Abstract
New approaches to treating periodontal diseases aim to balance sustaining the natural oral microbiota and modifying the host immune response. Gum Arabic (GA) is a natural polysaccharide rich in prebiotics.
The aim of this study was to assess the effect of GA on clinical (Plaque Index (PI), Gingival Index (GI)) and immunological (Gingival Crevicular Fluid Interleukin 1 Beta (GCF IL-1 β)) parameters in patients with plaque-induced gingivitis.
Materials and methods
This placebo-controlled, double-blinded randomised clinical trial was conducted at the Department of Periodontology at Khartoum Dental Teaching Hospital, Khartoum, Sudan, from July to October 2016. Patients diagnosed with plaque-induced gingivitis meeting the study eligibility criteria were enrolled. At baseline, PI, GI and GCF IL-1β were measured. Patients received full-mouth scaling and were randomly assigned to receive either GA powder (intervention group) or Microcrystalline cellulose powder (placebo group). The patients were instructed to apply the treatment twice a day throughout the study. The PI, GI and GCF IL-1β were reassessed after 30 and 60 days.
Results
A total of 60 patients were enrolled (30 in each group). Compared to the placebo group, the intervention group showed a statistically significant reduction in GI scores after 30 days and improved PI scores at 30 and 60 days. Between baseline and 60 days, patients who received GA exhibited a significant reduction in GCF IL-1β levels compared to the placebo group.
Conclusion
GA was found to be effective in controlling plaque and gingivitis.
Clinical Trial Registration. ISRCTN registry ISRCTN14209449.
Keywords: Antimicrobial, Chemical plaque control, Clinical trial, Gingivitis, Gum Arabic
1. Introduction
Periodontal disease is considered the 11th most prevalent disease worldwide (Tonetti et al., 2017). By causing teeth loss, severe periodontitis may affect aesthetics, nutrition, and speech and masticatory function, leading to low self-esteem and poor quality of life (Tonetti et al., 2017). Periodontitis is preceded by gingivitis, an inflammation of the gingiva caused by dental plaque. Gingivitis is associated with an increase in inflammatory mediators and cytokines, including Interleukin 1 beta (Heasman et al., 1993) and changes in the gingival crevicular fluid (GCF) (Greenstein, 1984). GCF is an inflammatory exudate that carries biological markers of inflammation (Stadler et al., 2016). GCF is a valuable diagnostic tool because it reflects the inflammatory status of the gingiva (Engebretson et al., 2002) and can be collected through a simple, non-invasive procedure.
Due to the direct association between plaque levels and gingivitis (Loe et al., 1965), treatment focuses on the mechanical removal of plaque through scaling and root planing. When this is insufficient, chemical plaque control agents are often considered adjuvant treatments. However, these remedies are often associated with side effects such as discolouration of teeth and unpleasant taste in the mouth (Brecx et al., 1993).
New approaches to treating periodontal diseases aim to balance sustaining the natural oral microbiota and modifying the host immune response cautiously. Probiotics and prebiotics are examples of this modality (Devine and Marsh, 2009, Teughels et al., 2011).
Probiotics are “live microorganisms that when administered in adequate amounts, confer a health benefit on the host” (Joint FAO/WHO Working Group, 2002). Prebiotics are non-digestible oligosaccharides that affect the proliferation of resident bacteria that may exert probiotic effects (Gibson and Roberfroid, 1995). The effectiveness of probiotics and prebiotics in improving gastrointestinal health has encouraged their use in treating oral diseases (Reid et al., 2003). Prebiotics can promote the growth of beneficial microorganisms within the oral microbiota (Devine and Marsh, 2009).
Gum Arabic (GA) is a natural polysaccharide rich in prebiotics (Mariod, 2018) and a Sudanese national commodity. It is a dried exudation obtained from the Acacia Senegal or Acacia Seyal trees. GA has documented prebiotic functionality in humans (Calame et al., 2008), as well as antimicrobial (Tambekar et al., 2009); Ali et al., 2020) and anti-plaque effects (Pradeep et al., 2012).
This study objective was to assess the effect of GA on clinical (Plaque Index (PI), Gingival Index (GI)) and immunological (Gingival Crevicular Fluid Interleukin 1 Beta (GCF IL-1 β)) parameters in patients with plaque-induced gingivitis.
2. Material and methods
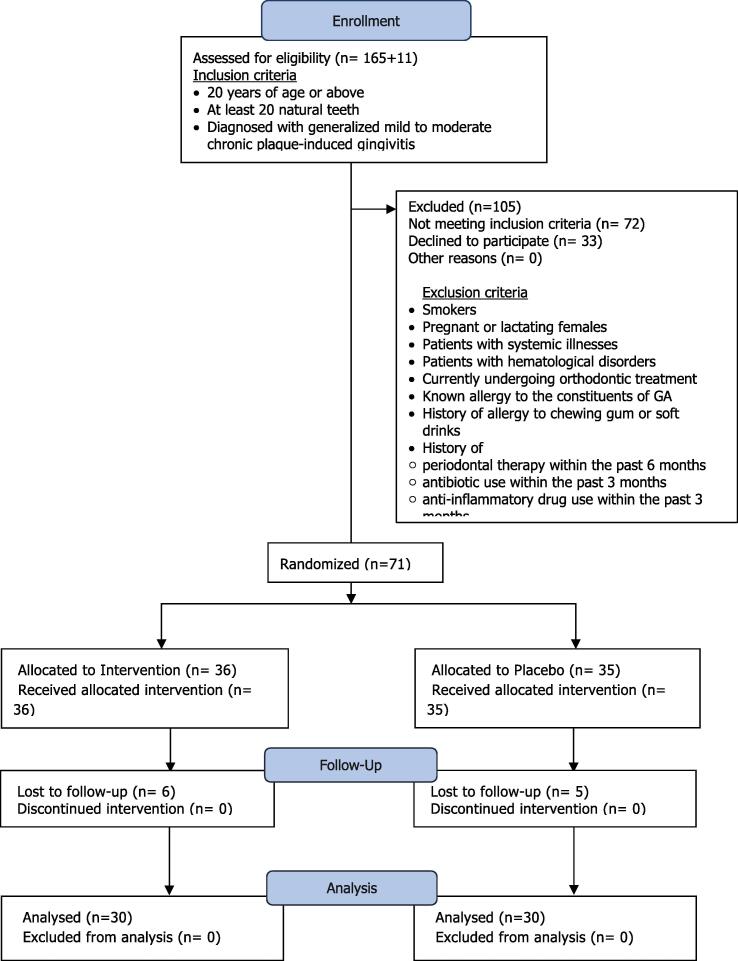
A placebo-controlled, double-blinded clinical trial was conducted at the Department of Periodontology at Khartoum Dental Teaching Hospital, Khartoum, Sudan, from July to October 2016. Patients diagnosed with plaque-induced gingivitis meeting the study eligibility criteria described in Fig. 1 were enrolled. Patients were arbitrarily assigned to the intervention or placebo group based on a computer-generated list.
Fig. 1.
Patient Flow chart.
The calculated sample size (Kelsey calculation) was 56, with 28 per group (95% CI, 80% power, 35% improved parameters, and the ratio of the exposed to unexposed was 1:1). Accounting for dropouts, 30 patients were recruited in each group. Patients were assigned by restricted randomisation (blocking) computer generation for allocation. The block size was six, and the allocation ratio was 1:1 (Pradeep et al., 2012, Pradeep et al., 2010).
2.1. Intervention procedures
All patients received a 10 ml normal saline preprocedural mouth rinse at baseline, followed by a clinical assessment. The GI and PI scores were first measured, and then GCF samples were collected, followed by full mouth scaling.
The intervention group received 150 g containers of GA in the form of a pale white powder. The placebo group received 150 g containers of microcrystalline cellulose (MC) in the form of a pale white powder, similar to GA. MC is a pharmacologically inactive substance composed of purified, partially depolymerised cellulose (FAO, 2017).
Patients were instructed to use their fingers to apply half a teaspoon of the powder to the buccal surfaces of all dento-gingival areas, without rubbing, for five minutes before rinsing with tap water, one hour after tooth brushing, twice daily (morning and evening) (Pradeep et al., 2010). The use of other mouthwashes was prohibited. Patients were advised to report any allergic reactions or complications and to stop using the powder immediately in such cases. No adverse effects were reported.
After 30 and 60 days from the baseline assessment, GI, PI and GCF IL-1β levels were assessed. Patients received another sealed container of 150 g (GA or MC) during the follow-up visits.
2.1.1. Clinical outcome measures
The primary outcome measures were the Plaque index (PI) (Silness and Löe 1964 – PI), the Gingival Index (GI) (Löe and Silness 1963 – GI) and the GCF Interleukin 1 Beta (GCF IL-1 β) level.
GI and PI scores were manually collected from four tooth surfaces (buccal, lingual, mesial and distal) using a graduated William’s periodontal probe (HuFreidy®, IL, US) and a dental mirror.
The measurements were conducted by the trained and calibrated principal investigator (AG). An intra-rater reliability test was performed (Rodrigues et al., 2009). Cohen’s Kappa for PI and GI were 0.854 and 0.776, respectively, indicating almost perfect to substantial agreement (Cohen, 1960).
GCF was collected from the mesiobuccal surface of the lateral incisor, canine and first premolar of the upper right quadrant. First, the GCF collection site was isolated using cotton rolls and air dried. The supragingival plaque was removed using a universal curette. A paper point was then inserted into the gingival crevice and stored for 30 s. The paper points were then stored in cryovial tubes at −80 °C.
The samples were analysed using enzyme-linked immunosorbent assays (ELISA). IL-1β was measured in aliquots of cell-free supernatants by a sandwich ELISA using a set of reagents provided by Bio Legends ELISA MAX Deluxe (Bio Legend®, US). The ELISA procedure was performed according to the supplier’s instructions. The optical density was measured using a microplate reader instrument at 450 nm (Thermo Fisher Scientific®, Finland). The IL-1β level was determined using plotted standard curves on Microsoft Excel 2016 software.
2.1.2. Data management and statistical analysis
Data was analysed using an intention-to-treat approach.
Diagnosis of generalised mild to moderate plaque-induced gingivitis was based on the following criteria: Gingival Index (GI) score > 1, Plaque Index (PI) score > 1, pocket probing depth < 3 mm, clinical attachment loss = 0 and no clinical evidence of bone loss.
A mean GI score (MGS) and a mean PI score (MPS) generated for the comparator groups were calculated by adding the GI and PI scores, respectively, and dividing by the total number of surfaces in each group. The changes in the GI and PI between measurement points (baseline or 0 days, 30 days, 60 days) are presented as GI percentage reduction (% ± SD) and PI percentage reduction (% ± SD), respectively. Percentage reduction was calculated using the following formula:
[(Score at previous time point − Score at current time point)/Absolute value of score at previous time point] * 100.
A dichotomous variable, the degree of plaque accumulation, was created by dividing the data into moderate (mean PI of patient < median PI) and severe (mean PI of patient ≥ median).
Similarly, a variable named the degree of gingival inflammation was created by dichotomising the GI data to mild (mean GI of patient < median GI) and moderate gingival inflammation (mean GI of patient ≥ median GI). Statistical analyses were conducted using SPSS® (SPSS, Inc, Somers, NY, USA) version 21.
3. Results
Seventy-one patients were recruited. Eleven patients were lost to follow-up, resulting in 60 patients, with 30 in each group (Fig. 1). This randomly selected sample showed no statistically significant differences in age or gender between the two groups (Table 1).
Table 1.
Percentage distribution of sociodemographic variables of the study population. Intervention (n = 30), Placebo(n = 30).
| Sociodemographic Variables | |||
|---|---|---|---|
| Intervention n = 30 Count (%) |
Placebo n = 30 Count (%) |
||
| Gender | Male | 5 (16.7) | 10 (33.3) |
| Female | 25 (83.3) | 20 (66.7) | |
| Age group | 20–29 years | 22 (73.3) | 27 (90) |
| 30–39 years | 8 (26.7) | 3 (10) | |
| Marital status | Single | 26 (86.7) | 30 (100) |
| Married | 4 (13.3) | 0 (0) | |
| Occupation | Employee | 10 (33.3) | 4 (13.3) |
| Student | 13 (43.3) | 23 (76.7) | |
| Unemployed | 7(23.3) | 3 (10) | |
| Education level |
Completed Secondary School |
17(56.7) | 24 (80) |
| Completed University Level |
13(43.3) | 6 (20) | |
| Oral hygiene tools used | Tooth Brush | 30(100) | 29 (96.7) |
| Tooth Brush & Miswak | 0(0) | 1 (3.3) | |
| Frequency of daily tooth brushing | Once | 12(40) | 18 (60) |
| Twice | 16(53.3) | 12 (40) | |
| Three times or more | 2(6.7) | 0 (0) | |
3.1. MGS and MPS
At baseline, no statistically significant differences existed between the MGS and MPS of the comparator groups (p < 0.446, p < 0.359, respectively) (Table 2).
Table 2.
Comparison of the Mean Gingival Index Score (MGS) and the Mean Plaque Index Score (MPS) for the Intervention and Placebo groups at the three measurement points. Intergroup comparison by Mann-Whitney Test. (Mean, Standard deviation).
| Mean Gingival Index Score (MGS) and the Mean Plaque Index Score (MPS) | ||||||
|---|---|---|---|---|---|---|
| Intervention (Mean, SD) | Placebo (Mean, SD) | P-value | ||||
| MGS | Baseline | 1.45 | 0.29 | 1.40 | 0.29 | 0.446 |
| 30 days | 1.09 | 0.22 | 1.20 | 0.16 | 0.012* | |
| 60 days | 0.95 | 0.26 | 1.10 | 0.22 | 0.052 | |
| MPS | Baseline | 1.37 | 0.48 | 1.48 | 0.50 | 0.359 |
| 30 days | 0.84 | 0.25 | 1.16 | 0.22 | 0.0001*** | |
| 60 days | 0.88 | 0.29 | 1.12 | 0.23 | 0.002** | |
*P < 0.05 ** P < 0.01 ***P < 0.001.
At 30 days, the intervention group’s MGS (1.09 ± 0.22) was significantly lower than the placebo’s (1.20 ± 0.16) (p < 0.012).
Similarly, at 30 days, the MPS in the intervention group (0.84 ± 0.25) was significantly lower than that of the placebo (1.16 ± 0.22) (p < 0.001).
At 60 days, the MPS of the intervention group showed a slight increase (0.88 ± 0.29) from the previous measurement at 30 days (0.84 ± 0.25). Despite this increase, the intervention group’s MPS at 60 days remained significantly lower than that of the placebo (1.12 ± 0.23) (p < 0.002).
3.1.1. GI percentage reduction
At 30 days, the intervention group exhibited a significant GI reduction when compared to the placebo (p < 0.019) (Table 3). Similarly, at 60 days, the intervention group showed a significantly greater GI reduction when compared to the placebo (p < 0.005).
Table 3.
Comparison of the GI percentage reduction in the Intervention and Placebo groups for all patients, patients with mild, moderate inflammation. Intergroup comparison by Mann-Whitney Test. (Percentage %, Standard Deviation SD).
| GI Percentage Reduction | ||||||
|---|---|---|---|---|---|---|
| Intervention |
Placebo |
|||||
| Interval | % | SD | % | SD | P-value | |
| All Patients |
Baseline to 30 days | 22.33 | 18.40 | 12.13 | 14.39 | 0.019* |
| 30 to 60 days | 10.74 | 20.17 | 7.34 | 15.62 | 0.395 | |
| Baseline to 60 days | 33.07 | 19.01 | 19.47 | 16.57 | 0.005** | |
| Patients with mild inflammation |
Baseline to 30 days | 17.15 | 17.15 | 5.57 | 10.55 | 0.007** |
| 30 to 60 days | 13.13 | 22.75 | 9.35 | 16.17 | 0.421 | |
| Baseline to 60 days | 30.28 | 21.54 | 14.92 | 14.61 | 0.012* | |
| Patients with moderate inflammation |
Baseline to 30 days | 34.40 | 16.03 | 27.46 | 9.72 | 0.122 |
| 30 to 60 days | 5.17 | 11.45 | 2.63 | 13.95 | 0.691 | |
| Baseline to 60 days | 39.57 | 9.02 | 30.09 | 16.75 | 0.077 | |
*P < 0.05 ** P < 0.01.
The GI percentage reduction was compared in patients with different degrees of gingival inflammation (mild or moderate).
In patients with mild gingival inflammation, the intervention group exhibited a significantly greater GI percentage reduction when compared to the placebo group at 30 days (p < 0.007) and 60 days (p < 0.012). There were no statistically significant differences between the two groups in patients with moderate gingival inflammation.
3.1.2. PI percentage reduction
At 30 days, the intervention group exhibited a significantly greater PI percentage reduction when compared to the placebo (p < 0.004) (Table 4). No significant differences were noted after 60 days.
Table 4.
Comparison of the PI percentage reduction in the Intervention and Placebo groups for all patients, patients with moderate, high plaque accumulation. Intergroup comparison by Mann-Whitney Test. (Percentage %, Standard Deviation SD).
| PI Percentage Reduction | ||||||
|---|---|---|---|---|---|---|
| Intervention |
Placebo |
P-value | ||||
| Interval | % | SD | % | SD | ||
| All Patients |
Baseline to 30 days | 35.12 | 22.10 | 16.37 | 23.08 | 0.004** |
| 30 to 60 days | −3.64 | 24.44 | 2.38 | 20.02 | 0.404 | |
| Baseline to 60 days | 31.48 | 25.11 | 18.75 | 23.41 | 0.059 | |
| Patients with moderate plaque | Baseline to 30 days | 35.22 | 23.68 | 12.92 | 21.99 | 0.003** |
| 30 to 60 days | −2.01 | 26.69 | 1.68 | 20.46 | 0.597 | |
| Baseline to 60 days | 33.21 | 26.92 | 14.60 | 22.85 | 0.019* | |
| Patients with high plaque | Baseline to 30 days | 34.89 | 19.18 | 24.44 | 24.85 | 0.310 |
| 30 to 60 days | −7.43 | 19.00 | 4.00 | 20.04 | 0.508 | |
| Baseline to 60 days | 27.46 | 21.16 | 28.44 | 23.02 | 0.691 | |
*P < 0.05 ** P < 0.01.
The PI percentage reduction in patients with different degrees of plaque accumulation was compared.
In patients with moderate plaque accumulation, the intervention group had a significant PI reduction when compared to the placebo at 30 days (p < 0.003) and 60 days (p < 0.019). No statistically significant differences were noted across the comparator groups for patients with high plaque accumulation.
3.1.3. GCF IL-1β
At 30 days, GCF IL-1β levels exhibited a significant drop in both the intervention (p < 0.009) and placebo groups (p < 0.007).
After 60 days, the intervention group exhibited a statistically significant drop in GCF IL-1β level (p < 0.002) (Table 5). However, the difference in the placebo group was not statistically significant (p < 0.056).
Table 5.
Changes in GCF IL-1β (Gingival Crevicular Fluid Interleukin 1 beta) levels in the Intervention and Placebo groups between the three measurement intervals measured in pictograms per millilitre (pg/ml). Intragroup comparison by Paired samples T-test. Intergroup comparison using independent sample’s T test.
| GCF IL-1β levels | ||||||
|---|---|---|---|---|---|---|
| Intervention | Mean | SD | Mean | SD | P-value | |
| 0 to 30 | 111.91 | 74.45 | 65.97 | 48.76 | 0.009** | −45.94 |
| 30 to 60 | 60.56 | 42.95 | 70.99 | 43.28 | 0.314 | 10.43 |
| 0 to 60 | 114.14 | 73.68 | 67.49 | 36.53 | 0.002** | −46.65 |
| Placebo | ||||||
| 0 to 30 | 97.83 | 64.72 | 61.48 | 42.93 | 0.007** | −36.35 |
| 30 to 60 | 67.74 | 48.70 | 67.48 | 37.20 | 0.983 | −0.26 |
| 0 to 60 | 101.38 | 65.45 | 70.99 | 43.28 | 0.056 | −30.39 |
|
Intervention |
Placebo |
|||||
| Mean | SD | Mean | SD | P-value | ||
| 0 | 112.20 | 73.17 | 97.83 | 64.72 | 0.424 | |
| 30 | 65.97 | 48.76 | 61.48 | 42.93 | 0.708 | |
| 60 | 67.49 | 36.53 | 70.99 | 43.28 | 0.742 | |
| 0 to 30 | 45.94 | 87.48 | 36.35 | 67.90 | 0.639 | |
| 30 to 60 | 0.26 | 64.57 | −10.43 | 53.75 | 0.504 | |
| 0 to 60 | 46.65 | 74.28 | 30.39 | 80.67 | 0.432 | |
*P < 0.05 ** P < 0.01.
A Pearson correlation further examined the relationship between the GCF IL-1β, PI and GI. However, all the findings were not statistically significant.
4. Discussion
Gum Arabic was found to be effective in reducing plaque and gingival inflammation. The intervention group exhibited a significantly lower MGS at 30 days, a significant drop in MPS at both 30 and 60 days and a significant drop in GCF IL-1β at 30 and at 60 days.
Our findings are in line with earlier studies. Prior research documented a significant reduction in PI and GI scores in plaque-induced gingivitis with Acacia Arabica (AA) use (Pradeep et al., 2012). These results were also evident with AA’s toothpaste formula (Tangade et al., 2012). The use of AA gel in mild to moderate plaque-induced periodontitis was associated with clinical attachment gain, a significant reduction in probing depth, and improved PI and GI outcomes (Singhal et al., 2018). However, when Acacia Senegal chewing gum was compared to sugar-free gum, no significant differences in PI and GI scores were noted after one week (Gazi, 1991).
There are several possible explanations for GA’s anti-plaque and anti-gingivitis properties (Singhal et al., 2018).
Evidence suggests that GA has an antibacterial effect (Baien et al., 2020). AA induces an inhibition zone on periodonpathogenic bacterial cultures (Saini et al., 2008, Tambekar et al., 2009) and suppresses P. gingivalis and P. intermedia (Clark et al., 1993). A recent genetic analysis has revealed emerging antibiotic-resistant genes (ARGs) and metal-resistant genes (MRGs) in the dental plaque microbiota after scaling and root planing, thus highlighting the need for novel antibacterial strategies to control periodontitis (Kang et al., 2021).
Scientists suggest that AA could be used as a chemical plaque control agent, as it is deemed comparable to 1% chlorhexidine (Pradeep et al., 2012). Chlorhexidine is considered the gold standard treatment for gingivitis, however, its use does not impact GCF IL-1β levels (Türkoǧlu et al., 2009), and neither does the use of triclosan dental gel (Sköld-Larsson et al., 2003).
GA also acts as an antioxidant agent (Ali et al., 2009, Hassanien, 2020). Although these claims have been contested (Al-Majed et al., 2003, Ali, 2004), studies suggest that GA protects cardiac (Abd-Allah et al., 2002), renal (Al-Yahya et al., 2009) and hepatic tissues (Gamal El-din et al., 2003) from the effects of cytotoxic drugs. These drugs cause damage by producing reactive oxygen species (ROS) and inducing oxidative stress (Hinson et al., 2004). This process is comparable to periodontal disease, where the host-microbial interaction increases ROS production, leading to oxidative stress and subsequent tissue damage (Pendyala et al., 2008, Waddington et al., 2000).
We observed that GA might have a differential impact based on the amount of plaque and the degree of gingival inflammation. GA appeared to reduce PI more effectively in moderate plaque than in high plaque accumulation. This finding aligns with prior observations that GA affects early plaque formation but has no long-term effects (Gazi, 1991).
We observed that GA had a significant effect in mild gingival inflammation, as opposed to moderate cases. It is possible that with heightened inflammation, increased sulcus depth limits GA’s accessibility, thus reducing its effect. Clark et al. (1993) noted the inability of Acacia gum to reach deep areas of periodontal pockets and recommended using a delivery system. Our results support this. Areas of heightened inflammation could benefit from the targeted application of GA through subgingival irrigation.
GA also has an anti-inflammatory effect (Kamal et al., 2018, Ali et al., 2020, Hassanien, 2020). The presence of high levels of inflammatory cytokines, IL-1β and TNF-α in diseased periodontal tissues has been well established (Kinane et al., 2008). A recent review by Cheng et al. (2020) suggests that targeting IL-1β could potentially have a therapeutic effect on periodontitis. To the best of our knowledge, no study to date has evaluated the impact of GA on GCF IL-1β levels.
The observed drop in GCF IL-1β levels in both groups after 30 days could be attributed to baseline nonsurgical periodontal therapy (De Lima Oliveira et al., 2012, Engebretson et al., 2002). However, the significant drop in GCF IL-1β between baseline and 60 days in the intervention group only is possibly due to GA. This finding, while preliminary, suggests that GA can suppress periodontal inflammation at a more profound level by suppressing local mediators.
We carefully interpret our GCF findings because although GCF IL-1β levels may reflect the severity of periodontal disease (Heasman et al., 1993), its progression cannot be evaluated by the GCF IL-1β level alone. The role of periodontal pathogens, host response and genetic factors should be all considered. Thus, we recommend further research to assess the effect of GA by evaluating plaque culture and incorporating a complete cytokine profile.
The strength of this study is in that we incorporated calibration, randomisation and double blinding to limit bias. At baseline, the patients’ daily brushing habits could have varied, and so during the trial, they were instructed to brush twice daily. Nevertheless, the Hawthorn effect may have masked the true extent of the differences between the groups.
A potential limitation of this study could have been that the baseline treatment may have contributed to the drop in GI and PI scores. We are not concerned by this as all participants underwent the same treatment at baseline. This means that the effect was the same across both groups, and any significant difference between the intervention and placebo groups can be related to the GA intervention.
The possible role of GA as an anti-plaque and anti-gingivitis agent is reemphasised throughout the study. GA may be poised to have future applications in preventing periodontitis because of its role in reducing GCF IL-1β. It could open the door for a local, natural and cost-effective product that could help alleviate the high burden of periodontal diseases in Sudan (Khalifa et al., 2012).
5. Conclusion
Gum Arabic was found to be effective in controlling plaque and gingivitis. Thus, GA may be a beneficial herbal formulation for chemical plaque control and a possible adjuvant treatment for plaque-induced gingivitis.
Ethical approval
Ethical approval was obtained from the Research Ethics Committee of the National Medicine and Poisons Board. Written informed consent was obtained from all participants. The data were coded and securely locked to ensure confidentiality.
CRediT authorship contribution statement
Arwa M. Gafar: Conceptualization, Methodology, Investigation, Writing – original draft. AbdelRahman M. Ramadan: Conceptualization, Methodology, Supervision. Nouar A. ElSaid: . Nazik M. Nurelhuda: Conceptualization, Methodology, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Nouar A. ElSaid, Email: nouar.elsaid@post.harvard.edu.
Nazik M. Nurelhuda, Email: n.nurelhuda@gmail.com.
References
- Abd-Allah A.R., Al-Majed A.A., Mostafa A.M., Al-Shabanah O.A., Din A.G., Nagi M.N. Protective effect of arabic gum against cardiotoxicity induced by doxorubicin in mice: A possible mechanism of protection. J. Biochem. Mol. Toxicol. 2002;16:254–259. doi: 10.1002/jbt.10046. [DOI] [PubMed] [Google Scholar]
- Al-Majed A.A., Abd-Allah A.R.A., Al-Rikabi A.C., Al-Shabanah O.A., Mostafa A.M. Effect of oral administration of arabic gum on cisplatin-induced nephrotoxicity in rats. J. Biochem. Mol. Toxicol. 2003;17:146–153. doi: 10.1002/jbt.10072. [DOI] [PubMed] [Google Scholar]
- Al-Yahya A.A., Al-Majed A.A., Gado A.M., Daba M.H., Al-Shabanah O.A., El-Azab A.S., Abd-Allah A.R.A. Acacia senegal gum exudate offers protection against cyclophosphamide- induced urinary bladder cytotoxicity. Oxid. Med. Cell. Longev. 2009;2:207–213. doi: 10.4161/oxim.2.4.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali B.H. Does Gum Arabic Have an Antioxidant Action in Rat Kidney? Ren. Fail. 2004;26:1–3. doi: 10.1081/JDI-120028536. [DOI] [PubMed] [Google Scholar]
- Ali B.H., Al Za’abi M., Al Suleimani Y., Manoj P., Ali H., Ribeiro D.A., Nemmar A. Gum arabic reduces inflammation, oxidative, and nitrosative stress in the gastrointestinal tract of mice with chronic kidney disease. Naunyn. Schmiedebergs. Arch. Pharmacol. 2020;393:1427–1436. doi: 10.1007/s00210-020-01844-y. [DOI] [PubMed] [Google Scholar]
- Ali B.H., Ziada A., Blunden G. Biological effects of gum arabic: A review of some recent research. Food Chem. Toxicol. 2009 doi: 10.1016/j.fct.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Baien S.H., Seele J., Henneck T., Freibrodt C., Szura G., Moubasher H., Nau R., Brogden G., Mörgelin M., Singh M., Kietzmann M., von Köckritz-Blickwede M., de Buhr N. Antimicrobial and Immunomodulatory Effect of Gum Arabic on Human and Bovine Granulocytes Against Staphylococcus aureus and Escherichia coli. Front. Immunol. 2020;10:3119. doi: 10.3389/fimmu.2019.03119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecx M., MacDonald L.L., Legary K., Cheang M. Long-term Effects of Meridol® and Chlorhexidine Mouthrinses on Plaque, Gingivitis, Staining, and Bacterial Vitality. J. Dent. Res. 1993;72:1194–1197. doi: 10.1177/00220345930720080601. [DOI] [PubMed] [Google Scholar]
- Calame W., Weseler A.R., Viebke C., Flynn C., Siemensma A.D. Gum arabic establishes prebiotic functionality in healthy human volunteers in a dose-dependent manner. Br. J. Nutr. 2008;100:1269–1275. doi: 10.1017/S0007114508981447. [DOI] [PubMed] [Google Scholar]
- Cheng R., Wu Z., Li M., Shao M., Hu T. Interleukin-1β is a potential therapeutic target for periodontitis: a narrative review. Int. J. Oral Sci. 2020;12:1–9. doi: 10.1038/s41368-019-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.T., Gazi M.I., Cox S.W., Eley B.M., Tinsley G.F. The effects of Acacia arabica gum on the in vitro growth and protease activities of periodontopathic bacteria. J. Clin. Periodontol. 1993;20:238–243. doi: 10.1111/j.1600-051X.1993.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960;20:37–46. doi: 10.1177/001316446002000104. [DOI] [Google Scholar]
- De Lima Oliveira A.P., De Faveri M., Gursky L.C., Mestnik M.J., Feres M., Haffajee A.D., Socransky S.S., Teles R.P. Effects of periodontal therapy on GCF cytokines in generalized aggressive periodontitis subjects. J. Clin. Periodontol. 2012;39:295–302. doi: 10.1111/j.1600-051X.2011.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine D.A., Marsh P.D. Prospects for the development of probiotics and prebiotics for oral applications. J. Oral Microbiol. 2009 doi: 10.3402/jom.v1i0.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebretson S.P., Grbic J.T., Singer R., Lamster I.B. GCF IL-1β profiles in periodontal disease. J. Clin. Periodontol. 2002;29:48–53. doi: 10.1034/j.1600-051x.2002.290108.x. [DOI] [PubMed] [Google Scholar]
- FAO, 2017. Microcrystalline Cellulose.
- Gamal El-din A.M., Mostafa A.M., Al-Shabanah O.A., Al-Bekairi A.M., Nagi M.N. Protective effect of arabic gum against acetaminophen-induced hepatotoxicity in mice. Pharmacol. Res. 2003;48:631–635. doi: 10.1016/S1043-6618(03)00226-3. [DOI] [PubMed] [Google Scholar]
- Gazi M.I. The finding of antiplaque features in Acacia Arabica type of chewing gum. J. Clin. Periodontol. 1991;18:75–77. doi: 10.1111/j.1600-051X.1991.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Greenstein G. The Role of Bleeding upon Probing in the Diagnosis of Periodontal Disease: A Literature Review. J. Periodontol. 1984;55:684–688. doi: 10.1902/jop.1984.55.12.684. [DOI] [PubMed] [Google Scholar]
- Hassanien M.A. The protective and antioxidant effects of gum arabic: a review of recent evidence using the new PubMed system. Int. J. Community Med. Public Heal. 2020;7:356–360. doi: 10.18203/2394-6040.ijcmph20195592. [DOI] [Google Scholar]
- Heasman P.A., Collins J.G., Offenbacher S. Changes in crevicular fluid levels of interleukin-1β, leukotriene B4, prostaglandin E2, thromboxane B2 and tumour necrosis factor α in experimental gingivitis in humans. J. Periodontal Res. 1993;28:241–247. doi: 10.1111/j.1600-0765.1993.tb02090.x. [DOI] [PubMed] [Google Scholar]
- Hinson J.A., Reid A.B., McCullough S.S., James L.P. Acetaminophen-induced hepatotoxicity: Role of metabolic activation, reactive oxygen/nitrogen species, and mitochondrial permeability transition. Drug Metab. Rev. 2004;36:805–822. doi: 10.1081/DMR-200033494. [DOI] [PubMed] [Google Scholar]
- Joint FAO/WHO Working Group, 2002. Probiotics in food Health and nutritional properties and guidelines for evaluation.
- Kamal E., Kaddam L.A., Dahawi M., Osman M., Salih M.A., Alagib A., Saeed A. Gum Arabic fibers decreased inflammatory markers and disease severity score among Rheumatoid Arthritis patients, Phase II Trial. Int. J. Rheumatol. 2018 doi: 10.1155/2018/4197537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y., Sun B., Chen Y., Lou Y., Zheng M., Li Z. Dental Plaque Microbial Resistomes of Periodontal Health and Disease and Their Changes after Scaling and Root Planing Therapy. mSphere. 2021;6 doi: 10.1128/MSPHERE.00162-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifa N., Allen P.F., Abu-bakr N.H., Abdel-Rahman M.E., Abdelghafar K.O. A survey of oral health in a Sudanese population. BMC Oral Health. 2012;12:5. doi: 10.1186/1472-6831-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinane D.F., Galicia J.C., Gorr S.U., Stathopoulou P.G., Benakanakere M. P. gingivalis interactions with epithelial cells. Front. Biosci. 2008;13:966–983. doi: 10.2741/2736. [DOI] [PubMed] [Google Scholar]
- Loe H., Silness J. Periodontal Disease in Pregnancy. I. Prevalence and Severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- Loe H., Theilade E., Jensen S.B. Experimental Gingivitis in Man. J. Periodontol. 1965;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- Mariod A.A. Gum Arabic: Structure, Properties, Application and Economics. Elsevier; 2018. Gum Arabic Dietary Fiber; pp. 237–243. [DOI] [Google Scholar]
- Pendyala G., Thomas B., Kumari S. The challenge of antioxidants to free radicals in periodontitis. J. Indian Soc. Periodontol. 2008;12:79. doi: 10.4103/0972-124x.44100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeep A.R., Agarwal E., Bajaj P., Naik S.B., Shanbhag N., Uma S.R. Clinical and microbiologic effects of commercially available gel and powder containing Acacia arabica on gingivitis. Aust. Dent. J. 2012;57:312–318. doi: 10.1111/j.1834-7819.2012.01714.x. [DOI] [PubMed] [Google Scholar]
- Pradeep A.R., Happy D., Garg G. Short-term clinical effects of commercially available gel containing Acacia arabica: A randomized controlled clinical trial. Aust. Dent. J. 2010;55:65–69. doi: 10.1111/j.1834-7819.2009.01180.x. [DOI] [PubMed] [Google Scholar]
- Reid G., Jass J., Sebulsky M.T., McCormick J.K. Potential Uses of Probiotics in Clinical Practice. Clin. Microbiol. Rev. 2003;16:658–672. doi: 10.1128/CMR.16.4.658-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues I.S., Tavares V.N., Pereira S.L., Costa F.N. Antiplaque and antigingivitis effect of Lippia sidoides. A double-blind clinical study in humans. J. Appl. Oral Sci. 2009;17:404–407. doi: 10.1590/S1678-77572009000500010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini M.L., Saini R., Roy S., Kumar A. Comparative pharmacognostical and antimicrobial studies of acacia species (Mimosaceae) J. Med. Plants Res. 2008;2:378–386. doi: 10.5897/JMPR.9000378. [DOI] [Google Scholar]
- Silness J., Loe H. Periodontal Disease in Pregnancy. Ii. Correlation between Oral Hygiene and Periodontal Condtion. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- Singhal R., Agarwal V., Rastogi P., Khanna R., Tripathi S. Efficacy of Acacia arabica gum as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A randomized controlled clinical trial. Saudi Dent. J. 2018;30:53–62. doi: 10.1016/j.sdentj.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sköld-Larsson K., Yucel-Lindberg T., Twetman S., Modéer T. Effect of a triclosan-containing dental gel on the levels of prostaglandin I2 and interleukin-1β in gingival crevicular fluid from adolescents with fixed orthodontic appliances. Acta Odontol. Scand. 2003;61:193–196. doi: 10.1080/00016350310003242. [DOI] [PubMed] [Google Scholar]
- Stadler A.F., Angst P.D.M., Arce R.M., Gomes S.C., Oppermann R.V., Susin C. Gingival crevicular fluid levels of cytokines/chemokines in chronic periodontitis: a meta-analysis. J. Clin. Periodontol. 2016;43:727–745. doi: 10.1111/jcpe.12557. [DOI] [PubMed] [Google Scholar]
- Tambekar D.H., Khante B.S., Chandak B.R., Titare A.S., Boralkar S.S., Aghadte S.N. Screening of antibacterial potentials of some medicinal plants from Melghat forest in India. African J. Tradit. Complement. Altern. Med. 2009;6:228–232. doi: 10.4314/ajtcam.v6i3.57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangade P.S., Mathur A., Tirth A., Kabasi S. Anti-gingivitis effects of Acacia arabica-containing toothpaste. Chinese J. Dent. Res. 2012;15:49–53. [PubMed] [Google Scholar]
- Teughels W., Loozen G., Quirynen M. Do probiotics offer opportunities to manipulate the periodontal oral microbiota? J. Clin. Periodontol. 2011;38:159–177. doi: 10.1111/j.1600-051X.2010.01665.x. [DOI] [PubMed] [Google Scholar]
- Tonetti M.S., Jepsen S., Jin L., Otomo-Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017;44:456–462. doi: 10.1111/jcpe.12732. [DOI] [PubMed] [Google Scholar]
- Türkoǧlu O., Becerik S., Emingil G., Kütükçüler N., Baylas H., Atilla G. The effect of adjunctive chlorhexidine mouthrinse on clinical parameters and gingival crevicular fluid cytokine levels in untreated plaque-associated gingivitis. Inflamm. Res. 2009;58:277–283. doi: 10.1007/s00011-008-8129-z. [DOI] [PubMed] [Google Scholar]
- Waddington R.J., Moseley R., Embery G. Reactive oxygen species: A potential role in the pathogenesis of periodontal diseases. Oral Dis. 2000;6:138–151. doi: 10.1111/j.1601-0825.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]