAs[19].
With application of ELISA, IFN-γ levels from plasma are reported as international units per milliliter (IU/mL). Similar to the technique used in the T-SPOT.TB test, a quantitative result is calculated by subtracting the estimated IFN-γ level in the negative (nil) control tube from that in the antigen-specific tubes. The interpretation of the results is summarized in Table 2. An estimated value of >0.35 IU/mL and that >25% of the negative control value found in either one or both TB antigen-coated tubes is considered positive. While values falling outside these prescribed limits are considered negative, a mitogen control level <0.5 IU/mL or a negative (nil) control value >8.0 IU/mL, is interpreted as an indeterminate report[22]. Affected patients may show poor response to the mitogen (positive control tube) due to two possible reasons. First, the test may not have been performed correctly, due to errors in specimen collection, delayed specimen processing, incubator malfunction, or technical issues. Second, anergy can lead to a persistently diminished response to mitogens. The reproducibility and reportability of an indeterminate result may provide clinically useful information[15]. Figure 2 illustrates the common commercial IGRAs, including T-SPOT.TB test, QFT-GIT, and QFT-Plus.
Table 2. Interpretation of results of interferon-gamma release assays.
| Negative control | Positive control minus negative control | First antigen tube minus negative control | Second antigen tube minus negative control |
| ≤8.00 | Not relevant | ≥0.35 and ≥25% of negative control value | Not relevant |
| ≤8.00 | Not relevant | Not relevant | ≥0.35 and ≥25% of negative control value |
| ≤8.00 | ≥0.50 | <0.35 | <0.35 |
| ≤8.00 | ≥0.50 | ≥0.35 and <25% of negative control value | ≥0.35 and <25% of negative control value |
| ≤8.00 | ≥0.50 | <0.35 | ≥0.35 and <25% of negative control value |
| ≤8.00 | ≥0.50 | ≥0.35 and <25% of negative control value | <0.35 |
| ≤8.00 | <0.50 | <0.35 | <0.35 |
| ≤8.00 | <0.50 | ≥0.35 and <25% of negative control value | ≥0.35 and <25% of negative control value |
| ≤8.00 | <0.50 | <0.35 | ≥0.35 and <25% of negative control value |
| >8.00 | Not relevant | Not relevant | Not relevant |
Adapted from Della Bella et al[22].
IU/mL
Figure 2. Current interferon-gamma release assays approved by the Food and Drug Administration for commercial use.
Currently, other products of IGRAs launched in the market include STANDARD E TB-Feron ELISA/STANDARD and F TB-Feron FIA (IFN-gamma; both SD Biosensor), LIOFERON TB/LTBI (LIONEX Diagnostics & Therapeutics GmbH), and Advansure TB IGRA and Avansure i3 TB-IGRA (both LG Chem). Additionally, products of IGRAs that are continuously developing include T-Track(R) TB (Lophius Biosciences GmbH, Germany), VIDAS TB-IGRA (bioMérieux, France), Access QuantiFERON®-TB (QIAGEN, USA), ichromaTM IGRA-TB (Boditech Med Inc., Republic of Korea) and IP-10 IGRA elisa/lateral flow (rBioPharm, Germany)[1].
Another adding antigen other than ESAT-6, CFP-10, and TB 7.7 used for LIOFeron TB/LTBI is alanine dehydrogenase, which can induce CD8 T-cell response. This antigen is not found in BCG and is reportedly involved in the adaptation of Mtb to an anaerobic dormant stage observed in LTBI. Like the QFT-Plus, LIOFeron TB/LTBI test includes four tubes (positive control, negative control, and two antigen-coated tubes). The first antigen tube, TB-A, contains long peptides of ESAT-6, CFP-10, and TB7.7. However, the second antigen tube called TB-B is coated only with highly purified recombinant alanine dehydrogenase, a different antigen from that used in QFT-Plus. No other antigens or peptides are included in the TB-B tube[22].
Besides IFN-γ, several markers such as interleukin (IL)-2, IFN-γ-inducible protein of 10 kDa (IP-10), IL-5, and IL-10 have been investigated to improve diagnostic performance and discrimination of TB status. These biomarkers are beyond our scope; thus, they are not included in this review[23]–[25].
Sensitivity, Specificity, and Accuracy of Interferon-Gamma Release Assays
The quality of evidence supporting the use of both IGRAs and the TST for diagnosing active TB infection is low[17]. IGRAs are insufficiently accurate diagnostic tests for active TB and show limited specificity in distinguishing an active infection from an immune response to LTBI[6]. Considering the lack of a gold-standard diagnostic test for LTBI, sensitivity and specificity of IGRAs are typically estimated using representative reference standards. Sensitivity was assessed in patients with microbial culture-confirmed TB, while specificity was evaluated in low-risk individuals from low-incidence areas without any known history of exposure to the disease[18]. Some studies compared sensitivity and specificity of IGRAs with that of the TST or between generations of the immunoassay itself. Table 3 summarizes the pooled sensitivity and specificity of IGRAs as reported in systematic reviews and Meta-analyses[6],[19],[26]. A systematic review and Meta-analysis reported a high agreement and no significant difference in sensitivity and specificity between QFT-GIT and QFT-Plus[27]. IGRA performance also varies between regions with high and those with low TB incidence, with lower sensitivity observed in the former endemic areas[17].
Table 3. Sensitivity and specificity of interferon-gamma release assays collated from systematic review and Meta-analysis.
| Tests | Pooled sensitivity (95%CI) | Pooled specificity (95%CI) |
| Sester et al[6], 2011 | ||
| TST | 0.65 (0.61-0.68) | 0.75 (0.72-0.78) |
| T-SPOT.TB | 0.81 (0.78-0.84) | 0.59 (0.56-0.62) |
| QFT-GIT | 0.80 (0.75-0.84) | 0.79 (0.75-0.82) |
| Metcalfe et al[26], 2011 | ||
| T-SPOT.TB | 0.88 (0.81-0.95) | 0.61 (0.40-0.79) |
| T-SPOT.TBa | 0.76 (0.45-0.92) | 0.52 (0.40-0.63) |
| QFT-GIT | 0.84 (0.78-0.91) | 0.52 (0.41-0.62) |
| QFT-GITa | 0.60 (0.34-0.82) | 0.50 (0.35-0.65) |
| Sotgiu et al[19], 2019 | ||
| QFT-Plus | 0.94 (0.90-0.98) | 0.95 (0.93-0.97) |
| QFT-Plusb | 0.91 (0.84-0.96) | |
| TB1 tube | 0.91 (0.79-0.98) | |
| TB2 tube | 0.95 (0.88-0.95) |
TB: Tuberculosis; TST: Tuberculin skin test; QFT-GIT: QuantiFERON-TB Gold in-tube; QFT-Plus: QuantiFERON-TB Gold Plus. aAmong HIV-infected individuals; bAmong latent tuberculosis infections.
The new generation of IGRAs is under development to improve test performance. Della Bella et al[22] compared test parameters of LIOFeron TB/LTBI test with QFT-Plus in application for diagnosis of active TB and LTBI. While the accuracy of both tests was comparable, LIOFeron TB/LTBI assay was more sensitive than the QFT-Plus for detecting LTBI. However, the former test was unable to distinguish active TB from LTBI.
IGRAs performed in patients with human immunodeficiency virus (HIV) infection demonstrated lower sensitivity than those performed in immunocompetent individuals. A Meta-analysis showed lower sensitivity for T.SPOT.TB and QFT-GIT, particularly in affected patients with low CD4 counts of <100 cells/mL[26],[28]. In low- and middle-income countries, IGRAs may have a role in identifying TB infection in HIV-infected patients due to the decreased utilization of TST in immunosuppressed individuals[17]. However, a study performed in a population with a high prevalence of HIV reported three times the number of ocular TB patients with a positive QFT tested positive on a TST. Therefore, the investigators concluded that QFT should not replace TST in a limited-resource setting[29].
Two studies reported the application of IGRAs in the diagnosis of TBU. A study from Korea evaluated the usefulness of QFT-G in diagnosing presumed TBU in 181 patients. The sensitivity and specificity were reported as 100% and 72%, respectively, with a high positive predictive value in younger patients (≤40y) presenting with posterior uveitis and retinal vasculitis[30]. Another prospective head-to-head study by Ang et al[31] reported that the QFT-GIT test was statistically more accurate in diagnosing true-positive TBU patients than the T-SPOT.TB test (98% vs 76%, respectively). Due to the greater positive predictive value of QFT-GIT compared to that of the T-SPOT.TB test, they suggested application of the former as a first-line diagnostic test rather than the latter or a TST.
Advantages and Disadvantages of Interferon-Gamma Release Assays
Features of IGRAs and the TST are compared in Table 4. The most important advantage of the former test over the latter is the elimination of false-positive results associated with prior BCG vaccination or with previous exposure to environmental mycobacteria. The time-frame of a patient's exposure to the BCG vaccine may impact the TST result. If the patient had only been inoculated at birth or during the infantile period without having received subsequent booster shots, the impact on TST specificity was minimal. False-positive TST interpretation usually decreases over time, although, it can persist in some individuals and reduce diagnostic accuracy of the test for LTBI[3].
Table 4. Comparison between tuberculin skin testing and interferon-gamma release assays.
| Test | Advantages | Disadvantages |
| TST | Low cost for reagents | Requires two visits |
| Easy to perform in field setting | Subjective results with inter-reader and intrareader variability in measurement | |
| Ability to test a large number of people quickly | Low specificity in BCG-vaccinated people | |
| Minimal test-retest variability in low-risk populations | ||
| IGRAs | Single-visit | Higher cost for reagents |
| Positive and negative controls built into the test | Requires transporting samples to a laboratory | |
| Objective results | More complicated settings (laboratory registration, drawing blood, labelling, and transporting tubes to a laboratory) | |
| Electronic laboratory reporting | ||
| More specific in BCG-vaccinated people | ||
| Both tests | Limited sensitivity for active disease and inability to distinguish active TB from LTBI | |
| Low ability to predict short-term progression to active TB | ||
| Reduced sensitivity in immunosuppressed populations | ||
| Inability to differentiate a resolved infection from a new or ongoing infection | ||
BCG: Bacillus Calmette-Guerin; IGRAs: Interferon-gamma release assays; LTBI: Latent tuberculosis infection; TB: Tuberculosis; TST: Tuberculin skin test. Adapted from Haas and Belknap[3].
TST also has limitations in its sensitivity. Approximately 75%-90% of patients with active TB show a positive TST. TST performed in patients with immunosuppressive conditions, including HIV infection and disseminated TB, is associated with a high rate of false-negative results (>50%) and, therefore, requires careful interpretation. In such patients, especially in the immunocompromised and in the pediatric age-group, IGRAs demonstrate higher sensitivity than the TST. In addition, IGRAs become more valuable because of the higher pretest probability for identification of TBU, particularly in uveitis patients from endemic areas[16].
Furthermore, the technique of IGRAs is more convenient for patients than that of the TST, as it requires only a single visit for blood sampling. Unlike TST, IGRAs are performed in vitro and can be repeated without risk of sensitization or a boosting effect. These characteristics make IGRAs practical tests for application in TB screening programs, particularly those conducted in an occupational setting[18].
A systematic review reported high variation in reversion rates of IGRAs in either active or latent TB among studies, which makes these tests unreliable for monitoring response to anti-tuberculous therapy (ATT)[32]. However, an advantage of QFT-Plus in monitoring the response to ATT was recently reported[33]–[34]. A decrease in IFN-γ response following QFT-Plus-TB2 stimulation was observed after subjects with active TB had received ATT. However, the study was conducted in a region with low endemicity. Further studies are indicated to confirm the utility of QFT-Plus in monitoring treatment response.
Similar to the TST, IGRAs perform an indirect assessment of immune response to Mtb. False-negative and indeterminate IGRA results have been reported in infants and young children <5 years of age, advance age, early infection phase (<6-8wk), low peripheral lymphocyte counts, in individuals with a recent history of viral vaccination, in immunosuppressed patients (e.g., HIV infection with ≤200 cells/µL CD4 T-cell count, disseminated TB), in those with recent viral and bacterial infections, and in those on immunosuppressant therapy (e.g., high-dose corticosteroids, TNF inhibitors) [15],[19],[35]–[38]. In addition, IGRAs demonstrate cross-reactivity and may give positive results due to presence of some mycobacterial antigens within the RD-1 locus, as mentioned before[19].
Nevertheless, interpretation of IGRAs is still complicated by the immunologic recall of preexisting hypersensitivity to TB (e.g., a booster BCG vaccine shot), conversions due to new infection, reversions from positive to negative results, lack of consensus on a cut-off value, and inconsistent test reproducibility[18]. Eventually, higher costs and the technically advanced lab facilities required to perform IGRAs become obstacles to their application in some settings. Therefore, the TST still remains a comparably cost-effective and technically easier alternative diagnostic test for TB[3],[16].
Role of Interferon-Gamma Release Assays in Tuberculous Uveitis
Some experts suggest performing TST or IGRAs for all patients with idiopathic uveitis, whereas others recommend testing patients with either suspected signs of TBU or with additional risks for TB, including birth outside the US, history of living in an endemic area, history of incarceration or homelessness, history of intravenous drug abuse, HIV positive status, failure to respond to oral corticosteroid or immunosuppressive therapy, or presence of granulomatous inflammation on presentation[16].
Several studies have provided evidence for the use of IGRAs in TBU. Most studies that were performed using the previous generation of IGRAs showed supportive evidence for TBU diagnosis. Groen-Hakan et al[39] described the QFT-G test as a helpful diagnostic tool for uveitis patients in non-endemic countries. They suggested that a positive IGRA result in uveitis of unexplained cause may indicate a possible benefit of initiating ATT, particularly in affected patients with severe and sight-threatening inflammation. Pathanapitoon et al[40] found that most patients with positive QFT-G tests experienced uveitis of unknown etiology and occlusive retinal vasculitis. A more significant percentage of positive IGRA results was reported in patients with posterior uveitis[29],[41]. Using QFT-G, Gineys et al[42] considered that the standard laboratory cut-off value of ≥0.35 IU/mL may lead to over-treatment. They proposed that a cut-off value of 2.00 IU/mL was recommended for the diagnosis of TBU.
Ang et al[43]–[44] demonstrated the usefulness of QFT-GIT. They found that while the sensitivity of QFT-GIT was not superior to that of the TST, the former was slightly more specific than the latter. Similarly, the T-SPOT.TB test was found to be more specific but less sensitive than the TST. They recommended using IGRAs together with the TST during screening for TBU, as the overall diagnostic accuracy increased when both tests were performed together. Additionally, utilizing both TST and IGRAs for patients at presentation was found to be cost-effective for the diagnosis of TBU. Nevertheless, negative IGRA and TST results in patients with clinical signs suggestive of TBU should be interpreted cautiously, as these do not exclude a diagnosis of the disease[41].
The utility of IGRAs was also proposed for the management of TBU in the Collaborative Ocular Tuberculosis Study consensus guidelines. Initiation of ATT is considered based on several supportive factors, including endemic/non-endemic geography, clinical findings suggestive of TBU, immunologic test (IGRAs or TST) results, and radiographic evidence of healed or active pulmonary TB[45]–[46].
To our knowledge, several studies have been conducted on the usefulness of monitoring the response to ATT in active or latent TB; however, the exact role of IGRAs in monitoring treatment response in TBU remains undefined. Additionally, no study has reported the accuracy and clinical application of QFT-Plus in TBU[47]. The evidence of using new generations of IGRAs in TBU is still lacking.
CONCLUSION
TBU comprises a broad spectrum of clinical presentations, and its diagnosis remains challenging. IGRAs are considered useful immunodiagnostic tests that may supplement TST findings to enable diagnosis of TBU in affected patients. Understanding the principles and careful interpretation of IGRAs is helpful for ophthalmologists in clinical settings. However, studies on the new versions of IGRAs in TBU are still lacking, and further studies are needed to determine the role of IGRAs in monitoring the response to ATT in TBU.
Acknowledgments
Conflicts of Interest: Tungsattayathitthan U, None; Boonsopon S, None; Tesavibul N, None; Dharakul T, None; Choopong P, None.
REFERENCES
- 1.Global tuberculosis report 2020. https://www.who.int/publications-detail-redirect/9789240013131.
- 2.World Health Organization. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. World Health Organization; 2018. p. x, 64 p. https://apps.who.int/iris/handle/10665/260233. [PubMed] [Google Scholar]
- 3.Haas MK, Belknap RW. Diagnostic tests for latent tuberculosis infection. Clin Chest Med. 2019;40(4):829–837. doi: 10.1016/j.ccm.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Abu El-Asrar AM, Abouammoh M, Al-Mezaine HS. Tuberculous uveitis. Int Ophthalmol Clin. 2010;50(2):19–39. doi: 10.1097/IIO.0b013e3181d2ccb9. [DOI] [PubMed] [Google Scholar]
- 5.Ang M, Chee SP. Controversies in ocular tuberculosis. Br J Ophthalmol. 2017;101(1):6–9. doi: 10.1136/bjophthalmol-2016-309531. [DOI] [PubMed] [Google Scholar]
- 6.Sester M, Sotgiu G, Lange C, et al. Interferon-γ release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2011;37(1):100–111. doi: 10.1183/09031936.00114810. [DOI] [PubMed] [Google Scholar]
- 7.Gupta V, Gupta A, Rao NA. Intraocular tuberculosis—an update. Surv Ophthalmol. 2007;52(6):561–587. doi: 10.1016/j.survophthal.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Gupta A, Sharma A, Bansal R, Sharma K. Classification of intraocular tuberculosis. Ocul Immunol Inflamm. 2015;23(1):7–13. doi: 10.3109/09273948.2014.967358. [DOI] [PubMed] [Google Scholar]
- 9.Testi I, Agrawal R, Mehta S, Basu S, Nguyen Q, Pavesio C, Gupta V. Ocular tuberculosis: where are we today? Indian J Ophthalmol. 2020;68(9):1808–1817. doi: 10.4103/ijo.IJO_1451_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Distia Nora R, van Velthoven ME, Ten Dam-van Loon NH, Misotten T, Bakker M, van Hagen MP, Rothova A. Clinical manifestations of patients with intraocular inflammation and positive QuantiFERON-TB gold in-tube test in a country nonendemic for tuberculosis. Am J Ophthalmol. 2014;157(4):754–761. doi: 10.1016/j.ajo.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Figueira L, Fonseca S, Ladeira I, Duarte R. Ocular tuberculosis: position paper on diagnosis and treatment management. Rev Port Pneumol (2006) 2017;23(1):31–38. doi: 10.1016/j.rppnen.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Abdisamadov A, Tursunov O. Ocular tuberculosis epidemiology, clinic features and diagnosis: a brief review. Tuberculosis (Edinb) 2020;124:101963. doi: 10.1016/j.tube.2020.101963. [DOI] [PubMed] [Google Scholar]
- 13.Ang M, Vasconcelos-Santos DV, Sharma K, Accorinti M, Sharma A, Gupta A, Rao NA, Chee SP. Diagnosis of ocular tuberculosis. Ocul Immunol Inflamm. 2018;26(2):208–216. doi: 10.1080/09273948.2016.1178304. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal A, Agrawal R, Gunasekaran DV, et al. The collaborative ocular tuberculosis study (COTS)-1 report 3:polymerase chain reaction in the diagnosis and management of tubercular uveitis: global trends. Ocul Immunol Inflamm. 2019;27(3):465–473. doi: 10.1080/09273948.2017.1406529. [DOI] [PubMed] [Google Scholar]
- 15.Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161(4 Pt 2):S221–S247. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 16.Albini TA, Karakousis PC, Rao NA. Interferon-gamma release assays in the diagnosis of tuberculous uveitis. Am J Ophthalmol. 2008;146(4):486–488. doi: 10.1016/j.ajo.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Use of tuberculosis interferon-gamma release assays (IGRAs) in low- and middle-income countries: policy statement. World Health Organization; 2011. https://apps.who.int/iris/handle/10665/44759. [PubMed] [Google Scholar]
- 18.Pai M, Denkinger CM, Kik SV, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27(1):3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sotgiu G, Saderi L, Petruccioli E, Aliberti S, Piana A, Petrone L, Goletti D. QuantiFERON TB Gold Plus for the diagnosis of tuberculosis: a systematic review and meta-analysis. J Infect. 2019;79(5):444–453. doi: 10.1016/j.jinf.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Rego K, Pereira K, MacDougall J, Cruikshank W. Utility of the T-SPOT®.TB test's borderline category to increase test resolution for results around the cut-off point. Tuberculosis. 2018;108:178–185. doi: 10.1016/j.tube.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Rozot V, Patrizia A, Vigano S, et al. Combined use of Mycobacterium tuberculosis-specific CD4 and CD8 T-cell responses is a powerful diagnostic tool of active tuberculosis. Clin Infect Dis. 2015;60(3):432–437. doi: 10.1093/cid/ciu795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Della Bella C, Spinicci M, Alnwaisri HFM, et al. LIOFeron®TB/LTBI: a novel and reliable test for LTBI and tuberculosis. Int J Infect Dis. 2020;91:177–181. doi: 10.1016/j.ijid.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Wei ZH, Li YT, Wei CJ, Li YH, Xu H, Wu Y, Jia YJ, Guo R, Jia J, Qi XM, Li ZH, Gao XL. The meta-analysis for ideal cytokines to distinguish the latent and active TB infection. BMC Pulm Med. 2020;20(1):248. doi: 10.1186/s12890-020-01280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wawrocki S, Seweryn M, Kielnierowski G, Rudnicka W, Wlodarczyk M, Druszczynska M. IL-18/IL-37/IP-10 signalling complex as a potential biomarker for discriminating active and latent TB. PLoS One. 2019;14(12):e0225556. doi: 10.1371/journal.pone.0225556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu X, Tang Y, Yue Y, Zeng Y, Li W, Qu Y, Mu D. Accuracy of interferon-γ-induced protein 10 for diagnosing latent tuberculosis infection: a systematic review and meta-analysis. Clin Microbiol Infect. 2019;25(6):667–672. doi: 10.1016/j.cmi.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Metcalfe JZ, Everett CK, Steingart KR, Cattamanchi A, Huang L, Hopewell PC, Pai M. Interferon-γ release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and meta-analysis. J Infect Dis. 2011;204(Suppl 4):S1120–S1129. doi: 10.1093/infdis/jir410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh CE, Ortiz-Brizuela E, Bastos ML, Menzies D. Comparing the diagnostic performance of QuantiFERON-TB gold plus to other tests of latent tuberculosis infection: a systematic review and meta-analysis. Clin Infect Dis. 2021;73(5):e1116–e1125. doi: 10.1093/cid/ciaa1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cattamanchi A, Smith R, Steingart KR, Metcalfe JZ, Date A, Coleman C, Marston BJ, Huang L, Hopewell PC, Pai M. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2011;56(3):230–238. doi: 10.1097/QAI.0b013e31820b07ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smit DP, Esterhuizen TM, Meyer D. The role of QuantiFERON®-TB gold and tuberculin skin test as diagnostic tests for intraocular tuberculosis in HIV-positive and HIV-negative patients in South Africa. Ocul Immunol Inflamm. 2018;26(6):853–858. doi: 10.1080/09273948.2017.1327078. [DOI] [PubMed] [Google Scholar]
- 30.Ahn SJ, Kim KE, Woo SJ, Park KH. The usefulness of interferon-gamma release assay for diagnosis of tuberculosis-related uveitis in Korea. Korean J Ophthalmol. 2014;28(3):226–233. doi: 10.3341/kjo.2014.28.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ang M, Wong WL, Kiew SY, Li X, Chee SP. Prospective head-to-head study comparing 2 commercial interferon gamma release assays for the diagnosis of tuberculous uveitis. Am J Ophthalmol. 2014;157(6):1306–1314. 1314.e1–1314.e4. doi: 10.1016/j.ajo.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 32.Clifford V, He Y, Zufferey C, Connell T, Curtis N. Interferon gamma release assays for monitoring the response to treatment for tuberculosis: a systematic review. Tuberculosis. 2015;95(6):639–650. doi: 10.1016/j.tube.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Ledesma JR, Ma JN, Zheng P, Ross JM, Vos T, Kyu HH. Interferon-gamma release assay levels and risk of progression to active tuberculosis: a systematic review and dose-response meta-regression analysis. BMC Infect Dis. 2021;21(1):467. doi: 10.1186/s12879-021-06141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pourakbari B, Mamishi S, Benvari S, Sauzullo I, Bedini A, Valentini P, Keicho N, Mahmoudi S. Can interferon-γ release assays Be useful for monitoring the response to anti-tuberculosis treatment?: a systematic review and meta-analysis. Arch Immunol Ther Exp (Warsz) 2020;68(1):4. doi: 10.1007/s00005-020-00568-4. [DOI] [PubMed] [Google Scholar]
- 35.Smith R, Cattamanchi A, Steingart KR, Denkinger C, Dheda K, Winthrop KL, Pai M. Interferon-γ release assays for diagnosis of latent tuberculosis infection: evidence in immune-mediated inflammatory disorders. Curr Opin Rheumatol. 2011;23(4):377–384. doi: 10.1097/BOR.0b013e3283474d62. [DOI] [PubMed] [Google Scholar]
- 36.Ferrara G, Losi M, D'Amico R, et al. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet. 2006;367(9519):1328–1334. doi: 10.1016/S0140-6736(06)68579-6. [DOI] [PubMed] [Google Scholar]
- 37.Talati NJ, Seybold U, Humphrey B, Aina A, Tapia J, Weinfurter P, Albalak R, Blumberg HM. Poor concordance between interferon-gamma release assays and tuberculin skin tests in diagnosis of latent tuberculosis infection among HIV-infected individuals. BMC Infect Dis. 2009;9:15. doi: 10.1186/1471-2334-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamasue M, Komiya K, Usagawa Y, Umeki K, Nureki SI, Ando M, Hiramatsu K, Nagai H, Kadota JI. Factors associated with false negative interferon-γ release assay results in patients with tuberculosis: a systematic review with meta-analysis. Sci Rep. 2020;10(1):1607. doi: 10.1038/s41598-020-58459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groen-Hakan F, van Laar JAM, Bakker M, van Hagen PM, Hardjosantoso H, Rothova A. Prevalence of positive QuantiFERON-TB gold In-tube test in uveitis and its clinical implications in a country nonendemic for tuberculosis. Am J Ophthalmol. 2020;211:151–158. doi: 10.1016/j.ajo.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Pathanapitoon K, Kunavisarut P, Sirirungsi W, Rothova A. Looking for ocular tuberculosis: prevalence and clinical manifestations of patients with uveitis and positive QuantiFERON®-TB gold test. Ocul Immunol Inflamm. 2018;26(6):819–826. doi: 10.1080/09273948.2016.1245760. [DOI] [PubMed] [Google Scholar]
- 41.Trad S, Bodaghi B, Saadoun D. Update on immunological test (quantiferon-TB gold) contribution in the management of tuberculosis-related ocular inflammation. Ocul Immunol Inflamm. 2018;26(8):1192–1199. doi: 10.1080/09273948.2017.1332232. [DOI] [PubMed] [Google Scholar]
- 42.Gineys R, Bodaghi B, Carcelain G, Cassoux N, Boutin LTH, Amoura Z, Lehoang P, Trad S. QuantiFERON-TB gold cut-off value: implications for the management of tuberculosis-related ocular inflammation. Am J Ophthalmol. 2011;152(3):433–440.e1. doi: 10.1016/j.ajo.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Ang M, Nguyen HV, Kiew SY, Chen S, Chee SP, Finkelstein E. Cost-effectiveness of alternative strategies for interferon-γ release assays and tuberculin skin test in tuberculous uveitis. Br J Ophthalmol. 2015;99(7):984–989. doi: 10.1136/bjophthalmol-2014-306285. [DOI] [PubMed] [Google Scholar]
- 44.Ang M, Wong W, Ngan CCL, Chee SP. Interferon-gamma release assay as a diagnostic test for tuberculosis-associated uveitis. Eye (Lond) 2012;26(5):658–665. doi: 10.1038/eye.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agrawal R, Testi I, Mahajan S, et al. Collaborative Ocular Tuberculosis Study Consensus Group. Collaborative ocular tuberculosis study consensus guidelines on the management of tubercular uveitis-report 1: guidelines for initiating antitubercular therapy in tubercular choroiditis. Ophthalmology. 2021;128(2):266–276. doi: 10.1016/j.ophtha.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Agrawal R, Testi I, Bodaghi B, et al. Collaborative Ocular Tuberculosis Study Consensus Group. Collaborative ocular tuberculosis study consensus guidelines on the management of tubercular uveitis-report 2: guidelines for initiating antitubercular therapy in anterior uveitis, intermediate uveitis, panuveitis, and retinal vasculitis. Ophthalmology. 2021;128(2):277–287. doi: 10.1016/j.ophtha.2020.06.052. [DOI] [PubMed] [Google Scholar]
- 47.Rahman S, Irfan M, Siddiqui MAR. Role of interferon gamma release assay in the diagnosis and management of Mycobacterium tuberculosis-associated uveitis: a review. BMJ Open Ophthalmol. 2021;6(1):e000663. doi: 10.1136/bmjophth-2020-000663. [DOI] [PMC free article] [PubMed] [Google Scholar]


