Abstract
As a one of the focuses of ecological research, understanding the regulation of plant diversity on community stability is helpful to reveal the adaption of plant to environmental changes. However, the relationship between plant diversity and community stability is still controversial due to the scale effect of its influencing factors. In this study, we compared the changes in community stability and different plant diversity (i.e., species, functional, and phylogenetic diversities) between three communities (i.e., riparian forest, ecotone community, and desert shrubs), and across three spatial scales (i.e., 100, 400, and 2500 m2), and then quantified the contribution of soil properties and plant diversity to community stability by using structural equation model (SEM) in the Ebinur Lake Basin Nature Reserve of the Xinjiang Uygur Autonomous Region in the NW China. The results showed that: (1) community stability differed among three communities (ecotone community > desert shrubs > riparian forest). The stability of three communities all decreased with the increase of spatial scale (2) species diversity, phylogenetic richness and the mean pairwise phylogenetic distance were higher in ecotone community than that in desert shrubs and riparian forest, while the mean nearest taxa distance showed as riparian forest > ecotone community > desert shrubs. (3) Soil ammonium nitrogen and total phosphorus had the significant direct negative and positive effects on the community stability, respectively. Soil ammonium nitrogen and total phosphorus also indirectly affected community stability by adjusting plant diversity. The interaction among species, functional and phylogenetic diversities also regulated the variation of community stability across the spatial scales. Our results suggested that the effect of plant diversities on community stability were greater than that of soil factors. The asynchronous effect caused by the changes in species composition and functional traits among communities had a positive impact on the stability. Our study provided a theoretical support for the conservation and management of biodiversity and community functions in desert areas.
Keywords: alpha diversity, beta diversity, diversity-stability relationship, stabilization mechanisms, spatial scale
Introduction
Stability is a comprehensive feature of plant community structure and function (Wang, 2002), which is directly related to biodiversity maintenance, water and carbon balance, climate regulation, and soil and water conservation (Li et al., 2022). Under the influence of climate change and biological invasion, community stability was decreasing globally (Wang C. et al., 2021). Assessing its change and the influencing factors has become one of the most important topics in the scientific community (Li et al., 2022).
Plant diversity is considered as a key indicator to measure community stability, because ecosystem function and structure are increasingly vulnerable to the threat of biodiversity loss (Evans et al., 2022). However, the vast majority of previous studies used species diversity to assess community stability (Sasaki and Lauenroth, 2011; Craven et al., 2018; Liu et al., 2019). Since species diversity ignores the differences in functional traits and phylogenetic classification among species, it may have some shortages in assessing community stability (Chao et al., 2014). With the deepening of the understanding of biodiversity, an increasing number of ecologists have realized that functional and phylogenetic diversity are more advantageous than species diversity in assessing community stability (Su et al., 2021; Token et al., 2022). In addition, although studies have demonstrated that diversity can affect community stability in many ways, the influencing mechanisms and their relative contribution remain unclear (Sasaki and Lauenroth, 2011; Hallett et al., 2014). To date, scientists have identified at least four potential mechanisms of diversity for maintaining community stability: portfolio effect (higher diversity or richness leads to higher stability) (Hector et al., 2010); Selection effect (higher advantages lead to higher stability) (Grman et al., 2010); Insurance effect (asynchronous or compensatory dynamics of larger species lead to higher stability) (Brown et al., 2016); and over-yielding effect (larger total biomass leads to higher stability) (Hector et al., 2010). However, these mechanisms are not fixed, which varies among ecosystems and their contributions varies with the environment. The relationship between plant diversity and community stability needs to be further explored in diverse ecosystems.
Biodiversity can be divided into α and β diversity based on the regional scale (Jost, 2007; Ricotta and Szeidl, 2009). α diversity represents the number and distribution uniformity of species, and the variation on their functional traits in a community (Rosauer et al., 2009; Mason and de Bello, 2013). β diversity refers to the differences in species composition, evolutionary relationship and functional attributes among communities across spatial or temporal scales (Tan et al., 2019; Wang S. et al., 2021). In terms of the relationship between plant diversity and community stability, most studies focus on α diversity while ignore β diversity because the former is easy to observe, and the influences of spatial and temporal changes are not considered (Wang and Loreau, 2016; Qiao et al., 2022). Although some recent studies have tested the effect of β diversity on community stability at multiple environmental gradients (Zhang et al., 2019; Hautier et al., 2020; Wang S. et al., 2021), they have been concentrated in resource-rich environments, such as tropical and subtropical forests (Hautier et al., 2020; Wang S. et al., 2021; Qiao et al., 2022). Most of them did not analyze the effect of diversity on community stability across different spatial scales.
Community ecologists have long been interested in unraveling the maintenance mechanism of community stability in highly variable environments (Loreau and de Mazancourt, 2013; Li and Stevens, 2017; Li et al., 2018a). The possible influence of abiotic factors on community stability may be increased seriously due to the increasing global environmental change (De Laender et al., 2016; Wu et al., 2021). Recent studies have shown that abiotic factors (i.e., soil properties) may obscure the extent and direction of biodiversity’s impacts on community function and stability (Coelho de Souza et al., 2019; Wang et al., 2020). Environmental changes not only directly affect the community stability, but also indirectly affect it through changes in plant diversity (Xu et al., 2015b). For example, environmental change can greatly alter the composition, abundance and diversity of communities, thus affecting the maintenance of community stability (Hallett et al., 2014; Donohue et al., 2016). However, most of these studies have focused on forests and grassland ecosystems in moist regions, while few studies have been carried out in arid deserts (Hautier et al., 2020; Wang C. et al., 2021; Qiao et al., 2022). It is well known that one of the most striking features of desert ecosystems, that distinguishes them from other systems, is that local plants are subjected to severe drought stress due to the lack of rainfall (Gong et al., 2019; Song et al., 2020; Yang X. et al., 2022). In this case, the relationship between diversity and community stability in arid desert may be different from other systems.
The Ebinur Lake Wetland National Nature Reserve (ELWNNR) is a treasure house of biodiversity in arid desert areas of the Xinjiang Uygur Autonomous Region in the NW China, which provides a good place for studying the maintenance of diversity on community stability in arid desert ecosystem (Jiang L. et al., 2022). Affected by arid climatic conditions, the local ecosystem is relatively sensitive and vulnerable to environmental changes (Yang et al., 2019; Token et al., 2022). Although the relationship between plant diversity and community stability in arid desert system have been reported, they mainly focus on the effects of species diversity and functional diversity on community stability under different water and salt gradients, whereas did not discuss the influences of phylogenetic and beta diversity. Also, they did not consider the variation on the relationship between diversity and community stability along different spatial scales (Wang et al., 2017; Hu et al., 2021; Token et al., 2022). In order to solve these problems, we investigated community characteristics and diversity (α and β diversities) on the River bank, the transition zone and the desert hinterland in the ELWNNR, respectively. Then, based on different spatial scales (10 m × 10 m, 20 m × 20 m, and 50 m × 50 m), we compared the differences in plant diversity and community stability among three sampling sites, and the variation across three scales. After that, the structural equation model (SEM) is used to analyze the direct and indirect effects of plant diversity and soil properties on the community stability. Our study is aim to: (1) reveal the variation on the relationship between plant diversity and community stability across spatial scales; and (2) expose the regulation mechanism of soil properties and plant diversity on community stability in arid desert ecosystem. Our study can not only enrich people’s knowledge of the influencing factors and maintenance mechanisms of community stability, but also have important practical significance for the restoration and reconstruction of degraded arid desert ecosystems.
Materials and methods
Study area
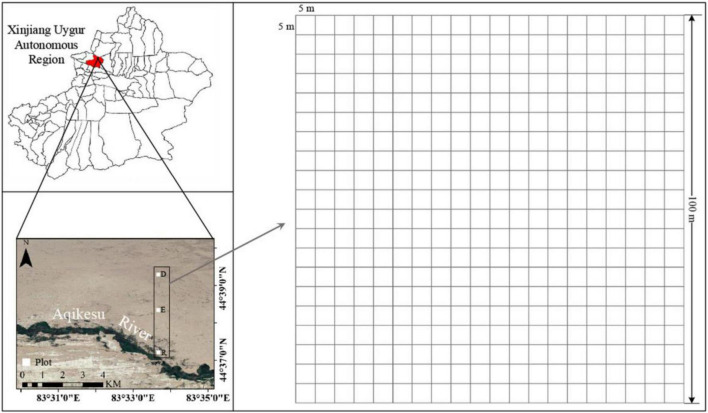
The ELWNNR is located in the southwest of the Junggar Basin in the Xinjiang Uygur Autonomous Region, NW China (Figure 1). It is the lowest depression and water-salt accumulation center in the southwest margin of the Junggar Basin. Affected by a typical temperate continental arid climate, the annual evaporation is more than 1600 mm, the annual rainfall is about 100 mm, and the sunshine duration is about 2800 h. The extreme maximum and minimum temperatures are 44 and -33°C, respectively (Hu et al., 2022). The local community is mainly consisted of desert plants (Yang et al., 2014). The main dominant plants include Populus euphratica, Haloxylon ammodendron, Halimodendron halodendron, Reaumuria soongarica, Nitraria roborowskii, Halocnemum strobilaceum, Alhagi sparsifolia, Apocynum venetum, Seriphidium terrae-albae, Phragmites australis, Salsola collina, Suaeda glauca, etc. (Jiang L. et al., 2022). Three large sample plots of 1 hm2 (100 m × 100 m) was set up in the river bank, the transition zone between riparian forest and desert shrubs (ecotone community), and the desert hinterland in the ELWNNR, respectively. Each sample plot was divided into 400 of 5 m × 5 m quadrats (Figure 1). P. euphratica was the dominant species in the riparian forest, H. ammodendron and P. euphratica were the co-dominant species in ecotone community, while H. ammodendron was the dominant species in the desert shrubs. The main species of three communities were shown in the appendix (Supplementary Tables 1–3).
FIGURE 1.
Study area and location of the sampling plots. R, E, and D represent riparian forest, ecotone community, and desert shrubs, which grow in the river bank, the transitional zone and desert hinterland, respectively.
Community investigation and leaf functional trait determination
Plant community characteristics (species composition, abundance, plant height, and crown area) were investigated in all quadrats. Then, 20 mature leaves were collected randomly from each individual in each quadrat. The leaf length, width and thickness were measured by vernier caliper. The leaf area was calculated by grid method. More specifically, the leaves were spread on a 1 mm2 grid paper and photographed with a camera. The images were then uploaded to Photoshop 7.0 to calculate the leaf area. After all leaves were brought back to the laboratory, an electronic analytical balance with accuracy < 0.0001 g was used to measure leaf fresh weight. The drying method was used to measure leaf dry weight and then calculated the specific leaf area and dry matter content. After all dry leaves were ground and sieved into powder. The contents of leaf carbon, nitrogen and phosphorus were determined using the potassium dichromate dilution heat method, the Mo-Sb colorimetric method and the Kjeldahl digestion method.
Collection and measurement of soil samples
In each quadrat, the diagonal method was used to select the center position. After considering that 0–20 cm topsoil can well reflect soil nutrient status in arid desert (Tian et al., 2010), we drilled this topsoil in the center position in this study. Two soil samples were collected from each quadrat, one of which was collected with a ring knife to measure its fresh weight and soil bulk density, and then brought back to the laboratory for drying to calculate soil water content (SWC). The other sample was brought back to the laboratory to dry naturally for later soil properties determination. SWC was determined by the drying method (Zhang et al., 2015). Soil salinity content (SA) and soil pH was determined using the weight method and a pH meter (Leici PHS-3C, Shanghai Yidian Scientific Instrument Co., Ltd, China), respectively. Soil organic carbon (SOC), total nitrogen (TN), ammonium nitrogen (AN), and nitrate nitrogen (NN) was determined by the potassium dichromate dilution heat method, the Kjeldahl digestion method (BUCHI-K370, BUCHI Labortechnik AG, Switzerland) (Dalai et al., 1984), the indophenol blue colorimetry (Bao, 2000), and the dual-wavelength ultraviolet spectrophotometry (Norman et al., 1985). The total phosphorus (TP) and available phosphorus (AP) was determined by the Mo-Sb colorimetric method (Spectrophotometer UV1200, Shanghai AOE Instruments Co., Ltd., China) (Li et al., 2019a).
Statistical analysis
Spatial scales
In order to test whether the relationship between community stability and diversity varied with spatial scales (scale-dependence), 5 m × 5 m quadrat was used as the basic unit and then randomly generated plots with area of 10 m × 10 m, 20 m × 20 m, and 50 m × 50 m, respectively. The number of each new plot types was 100. The values of soil properties and plant functional traits of each newly generated plot were expressed by means of the average of 5 m × 5 m basic quadrats (Hu et al., 2022). All the above processes were completed in R4.1.2 software.
Community stability
Plant community stability was determined by using inverse of coefficient of variation (ICV) (Eq. 1). Where μ is the average density of each species in the quadrat; σ is the standard deviation of each species density. Larger ICV value suggest higher community stability and small variability of species density (Wu et al., 2014; Yang et al., 2017).
| (1) |
Plant diversity
Species and functional diversities were completed in Vegan and FD package. The phylogenetic trees were firstly generated using the maximum likelihood (ML) method and the Bayesian inference (BI) method implemented MEGA 6.0 and BEAST 1.7, respectively (Drummond et al., 2012). The results of the phylogenetic trees of the three communities were presented in the appendix (Supplementary Figures 1–3). Then, the picante software package was used to calculate phylogenetic diversity. As suggested from Tan et al. (2019), species β diversity was divided into the Local Contribution to Beta Diversity (LCBD) and the Species Contribution to Beta Diversity (SCBD). Species β diversity, LCBD and SCBD were completed in adespatial package. All calculations of diversity were conducted in R 4.1.2.
Influencing factors of diversity-stability relationship
Differences in community stability and diversity among three communities (riparian forest, ecotone community, and desert shrubs), as well as among three spatial scales were analyzed by the one-way ANOVA. The correlations among diversity, soil properties and community stability were analyzed by cor.test function. The relative contributions of soil properties (soil water content, salt content, organic carbon, pH and total nitrogen, etc.) and plant diversity (species diversity, functional diversity, phylogenetic diversity index, etc.) on community stability were analyzed using SEM. Before the calculation of SEM, the multiple linear regression model (MLRM) was used to reduce the number of variables while screen the influencing factors of community stability. The factors with VIF > 10 will be eliminated. However, since there were still many factors left over after the selection of the multiple linear regression model, the Principal Component Analysis (PCA) was continued to screen out the final influencing factors. One-way ANOVA, cor.test, MLRM and PCA were conducted using R 4.1.2, whereas SEM was completed in Amos 24.0.
Results
Change in community stability across spatial scales
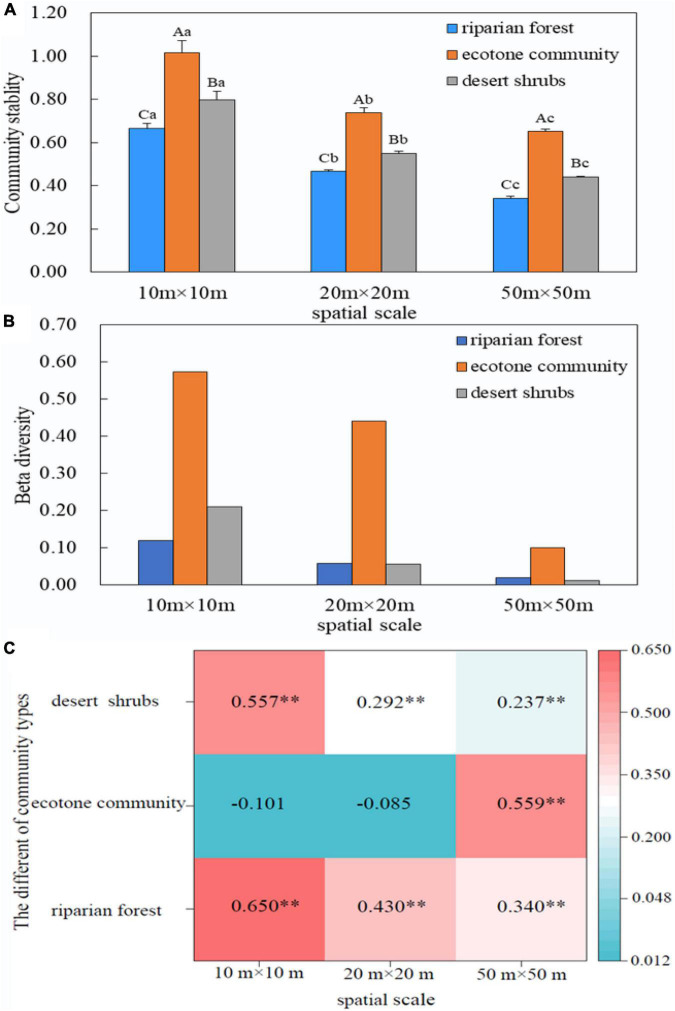
Our results found that community stability decreased significantly with the spatial scales (100 m2 > 400 m2 > 2500 m2) in all sampling positions (P < 0.05). The order of community stability among three communities was as follows: ecotone community > desert shrubs > riparian forest (Figure 2).
FIGURE 2.
Relationship between community stability and β diversity. (A) Variation of community stability across spatial scales; (B) Variation of β diversity across spatial scales; (C) Relationship between LCBD and community stability. The capital letters indicate the difference of the stability between different communities at the same spatial scale, while the lower letters suggest the difference of the stability of the same community among different spatial scales. P < 0.05. **P < 0.01.
Change in plant diversity across spatial scales
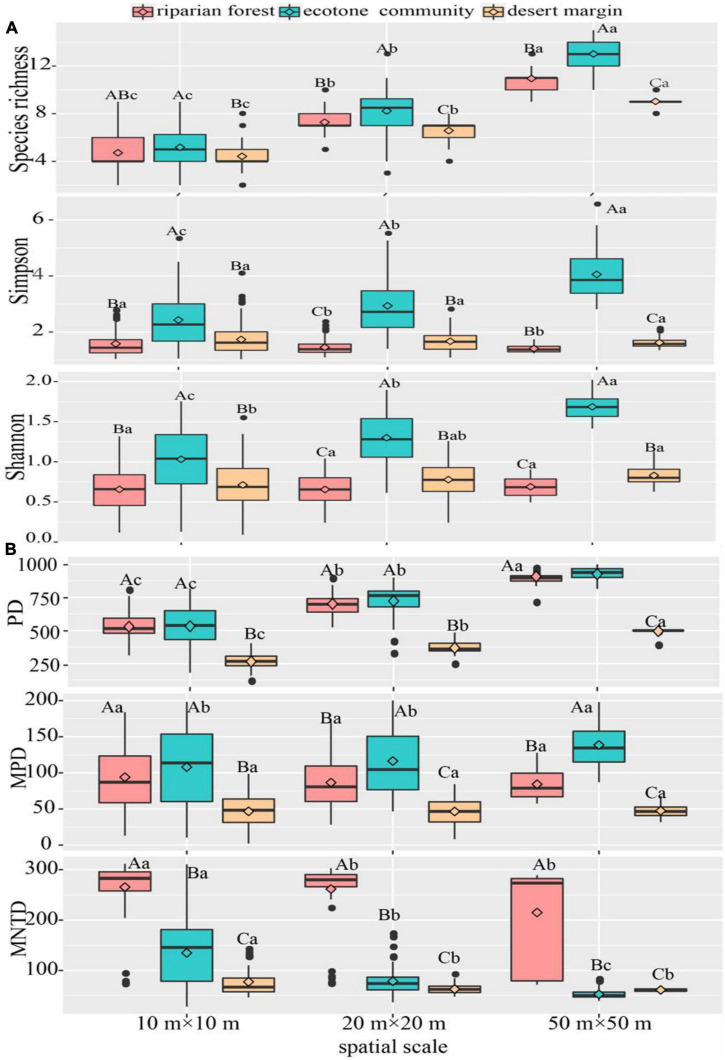
Species richness and Shannon Wiener index increased with spatial scales in all sampling communities. The Simpson index showed the different pattern among three communities, which increased with the spatial scales in the ecotone community, but showed the opposite results in desert shrubs and riparian forest. Among three sampling positions, all species diversity (i.e., richness, Shannon Wiener, and Simpson indexes) were higher in the ecotone community than desert shrubs and riparian forest across three spatial scales (Figure 3).
FIGURE 3.
Variation of species and phylogenetic diversities across spatial scales. (A) Species diversity; (B) phylogenetic diversity. The instruction of capital and lower-case letters see Figure 2. P < 0.05.
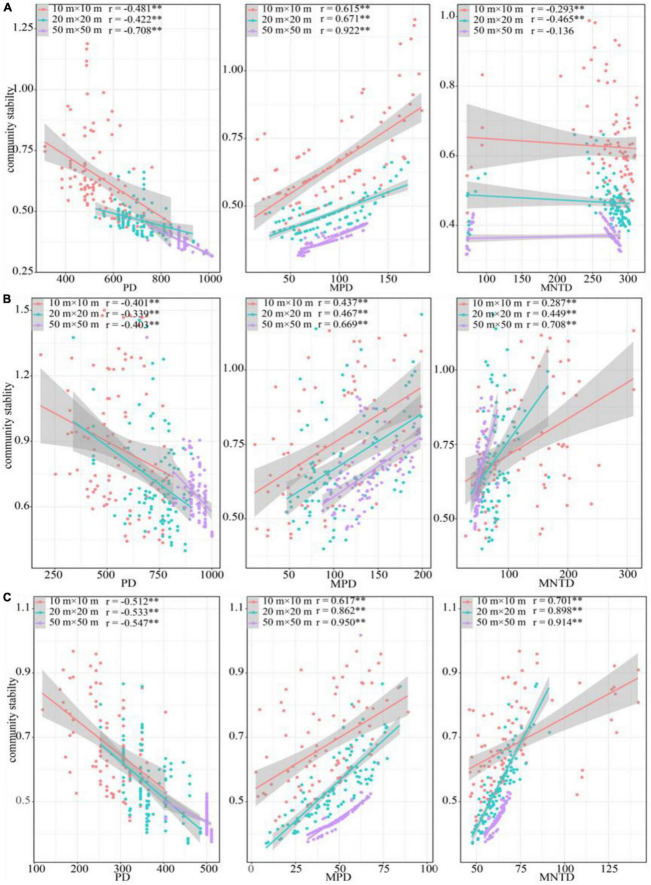
With regarding to phylogenetic diversity, the phylogenetic richness (PD) showed an increasing trend with spatial scale in all sampling positions, while the mean nearest taxa distance (MNTD) decreased. However, the MPD increased with spatial scale in the ecotone community, while not changed in the riparian forest and desert shrubs. Among three sampling positions, PD and MPD were higher in the ecotone community and riparian forest than desert shrubs across three spatial scales, whereas MNTD showed the opposite pattern (Figure 3).
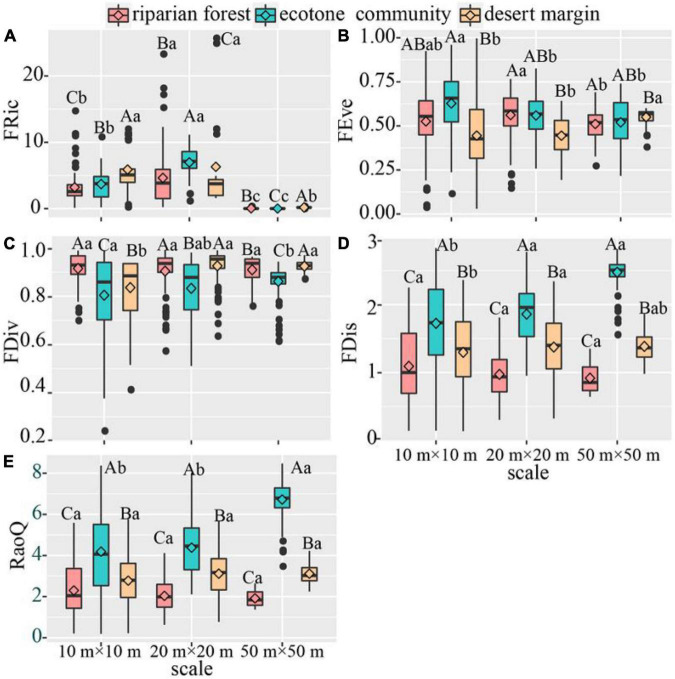
In terms of functional diversity, our results showed that functional richness (FRic) of three sampling positions firstly increased and then decreased with the increase of spatial scales. The functional evenness (FEve) of riparian forest and ecotone community decreased with the increase of spatial scale, whereas desert shrubs showed an opposite pattern. Functional divergence (FDiv) and functional dispersion (FDis) increased with spatial scale in the ecotone community and desert shrubs, while not changed in the riparian forest. The RaoQ increased in the ecotone community, while not changed in the riparian forest and desert shrubs. Among three sampling positions, RaoQ, FDis and FEve were higher in the ecotone community than desert shrubs and riparian forest across three spatial scales, whereas FDiv showed the opposite pattern. FRic at 20 m × 20 m in the ecotone community was higher than that in desert shrubs and riparian forest, while showed the opposite pattern at 10 m × 10 m and 50 m × 50 m (Figure 4).
FIGURE 4.
Variation of functional diversity across spatial scales. The instruction of capital and lower-case letters see Figure 2. (A) FRic. (B) FEve. (C) FDiv. (D) FDis. (E) RaoQ. P < 0.05.
The β diversity decreased with the increase of spatial scales in all sampling positions. The β diversity in the ecotone community was larger than that in riparian forest and desert shrubs (Figure 2).
Correlation between plant diversity and community stability
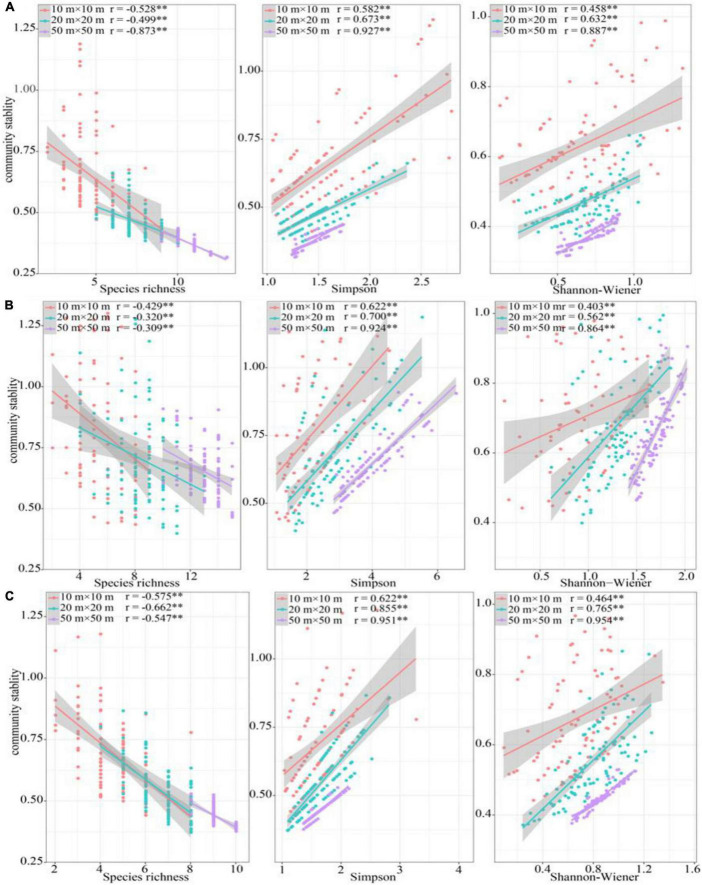
Richness has a negative correlation with community stability in all sampling positions across three spatial scales, while the Simpson and Shannon-Wiener indices were positively correlated (Figure 5). In most cases, the correlation coefficient between species diversity and community stability increased with increasing spatial scale (Figure 5).
FIGURE 5.
Relationship between species diversity and community stability. (A) riparian forest, (B) ecotone community, (C) desert shrubs. *P < 0.05, **P < 0.01, ***P < 0.001.
Expect 50 m × 50 m in riparian forest, FRic had the significant negative relationship with community stability in the other sampling positions across three spatial scales. The relation of FDiv, FDis, and RaoQ to community stability was completely opposite. FDiv negatively correlated with community stability in all sampling positions across three spatial scales, but FDis and RaoQ were positive correlated (Table 1). The relationship of FEve with community stability showed no significant trend at different sampling positions and different scales. For example, it was significantly correlated with community stability in the ecotone community at 50 m × 50 m. However, in at the same size, it was significantly positively correlated with stability in the riparian forest. Unlike species diversity, the correlation coefficients between functional diversity and community stability had not showed a consistent change trend with the increase of spatial scale (Table 1).
TABLE 1.
Relationship between functional diversity and community stability.
| Types | Scale | FRic | FEve | FDiv | FDis | RaoQ |
| Riparian forest | 10 m × 10 m | −0.421** | −0.142 | −0.527** | 0.597** | 0.538** |
| 20 m × 20 m | −0.420** | −0.084 | −0.254** | 0.346** | 0.304** | |
| 50 m × 50 m | 0.306** | 0.332** | −0.126 | 0.278** | 0.246** | |
| Ecotone community | 10 m × 10 m | −0.336** | 0.313** | −0.475** | 0.148 | 0.205* |
| 20 m × 20 m | −0.175 | 0.071 | −0.394** | 0.357** | 0.266** | |
| 50 m × 50 m | −0.412** | −0.507** | −0.658** | −0.050 | −0.139 | |
| Desert shrubs | 10 m × 10 m | −0.456** | 0.194 | −0.364** | 0.568** | 0.485** |
| 20 m × 20 m | −0.510** | −0.138 | −0.535** | 0.885** | 0.853** | |
| 50 m × 50 m | −0.547** | −0.068 | −0.248* | 0.933** | 0.908** |
*P < 0.05, **P < 0.01, ***P < 0.001.
In terms of phylogenetic diversity, PD was significant negative correlation with community stability in all sampling positions across three spatial scales, while MPD was positive related. MNTD was negatively correlated with community stability in the ecotone community and the desert shrubs, and positively correlated with community stability in the riparian forest (Figure 6). Similar to species diversity, the correlation coefficient between phylogenetic diversity and community stability increased with increasing spatial scale in most cases (Figure 6).
FIGURE 6.
Relationship between phylogenetic diversity and community stability. (A) Riparian forest. (B) Ecotone community. (C) Desert shrubs. **P < 0.01.
There was a significant positive correlation between LCBD and community stability in riparian forest and desert shrubs. The correlation coefficient between LCBD and stability in these two positions decreased with the increase of spatial scale. In the ecotone community, the relationship between beta diversity and stability varied greatly with scale, which showed no significant negative correlation at small and medium scales (10 m × 10 m and 20 m × 20 m), but significant positive correlation at 50 m × 50 m (Figure 2).
As shown above, beta diversity included LCBD and SCBD. Where SCBD represented the contribution of species to beta diversity. Within each scale, there was only one value of SCBD for each species. Thus there were only 3 values at 3 spatial scales, resulting in that the correlation analysis could not be used to judge the relationship between SCBD and community stability. In this study, however, we classified species into woody plants and herbaceous using life forms. Then, the changes of SCBD of these two types with spatial scale were used to determine whether they had an impact on community stability. Our results showed that the uniqueness of herbaceous and woody plants across spatial scales showed an opposite change in all sampling positions (Supplementary Figure 4). This indicated that SCBD can also affect community stability in arid and desert areas.
The relative effects of plant diversity and soil factors on the stability of desert plant communities
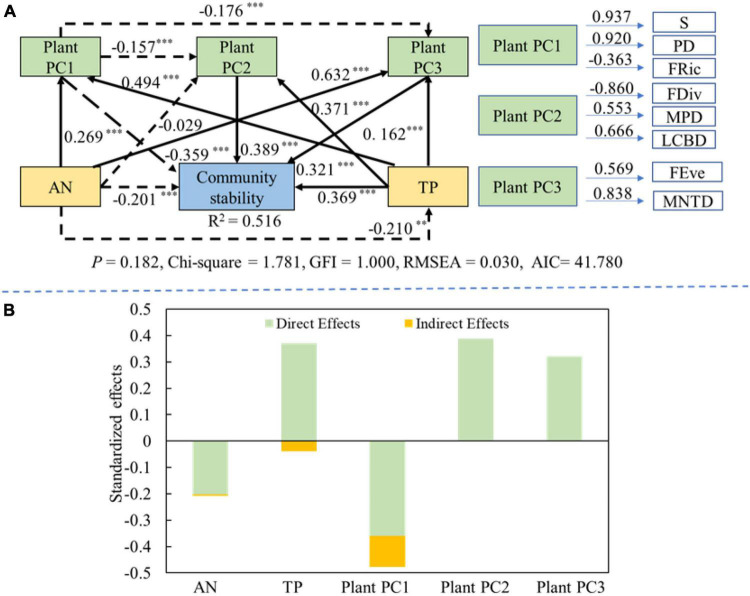
The collinearity results of the MLRM showed that the following factors (species richness, PD, FRic, FEve, FDiv, MPD, MNTD, LCBD, total phosphorus and ammonium nitrogen) were filtered to be the influencing variances of diversity-community stability relationship (VIF<10) (Supplementary Table 4). After that, in order to further reduce the number of variables about plant diversity, the PCA was used to compress the latitude of variables. The results showed that the diversity was reduced to three principal components. PC1 was mainly composed of FRic, PD and species richness (Plant PC1). PC2 included FDiv, MPD, and LCBD (Plant PC2), while PC3 included MNTD and FEve (Plant PC3) (Supplementary Tables 5, 6).
The SEM results showed that soil ammonium nitrogen had a significant direct negative effect on community stability (path coefficient = −0.201; P < 0.01). Soil total phosphorus also significant indirectly affected plant PC1 and plant PC3 to regulate community stability. Soil total phosphorus had a significant direct positive effect on the community stability (path coefficient = 0.369; P < 0.001), it also indirectly regulated community stability via affecting plant PC1, plant PC2 and plant PC3. Plant PC2 and plant PC3 had the significantly positive directly influences on the community stability (path coefficient = 0.389 and 0.321; P < 0.001), while plant PC1 had the significant directly negative effect (path coefficient = −0.359; P < 0.001) (Figure 7A). The contribution order of the influencing factors was as follows: plant PC1 (−0.477) > soil PC2 (0.389) > soil total phosphorus (0.331) > plant PC3 (0.321) > soil ammonium nitrogen (−0.208) (Figure 7B).
FIGURE 7.
The effects of plant diversity and soil properties on the community stability. Black solid and black dashed lines indicate the positive and negative effects, respectively. Values on lines denote the standardized effect size. (A) Structural equation model showing the direct and indirect effects of plant diversity and soil factors on community stability. (B) The standardized direct and indirect effects about structural equation model. **P < 0.01; ***P < 0.001.
Discussion
Moderate disturbance and drought-salt stress resulted in higher stability and diversity in ecotone community than desert shrubs and riparian forest
Our results found that the stability in the ecotone community was higher than desert shrubs, and they were higher than riparian forest (Figure 2). As suggested by the “Moderate Disturbance Hypothesis,” since ecotone community was located in the transition zone between riparian forest and desert shrubs, plant diversity (species, functional diversity, and phylogenetic diversities) in this area was higher than the other two communities due to moderate disturbance (Zhao et al., 2017; Figures 3, 4). The complex community structure and relationships among plants made it more stable. Compared with riparian forest, desert shrub community mainly consisted of shrubs and herbaceous plants with stronger drought resistance. They have a short life history, abundant seeds, and high reproductive capacity, which allowed them to recover quickly after community stability is destroyed. Therefore, the stability of desert shrub community was higher than that of the riparian forest community (Chen et al., 2012). This result was consistent with the finding of the Pfisterer and Schmid on the resistance and resilience of ecosystems to drought disturbance (Pfisterer and Schmid, 2002). They found that systems with fewer species were more resistant or adaptable to environmental stresses in the arid desert regions.
Another reason for the difference in diversity among the three communities was drought and salt stress. In arid desert, the water content in the soil in the riparian forest was high due to the water replenishment from river water to the groundwater. This was beneficial to the growth for shallow-rooted herbs (Yang et al., 2017). However, on the other hand, the high groundwater level made salt more easily migrate to the soil surface with water evaporation, which leaded to the increasing degree of salt stress suffered by plants (Zheng et al., 2005). This was not conducive to the growth of shallow-rooted and salt-tolerant herbs or small shrubs (Li et al., 2019b). The trade-off between drought and salt stress limited the growth of some salt-tolerant and wet-tolerant herbaceous plants, leading to a decline in species richness in riparian forest. In terms of desert habitat, only few drought-tolerant herbs and shrubs can survive due to the water deficient, thus maintaining low biodiversity. In contrast, the ecotone community was not affected by high salinity and low soil water content (more drought stress), thus many drought-tolerant and alkali-tolerant shrubs and herbs could survive, thus leading to the highest species richness and diversity in this community (Figure 3).
Except for functional richness (FRic), the other diversity in the three communities all increased with the increase of spatial scale. This may be because larger sampling areas include more species, thus increasing the diversity. FRic reached the maximum in ecotone community at 20 m × 20 m. This may be due to functional desaturation at smaller spatial scales (10 m × 10 m), while overabundance of species may lead to functional redundancy at larger scales (50 m × 50 m). Although the number of species and phylogenetic diversity was the largest at 50 m × 50 m, the difference in response to environmental change among species may lead to a significant decline in functional richness under the influence of disturbance (Díaz and Cabido, 2001; Qiao et al., 2022; Figure 4).
Asynchronous effect of species composition and functional traits increased community stability
An increasing number of scholars believed that higher species diversity can stabilize the ecosystem functions (Gross et al., 2014). In this study, the relationship between diversity and community stability have been studied across three spatial scales (10 m × 10 m, 20 m × 20 m, and 50 m × 50 m). The results showed that the correlation coefficient of Simpson dominance indexes to community stability were significantly larger than that of species richness (Figure 5), which indicated that the influence of dominance on community stability was greater than that of species number. This may be because the contribution of each species was uneven to community stability, and the increase of species number had not necessarily in promoting community stability (Li et al., 2022). As suggested by the “Selection Effect,” the dominant species determined ecosystem recovery process in a short time after stability disequilibrating due to their special characteristics such as the high resource utilization. An increasing of the relative abundance of dominant species will greatly increase community stability (Grman et al., 2010), because the contribution of dominant species to stability was higher than that of other species (Wayne Polley et al., 2007). Moreover, according to the “Complementary Effect,” the increase of biodiversity was beneficial to the functional complementation among species due to different specific niches among species. Niche differentiation among species can enable community to capture more resources, thus achieving stability under the premise of rational allocation of resource (Loreau and Hector, 2001). This compensatory effect can be confirmed in our results. The diversity of the ecotone community was significantly higher than those in other communities. The advantage in diversity made its community stability was higher than the other two communities.
Studies have shown that functional and phylogenetic diversities may be more closely related to community stability than species diversity (Reiss et al., 2009; Cadotte et al., 2011; Naeem et al., 2012). Our results also supported this view. There was a significant positive correlation between the MPD species and community stability in all communities (Figure 6). This suggested that high phylogenetic diversity provided more complementary functions (Ritchie et al., 2021), and higher nutrient utilization efficiency (Shang and Yan, 2017), thus improving community stability. Additionally, our results found that the RaoQ index was positively correlated with the community stability. This may indicate that the differentiation of functional niche within community can promote community stability (Mason et al., 2010). The greater difference of traits among species ensured community obtain more resources and reduce intraspecific competition, which in turn increased community stability. The functional divergence reflected the degree of niche complementarity among species in a community. Higher values of such index suggested less niche overlap among species in the community. An increase of functional divergence was conducive to maintain the community stability.
Apart from alpha diversity, beta diversity also had an non-negligible impact on community stability. It has been found that the spatial similarity of community composition (biological homogenization) may increase the spatial synchronization of ecosystem dynamics, thus reducing community stability (Olden et al., 2004; France and Duffy, 2006). Synchronization within and between communities may explain the positive biodiversity-stability relationship (Zhang et al., 2018). In this study, the changes in alpha diversity was consistent with beta diversity among three communities (ecotone community > desert shrubs > riparian forest). The consistent change between those two types of diversity was more conducive to maintaining the positive relationship between diversity and stability. This was because alpha and beta diversity can enhance the stability of regional ecosystems through “local and spatial insurance effects” (Wang and Loreau, 2016). The “local insurance effect” was originated from the asynchronous response of species to the local environment due to functional trait differentiation among species (Yachi and Loreau, 1999). The “spatial insurance effect” was caused by the asynchronous response of different communities with different species composition to the related spatial environment (Wang and Loreau, 2016). In this study, such response can be demonstrated by the performance of different species. Our results found that the responses of woody plants and herbaceous to scale changes were differed. The unsynchronized responses between these two life forms to the spatial scale were helpful to increase community stability (Figure 6). Moreover, previous studies have found that the responses of resource investment and distribution pattern to drought-salt stress differed between different plant growth types in arid desert region (Qie et al., 2018). These suggested that the asynchronous effect caused by the changes in species composition and functional traits among communities had a positive impact on the stability. The biodiversity loss and biological homogenization may damage the community stability in the arid desert ecosystem (Wang S. et al., 2021).
Soil ammonium nitrogen and phosphorus promoted community stability by affecting plant diversity
Many studies have shown that, climatic conditions and topography are the main factors affecting plant diversity at the global scale, but the regional scale, environmental factors such as soil nutrients are considered to determine distributions of plant communities (Yu et al., 2009; Zhang et al., 2016). Such difference in scale leaded to an extremely complex relationships among environmental factors, diversity and stability, The change of community stability with spatial scale was not caused by a single environmental factor, but by the complex interaction of multiple factors (Pyšek and Lepš, 1991). Current research shown that the influence of environmental factors on the diversity-community stability relationship have the following types: positive promotion (Adams and Zhang, 2009), the effect of “single peak” curve (Zhao et al., 2007), negative restrictions (Zhang et al., 2011) and no no-significant effect. However, all of these results occurred in humid region, we did not known how relationship are existed in the arid desert region.
Our study found that soil factors not only directly affected the community stability, but also indirectly influenced it by regulating plant diversity. Moreover, the interaction among plant diversity in each dimension also regulated the variation of community stability (Figure 7). Soil ammonium nitrogen directly reduced community stability. This may be because the increased nitrogen improved the efficiency of plant use of soil nutrients, further giving some species that can use additional nitrogen an advantage in interspecific competition (Xu et al., 2015a). Soil ammonium nitrogen can positively affect the MNTD in promoting community stability, which may be because phylogenetic diversity was significantly enhanced with the increase of soil ammonium nitrogen (Sun et al., 2021). As interspecific differences in functional traits are regulated by phylogeny, the enhanced phylogenetic diversity may lead to greater niche complementarity, thus promoting community stability (Thompson et al., 2015). Many studies had found that soil phosphorus content was a key determinant of biodiversity, because it affected the structure and composition of plant communities (Lambers et al., 2011; Li et al., 2018b; Yang X. T. et al., 2022). In semi-arid and arid areas soil contained a large amount of calcium salts due to water shortage, which adsorbed and fixed phosphorus, making it difficult for plants to absorb and use phosphorus. Therefore, phosphorus was considered to be one of the major limiting factors of the ecosystem in these areas (Wang et al., 2008). In this study, soil total phosphorus had a significant positive effect on plant community stability. This may be because found the increased soil phosphorus concentration accelerated phosphorus the uptake of the phosphorus by plants (Hedin, 2004), allowing species to occupy more ecological niches, and thus increasing community stability.
As a dimension of beta diversity, LCBD represented the contribution of community composition to beta diversity. Larger LCBD showed that the community owned the high ecological specificity, and its composition greatly changed with environmental gradient or spatial scale (Dubois et al., 2020). This study found that the LCBD had a significant positive impact on community stability, suggesting that communities with high ecological uniqueness had higher stability. This may be because communities with high ecological uniqueness often had obvious differences with other communities (Dubois et al., 2020), High ecological uniqueness may utilize more resources and increase the differences of plant functional traits within the community, which in turn was more conducive to community stability. This also can be confirmed by the FDiv results. FDiv reflected the contribution of rare species to community diversity (Song et al., 2011), Higher FDiv suggested the rare species played more obviously role in community stability. In this study, FDiv had a negative and significant impact on community stability, indicating that the increase of rare species dominance would inhibit community stability. On the contrary, it confirmed the positive effect of dominant species on community stability from another aspect.
Conclusion
Our results found community stability decreased significantly with the increase of spatial scale in the arid desert ecosystem. Community stability in the ecotone community was higher than that in desert shrubs and riparian forest. The correlation coefficient of Simpson index with community stability was significantly larger than that of species richness in all sampled communities. These suggested that the moderate disturbance and drought-salt stress resulted in higher stability and diversity in ecotone community than desert shrubs and riparian forest. The influence of species dominance on community stability was greater than the species number. Our results found that there existed obvious relationship between community stability and diversity in three sampled communities, suggested that the increase of biodiversity was beneficial to community stability due to the “Complementary Effect” of functional niches. The SEM results showed that soil ammonium nitrogen and total phosphorus not only directly affected the community stability, but also have the indirect influences by regulating the change of plant diversity. Moreover, the interaction of plant diversity in different dimension also regulated the change of community stability. Our study also found that local community with high specificity were more stable, which suggested that high specificity communities should be emphasized in diversity conservation. Although increasing plant diversity is considered an important way to maintain community stability, our results suggested that selecting suitable dominant species to enhance community stability may be a better approach.
There are also some deficiencies in this study. The functional traits only include leaf traits, but not root, hydraulic and stem traits. In arid desert, root and hydraulic traits are more advantageous than leaf traits in expressing plant adaptability to environment, especially to drought stress. In addition, only interspecific differences were used to analyze the effect of trait differentiation on community stability in this study, while intraspecific variation of traits was ignored. As many studies have confirmed that the intraspecific variation of traits also plays an important role in ecosystem functioning (Arshad and Yan, 2018; Bello et al., 2021; Jiang M. et al., 2022). Moreover, this study only analyzed the changes of community stability with spatial scale and its influencing factors. It is well known that functional traits, niche and species turnover also vary across temporal scales (Furness et al., 2021; Xu et al., 2021). For example, ephemeral plants are common in desert communities in early spring. The higher abundance of these plants leads to higher plant diversity of community at that time than at other times of year. But after spring, their mortality greatly reduces the diversity and stability of the community. Therefore, the temporal and spatial changes of the relationship between community stability and plant diversity should be synchronous analyzed in future studies. It has been found in recent years that the soil microorganisms have a particularly important effect on plant community composition, thus may also play an important role in maintaining community stability (Zhang et al., 2021). Therefore, the influence of soil microbe-plant interaction on community stability also need to be considered in future studies.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
L-MJ, KS, DH, JZ, and G-HL conceived and designed the experiments. L-MJ, KS, DH, and JZ performed the experiments. L-MJ analyzed the data and wrote the manuscript. X-DY improved the quality of the article. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank Xue-Min He, Heng-Fang Wang, Jing-Long Wang, Shi-Yun Wang, Wen-Jing Li, Zhou-Kang Li, Na Li, Yu-Dong Chen, Zhu-Feng Hou, and Han-Peng Li in Key Laboratory of Oasis Ecology of Xinjiang University for their indispensable help in fieldwork and laboratory analysis.
Funding
This study was financially supported by the Xinjiang Uygur Autonomous Region Graduate Research and Innovation Project (XJ2020G011), National Natural Science Foundation of China (42171026), Natural Science Foundation of Xinjiang (2022D01C42), and Xinjiang Uygur Autonomous Region Innovation Environment Construction Special Project & Science and Technology Innovation base Construction Project (PT2107).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.969852/full#supplementary-material
References
- Adams J. M., Zhang Y. (2009). Is there more insect folivory in warmer temperate climates? A latitudinal comparison of insect folivory in eastern North America. J. Ecol. 97 933–940. 10.1111/j.1365-2745.2009.01523.x [DOI] [Google Scholar]
- Arshad A., Yan E. R. (2018). The mediation roles of intraspecific and interspecific functional trait diversity for linking the response of aboveground biomass to species richness across forest strata in a subtropical forest. Ecol. Indic. 85 493–501. [Google Scholar]
- Bao S. D. (2000). Soil Agrochemical Analysis, 3rd Edn. Beijing: China Agricultural Press. [Google Scholar]
- Bello F. D., Lavorel S., Hallett L. M., Valencia E., Garnier E., Roscher C., et al. (2021). Functional trait effects on ecosystem stability: Assembling the jigsaw puzzle. Trends Ecol. Evol. 36 822–836. 10.1016/j.tree.2021.05.001 [DOI] [PubMed] [Google Scholar]
- Brown B. L., Downing A. L., Leibold M. A. (2016). Compensatory dynamics stabilize aggregate community properties in response to multiple types of perturbations. Ecology 97 2021–2033. 10.1890/15-1951.1 [DOI] [PubMed] [Google Scholar]
- Cadotte M. W., Carscadden K., Mirotchnick N. (2011). Beyond species: Functional diversity and the maintenance of ecological processes and services: Functional diversity in ecology and conservation. J. Appl. Ecol. 48 1079–1087. 10.1111/j.1365-2664.2011.02048.x [DOI] [Google Scholar]
- Chao A., Chiu C.-H., Jost L. (2014). Unifying Species Diversity, Phylogenetic Diversity, Functional Diversity, and Related Similarity and Differentiation Measures Through Hill Numbers. Annu. Rev. Ecol. Evol. Syst. 45 297–324. 10.1146/annurev-ecolsys-120213-091540 [DOI] [Google Scholar]
- Chen J., Guo Y. L., Lu X. L., Ding S. Y., Su S., Guo J. J., et al. (2012). Species diversity of herbaceous communities in the Yiluo River Basin. Acta Ecol. Sin. 32 3021–3030. 10.5846/stxb201104270556 [DOI] [Google Scholar]
- Coelho de Souza F., Dexter K. G., Phillips O. L., Pennington R. T., Neves D., Sullivan M. J. P., et al. (2019). Evolutionary diversity is associated with wood productivity in Amazonian forests. Nat. Ecol. Evol. 3 1754–1761. 10.1038/s41559-019-1007-y [DOI] [PubMed] [Google Scholar]
- Craven D., Eisenhauer N., Pearse W. D., Hautier Y., Isbell F., Roscher C., et al. (2018). Multiple facets of biodiversity drive the diversity–stability relationship. Nat. Ecol. Evol. 2 1579–1587. 10.1038/s41559-018-0647-7 [DOI] [PubMed] [Google Scholar]
- Dalai R. C., Sahrawat K. L., Myers R. J. K. (1984). Inclusion of nitrate and nitrite in the Kjeldahl nitrogen determination of soils and plant materials using sodium thiosulphate. Commun. Soil Sci. Plant Anal. 15 1453–1461. [Google Scholar]
- De Laender F., Rohr J. R., Ashauer R., Baird D. J., Berger U., Eisenhauer N., et al. (2016). Reintroducing Environmental Change Drivers in Biodiversity–Ecosystem Functioning Research. Trends Ecol. Evol. 31 905–915. 10.1016/j.tree.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz S., Cabido M. (2001). Vive la différence: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 16 646–655. [Google Scholar]
- Donohue I., Hillebrand H., Montoya J. M., Petchey O. L., Pimm S. L., Fowler M. S., et al. (2016). Navigating the complexity of ecological stability. Ecol. Lett. 19 1172–1185. 10.1111/ele.12648 [DOI] [PubMed] [Google Scholar]
- Drummond A. J., Suchard M. A., Xie D., Rambaut A. (2012). Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29 1969–1973. 10.1093/molbev/mss075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois R., Proulx R., Pellerin S. (2020). Ecological uniqueness of plant communities as a conservation criterion in lake-edge wetlands. Biol. Conserv. 243:108491. 10.1016/j.biocon.2020.108491 [DOI] [Google Scholar]
- Evans L. C., Melero Y., Schmucki R., Boersch-Supan P. H., Brotons L., Fontaine C., et al. (2022). Bioclimatic context of species’ populations determines community stability. Glob. Ecol. Biogeogr. 31 1542–1555. 10.1111/geb.13527 [DOI] [Google Scholar]
- France K. E., Duffy J. E. (2006). Diversity and dispersal interactively affect predictability of ecosystem function. Nature 441 1139–1143. 10.1038/nature04729 [DOI] [PubMed] [Google Scholar]
- Furness E. N., Garwood R. J., Mannion P. D. D., Sutton M. (2021). Productivity, niche availability, species richness, and extinction risk: Untangling relationships using individual-based simulations[J]. Ecol Evol. 11 8923–8940. 10.1002/ece3.7730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y., Ling H., Lv G., Chen Y., Guo Z., Cao J. (2019). Disentangling the influence of aridity and salinity on community functional and phylogenetic diversity in local dryland vegetation. Sci. Total Environ. 653 409–422. 10.1016/j.scitotenv.2018.10.358 [DOI] [PubMed] [Google Scholar]
- Grman E., Lau J. A., Schoolmaster D. R., Gross K. L. (2010). Mechanisms contributing to stability in ecosystem function depend on the environmental context: Stabilizing mechanisms in grasslands. Ecol. Lett. 13 1400–1410. 10.1111/j.1461-0248.2010.01533.x [DOI] [PubMed] [Google Scholar]
- Gross K., Cardinale B. J., Fox J. W., Gonzalez A., Loreau M., Wayne Polley H., et al. (2014). Species Richness and the Temporal Stability of Biomass Production: A New Analysis of Recent Biodiversity Experiments. Am. Nat. 183 1–12. 10.1086/673915 [DOI] [PubMed] [Google Scholar]
- Hallett L. M., Hsu J. S., Cleland E. E., Collins S. L., Dickson T. L., Farrer E. C., et al. (2014). Biotic mechanisms of community stability shift along a precipitation gradient. Ecology 95 1693–1700. 10.1890/13-0895.1 [DOI] [PubMed] [Google Scholar]
- Hautier Y., Zhang P., Loreau M., Wilcox K. R., Seabloom E. W., Borer E. T., et al. (2020). General destabilizing effects of eutrophication on grassland productivity at multiple spatial scales. Nat. Commun. 11:5375. 10.1038/s41467-020-19252-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector A., Hautier Y., Saner P., Wacker L., Bagchi R., Joshi J., et al. (2010). General stabilizing effects of plant diversity on grassland productivity through population asynchrony and overyielding. Ecology 91 2213–2220. 10.1890/09-1162.1 [DOI] [PubMed] [Google Scholar]
- Hedin L. O. (2004). Global organization of terrestrial plant-nutrient interactions. Proc. Natl. Acad. Sci. U.S.A. 101, 10849–10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D., Jiang L., Hou Z., Zhang J., Wang H., Lv G. (2022). Environmental filtration and dispersal limitation explain different aspects of beta diversity in desert plant communities. Glob. Ecol. Conserv. 33:e01956. 10.1016/j.gecco.2021.e01956 [DOI] [Google Scholar]
- Hu D., Lü G. H., Wang H. F., Yang Q., Cai Y. (2021). Response of desert plant diversity and stability to soil factors based on water gradient. Acta Ecol. Sin. 41 6738–6748. 10.5846/stxb202006171581 [DOI] [Google Scholar]
- Jiang L., Hu D., Wang H., Lv G. (2022). Discriminating ecological processes affecting different dimensions of α− and β−diversity in desert plant communities. Ecol. Evol. 12 1–13. 10.1002/ece3.8710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., He L. Y., Fan B. J., Wang T., Yang N., Liu Y. L., et al. (2022). Intraspecific more than interspecific diversity plays an important role on Inner Mongolia grassland ecosystem functions: A microcosm experiment. Sci. Total Environ. 826:154134. 10.1016/j.scitotenv.2022.154134 [DOI] [PubMed] [Google Scholar]
- Jost L. (2007). Partitioning diversity into independent alpha and beta components. Ecology 88 2427–2439. 10.1890/06-1736.1 [DOI] [PubMed] [Google Scholar]
- Lambers H., Brundrett M. C., Raven J. A., Hopper S. D. (2011). Plant mineral nutrition in ancient landscapes: High plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 348 7–27. 10.1007/s11104-011-0977-6 [DOI] [Google Scholar]
- Li Q., Shi X., Zhao Z., Wu Q. (2022). Ecological restoration in the source region of Lancang River: Based on the relationship of plant diversity, stability and environmental factors. Ecol. Eng. 180:106649. 10.1016/j.ecoleng.2022.106649 [DOI] [Google Scholar]
- Li Q., Song X., Chang S. X., Peng C. G., Xiao W. F., Zhang G. B., et al. (2019a). Nitrogen depositions increase soil respiration and decrease temperature sensitivity in a Moso bamboo forest. Agric. For. Meteorol. 268 48–54. [Google Scholar]
- Li Q., Zhu J. H., Xiao W. F. (2019b). Relationships and trade-offs between, and management of biodiversity and ecosystem services. Acta Ecol. Sin. 39 2655–2666. 10.5846/stxb201803160519 [DOI] [Google Scholar]
- Li W., Stevens M. H. H. (2017). Community temporal variability increases with fluctuating resource availability. Sci. Rep. 7:45280. 10.1038/srep45280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Li X., Zhao Y., Zheng S., Bai Y. (2018a). Ecosystem structure, functioning and stability under climate change and grazing in grasslands: Current status and future prospects. Curr. Opin. Environ. Sustain. 33 124–135. [Google Scholar]
- Li W., Liu Y., Wang J., Shi S., Cao W. (2018b). Six years of grazing exclusion is the optimum duration in the alpine meadow-steppe of the north-eastern Qinghai-Tibetan Plateau. Sci. Rep. 8:17269. 10.1038/s41598-018-35273-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li X., Ma Q., Zhang X., Chen Y., Isbell F., et al. (2019). Nitrogen addition reduced ecosystem stability regardless of its impacts on plant diversity. J. Ecol. 107 2427–2435. 10.1111/1365-2745.13187 [DOI] [Google Scholar]
- Loreau M., de Mazancourt C. (2013). Biodiversity and ecosystem stability: A synthesis of underlying mechanisms. Ecol. Lett. 16 106–115. 10.1111/ele.12073 [DOI] [PubMed] [Google Scholar]
- Loreau M., Hector A. (2001). Partitioning selection and complementarity in biodiversity experiments. Nature 412 72–76. 10.1038/35083573 [DOI] [PubMed] [Google Scholar]
- Mason N. W. H., de Bello F. (2013). Functional diversity: A tool for answering challenging ecological questions. J. Veg. Sci. 24 777–780. 10.1111/jvs.12097 [DOI] [Google Scholar]
- Mason N. W. H., Peltzer D. A., Richardson S. J., Bellingham P. J., Allen R. B. (2010). Stand development moderates effects of ungulate exclusion on foliar traits in the forests of New Zealand: Ungulate impacts on foliar traits. J. Ecol. 98 1422–1433. 10.1111/j.1365-2745.2010.01714.x [DOI] [Google Scholar]
- Naeem S., Duffy J. E., Zavaleta E. (2012). The Functions of Biological Diversity in an Age of Extinction. Science 336 1401–1406. 10.1126/science.1215855 [DOI] [PubMed] [Google Scholar]
- Norman R., Edberg J., Stucki J. (1985). Determination of Nitrate in Soil Extracts by Dual-wavelength Ultraviolet Spectrophotometry. Soil Sci. Soc. Am. J. 49 1182–1185. [Google Scholar]
- Olden J. D., LeRoy Poff N., Douglas M. R., Douglas M. E., Fausch K. D. (2004). Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 19 18–24. 10.1016/j.tree.2003.09.010 [DOI] [PubMed] [Google Scholar]
- Pfisterer A. B., Schmid B. (2002). Diversity-dependent production can decrease the stability of ecosystem functioning. Nature 416 84–86. 10.1038/416084a [DOI] [PubMed] [Google Scholar]
- Pyšek P., Lepš J. (1991). Response of a weed community to nitrogen fertilization: A multivariate analysis. J. Veg. Sci. 2 237–244. 10.2307/3235956 [DOI] [Google Scholar]
- Qiao X., Geng Y., Zhang C., Han Z., Zhang Z., Zhao X., et al. (2022). Spatial asynchrony matters more than alpha stability in stabilizing ecosystem productivity in a large temperate forest region. Glob. Ecol. Biogeogr. 31 1133–1146. 10.1111/geb.13488 [DOI] [Google Scholar]
- Qie Y. D., Jiang L. M., Lv G. H., Yang X. D., Wang H. F., Teng D. X. (2018). Response of plant leaf functional traits to soil aridity and salinity in temperate desert ecosystem. Ecol. Environ. Sci. 27 2000–2010. [Google Scholar]
- Reiss J., Bridle J. R., Montoya J. M., Woodward G. (2009). Emerging horizons in biodiversity and ecosystem functioning research. Trends Ecol. Evol. 24 505–514. 10.1016/j.tree.2009.03.018 [DOI] [PubMed] [Google Scholar]
- Ricotta C., Szeidl L. (2009). Diversity partitioning of Rao’s quadratic entropy. Theor. Popul. Biol. 76 299–302. 10.1016/j.tpb.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Ritchie A. M., Hua X., Cardillo M., Yaxley K. J., Dinnage R., Bromham L. (2021). Phylogenetic diversity metrics from molecular phylogenies: Modelling expected degree of error under realistic rate variation. Divers. Distrib. 27 164–178. 10.1111/ddi.13179 [DOI] [Google Scholar]
- Rosauer D., Laffan S. W., Crisp M. D., Donnellan S. C., Cook L. G. (2009). Phylogenetic endemism: A new approach for identifying geographical concentrations of evolutionary history. Mol. Ecol. 18 4061–4072. 10.1111/j.1365-294X.2009.04311.x [DOI] [PubMed] [Google Scholar]
- Sasaki T., Lauenroth W. K. (2011). Dominant species, rather than diversity, regulates temporal stability of plant communities. Oecologia 166 761–768. 10.1007/s00442-011-1916-1 [DOI] [PubMed] [Google Scholar]
- Shang H., Yan Y. H. (2017). Natural hybridization and biodiversity conservation. Biodiver. Sci. 25 683–688. [Google Scholar]
- Song W., Chen S., Zhou Y., Lin G. (2020). Rainfall amount and timing jointly regulate the responses of soil nitrogen transformation processes to rainfall increase in an arid desert ecosystem. Geodema 364:114197. [Google Scholar]
- Song Y. T., Wang P., Zhou D. W. (2011). Methods for calculating functional diversity of plant communities Chinese. J. Ecol. 30 2053–2059. 10.13292/j.1000-4890.2011.0356 [DOI] [Google Scholar]
- Su G., Logez M., Xu J., Tao S., Villéger S., Brosse S. (2021). Human impacts on global freshwater fish biodiversity. Science 371 835–838. 10.1126/science.abd3369 [DOI] [PubMed] [Google Scholar]
- Sun W., Li S. W., Wang J. H., Fu G. (2021). Effects of grazing on plant species and phylogenetic diversity in alpine grasslands, Northern Tibet. Ecol. Eng. 170:106331. [Google Scholar]
- Tan L., Fan C., Zhang C., Zhao X. (2019). Understanding and protecting forest biodiversity in relation to species and local contributions to beta diversity. Eur. J. For. Res. 138 1005–1013. 10.1007/s10342-019-01220-3 [DOI] [Google Scholar]
- Thompson P. L., Davies T. J., Gonzalez A. (2015). Ecosystem Functions across Trophic Levels Are Linked to Functional and Phylogenetic Diversity. PLoS One 10:e0117595. 10.1371/journal.pone.0117595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H., Chen G., Zhang C., Melillo J. M., Hall C. A. S. (2010). Pattern and variation of C: N: P ratios in China’s soils: A synthesis of observational data. Biogeochemistry 98 139–151. [Google Scholar]
- Token S., Jiang L., Zhang L., Lv G. (2022). Effects of plant diversity on primary productivity and community stability along soil water and salinity gradients. Glob. Ecol. Conserv. 36:e02095. 10.1016/j.gecco.2022.e02095 [DOI] [Google Scholar]
- Wang C., Cheng H., Wang S., Mei W., Du D. L. (2021). Plant community and the influence of plant taxonomic diversity on community stability and invasibility: A case study based on Solidago canadensis L. Sci. Total Environ. 768:144518. 10.1016/j.scitotenv.2020.144518 [DOI] [PubMed] [Google Scholar]
- Wang G. (2002). Further thoughts on diversity and stability in ecosystems. Biodivers. Sci. 10 126–134. 10.17520/biods.2002015 34063014 [DOI] [Google Scholar]
- Wang H. F., Lü G. H., Zhou Y. Z., Cao J. (2017). Effects of functional diversity and functional redundancy on the stability of desert plant communities under different water and salt gradients. Acta Ecol. Sin. 37 7928–7937. 10.5846/stxb201610192139 [DOI] [Google Scholar]
- Wang S., Loreau M. (2016). Biodiversity and ecosystem stability across scales in metacommunities. Ecol. Lett. 19 510–518. 10.1111/ele.12582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Loreau M., Mazancourt C., Isbell F., Beierkuhnlein C., Connolly J., et al. (2021). Biotic homogenization destabilizes ecosystem functioning by decreasing spatial asynchrony. Ecology 102:e03332. 10.1002/ecy.3332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Yang Y. H., Ma W. H. (2008). Storage, patterns and environmental controls of soil phosphorus in China. Acta Sci. Nat. Univ. Pekinensis 44 945–952. [Google Scholar]
- Wang Y., Niu X., Zhao L., Liang C., Miao B., Zhang Q., et al. (2020). Biotic stability mechanisms in Inner Mongolian grassland. Proc. R. Soc. B Biol. Sci. 287:20200675. 10.1098/rspb.2020.0675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne Polley H., Wilsey B. J., Derner J. D. (2007). Dominant species constrain effects of species diversity on temporal variability in biomass production of tallgrass prairie. Oikos 116 2044–2052. 10.1111/j.2007.0030-1299.16080.x [DOI] [Google Scholar]
- Wu G.-L., Zhang Z.-N., Wang D., Shi Z.-H., Zhu Y.-J. (2014). Interactions of soil water content heterogeneity and species diversity patterns in semi-arid steppes on the Loess Plateau of China. J. Hydrol. 519 1362–1367. 10.1016/j.jhydrol.2014.09.012 [DOI] [Google Scholar]
- Wu Z., Chen S., De Boeck H. J., Stenseth N. C., Tang J., Vitasse Y., et al. (2021). Atmospheric brightening counteracts warming-induced delays in autumn phenology of temperate trees in Europe. Glob. Ecol. Biogeogr. 30 2477–2487. 10.1111/geb.13404 [DOI] [Google Scholar]
- Xu F. W., Li J. J., Wu L. J., Su J. S., Wang Y., Chen D. M., et al. (2021). Linking leaf traits to the temporal stability of above- and belowground productivity under global change and land use scenarios in a semi-arid grassland of Inner Mongolia. Sci. Total Environ. 818:151858. [DOI] [PubMed] [Google Scholar]
- Xu Z., Ren H., Cai J., Wang R., He P., Li M.-H., et al. (2015a). Antithetical effects of nitrogen and water availability on community similarity of semiarid grasslands: Evidence from a nine-year manipulation experiment. Plant Soil 397 357–369. 10.1007/s11104-015-2634-y [DOI] [Google Scholar]
- Xu Z., Ren H., Li M.-H., van Ruijven J., Han X., Wan S., et al. (2015b). Environmental changes drive the temporal stability of semi-arid natural grasslands through altering species asynchrony. J. Ecol. 103 1308–1316. 10.1111/1365-2745.12441 [DOI] [Google Scholar]
- Yachi S., Loreau M. (1999). Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proc. Natl. Acad. Sci. U.S.A. 96 1463–1468. 10.1073/pnas.96.4.1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. T., Fan J., Ge J. M., Du M. G., Jing M. (2022). Soil physical and chemical properties and vegetation characteristics of different types of grassland in Qilian Mountains, China. Chinese J. Appl. Ecol. 33 878–886. 10.13287/j.1001-9332.202204.019 [DOI] [PubMed] [Google Scholar]
- Yang X., Anwar E., Zhou J., He D., Gao Y., Cao Y. E., et al. (2022). Higher association and integration among functional traits in small tree than shrub in resisting drought stress in an arid desert. Environ. Exp. Bot. 201:104993. 10.1002/eco.1858 [DOI] [Google Scholar]
- Yang X., Lv G., Ali A., Ran Q., Gong X., Wang F., et al. (2017). Experimental variations in functional and demographic traits of Lappula semiglabra among dew amount treatments in an arid region. Ecohydrology 10:e1858. 10.1002/eco.1858 [DOI] [Google Scholar]
- Yang X., Qie Y., Teng D., Ali A., Bolan N., Xu Y., et al. (2019). Prediction of groundwater depth in an arid region based on maximum tree height. J. Hydrol. 574 46–52. 10.1016/j.jhydrol.2019.04.022 [DOI] [Google Scholar]
- Yang X., Zhang X., Lv G., Ali A. (2014). Linking Populus euphratica Hydraulic Redistribution to Diversity Assembly in the Arid Desert Zone of Xinjiang, China. PLoS One 9:e109071. 10.1371/journal.pone.0109071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Zhang Q., Su F., Zhang C., Pu Z., Xia J., et al. (2017). Daytime warming lowers community temporal stability by reducing the abundance of dominant, stable species. Glob. Change Biol. 23 154–163. 10.1111/gcb.13391 [DOI] [PubMed] [Google Scholar]
- Yu H. Y., Chen Y. N., Li W. H. (2009). Relationship between Soil Properties and Plant Diversity in a Desert Riparian Forest in the lower Reaches of the Tarim River, Xinjiang, China. Arid Soil Res. Rehabil. 23 283–296. [Google Scholar]
- Zhang R. Y., Wang Z. W., Niu S. L., Tian D. S., Wu Q., Gao X. F. P., et al. (2021). Diversity of plant and soil microbes mediates the response of ecosystem multifunctionality to grazing disturbance. Sci. Total Environ. 776:14570. 10.1016/j.scitotenv.2021.145730 [DOI] [PubMed] [Google Scholar]
- Zhang R., Liu T., Zhang J. L., Sun Q. M. (2016). Spatial and environmental determinants of plant species diversity in a temperate desert. J. Plant Ecol. 9 124–131. [Google Scholar]
- Zhang Y., Adams J., Zhao D. (2011). Does insect folivory vary with latitude among temperate deciduous forests? Ecol. Res. 26 377–383. 10.1007/s11284-010-0792-1 [DOI] [Google Scholar]
- Zhang Y., Feng J., Loreau M., He N., Han X., Jiang L. (2019). Nitrogen addition does not reduce the role of spatial asynchrony in stabilising grassland communities. Ecol. Lett. 22 563–571. 10.1111/ele.13212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., He N., Loreau M., Pan Q., Han X. (2018). Scale dependence of the diversity-stability relationship in a temperate grassland. J. Ecol. 106 1277–1285. 10.1111/1365-2745.12903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. S., Dong X. J., Xu B. X., Dong X. J., Zhang Z. S., Gao Y. H., et al. (2015). Soil respiration sensitivities to water and temperature in a revegetated desert. J. Geophys. Res. Biogeosci. 120 773–787. [Google Scholar]
- Zhao X. Y., He X. M., Yang X. D., Zhang X. N., Lv G. H. (2017). Effects of soil moisture and salt on desert plant biodiversity in Ebinur Lake Basin of Xinjiang, China. J. Arid Land Resour. Environ. 31 76–82. 10.13448/j.cnki.jalre.2017.182 10 [DOI] [Google Scholar]
- Zhao X., Zhao H., Li Y., Guo Y., Zhao Y. (2007). Changes of species diversity and productivity in relation to soil properties in sandy grassland in Horqin Sand Land. Environ. Sci. 28 945–951. [PubMed] [Google Scholar]
- Zheng D., Li W. H., Chen Y. P., Liu J. Z. (2005). Review on the relationship between groundwater and natural vegetation in arid areas. Resour. Sci. 4 160–167. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.