Abstract
AIM
To evaluate the role of internal limiting membrane (ILM) peeling in preventing secondary epiretinal membrane (ERM) formation in pars plana vitrectomy (PPV) for proliferative diabetic retinopathy (PDR).
METHODS
This retrospective study analyzed the medical records of patients who underwent PPV for PDR and were followed up for minimum 3mo. ILM peeling was performed based on the intraoperative surgeons' judgments. ERM was assessed by optical coherence tomography photography. The relationship between ILM peeling and postoperative ERM was analyzed.
RESULTS
In total, 212 eyes from 197 patients were included in this study. The incidence of secondary ERM in the ILM non-peeling group was significantly higher than that in the ILM peeling group (37.0% vs 14.0%; P<0.001). Multivariate logistical regression revealed that ILM peeling was highly associated with the prevention of secondary ERM development [odds ratio 0.38; 95% confidence interval 0.17-0.86; P<0.05].
CONCLUSION
ILM peeling during PPV for PDRs can effectively reduce the incidence of secondary ERM development and is worth consideration by vitreoretinal surgeons.
Keywords: internal limiting membrane, secondary epiretinal membrane, proliferative diabetic retinopathy
INTRODUCTION
Secondary epiretinal membrane (ERM) is not a rare complication after vitrectomy. About 8.97% to 47.7% patients occurred secondary ERM after vitrectomy without internal limiting membrane (ILM) peeling for rhegmatogenous retinal detachment (RRD)[1]–[5]. The location of retinal breaks at the equator is a significant risk factor [odds ratio (OR), 3.9; 95% confidence interval (CI), 1.3-11.2][4]. It might cause visual impairment or metamorphopsia[6], thus requiring further membrane peeling surgery. A series of clinical studies found ILM peeling prevent the development of secondary ERM for RRD, although better best-corrected visual acuity (BCVA) among ILM peeling patients was not found[2],[7]–[8]. There were also some studies found ILM peeling can be as a prophylactic treatment to prevent macular pucker formation in patients who underwent retinectomy[9].
Regarding secondary ERM after vitrectomy for proliferative diabetic retinopathy (PDR), two pilot studies have reported the incidence to be 38.5% and 49%[10]–[11], respectively. ILM peeling can significantly minimize this frequency. Recently, Mehta et al[12] reported that ILM cleaning without ILM peeling can reduce the incidence of ERM formation after vitrectomy for PDR compared with that after standard procedure (4% vs 20%; P=0.01). Lin et al[13] had reported that ILM peeling can treat diabetic macular edema, although this has been refuted by some studies[14]–[16].
However, in practice, there are no uniform criteria for the surgical removal of the secondary ERM after vitrectomy for PDR. We encountered rapid and apparent development of secondary ERM in some cases with a mild postoperative reaction. We investigated the original ILM peeling for the prevention of secondary ERM and postoperative visual acuity (VA) and risk factors for secondary ERM development.
SUBJECTS AND METHODS
Ethical Approval
The research protocol complies with the ethical guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of Wenzhou Medical Eye Hospital. The informed consent was waived. Patients' clinical information was obtained from archived electronic medical records.
We retrospectively reviewed patients' information who underwent pars plana vitrectomy (PPV) for PDR at the Eye Hospital of Wenzhou Medical University between January 2018 and December 2019. PDR was defined by the presence of neovascularization of the disc (NVD) or elsewhere (NVE) or vitreous hemorrhage (VH) or preretinal hemorrhage[17]. Patients who underwent PPV for PDR and had readable postoperative (≥3mo after operation) OCT photographs were included. The exclusion criteria were as follows: 1) history of PPV surgery; 2) history of ocular penetrating trauma; 3) history of retinal vein occlusion, uveitis, age-related macular degeneration, or other fundus disease that may influence the macula; 4) traction retinal detachment involving the posterior pole; 5) silicone oil tamponade; 6) postoperative endophthalmitis. Pre- and postoperative retinal photocoagulation and intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF) or triamcinolone acetonide were considered risk factors for postoperative ERM development but were not exclusion criteria.
The 23- or 25-gauge PPVs were performed under retrobulbar (50% mixture of 2% lidocaine and 0.75% bupivacaine) or general anesthesia using three different vitrectomy machines (Accurus Surgical System, Alcon Laboratories, Fort Worth, TX; Stellaris PC, Bausch & Lomb, Bridgewater Township, NJ; Constellation Vision System, Alcon Laboratories) by 5 surgeons. The ERM will be peeled off if there was already ERM at the time of first vitreous surgery. But the removal of ILM was depended on the doctor's judgment. The ILM was stained with indocyanine green (Dandong Yi Chuang Pharmaceutical Co., Ltd, Liaoning Province, China) and then removed by ILM peeling forceps. ILM was peeled in the entire macular area (at least 2 disc diameters around the fovea). A combined surgery (PPV + phacoemulsification or intraocular lens implantation), pan retinal photocoagulation (PRP), and intraocular tamponade with air were performed, if necessary, depending on the surgeons' experience.
ERMs were assessed by two experienced ophthalmologists (Wen H and Lin Z) using the OCT (Heidelberg Spectralis OCT, Heidelberg, Germany) photographs. In the OCT scan, postoperative ERM was defined as hyper-reflective line internal to the ILM. If there were inconsistencies on the ERMs, the photographs were sent to the 3rd senior vitreoretinal surgeon with equal or more experience for consultation (Wu RH). To reduce subjective bias, the blind method was applied during assessment.
Statistical Analysis
The VA in decimal fraction was converted into logarithm of the minimum angle of resolution (logMAR) values. A BCVA of <0.02 was recorded as a logMAR value of 1.7. The normally distributed parameters were presented as mean±standard deviation, whereas median and quartile range was used for non-normally distributed parameters. The Chi-square or Fisher's exact test, as appropriate, was performed to compare discrete categorized data. Univariate logistic regressions were used to assess the relationship between postoperative ERM development and risk factors, including age, sex, history of retinal laser treatment, and ILM peeling. Next, multivariate logistic regression was performed for postoperative ERM, adjusted age and sex, and factors with a P value of <0.1 in the univariate analysis. The OR, hazard ratio, and 95%CIs were presented. A Cox proportional hazard regression was conducted for postoperative ERMs with adjustment of covariates. Statistical analysis was performed using SAS for Windows (Statistical Analysis System, version 9.1.3, SAS Inc., Cary, NC, USA).
RESULTS
Total 212 eyes of 197 patients (average age, 55.8±11.6y; 49.8% men) were enrolled in our study. The overall duration of follow-up was 8.7±4.9mo. There were 93 eyes in ILM peeling group and 119 in ILM non-peeling group. The baseline clinical characteristics of all patients are shown in Table 1. There were no differences in patient age, sex, status of lens, BCVA, and intraocular pressure between the groups. Patients in ILM peeling group had a higher proportion of preoperative ERM (39.8% vs 16.0%; P<0.001) and preoperative intravitreal anti-VEGF injection (61.3% vs 17.6%; P<0.001), a lower proportion of VH (19.4% vs 63.9%; P<0.001) and retinal laser treatment history (3.2% vs 15.1%; P=0.004) compared with those in ILM non-peeling group.
Table 1. Patient baseline characteristics in both groups.
| Parameters | ILM non-peeling group (n=119) | ILM peeling group (n=93) | P |
| Age (y) | 55.0±11.9 | 56.9±11.1 | 0.24 |
| Male | 57 (47.9) | 52 (55.9) | 0.25 |
| Right eye | 57 (47.9) | 46 (49.5) | 0.82 |
| Lens status | 0.68 | ||
| Phakic eyes | 104 (87.4) | 83 (89.2) | |
| Pseudophakic eyes | 15 (12.6) | 10 (10.8) | |
| BCVA (logMAR)a | 1.70 (0.80, 1.70) | 1.70 (0.82, 1.70) | 0.84 |
| IOP (mm Hg) | 12.7±4.1 | 12.6±3.0 | 0.76 |
| ERM | 19 (16.0) | 37 (39.8) | <0.001 |
| VH | 76 (63.9) | 18 (19.4) | <0.001 |
| History of retinal laser | 18 (15.1) | 3 (3.2) | 0.004 |
| Preoperative IVI of anti-VEGF | 21 (17.6) | 57 (61.3) | <0.001 |
ILM: Internal limiting membrane; BCVA: Best-corrected visual acuity; IOP: Intraocular pressure; ERM: Epiretinal membrane; VH: Vitreous hemorrhage; IVI: Intravitreal injection; VEGF: Vascular endothelial growth factor. aPresented by median (quartile range) and tested by Wilcoxon tests.
n (%)
The intra- and postoperative details for both groups are presented in Table 2. More patients in ILM peeling group underwent combined surgery with phacoemulsification (89.2% vs 73.1%; P=0.006), fibrovascular membrane peeling (57.0% vs 33.6%; P<0.001), retinal attachment (22.6% vs 11.8%; P=0.04), PRP (76.3% vs 61.3%; P=0.02), and air tamponade (57.0% vs 33.6%; P<0.001). The follow-up duration was 9.3±5.4mo in ILM non-peeling group and 8.0±4.1mo in ILM peeling group (P=0.06). During the follow-up period, supplemental PRP treatment was more common in ILM non-peeling group than in ILM peeling group (12.6% vs 4.3%; P=0.04). At the final follow-up, in ILM non-peeling group, the median logMAR VA was statistically better than in ILM peeling group (0.40 vs 0.52; P=0.04). The median logMAR VA improvement was 0.90 in ILM non-peeling group and 0.49 in ILM peeling group with significant difference (P=0.03). Compared with patients in ILM peeling group, patients in ILM non-peeling group had higher incidence of secondary ERM (37.0% vs 14.0%; P<0.001; Table 2). There was no difference between ILM non-peeling group [3.63mo (interquartile range, 1.45-5.88mo)] and ILM peeling group [3.39mo (interquartile range, 3.21-4.64mo)] in the timing of secondary ERM development during the follow-up (P=0.80). Figure 1 shows a typical example of ERM development in ILM non-peeling group.
Table 2. Operative and postoperative information for patients with or without ILM peeling.
| Parameters | ILM non-peeling group (n=119) | ILM peeling group (n=93) | P |
| Operative information | |||
| Combined PHACO | 76 (73.1) | 74 (89.2) | 0.006 |
| FVM peeling | 40 (33.6) | 53 (57.0) | <0.001 |
| Retinal attachment | 14 (11.8) | 21 (22.6) | 0.04 |
| PRP | 73 (61.3) | 71 (76.3) | 0.02 |
| Intraocular tamponade | |||
| BSS | 79 (66.4) | 40 (43.0) | <0.001 |
| Air | 40 (33.6) | 53 (57.0) | <0.001 |
| IVI at the end of surgery | 44 (37.0) | 15 (16.1) | <0.001 |
| Postoperative information | |||
| Follow-up time (mo) | 9.3±5.4 | 8.0±4.1 | 0.06 |
| Supplemental retinal laser | 15 (12.6) | 4 (4.3) | 0.04 |
| IVI of anti-VEGF | 22 (18.5) | 27 (29.0) | 0.07 |
| VH | 8 (6.7) | 4 (4.3) | 0.45 |
| NVGa | 5 (4.2) | 1 (1.1) | 0.23 |
| VA at final visit (logMAR)b | 0.40 (0.22, 0.80) | 0.52 (0.30, 1.00) | 0.04 |
| VA improvement (logMAR)b | 0.90 (0.30, 1.30) | 0.49 (0.22, 1.00) | 0.03 |
| IOP at final visit (mm Hg) | 15.1±6.7 | 13.6±3.9 | 0.05 |
| Secondary ERM | |||
| No preoperative ERM | 35 (35.0) | 5 (8.9) | <0.001 |
| Preoperative ERM | 9 (47.4) | 8 (21.6) | 0.047 |
| Total | 44 (37.0) | 13 (14.0) | <0.001 |
| Time for secondary ERM (mo)b | |||
| No preoperative ERM | 3.68 (1.79, 6.46) | 3.39 (3.29, 9.21) | 0.56 |
| Preoperative ERM | 3.21 (1.21, 5.29) | 3.50 (2.27, 4.34) | 0.92 |
| Total | 3.63 (1.45, 5.88) | 3.39 (3.21, 4.64) | 0.80 |
ILM: Internal limiting membrane; PHACO: Phacoemulsification; VH: Vitreous hemorrhage; FVM: Fibrovascular membrane; PRP: Pan retinal photocoagulation; BSS: Balanced salt solution; IVI: Intravitreal injection; VEGF: Vascular endothelial growth factor; NVG: Neovascular glaucoma; VA: Visual acuity; IOP: Intraocular pressure; ERM: Epiretinal membrane. aFisher's exact test; bPresented by median (quartile range) and tested by Wilcoxon tests.
n (%)
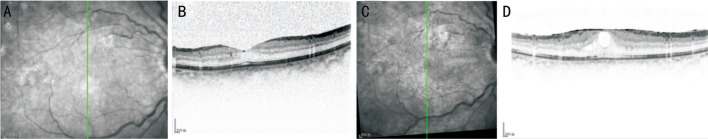
Figure 1. A typical case of secondary epiretinal membrane developed in the group without inner limiting membrane peeling.
A: SD-OCT scan, taken at the 7d follow-up of a patient with a proliferative diabetic retinopathy who underwent a vitrectomy without internal limiting membrane peeling; B: A scan along the green line in “A”; C: The 3mo follow-up SD-OCT scan of this patient shows a macular pucker; D: An OCT image along the green line in “C” shows a hyper-reflective line at the foveal surface. SD-OCT: Spectral domain optical coherence tomography.
We evaluated perioperative factors to identify their potential effect on the postoperative development of secondary ERM (Table 3). Univariate logistical regression showed that the incidence of secondary ERM significantly increased in cases without ILM peeling, preoperative intravitreal injection of anti-VEGF, preoperative VH, and history of retinal laser treatment. Multivariate analysis revealed that ILM peeling was highly associated with the prevention of secondary ERM development (OR, 0.38; 95%CI, 0.17-0.86, P<0.05).
Table 3. Risk factors for secondary ERM post vitrectomy of diabetic retinopathy.
| Parameters | Univariate, OR (95%CI) | Multivariate, OR (95%CI) |
| Preoperative factors | ||
| Age | 1.02 (0.99, 1.04) | 1.02 (0.99, 1.05) |
| Sex | 1.67 (0.90, 3.08) | 1.61 (0.84, 3.10) |
| Lens status | 0.65 (0.23, 1.82) | - |
| Baseline BCVA | 0.85 (0.46, 1.55) | - |
| Baseline IOP | 0.96 (0.88, 1.04) | - |
| Preoperative ERM | 1.26 (0.64, 2.48) | - |
| Preoperative VH | 2.12 (1.14, 3.93) | 1.15 (0.55, 2.38) |
| History of retinal laser | 2.79 (1.11, 6.97) | 2.10 (0.78, 5.67) |
| Preoperative IVI of anti-VEGF | 0.36 (0.18, 0.73) | 0.58 (0.26, 1.31) |
| Operative and postoperative factors | ||
| Surgeon | 0.95 (0.79, 1.14) | - |
| Combined PHACO | 0.89 (0.40, 1.96) | - |
| FVM peeling | 1.21 (0.66, 2.23) | - |
| ILM peeling | 0.28 (0.14, 0.55) | 0.38 (0.17, 0.86) |
| Retinal attachment | 1.11 (0.49, 2.48) | - |
| Intraocular tamponade | 1.03 (0.54, 1.98) | - |
| IVI at the end of surgery | 0.61 (0.32, 1.14) | - |
| Supplemental retinal laser | 1.44 (0.74, 2.78) | - |
| IVI of anti-VEGF | 1.29 (0.46, 3.56) | - |
| VH | 0.54 (0.24, 1.20) | - |
| NVG | 2.03 (0.62, 6.69) | - |
BCVA: Best-corrected visual acuity; IOP: Intraocular pressure; ERM: Epiretinal membrane; VH: Vitreous hemorrhage; IVI: Intravitreal injection; VEGF: Vascular endothelial growth factor; PHACO: Phacoemulsification; FVM: Fibrovascular membrane; ILM: Internal limiting membrane; NVG: Neovascular glaucoma.
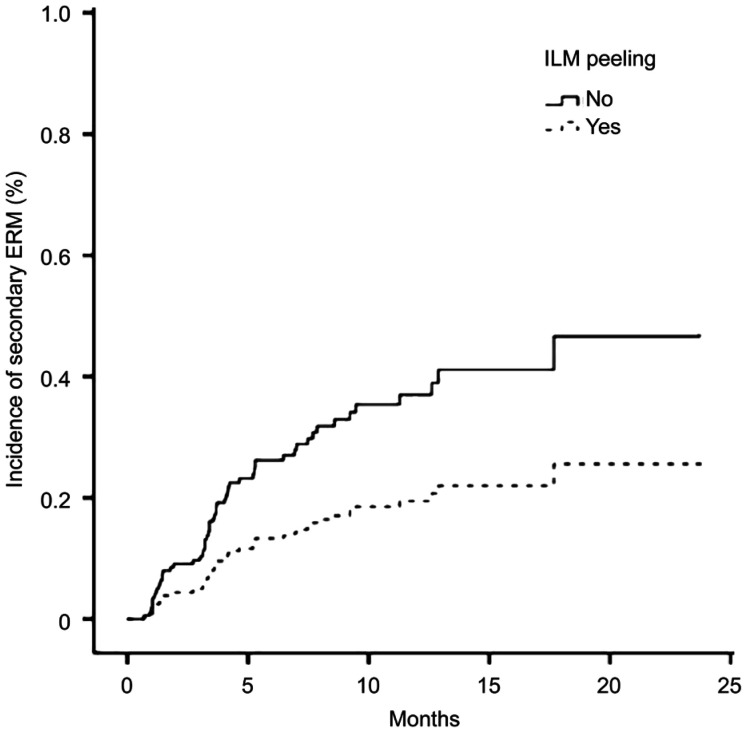
As showed in Figure 2, after adjusting for the factors screened from the univariate model, a Cox proportional hazard regression for the incidence of secondary ERM was performed. which showed only ILM peeling remained significant (hazard ratio, 0.47; 95%CI, 0.23-0.98; P=0.04).
Figure 2. Cox proportional curve of inner limiting membrane peeling on the incidence of secondary epiretinal membrane.
Adjusted for age, sex, preoperative vitreous hemorrhage, history of retinal laser treatment, and preoperative intravitreal injection.
DISCUSSION
Secondary ERM development causes visual deterioration and macular disorders such as macular cysts or macular thickening after successful surgical treatment of PDRs[17]–[19]. Recently, it has been reported that ILM peeling might decrease the ERM formation and diabetic macular edema[13],[19]–[20]. Although a previous randomized clinical trial that enrolled 207 patients with PDR reported a beneficial role of ILM peeling in patients with PDR for improvement in VA[19], ILM peeling was evaluated in patients with PDR undergoing PPV for the primary indication of VH as the primary objective. Most of these patients had VH, making the cohort different from our patient population. This study examined the effectiveness of ILM peeling during vitrectomy for PDR in reducing secondary ERM as well as identifying risk factors.
Although the occurrence of secondary ERM after diabetic vitrectomy is common, its mechanism remains unclear. Several factors are likely contributed to the high incidence of ERMs. First, residual native vitreous collagen and ILM in the macular area may act as a scaffold for cellular proliferation. Second, VH, postoperative inflammation, and the damaged retinal surface may provide an ideal environment for the growth of secondary ERMs. ILM peeling may therefore decrease the incidence of secondary ERM because it removes the scaffold that proliferates astrocytes, myofibroblasts, and retinal pigment epithelium cells[21]. Our results confirm that ILM peeling could effectively reduce the formation of secondary ERM. This result is consistent with previous studies that ILM peeling significantly reduced postoperative ERM after PPV for PDR[19]. In the current study, we observed that the overall incidence of secondary ERM was 26.9%. Furthermore, from the Cox proportional curve, we observed that the incidence of secondary ERM was lower in ILM peeling group that in ILM non-peeling group at any follow-up point. The incidence of ERM was 37.0% in ILM non-peeling group and 14.0% in ILM peeling group. Several previous studies have reported similar incidences of secondary ERM in ILM non-peeling groups, ranging from 20% to 52.8%, depending on the case mix and the methods of detection[22]–[23]. In fact, more factors associated with ERM formation were presented in ILM peeling group. In ILM peeling group, the patients had a higher rate of preoperative ERM, fibrovascular membrane, retinal attachment, PRP, and air tamponade. PDR eyes with more severe preoperative vitreoretinal changes may be more prone to secondary ERM[23]. Under diabetic conditions, a local proinflammatory and proangiogenic environment in eyes provides a strong stimulation for tissue proliferation, which is associated with the frequent incidence of ERM[23]. As well as head-down tilt after gas injection in the postoperative period, a higher number of cells on the macular surface would also result in an increased risk of ERM. In the current study, when considering the comprehensive risk factors, ILM peeling was the only independent risk factor for postoperative secondary ERM, which strongly emphasized the importance of ILM peeling during vitrectomy for patients with PDR.
For this study, we have also analyzed the time of secondary ERM. The median timing of secondary ERM development was similar at approximately 3mo for both groups. Most of the cases developed secondary ERM between 3 and 6mo postoperatively, similar to that observed in a study with a mean interval of 2.3mo (range, 1-4mo)[24].
According to our study, history of retinal laser treatment and preoperative VH was associated with an increased risk of secondary ERM. Chehaibou et al[25] found that the eyes with laser treatment developed proliferative tissue along the border of the laser scar, which may induce a gliotic reaction. Furthermore, intravitreal anti-VEGF injection prior to surgery decreased the risk of secondary ERM. Preoperative intravitreal anti-VEGF injection may enhance the absorption of VH by reducing the dissemination of blood cells, thereby decreasing the formation of ERM. However, these three factors were no longer significant in the multivariate analysis, after adjusting for ILM peeling. This may be because of the higher proportion of preoperative VH and laser treatment in ILM non-peeling group and the higher proportion of preoperative intravitreal anti-VEGF injection in ILM peeling group. Additionally, both preoperative retinal laser treatment and PRP were performed away from the retinal vessel arch/posterior polar area (no macular grid pattern laser treatment was performed), rather than adjacent to the macular area.
Considering the visual prognosis after surgery to treat postoperative ERMs, there might be a case for not peeling the ILM[1],[26]. Meanwhile, several studies have demonstrated that the ILM peeling group had a better BCVA than the ILM non-peeling group[10]–[11],[19]. In our study, although the improvement in VA was significant in both groups, the final median VA in ILM non-peeling group was better than that in ILM peeling group, and the most significant reason was an apparent higher proportion of VH in the former than that in latter (63.9% vs 19.4%); therefore, a more apparent VA improvement was gained in ILM non-peeling group.
Postoperative complications were similar between the two groups. Compared to previous studies, we found that the incidence of postoperative VH and neovascular glaucoma (NVG) was relatively lower at 5.6% and 2.8%, respectively (VH: 8%-25%[27]–[28]; NVG: 1%-9%[19],[29]). In our study, PRP was extensively applied during PPV, and anti-VEGF was injected preoperatively, which may explain the relatively low rate of VH and NVG[30].
Although the current study had a relatively large sample size, there are some limitations. First, because the study was retrospective in nature, there was certain to be some degree of subject heterogeneity. Second, pre- and postoperative intervention and operative procedures varied among different surgeons. Third, the length of follow-up was relatively short. Nonetheless, this is the first study to examine the prevention of secondary ERM for patients with PDR after ILM peeling. It would be beneficial to conduct further randomized clinical trials to support the efficacy of initial ILM peeling for PDR. In conclusion, ILM peeling might prevent secondary ERM development during PPV for PDRs and should be considered by sophisticated vitreoretinal surgeons.
Acknowledgments
Authors' contributions: The study was designed by Wu RH, Xu MN and Lin Z; Xu MN, Feng KM and Zhou HJ did data analysis; Wu RH, Xu MN, and Lin Z drafted the manuscript; all authors conducted the acquisition of data; the final manuscript has been read and approved by all authors.
Conflicts of Interest: Wu RH, None; Xu MN, None; Lin K, None; Ren MX, None; Wen H, None; Feng KM, None; Zhou HJ, None; Moonasar N, None; Lin Z, None.
REFERENCES
- 1.Fallico M, Russo A, Longo A, Pulvirenti A, Avitabile T, Bonfiglio V, Castellino N, Cennamo G, Reibaldi M. Internal limiting membrane peeling versus no peeling during primary vitrectomy for rhegmatogenous retinal detachment: a systematic review and Meta-analysis. PLoS One. 2018;13(7):e0201010. doi: 10.1371/journal.pone.0201010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyama K, Fujinami K, Watanabe K, Tsunoda K, Noda T. Internal limiting membrane peeling to prevent post-vitrectomy epiretinal membrane development in retinal detachment. Am J Ophthalmol. 2016;171:1–10. doi: 10.1016/j.ajo.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Sousa K, Calvão-Santos G, Costa J, Ferreira L, Mendonça L, Gentil R, Gomes NL. Anatomical and functional results of ILM peeling vs non-peeling in macula-off rhegmatogenous retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2020;258(10):2105–2110. doi: 10.1007/s00417-020-04775-9. [DOI] [PubMed] [Google Scholar]
- 4.Martínez-Castillo V, Boixadera A, Distéfano L, Zapata M, García-Arumí J. Epiretinal membrane after pars Plana vitrectomy for primary pseudophakic or aphakic rhegmatogenous retinal detachment: incidence and outcomes. Retina. 2012;32(7):1350–1355. doi: 10.1097/IAE.0b013e318242b965. [DOI] [PubMed] [Google Scholar]
- 5.Arias L, Padrón-Pérez N, Flores-Moreno I, Giralt L, Cobos E, Lorenzo D, García-Bru P, Dias B, Caminal JM. Internal limiting membrane peeling versus nonpeeling to prevent epiretinal membrane development in primary rhegmatogenous retinal detachment: a swept-source optical coherence tomography study with a new postoperative classification system. Retina. 2020;40(7):1286–1298. doi: 10.1097/IAE.0000000000002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akiyama K, Fujinami K, Watanabe K, Fukui M, Tsunoda K, Noda T. Validity and efficacy of internal limiting membrane peeling during initial vitrectomy for rhegmatogenous retinal detachment: visual outcomes in macula-sparing cases. Retin Cases Brief Rep. 2021;15(2):114–119. doi: 10.1097/ICB.0000000000000758. [DOI] [PubMed] [Google Scholar]
- 7.Mahmood SA, Rizvi SF, Khan BAM, Khan TH. Role of concomitant internal limiting membrane (ILM) peeling during rhegmatogenous retinal detachment (RRD) surgery in preventing postoperative epiretinal membrane (ERM) formation. Pak J Med Sci. 2021;37(3):651–656. doi: 10.12669/pjms.37.3.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanco-Teijeiro MJ, Bande Rodriguez M, Mansilla Cuñarro R, Paniagua Fernández L, Ruiz-Oliva Ruiz F, Piñeiro Ces A. Effects of internal limiting membrane peeling during vitrectomy for macula-off primary rhegmatogenous retinal detachment. Eur J Ophthalmol. 2018;28(6):706–713. doi: 10.1177/1120672117750055. [DOI] [PubMed] [Google Scholar]
- 9.Kohli GM, Shenoy P, Shetty S, Sangole A, Sen A. Macular internal limiting membrane peel for eyes undergoing vitrectomy for retinal detachment: rationalizing selection based on the severity of proliferative vitreoretinopathy. Indian J Ophthalmol. 2021;69(5):1356–1357. doi: 10.4103/ijo.IJO_500_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michalewska Z, Bednarski M, Michalewski J, Jerzy N. The role of ILM peeling in vitreous surgery for proliferative diabetic retinopathy complications. Ophthalmic Surg Lasers Imaging Retina. 2013;44(3):238–242. doi: 10.3928/23258160-20130503-05. [DOI] [PubMed] [Google Scholar]
- 11.Chang PY, Yang CM, Yang CH, Chen MS, Wang JY. Pars plana vitrectomy for diabetic fibrovascular proliferation with and without internal limiting membrane peeling. Eye (Lond) 2009;23(4):960–965. doi: 10.1038/eye.2008.334. [DOI] [PubMed] [Google Scholar]
- 12.Mehta A, Rana-Rahman R, Klaassen I, Rees J, Steel DH. The effect of internal limiting membrane cleaning on epiretinal membrane formation after vitrectomy for proliferative diabetic retinopathy. Ophthalmologica. 2020;243(6):426–435. doi: 10.1159/000509878. [DOI] [PubMed] [Google Scholar]
- 13.Lin HC, Yang CM, Chen SN, Hsieh YT. Vitrectomy with internal limiting membrane peeling versus nonsurgical treatment for diabetic macular edema with massive hard exudates. PLoS One. 2020;15(7):e0236867. doi: 10.1371/journal.pone.0236867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rinaldi M, Dell'Omo R, Morescalchi F, Semeraro F, Gambicorti E, Cacciatore F, Chiosi F, Costagliola C. ILM peeling in nontractional diabetic macular edema: review and metanalysis. Int Ophthalmol. 2018;38(6):2709–2714. doi: 10.1007/s10792-017-0761-6. [DOI] [PubMed] [Google Scholar]
- 15.Ghassemi F, Bazvand F, Roohipoor R, Yaseri M, Hassanpoor N, Zarei M. Outcomes of vitrectomy, membranectomy and internal limiting membrane peeling in patients with refractory diabetic macular edema and non-tractional epiretinal membrane. J Curr Ophthalmol. 2016;28(4):199–205. doi: 10.1016/j.joco.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamura Y, Sato Y, Isomae T, Shimada H. Effects of internal limiting membrane peeling in vitrectomy on diabetic cystoid macular edema patients. Jpn J Ophthalmol. 2005;49(4):297–300. doi: 10.1007/s10384-005-0199-7. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson CP, Ferris FL, III, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 18.Guo J, Bi X, Chen SN, Chen S, He GH, Wu B, Zhang W, Wang J. Efficacy of internal limiting membrane peeling for diabetic macular edema after preoperative anti-vascular endothelial growth factor injection. Int J Ophthalmol. 2020;13(11):1758–1764. doi: 10.18240/ijo.2020.11.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rush RB, Del Valle Penella A, Reinauer RM, Rush SW, Bastar PG. Internal limiting membrane peeling during vitrectomy for diabetic vitreous hemorrhage: a randomized clinical trial. Retina. 2021;41(5):1118–1126. doi: 10.1097/IAE.0000000000002976. [DOI] [PubMed] [Google Scholar]
- 20.Rush RB, Rush SW. Pars plana vitrectomy with internal limiting membrane peeling for treatment-Naïve diabetic macular edema: a prospective, uncontrolled pilot study. Clin Ophthalmol. 2021;15:2619–2624. doi: 10.2147/OPTH.S320214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Someya H, Takayama K, Takeuchi M, Yokoyama H, Kimura T, Morioka M, Takamura Y, Sameshima S, Ueda T, Ogata N, Tashiro M, Kitano S, Sakamoto T. Outcomes of 25-gauge vitrectomy for tractional and nontractional diabetic macular edema with proliferative diabetic retinopathy. J Ophthalmol. 2019;2019:5304524. doi: 10.1155/2019/5304524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Im JC, Kim JH, Park DH, Shin JP. Structural changes of the macula on optical coherence tomography after vitrectomy for proliferative diabetic retinopathy. Ophtalmologica. 2017;238(4):186–195. doi: 10.1159/000477826. [DOI] [PubMed] [Google Scholar]
- 23.Yang CM, Yeh PT, Cheng SF, Yang CH, Chen MS. Macular appearance after diabetic vitrectomy for fibrovascular proliferation: an optical coherence tomography study. Acta Ophthalmol. 2010;88(2):193–198. doi: 10.1111/j.1755-3768.2008.01420.x. [DOI] [PubMed] [Google Scholar]
- 24.Hsu YR, Yang CM, Yeh PT. Clinical and histological features of epiretinal membrane after diabetic vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2014;252(3):401–410. doi: 10.1007/s00417-013-2479-0. [DOI] [PubMed] [Google Scholar]
- 25.Chehaibou I, Pettenkofer M, Govetto A, Rabina G, Sadda SR, Hubschman JP. Identification of epiretinal proliferation in various retinal diseases and vitreoretinal interface disorders. Int J Retina Vitreous. 2020;6:31. doi: 10.1186/s40942-020-00233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelman R, Stevenson W, Prospero Ponce C, Agarwal D, Christoforidis JB. Retinal damage induced by internal limiting membrane removal. J Ophthalmol. 2015;2015:939748. doi: 10.1155/2015/939748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn J, Woo SJ, Chung H, Park KH. The effect of adjunctive intravitreal bevacizumab for preventing postvitrectomy hemorrhage in proliferative diabetic retinopathy. Ophthalmology. 2011;118(11):2218–2226. doi: 10.1016/j.ophtha.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 28.Hernández-Da Mota SE, Nuñez-Solorio SM. Experience with intravitreal bevacizumab as a preoperative adjunct in 23-G vitrectomy for advanced proliferative diabetic retinopathy. Eur J Ophthalmol. 2010;20(6):1047–1052. doi: 10.1177/112067211002000604. [DOI] [PubMed] [Google Scholar]
- 29.Takayama K, Someya H, Yokoyama H, Takamura Y, Morioka M, Sameshima S, Ueda T, Kitano S, Tashiro M, Sugimoto M, Kondo M, Sakamoto T, Takeuchi M. Risk factors of neovascular glaucoma after 25-gauge vitrectomy for proliferative diabetic retinopathy with vitreous hemorrhage: a retrospective multicenter study. Sci Rep. 2019;9(1):14858. doi: 10.1038/s41598-019-51411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guan J, Cai N, Liu LM, Zhao N, Liu NN. Ranibizumab pretreatment in vitrectomy with internal limiting membrane peeling on diabetic macular edema in severe proliferative diabetic retinopathy. Diabetes Ther. 2020;11(6):1397–1406. doi: 10.1007/s13300-020-00822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]