Key Points
Question
Is occupational exposure to formaldehyde (FA) in males associated with semen quality?
Findings
In this cohort study of 205 men, long-term personal occupational exposure to FA was associated with lower semen quality. Additionally, this deterioration was dose and time dependent and might be induced by oxidative stress.
Meaning
These findings may help researchers, physicians, and policy makers evaluate the hazardous effects of FA more completely and design targeted strategies for primary prevention of male infertility.
This cohort study assesses whether long-term occupational exposure to formaldehyde is associated with semen quality.
Abstract
Importance
The potential effects of long-term occupational exposure to formaldehyde (FA) on human semen quality is not clear.
Objective
To assess whether long-term occupational exposure to FA is associated with semen quality.
Design, Setting, and Participants
This population-based cohort study was conducted from June 1 to June 30, 2021, in Xi’an, China. Participants were adults aged 23 to 40 years who had lived in the study area for 24 months or longer. Data analysis was performed from September 1 to October 1, 2021.
Exposures
Long-term occupational exposure to FA was measured using a formaldehyde detector, and the FA exposure index (FEI) was calculated as follows: FEI = final concentration of FA (mg/m3) × work time during a workday (hour) × cumulative workdays (year).
Main Outcomes and Measures
Semen samples were collected by masturbation after 3 to 7 days of abstinence and were then assessed by the computer-automated semen analysis system, Baso-Papanicolaou staining, and sperm-chromatin structure assay.
Results
A total of 205 men (mean [SD] age, 29.49 [3.64] years), with 124 individuals in the FA exposure group (mean [SD] FEI, 73.72 [54.86]) and 81 age-matched controls, were included in the final analysis. Long-term personal occupational exposure to FA was significantly associated with poor semen quality. Specifically, a 1-unit increase in FEI was associated with a change of −0.99% (95% CI, −1.00% to −0.98%) in total sperm motility, −0.99% (95% CI, −0.99% to −0.97%) in progressive sperm motility, −0.05% (95% CI, −0.08% to −0.02%) in curvilinear velocity, −0.07% (95% CI, −0.10% to −0.04%) in straight line velocity, −0.07% (95% CI, −0.10% to −0.04%) in time-average velocity, −0.98% (95% CI, −0.99% to −0.93%) in normal sperm morphology, −0.24% (95% CI, −0.35% to −0.11%) in seminal neutral glucosidase, −0.61% (95% CI, −0.66% to −0.56%) in seminal plasma zinc, 0.52% (95% CI, 0.15% to 1.02%) in beat cross frequency, and 0.10% (95% CI, 0.06% to 0.14%) in the DNA fragmentation index. These associations remained significant after adjusting for confounding factors. Furthermore, subgroup analysis found that high levels of oxidative stress might promote the associations between FA exposure and semen quality.
Conclusions and Relevance
This study found an association between long-term occupational exposure to FA and semen quality. This deterioration was dose and time dependent and might be induced by oxidative stress.
Introduction
Infertility is a nonnegligible public health issue in male reproduction that is garnering increasing attention globally. Approximately 14% of couples in some high-resource countries and 25% of couples in some low-resource countries experience infertility.1,2 Male factors, mainly poor semen quality, account for nearly half of the infertility cases.3 A conspicuous decline in semen quality around the world4,5,6,7 confirmed this male reproductive crisis and triggered studies into the reason for decreases in semen quality. The remarkable changes in semen quality over a relatively short period is likely to be related to environmental factors.8,9
Formaldehyde (FA), a ubiquitous environmental contaminant, has been reported to be associated with various diseases, such as neurological, dermal, and respiratory disorders as well as cancer.10,11 As for the association with semen quality, numerous animal studies have found that FA exposure contributes to damage on seminiferous tubules12,13,14,15,16,17 and declines in semen quality18,19,20 in rats and mice. Unfortunately, human studies remained limited, and findings have been inconsistent. Ward et al21 reported that FA exposure showed no association with sperm count and morphology in Finnish males. However, this study was retrospective research relying on self-reported data and could be confounded by recall and selection biases. On the contrary, our previous study22 found that FA exposure was associated with decreased sperm motility in Chinese males. However, the uncomprehensive semen parameters in this previous study had limited capability to fully reflect the associations between FA exposure and semen quality.
Considering severe FA exposure22,23,24,25 and accelerating decline in semen quality in China,7 more studies are needed to explore whether occupational exposure to FA in males is associated with poor semen quality. Therefore, our goals were (1) to assess the association between long-term occupational exposure to FA and poor semen quality using the most comprehensive semen parameters including 20 parameters (5 conventional sperm parameters, 9 kinematic sperm parameters, sperm morphology, DNA fragmentation index [DFI], and 4 biochemistry parameters in seminal plasma) and (2) to explore the potential mechanism of associations between FA exposure and poor semen quality.
Methods
Study Participants
This population-based cohort study was conducted in the Shaanxi province of northwestern China from June 1 to June 30, 2021. The aim was to evaluate the associations of long-term occupational exposure to FA with semen quality. The study was organized by Xi’an Jiaotong Unversity. This work followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline and received approval for research ethics from the Medical Ethics Committee of Xi’an Jiaotong University. The study was conducted according to the Declaration of Helsinki.26 Volunteers were recruited from wood material industries in Xi’an. Participants were informed of the purpose of the present study and signed informed consent forms.
Inclusion criteria were as follows: (1) Chinese Han ethnicity; (2) aged 23 to 40 years; (3) worked in the FA exposure environment for at least 24 months and whose lifestyles did not change for at least 6 months prior to semen collection. To reduce the influence of confounding factors, exclusion criteria were carried out as follows: (1) living in newly built or decorated rooms; (2) having genital abnormalities or other chronic diseases including pelvic or spinal injuries, cytogenetic aberrations, testicular dysgenesis syndrome, sexually transmitted infections, chronic hepatitis B, hypertension, diabetes, and others; and (3) refusing to finish questionnaires and physical examination or to provide semen samples.
Reference group volunteers were recruited from the age-matched male Han population who had lived in same place and had no exposure experience to FA or other reproductive toxicants. Reference group volunteers were screened in accordance with the exclusion criteria.
Questionnaire and Physical Examination
Questionnaires were designed based on our previous study27 and were completed by all participants, containing basic information (age, nationality, and income), lifestyle (smoking status, alcohol consumption), working information (workplace, work time during a workday, and cumulative workdays), occupational history, previous diseases, and abstinence duration. Meanwhile, a physical examination was conducted for every participant, including body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) and genital examination.
Exposure Assessment
According to the methods used in our previous study,22 the FA exposure index (FEI) was measured for every participant. The concentration of FA was measured for each participant using a formaldehyde detector. To reduce possible risks, we conducted the measurements at 3 different times during a workday (9:00 am, 12:00 pm, and 3:00 pm), and the mean value of the 3 measurements was used as the final concentration of FA. Then, combined with the information collected from the questionnaires, FEI was calculated as follows: FEI = the final concentration of FA (mg/m3) × work time during a workday (hour) × cumulative workdays (year).
Semen Collection
Within 2 weeks after the FA exposure measurements, semen samples were collected by masturbation after 3 to 7 days of abstinence. All samples were incubated at 37 °C and analyzed within 60 minutes after collection.
Determination of Semen Quality
Conventional Sperm Parameters
Semen analyses were conducted according to the World Health Organization (WHO) guidelines.28 A total of 5 conventional sperm parameters, including semen volume (SV), sperm concentration (SC), total sperm count (TSC), total sperm motility (TSM), and progressive sperm motility (PSM), were assessed by a well-trained technician using the computer automated semen analysis system (CASA [WLJY-9000]) according to the manufacturer’s guidelines. The detection method was described in our previous study.22 At least 200 sperm were detected from each sample, and the total sampling time for each sample lasted about 5 minutes. Among the 5 conventional sperm parameters, SV, SC, and TSC represented the sperm count, while TSM and PSM represented sperm motility.
Kinematic Sperm Parameters
Similar to the analysis of conventional sperm parameters, a total of 9 kinematic sperm parameters, including curvilinear velocity (VCL), straight line velocity (VSL), time-average velocity (VAP), mean angular displacement (MAD), linearity, straightness, wobble, amplitude of lateral head displacement (ALH), and beat cross frequency (BCF), were also estimated by the same CASA system according to the manufacturer’s guidelines.22 At least 200 sperm were detected from each sample and the total sampling time for each sample, lasted about 5 minutes. The 9 kinematic sperm parameters represented sperm motility.
Sperm Morphology
Sperm morphology analysis was performed using smears. After fixing semen in methanol, a Baso-Papanicolaou staining kit was used for staining the slides. According to the WHO guidelines,28 at least 200 sperm were examined from each sample with an optical microscope (Olympus) at ×400 magnification. The result of sperm morphology was calculated by the percentage of normal spermatozoa and was expressed as normal sperm morphology (NSM). The evaluations were performed by 2 independent researchers. Each researcher assessed the same smear independently, and the final NSM was the mean value of the 2 researchers. Any discrepancies greater than 10% were reexamined by a third researcher to reach a final consensus.
DFI
DFI evaluation was conducted using the sperm chromatin structure assay.29 Acridine orange was used to stain the sperm possessing unstable chromatin, and flow cytometers were used to collect fluorescence signals. A minimum of 10 000 events were recorded for each sample.
Biochemistry Parameters in Seminal Plasma
Seminal plasma was collected from each participant. The 4 biochemistry parameters, including acid phosphatase (ACP), neutral glucosidase (NG), fructose, and zinc were determined using commercial kits (Huakang) according to the manufacturer manual.
Detection of Antioxidant Defenses in Semen
The antioxidant defenses in semen, including superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione peroxidase (GSHPx), were determined using commercial kits (JianCheng) according to the manufacturer manual.
Statistical Analysis
First, mean (SD) or median (IQR) for continuous variables and proportions for categorical variables were used to delineate demographic characteristics and semen quality. Second, the quantile-quartile plots were used to verify the normality of data, and any nonnormally distributed data was transformed into normally distributed data by the logarithmic transformation. Third, t and χ2 tests were used to explore the differences in all the characteristics aforementioned between the FA exposure group and control group. Fourth, a linear regression model was used to assess the associations between FEI and semen quality, controlling for confounding factors including age (years), BMI, income (yuan), smoking status, and alcohol consumption. Regression coefficients and 95% CIs were back-transformed and expressed as percentage change in each semen quality parameter for a 1-unit increase in FEI. Fifth, to investigate whether oxidative stress was a potential factor modifying the associations between FEI and semen quality, subgroup analyses were performed. The median level of SOD was identified and used as an empirical cutoff to dichotomize individuals into 2 groups: high and low levels of SOD. Linear regression models in low and high levels of SOD were conducted independently. Similarly, further subgroup analyses based on MDA and GSHPx were also conducted. Sixth, considering that smoking and drinking are known risk factors for poor sperm motility,30,31,32 a sensitivity analysis was performed by including and excluding them as confounding factors in linear regression models. In addition, we also conducted further analyses stratified by age and BMI to explore the potential effect modifiers.
All statistical analyses were performed in SPSS statistical software version 18.0 (IBM Corp). All tests were 2-sided, and P < .05 was considered statistically significant.
Results
Participant Characteristics
As shown in Table 1 and eFigure 1 in the Supplement, a total of 205 individuals were included in the final analysis, with a mean (SD) age of 29.49 (3.64) years, a mean (SD) income of 2463.42 (649.66) yuan ($364.90 [$96.23]), and a mean (SD) BMI of 24.01 (1.61); 174 men (84.88%) smoked, whereas 125 (60.98%) reported alcohol consumption. Among the 205 individuals, including 124 men with FA exposure and 81 in the control group, no differences between exposure group and control group were seen in age, income, BMI, smoking status, or alcohol consumption (eFigure 2 in the Supplement).
Table 1. Main Characteristics and Semen Parameters of the Study Participants.
| Characteristic | Participants, No. (%) (N = 205) |
|---|---|
| Age, mean (SD), y | 29.49 (3.64) |
| BMI, mean (SD) | 24.01 (1.61) |
| Income, yuan/working period, mean (SD)a | 2463.42 (649.66) |
| Smoking status | |
| Nonsmoker | 31 (15.12) |
| Smoker | 174 (84.88) |
| Alcohol consumption | |
| Nondrinker | 80 (39.02) |
| Drinker | 125 (60.98) |
| Conventional sperm parameters, median (IQR) | |
| SV, mL | 2.5 (2.00-3.60) |
| SC, million/mL | 54.07 (34.49-74.73) |
| TSC, million | 128.79 (79.20-206.33) |
| TSM, % | 51.01 (40.53-66.54) |
| PSM, % | 40.13 (29.92-54.28) |
| Kinematic sperm parameters, mean (SD), μm/s | |
| VCL | 49.74 (10.14) |
| VSL | 33.16 (7.86) |
| VAP | 36.13 (7.96) |
| MAD, mean (SD), ° | 53.86 (8.05) |
| Linearity, mean (SD) | 65.21 (8.23) |
| Straightness, mean (SD) | 88.12 (4.07) |
| Wobble, mean (SD) | 72.24 (6.74) |
| ALH, mean (SD), μm | 3.44 (1.12) |
| BCF, mean (SD) | 5.09 (1.03) |
| NSM, median (IQR), % | 10 (7.00-15.00) |
| DFI, mean (SD), % | 18.85 (7.99) |
| Biochemistry parameters in seminal plasma, mean (SD) | |
| ACP, U/ejaculate | 71.05 (21.08) |
| NG, mU/ejaculate | 7.4 (1.8) |
| Fructose, μmol/ejaculate | 4.96 (0.86) |
| Zinc, μmol/ejaculate | 4.76 (1.62) |
Abbreviations: ACP, acid phosphatase; ALH, amplitude of lateral head displacement; BCF, beat cross frequency; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DFI, DNA fragmentation index; MAD, mean angular displacement; NG, neutral glucosidase; NSM, normal sperm morphology; PSM, progressive sperm motility; SC, sperm concentration; SV, semen volume; TSC, total sperm count; TSM, total sperm motility; VAP, average path velocity; VCL, curvilinear velocity; VSL, straight line velocity.
To convert yuan to US dollars, multiply by 0.15.
FA Exposure Level
Among 124 individuals in the FA exposure group, the mean (SD) concentration of FA was 1.49 (0.61) mg/m3, and the mean (SD) FEI was 73.72 (54.86). For the 81 men in the control group, FA occupational exposure could be ignored because of the low concentration of FA in the air (0-0.02 mg/m3).
Semen Quality With and Without Occupational Exposure to FA
Conventional Sperm Parameters
As shown in Figure 1, men in the FA exposure group, compared with the control group, had lower median (IQR) TSM (46.54% [35.15%-58.84%] vs 58.33% [45.21%-73.72%]; P < .001) and PSM (37.12% [27.75%-46.08%] vs 50.11% [30.66%-64.42%]; P < .001). On the other hand, the differences in SV (2.60 [1.75-3.60] mL vs 2.50 [2.10-3.50] mL; P = .45), SC (54.25 [33.26-73.5] million/mL vs 53.94 [34.08-77.83] million/mL; P =.65), and TSC (125.20 [73.69-192.30] million vs 129.11 [76.62-221.29] million; P = .42) were not statistically significant (eFigure 5 in the Supplement).
Figure 1. Semen Quality With and Without Occupational Formaldehyde Exposure.
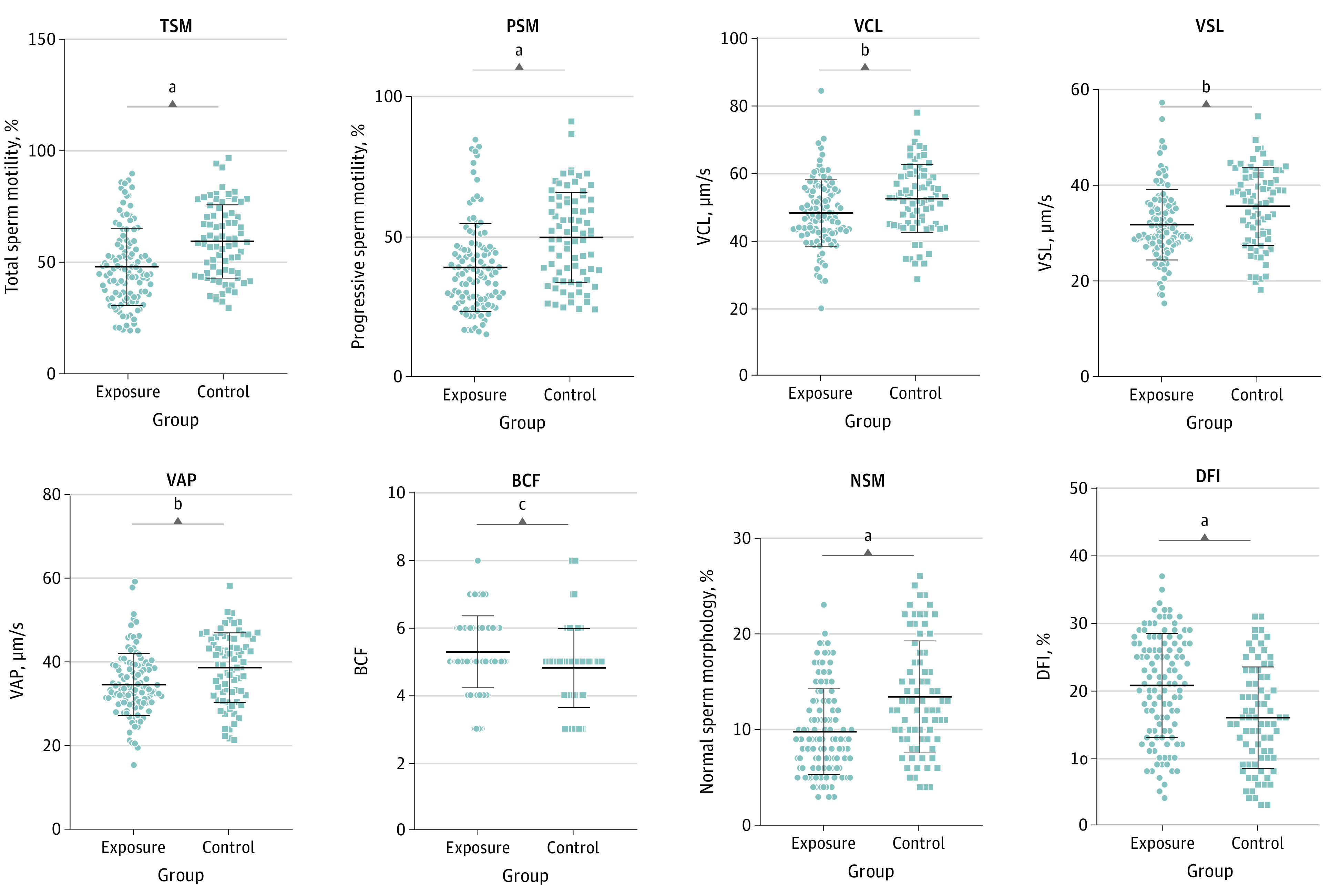
The center line is the mean (for curvilinear velocity [VCL], straight line velocity [VSL], average path velocity [VAP], beat cross frequency [BCF], and DNA fragmentation index [DFI]) or median (for total sperm motility [TSM], progressive sperm motility [PSM], and normal sperm morphology [NSM]), with outer lines indicating SD (for VCL, VSL, VAP, BCF, and DFI) or IQR (for TSM, PSM, and NSM). Each marker represents an individual’s data.
aP < .001.
bP < .005.
cP < .01.
Kinematic Sperm Parameters
Comparing men with vs without FA exposure, mean (SD) VCL (48.02 [9.89] μm/s vs 53.19 [9.77] μm/s; P = .004), VSL (31.62 [7.33] μm/s vs 34.99 [7.85] μm/s; P = .001), and VAP (34.52 [7.38] μm/s vs 39.22 [9.60] μm/s; P = .001) were lower; however, BCF (5.25 [0.97] vs 4.85 [1.09]; P = .009) was higher in the FA exposure group vs the control group (Figure 1). MAD, linearity, straightness, wobble, and ALH showed no changes between the exposure and control groups (eFigure 5 in the Supplement).
Sperm Morphology and DFI
The median (IQR) value of normal sperm morphology (NSM) in the FA exposure group and control group was 9.79% (6.00%-13.00%) and 13.41% (9.00%-17.75%), respectively, indicating that lower NSM was associated with exposure to FA (P < .001) (Figure 1). On the contrary, the mean value of DFI in FA exposure group and control group was 20.76% (7.75%) and 15.97% (7.53%), respectively, suggesting that FA is associated with increased DFI (P < .001) (Figure 1).
Biochemistry Parameters in Seminal Plasma
In the FA exposure group, zinc was lower than in the control group (eFigure 5 in the Supplement). ACP, NG, and fructose did not indicate any significant differences (eFigure 5 in the Supplement).
Association Between FEI and Semen Quality
Association Between FEI and Conventional Sperm Parameters
Figure 2 and Table 2 summarize the results of linear regression. The FEI was negatively associated with TSM and PSM, meaning that a change of −0.99% (95% CI, −1.00% to −0.98%; P <.001) in TSM and −0.99% (95% CI, −0.99% to −0.97%; P <.001) in PSM were associated with a 1-unit increase in FEI. SV, SC, and TSC had no statistically significant association with FEI (Table 2). In addition, after adjusting for confounding factors, including age, smoking, alcohol consumption, body mass index, and income, the associations between FEI and conventional sperm parameters showed the same trends (Table 2; eFigure 3 in the Supplement).
Figure 2. Associations of Formaldehyde Exposure Index (FEI) With Semen Quality.
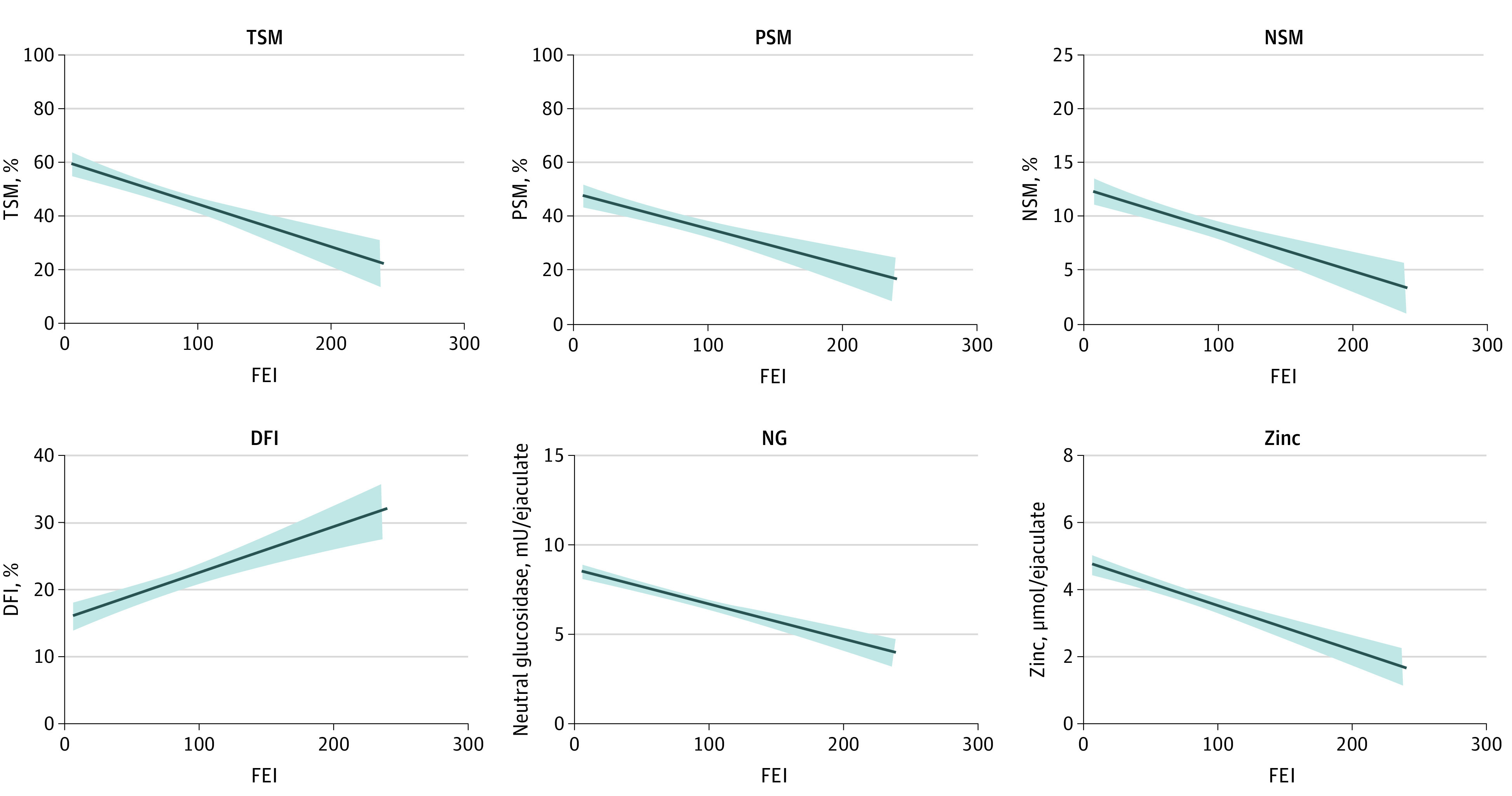
Solid curves indicate the effect estimate; shaded areas, 95% CIs; DFI, DNA fragmentation index; NG, neutral glucosidase; NSM, normal sperm morphology; PSM, progressive sperm motility; and TSM, total sperm motility.
Table 2. Associations of FEI With Semen Quality and Biochemistry Parameters of Seminal Plasma Before and After Adjusting for Confounding Factorsa.
| Parameter | All | |||
|---|---|---|---|---|
| Crude | Adjusted | |||
| Effect estimate, % (95% CI)b | P value | Effect estimate, % (95% CI)b | P value | |
| SV | −0.56 (−0.90 to 0.89) | .26 | −0.45 (−0.87 to 1.42) | .42 |
| SC | −0.11 (−0.69 to 1.58) | .81 | −0.01 (−0.66 to 1.91) | .98 |
| TSC | −0.34 (−0.74 to 0.65) | .36 | −0.22 (−0.69 to 1.00) | .60 |
| TSM | −0.99 (−1.00 to −0.98) | <.001c | −0.99 (−1.00 to −0.98) | <.001c |
| PSM | −0.99 (−0.99 to −0.97) | <.001c | −0.99 (−0.99 to −0.98) | <.001c |
| VCL | −0.05 (−0.08 to −0.02) | <.001c | −0.05 (−0.08 to −0.02) | <.001c |
| VSL | −0.07 (−0.10 to −0.04) | <.001c | −0.08 (−0.11 to −0.04) | <.001c |
| VAP | −0.07 (−0.10 to −0.04) | <.001c | −0.08 (−0.11 to −0.04) | <.001c |
| MAD | 0.01 (−0.02 to 0.05) | .47 | 0.02 (−0.01 to 0.05) | .27 |
| Linearity | −0.02 (−0.06 to 0.00) | .09 | −0.03 (−0.07 to 0.00) | .05 |
| Straightness | −0.06 (−0.12 to 0.00) | .09 | −0.07 (−0.13 to 0.00) | .05 |
| Wobble | −0.03 (−0.07 to 0.00) | .10 | 0.00 (−0.08 to 0.00) | .05 |
| ALH | −0.06 (−0.28 to 0.22) | .62 | −0.06 (−0.28 to 0.21) | .60 |
| BCF | 0.52 (0.15 to 1.02) | .003c | 0.57 (0.18 to 1.08) | .002c |
| NSM | −0.98 (−0.99 to −0.93) | <.001c | −0.98 (−0.99 to −0.93) | <.001c |
| DFI | 0.10 (0.06 to 0.14) | <.001c | 0.10 (0.06 to 0.14) | <.001c |
| ACP | 0.00 (−0.01 to 0.01) | .71 | 0.00 (−0.01 to 0.01) | .72 |
| NG | −0.24 (−0.35 to −0.11) | .001c | −0.24 (−0.35 to −0.10) | .001c |
| Fructose | 0.19 (−0.15 to 0.69) | .30 | 0.27 (−0.09 to 0.79) | .16 |
| Zinc | −0.61 (−0.66 to −0.56) | <.001c | −0.61 (−0.65 to −0.56) | <.001c |
Abbreviations: ACP, acid phosphatase; ALH, amplitude of lateral head displacement; BCF, beat cross frequency; DFI, DNA fragmentation index; FEI, formaldehyde exposure index; MAD, mean angular displacement; NG, neutral glucosidase; NSM, normal sperm morphology; PSM, progressive sperm motility; SC, sperm concentration; SV, semen volume; TSC, total sperm count; TSM, total sperm motility; VAP, average path velocity; VCL, curvilinear velocity; VSL, straight line velocity.
Confounding factors used in the adjusted model contained age, body mass index, income, smoking status, and alcohol consumption.
Effect estimates represent percentage changes in association with a 1-unit increase in FEI.
P < .05.
Association Between FEI and Kinematic Sperm Parameters
As shown in eFigure 6 in the Supplement and Table 2, FEI was significantly inversely associated with VCL, VSL, and VAP and positively associated with BCF. Specifically, a 1-unit increase in FEI was associated with a change of −0.05% (95% CI, −0.08% to −0.02%; P < .001) in VCL, −0.07% (95% CI, −0.10% to −0.04%; P < .001) in VSL, −0.07% (95% CI, −0.10% to −0.04%; P < .001) in VAP, and 0.52% (95% CI, 0.15% to 1.02%; P = .003) in BCF. However, MAD, linearity, straightness, wobble, and ALH had no significant association with FEI (Table 2). Additionally, the associations with the same trends between FEI and kinematic sperm parameters were observed after adjusting for confounding factors (Table 2; eFigure 3 in the Supplement).
Association Between FEI and Sperm Morphology and DFI
We found a negative association between FEI and NSM and a positive association between FEI and DFI (Figure 2 and Table 2). Specifically, a 1-unit increase in FEI was associated with a change of −0.98% (95% CI, −0.99% to −0.93%; P < .001) in NSM and an increase of 0.10% (95% CI, 0.06% to 0.14%; P < .001) in DFI. After adjusting for confounding factors, the 2 associations remained significant (Table 2; eFigure 3 in the Supplement).
Association Between FEI and Biochemistry Parameters in Seminal Plasma
As shown in Figure 2 and Table 2, NG and zinc were negatively associated with FEI. Specifically, a 1-unit increase in FEI was associated with a change of −0.24% (95% CI, −0.35% to −0.11%; P = .001) in NG and −0.61% (95% CI, −0.66% to −0.56%; P < .001) in zinc. However, ACP and fructose were not associated with FEI (Table 2). After adjusting for confounding factors, similar associations with narrower 95% CIs were observed for biochemistry parameters (Table 2; eFigure 3 in the Supplement).
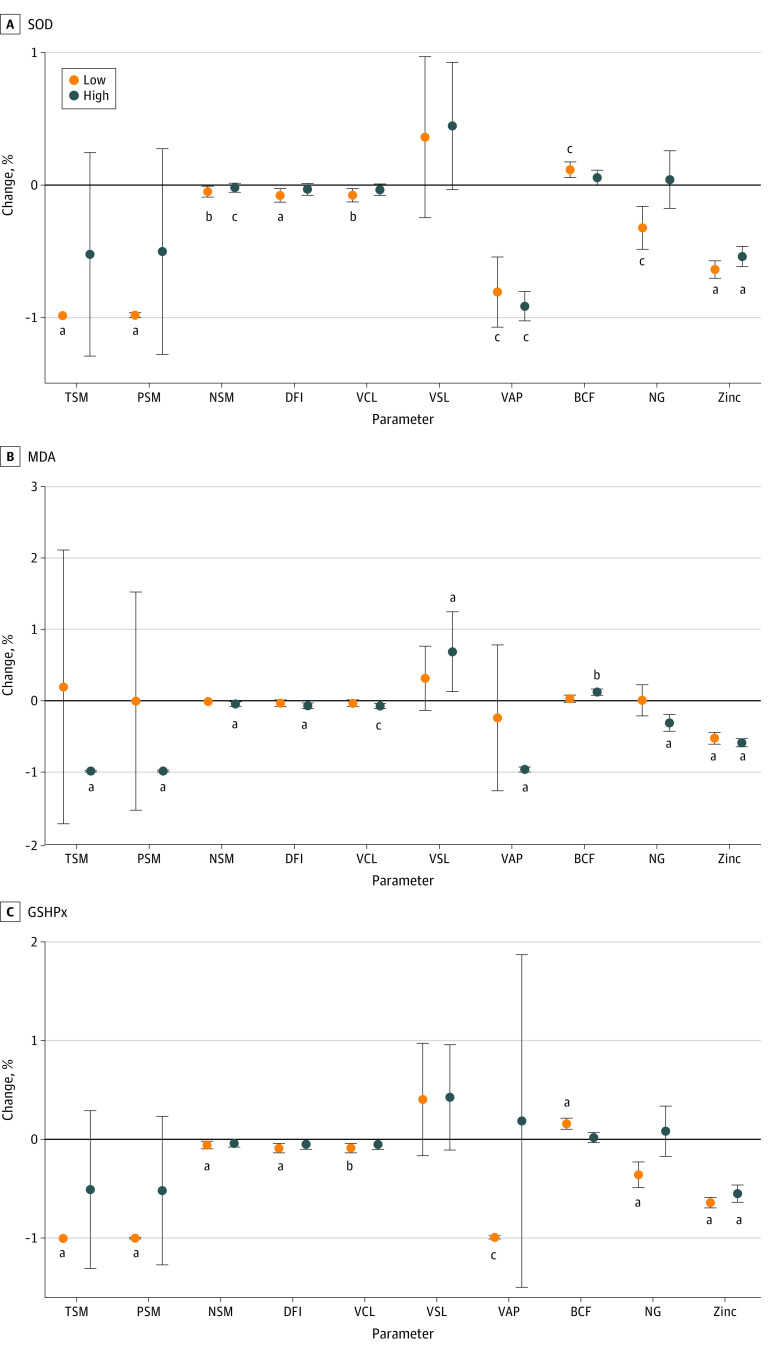
Subgroup Analyses of Semen Quality Based on Different Levels of Antioxidant Defenses
We further divided the men with FA exposures into groups based on antioxidant defenses. They were divided into low and high categories based on the median level of SOD (134.87 U/ml), MDA (6.49 nmol/ml), and GSHPx (54.44 U/ml).
Subgroup Analyses of Semen Quality Based on Different Levels of SOD
In the subgroup analysis of SOD levels, the associations between semen quality and FEI remained significant in the group with low levels of SOD but not in the group with high levels (Figure 3; eTable 1 in the Supplement). Specifically, the associations between the FEI and some measures of semen quality were significant in the subgroup with low SOD (TSM: percentage change, −0.99%; 95% CI, −1.00% to −0.97%; P < .001; PSM: percentage change, −0.99%; 95% CI, −1.00% to −0.96%; P < .001; VCL: percentage change, −0.05%; 95% CI, −0.09% to −0.01%; P = .009; VSL: percentage change, −0.08%; 95% CI, −0.13% to −0.03; P = .003; VAP: percentage change, −0.08%; 95% CI, −0.13% to −0.03%; P = .002; NG: percentage change, −0.34%; 95% CI, −0.48% to −0.16%; P = .001; DFI: percentage change, 0.10%; 95% CI, 0.05% to 0.16%; P < .001). NSM and zinc had statistically significant negative associations in both groups (NSM, low level of SOD: percentage change, −0.93%; 95% CI, −0.99% to −0.50%; P = .009; NSM, high level of SOD: percentage change, −0.97%; 95% CI, −0.99% to −0.79%; P = .001; zinc, low level of SOD: percentage change, −0.64%; 95% CI, −0.70% to −0.57%; P < .001; zinc, high level of SOD: percentage change, −0.54%; 95% CI, −0.62% to −0.46%; P < .001). BCF had no association in either group for SOD (eFigure 4 in the Supplement).
Figure 3. Percentage Changes in Semen Quality Associated With a 1-Unit Increase in Formaldehyde Exposure Index in Different Level of Antioxidant Defenses.
Dots indicate the percentage changes associated with a 1-unit increase in the formaldehyde exposure index; error bars, 95% CIs; BCF, beat cross frequency; DFI, DNA fragmentation index; GSHPx, glutathione peroxidase; MDA, malondialdehyde; NSM, normal sperm morphology; NG, neutral glucosidase; PSM, progressive sperm motility; SOD, superoxide dismutase; TSM, total sperm motility; VAP, average path velocity; VCL, curvilinear velocity; and VSL, straight line velocity.
aP < .001.
bP < .01.
cP < .005.
Subgroup Analyses of Semen Quality Based on Different Levels of MDA
Contrary to the subgroup analysis of SOD, nearly all the parameters mentioned previously were associated with FEI in the group with higher levels of MDA but not in the group with lower levels (Figure 3; eTable 2 in the Supplement). In particular, the negative associations in TSM (percentage change, −0.99%; 95% CI, −0.99% to −0.97%; P < .001), PSM (percentage change, −0.99%; 95% CI, −0.99% to −0.97%; P < .001), VCL (percentage change, −0.05%; 95% CI, −0.08% to −0.02%; P = .002), VSL (percentage change, −0.07%; 95% CI, −0.11% to −0.03%; P < .001), VAP (percentage change, −0.08%; 95% CI, −0.11% to −0.04%; P < .001), NSM (percentage change, −0.98%; 95% CI, −0.99% to −0.92%; P < .001), and NG (percentage change, −0.32%; 95% CI, −0.42% to −0.19%; P < .001) as well as the positive associations with DFI (percentage change, 0.11%; 95% CI, 0.07% to 0.15%; P < .001) and BCF (percentage change, 0.61%; 95% CI, 0.15% to 1.26%; P = .006) remained statistically significant in the group with higher levels but not in the group with lower levels. Zinc showed converse associations in both low and high groups of MDA (low level of MDA: percentage change, −0.53%; 95% CI, −0.61% to −0.44%; P < .001; high level of MDA: percentage change, −0.59%; 95% CI, −0.64% to −0.53%; P < .001) (eFigure 4 in the Supplement).
Subgroup Analyses of Semen Quality Based on Different Levels of GSHPx
Similar to the subgroup analysis of SOD, most parameters mentioned previously had significant associations with FEI in the group with low levels of GSHPx but not in the group with high levels (Figure 3; eTable 3 in the Supplement). The negative associations with TSM (percentage change, −0.99%; 95% CI, −0.99% to −0.98%; P < .001), PSM (percentage change, −0.99%; 95% CI, −0.99% to −0.98%; P < .001), VCL (percentage change, −0.05%; 95% CI, −0.09% to −0.01%; P = .007), VSL (percentage change, −0.08%; 95% CI, −0.13% to −0.03%; P = .001), VAP (percentage change, −0.08%; 95% CI, −0.13% to −0.03%; P = .001), NSM (percentage change, −0.99%; 95% CI, −0.99% to −0.96%; P < .001), and NG (percentage change, −0.36%; 95% CI, −0.47% to −0.22%; P < .001) as well as the positive association with DFI (percentage change, 0.16%; 95% CI, 0.10% to 0.21%; P < .001) were statistically significant in the group with low levels of GSHPx but not in the group with high levels. Zinc showed statistically negative associations in both groups; however, BCF was not associated with FEI in either group (low level of GSHPx: percentage change, −0.63%; 95% CI, −0.68% to −0.58%; P < .001; high level of GSHPx: percentage change, −0.55%; 95% CI, −0.62% to −0.45%; P < .001) (eFigure 4 in the Supplement). The results in further subgroup analyses adjusting for confounding factors were almost unchanged (eFigure 4, eTable 1, eTable 2, and eTable 3 in the Supplement).
Sensitivity Analyses and Stratified Analyses
Sensitivity analyses showed that results of the unadjusted analysis (smoking and alcohol consumption) were generally similar to those of adjusted analysis, although effect estimates became relatively larger (eTable 4 in the Supplement). Stratified analyses indicated stronger associations between FEI and BCF in men younger than 30 years or with a BMI lower than 24. However, these differences in estimated effects were generally nonsignificant (ie, had overlapping 95% CIs). Associations between FEI and other semen quality parameters in different stratified groups were generally similar to those in overall groups (eTable 5 in the Supplement).
Discussion
The present study assessed the associations between FA exposure and semen quality using 20 semen parameters, which we believe to be the most comprehensive evaluation system. Our results indicated that a long-term occupational exposure to FA was associated with a serious deterioration in sperm motility, morphology, and DNA integrity. Specifically, an increase in FEI was associated with decreases in TSM, PSM, VCL, VSL, VAP, NSM, and seminal plasma zinc as well as increases in BCF and DFI before and after adjusting for confounding factors, indicating that the associations were dose and time dependent. Furthermore, oxidative stress in semen seemed to enhance the association of FA exposure with semen quality, suggesting that the negative associations of occupational exposure to FA with semen quality might be induced by oxidative stress. To our knowledge, this is the first population-based cohort study to explore the potential mechanism of the associations between FA exposure and semen quality.
Studies on the associations between FA exposure and semen quality have been limited, and findings have remained inconsistent. In Finland, FA exposure was not associated with changes in sperm count and morphology.21 However, in China, our previous study22 found that FA exposure in males was associated with decreased sperm motility. The differences might result from racial and ethnic differences, representing various gene phenotypes and lifestyles that may affect semen quality.33 The present findings were coincident with our previous results22; the participants we recruited in this study had similar ethnicity and residence and came from the similar population. In addition, our previous study evaluated the semen quality with 5 conventional and kinematic parameters; however, these parameters were insufficient given that sperm morphology and sperm DNA integrity, essential assessors of semen quality and fertilization potential, were not added in the study. Our present study made up for this deficiency and found that sperm morphology and sperm DNA integrity were also associated with occupational FA exposure.
The present findings, ie, long-term exposure to FA was associated with significant reductions in sperm motility, morphology, and DFI, were in agreement with animal studies.34,35,36,37,38 Significantly, nearly all the animal studies reported that FA exposure could decrease sperm number34,35,36,37,38; however, similar declines were not observed in our study nor in the study by Wang et al.22 The differences might result from different doses and time of exposure. Animals were exposed to a relative high dose (10 mg/m3) for a short period (<35 d),36 while humans were exposed to a relatively low dose (0.22-5.43 mg/m3) for a longer period (>24 months). Moreover, a histological study of testis in animals revealed destruction of germinal epithelium, spermatogenic arrest, vacuolization of the tubules, and degeneration of Leydig cells.34,36,37 The disorder in spermatogenesis might be the source of weak quality in sperm.
Our study further explored the possible mechanism of the association of FA exposure with poor semen quality. Previous studies indicated that exposure to FA might increase the level of oxidative stress, resulting in the tissue damage and inflammation.39 In addition, some environmental pollutants and viruses induce poor semen quality through increasing levels of oxidative stress.40,41,42 Additionally, antioxidant treatment has been shown to have a positive effect on semen quality.43,44 Moreover, several antioxidant or oxidant compounds were found to alleviate oxidative damage in sperm caused by FA exposure.34,37 SOD and GSHPx are antioxidant enzymes protecting cells against oxidative damage, and MDA is a oxidation parameter measuring lipid peroxidation.45 It has been reported that FA exposure significantly decreases the levels of SOD and GSHPx, while it increased the level of MDA in the testicular tissue, suggesting that FA is associated with testicular oxidative stress and impaired testicular tissue in rodents.46 This result is consistent with our findings. Our study, to our knowledge, was the first population-based cohort study to investigate the association of oxidative stress with the association between FA exposure and semen quality. We found that in individuals with low SOD, high MDA, or low GSHPx, which indicate high levels of oxidative stress, FEI was negatively associated with semen quality; otherwise, in individuals with low levels of oxidative stress, no association was observed. This finding suggests that associations between FA exposure and poor semen quality might be induced by oxidative stress. Further studies are expected to add an intervention group receiving antioxidants.
Strengths and Limitations
The present study had several novel strengths. First, our research formulated strict inclusion and exclusion criteria to eliminate the selection bias coming from volunteers who had insufficient exposure time (<24 months); who lived in residences with high levels of indoor FA, such as a newly built or remodeled apartment; and who showed vast differences in educational levels and socioeconomic status as control group. These individuals were excluded given potential selection bias.22 Second, to our knowledge, our study used the most comprehensive set of 20 semen parameters, including 5 conventional sperm parameters (SV, SC, TSC, TSM, and PSM), 9 kinematic sperm parameters (VCL, VSL, VAP, MAD, linearity, straightness, wobble, ALH, and BCF), sperm morphology, DNA fragmentation index, and 4 biochemistry parameters of seminal plasma (ACP, NG, fructose, and zinc), to examine the associations between long-term occupational FA exposure and male reproductive health. Third, the present study used precise exposure monitoring equipment and a scientific exposure method to obtain accurate exposure data for participants. In addition, exposure durations were added in our analysis to calculate FEI according to both exposure duration and concentration. This technique was more precise in reflecting the true occupational FA exposure level. Fourth, our study was the first population-based cohort study to explore the possible mechanism of inverse associations between FA exposure and semen quality, and our result verified that oxidative stress in semen could promote the negative effect of FA occupational exposure on semen quality, which had been reported in animal studies.34,37
Although strict and rigorous methods were used to reduce bias, our study also had an unavoidable limitation. Only the concentration of FA in the workplace was measured and analyzed in our study. However, the background level of each individual was not measured. We recruited exposure and control groups from the same living place to alleviate these potential sources of bias. The varying metabolic capacity of FA was another factor contributing to the different background levels. The same concentration of FA in air could have various impacts in different participants. More precise indicators, such as formic acid in urine, a formaldehyde metabolite, should be analyzed in further studies to understand the association between FA in body and semen quality. In addition, the present study was conducted in a single region with a limited sample size, which may contribute to selection bias. This limited population may affect the generalizability of the study results. Further nationally retrospective cohort studies with larger sample size are in needed.
Conclusions
In this study, long-term occupational exposure to FA was negatively associated with semen quality, especially with sperm motility, morphology, and DNA integrity. In addition, our results indicated that the negative associations were dose and time dependent. Moreover, the associations of FA occupational exposure with poor semen quality might be induced by oxidative stress. The present study strengthened the molecular epidemiological understanding of FA exposure and semen quality and increased the objective evidence of the negative association of FA exposure with male reproduction.
eTable 1. Associations of FEI With Semen Quality and Biochemistry Parameters of Seminal Plasma in Different Level of SOD Before and After Adjusting for Confounding Factors
eTable 2. Associations of FEI With Semen Quality and Biochemistry Parameters of Seminal Plasma in Different Level of MDA Before and After Adjusting for Confounding Factors
eTable 3. Associations of FEI With Semen Quality and Biochemistry Parameters of Seminal Plasma in Different Level of GSHPx Before and After Adjusting for Confounding Factors
eTable 4. Effect Estimates and 95% CIs of Semen Quality Parameters With a 1-Unit Increase in FEI With and Without Adjustment for Smoking and Alcohol Consumption
eTable 5. Effect Estimates and 95% CIs of Semen Quality Parameters Associated With a 1-Unit Increase in FEI in Each Subgroup
eFigure 1. Flowchart of Participant Recruitment
eFigure 2. Distribution of Sociodemographic Characteristics With and Without FA Exposure
eFigure 3. Percentage Changes in Semen Quality Associated With a 1-Unit Increase in FEI Before and After Adjusting for Confounding Factors
eFigure 4. Heatmap of the Associations Between FEI and Semen Quality in Different Groups of Antioxidant Defenses Before and After Adjusting for Confounding Factors
eFigure 5. Distribution of Semen Quality With and Without Occupational FA Exposure
eFigure 6. Significant Associations of FEI With Kinematic Sperm Parameters
References
- 1.Vahidi S, Ardalan A, Mohammad K. Prevalence of primary infertility in the Islamic Republic of Iran in 2004-2005. Asia Pac J Public Health. 2009;21(3):287-293. doi: 10.1177/1010539509336009 [DOI] [PubMed] [Google Scholar]
- 2.Wilkes S, Chinn DJ, Murdoch A, Rubin G. Epidemiology and management of infertility: a population-based study in UK primary care. Fam Pract. 2009;26(4):269-274. doi: 10.1093/fampra/cmp029 [DOI] [PubMed] [Google Scholar]
- 3.Irvine DS. Epidemiology and aetiology of male infertility. Hum Reprod. 1998;13(suppl 1):33-44. doi: 10.1093/humrep/13.suppl_1.33 [DOI] [PubMed] [Google Scholar]
- 4.Levine H, Jørgensen N, Martino-Andrade A, et al. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update. 2017;23(6):646-659. doi: 10.1093/humupd/dmx022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sengupta P, Nwagha U, Dutta S, Krajewska-Kulak E, Izuka E. Evidence for decreasing sperm count in African population from 1965 to 2015. Afr Health Sci. 2017;17(2):418-427. doi: 10.4314/ahs.v17i2.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sengupta P, Dutta S, Krajewska-Kulak E. The disappearing sperms: analysis of reports published between 1980 and 2015. Am J Mens Health. 2017;11(4):1279-1304. doi: 10.1177/1557988316643383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lv MQ, Ge P, Zhang J, Yang YQ, Zhou L, Zhou DX. Temporal trends in semen concentration and count among 327 373 Chinese healthy men from 1981 to 2019: a systematic review. Hum Reprod. 2021;36(7):1751-1775. doi: 10.1093/humrep/deab124 [DOI] [PubMed] [Google Scholar]
- 8.Rahban R, Priskorn L, Senn A, et al. ; NICER Working Group . Semen quality of young men in Switzerland: a nationwide cross-sectional population-based study. Andrology. 2019;7(6):818-826. doi: 10.1111/andr.12645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mima M, Greenwald D, Ohlander S. Environmental toxins and male fertility. Curr Urol Rep. 2018;19(7):50. doi: 10.1007/s11934-018-0804-1 [DOI] [PubMed] [Google Scholar]
- 10.Nadalutti CA, Prasad R, Wilson SH. Perspectives on formaldehyde dysregulation: mitochondrial DNA damage and repair in mammalian cells. DNA Repair (Amst). 2021;105:103134. doi: 10.1016/j.dnarep.2021.103134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soltanpour Z, Mohammadian Y, Fakhri Y. The exposure to formaldehyde in industries and health care centers: A systematic review and probabilistic health risk assessment. Environ Res. 2022;204(Pt B):112094. doi: 10.1016/j.envres.2021.112094 [DOI] [PubMed] [Google Scholar]
- 12.Golalipour MJ, Azarhoush R, Ghafari S, Gharravi AM, Fazeli SA, Davarian A. Formaldehyde exposure induces histopathological and morphometric changes in the rat testis. Folia Morphol (Warsz). 2007;66(3):167-171. [PubMed] [Google Scholar]
- 13.Ozen OA, Akpolat N, Songur A, et al. Effect of formaldehyde inhalation on Hsp70 in seminiferous tubules of rat testes: an immunohistochemical study. Toxicol Ind Health. 2005;21(10):249-254. doi: 10.1191/0748233705th235oa [DOI] [PubMed] [Google Scholar]
- 14.Zeng CE, Peng X, Qiao Y. Reproductive toxicity of formaldehyde to male mice. Article in Chinese. Chinese J Hum Sexuality. 2003;12:1-4. [Google Scholar]
- 15.Ge P, Zhang X, Yang YQ, et al. Rno_circRNA_016194 might be involved in the testicular injury induced by long-term formaldehyde exposure via rno-miR-449a-5p mediated Atg4b activation. Food Chem Toxicol. 2021;155:112409. doi: 10.1016/j.fct.2021.112409 [DOI] [PubMed] [Google Scholar]
- 16.Ge P, Zhang X, Yang YQ, Lv MQ, Zhou DX. Long-term exposure to formaldehyde induced down-regulation of SPO11 in rats. Inhal Toxicol. 2021;33(1):8-17. doi: 10.1080/08958378.2020.1859652 [DOI] [PubMed] [Google Scholar]
- 17.Han SP, Zhou DX, Lin P, et al. Formaldehyde exposure induces autophagy in testicular tissues of adult male rats. Environ Toxicol. 2015;30(3):323-331. doi: 10.1002/tox.21910 [DOI] [PubMed] [Google Scholar]
- 18.Zhou DX, Qiu SD, Zhang J, Wang ZY. Reproductive toxicity of formaldehyde to adult male rats and the functional mechanism concerned. Article in Chinese. Sichuan Da Xue Xue Bao Yi Xue Ban. 2006;37(4):566-569. [PubMed] [Google Scholar]
- 19.Majumder PK, Kumar VL. Inhibitory effects of formaldehyde on the reproductive system of male rats. Indian J Physiol Pharmacol. 1995;39(1):80-82. [PubMed] [Google Scholar]
- 20.Xie Y, Yi Y, Tang M. Toxicity of formaldehyde on germ cells of male mice. Article in Chinese. J Environ Health. 2003;20:84-85. [Google Scholar]
- 21.Ward JB Jr, Hokanson JA, Smith ER, et al. Sperm count, morphology and fluorescent body frequency in autopsy service workers exposed to formaldehyde. Mutat Res. 1984;130(6):417-424. doi: 10.1016/0165-1161(84)90014-1 [DOI] [PubMed] [Google Scholar]
- 22.Wang HX, Li HC, Lv MQ, et al. Associations between occupation exposure to formaldehyde and semen quality, a primary study. Sci Rep. 2015;5:15874. doi: 10.1038/srep15874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang X, Bai Y, Duong A, Smith MT, Li L, Zhang L. Formaldehyde in China: production, consumption, exposure levels, and health effects. Environ Int. 2009;35(8):1210-1224. doi: 10.1016/j.envint.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 24.Tian GL, Wang YB. The development status and trend of formaldehyde industry in China. Chem Industry. 2018;36(05):19-22, 44. [Google Scholar]
- 25.Huang S, Xiong J, Zhang Y. Impact of temperature on the ratio of initial emittable concentration to total concentration for formaldehyde in building materials: theoretical correlation and validation. Environ Sci Technol. 2015;49(3):1537-1544. doi: 10.1021/es5051875 [DOI] [PubMed] [Google Scholar]
- 26.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 27.Wang HX, Zhou DX, Zheng LR, et al. Effects of paternal occupation exposure to formaldehyde on reproductive outcomes. J Occup Environ Med. 2012;54(5):518-524. doi: 10.1097/JOM.0b013e31824e6937 [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. WHO Press; 2010. [Google Scholar]
- 29.Evenson D, Jost L. Sperm chromatin structure assay is useful for fertility assessment. Methods Cell Sci. 2000;22(2-3):169-189. doi: 10.1023/a:1009844109023 [DOI] [PubMed] [Google Scholar]
- 30.De Brucker S, Drakopoulos P, Dhooghe E, et al. The effect of cigarette smoking on the semen parameters of infertile men. Gynecol Endocrinol. 2020;36(12):1127-1130. doi: 10.1080/09513590.2020.1775195 [DOI] [PubMed] [Google Scholar]
- 31.Sharma R, Harlev A, Agarwal A, Esteves SC. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur Urol. 2016;70(4):635-645. doi: 10.1016/j.eururo.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 32.Ricci E, Al Beitawi S, Cipriani S, et al. Semen quality and alcohol intake: a systematic review and meta-analysis. Reprod Biomed Online. 2017;34(1):38-47. doi: 10.1016/j.rbmo.2016.09.012 [DOI] [PubMed] [Google Scholar]
- 33.Huang C, Liu Q, Wang ZW, et al. Sperm donor lifestyle survey: modifiable risk factors for potential sperm donors. J Assist Reprod Genet. 2021;38(11):2965-2974. doi: 10.1007/s10815-021-02322-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zare M, Haghpanah T, Shekari MA, Eftekhar-Vaghefi SH. The prophylactic effect of date palm (Phoenix dactylifera L.) fruit extract on testicular toxicity induced by formaldehyde: an experimental study. Int J Reprod Biomed. 2020;18(4):275-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Afrigan L, Jafari Anarkooli I, Sohrabi D, et al. The effect of hydroethanolic extract of Matricaria chamomilla on the reproductive system of male rats exposed to formaldehyde. Andrologia. 2019;51(9):e13362. doi: 10.1111/and.13362 [DOI] [PubMed] [Google Scholar]
- 36.Vosoughi S, Khavanin A, Salehnia M, Asilian Mahabadi H, Shahverdi A, Esmaeili V. Adverse effects of formaldehyde vapor on mouse sperm parameters and testicular tissue. Int J Fertil Steril. 2013;6(4):250-267. [PMC free article] [PubMed] [Google Scholar]
- 37.Naghdi M, Maghbool M, Seifalah-Zade M, et al. Effects of common fig (Ficus carica) leaf extracts on sperm parameters and testis of mice intoxicated with formaldehyde. Evid Based Complement Alternat Med. 2016;2016:2539127. doi: 10.1155/2016/2539127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Betancourt-Martínez ND, Jiménez-Villarreal J, Carranza-Rosales P, et al. Sperm chromatin dispersion by formaldehyde in Wistar rats. Genet Mol Res. 2015;14(3):10816-10826. doi: 10.4238/2015.September.9.20 [DOI] [PubMed] [Google Scholar]
- 39.Lima LF, Murta GL, Bandeira AC, Nardeli CR, Lima WG, Bezerra FS. Short-term exposure to formaldehyde promotes oxidative damage and inflammation in the trachea and diaphragm muscle of adult rats. Ann Anat. 2015;202:45-51. doi: 10.1016/j.aanat.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Bai L, Li H, Yang W, Li M. Protective effects of Lepidium draba L. leaves extract on testis histopathology, oxidative stress indicators, serum reproductive hormones and inflammatory signalling in oxymetholone-treated rat. Andrologia. 2021;53(11):e14239. doi: 10.1111/and.14239 [DOI] [PubMed] [Google Scholar]
- 41.Hosseinzadeh A, Mehrzadi S, Siahpoosh A, Basir Z, Bahrami N, Goudarzi M. The ameliorative effect of ellagic acid on di-(2-ethylhexyl) phthalate-induced testicular structural alterations, oxidative stress, inflammation and sperm damages in adult mice. Reprod Biol Endocrinol. 2021;19(1):146. doi: 10.1186/s12958-021-00830-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pérez-Soto E, Fernández-Martínez E, Oros-Pantoja R, Medel-Flores O, Miranda-Covarrubias JC, Sánchez-Monroy V. Proinflammatory and oxidative stress states induced by human papillomavirus and Chlamydia trachomatis coinfection affect sperm quality in asymptomatic infertile men. Medicina (Kaunas). 2021;57(9):862. doi: 10.3390/medicina57090862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yaris M, Akdogan N, Öztürk M, Bozkurt A, Karabakan M. The effects of two different antioxidant combinations on sperm parameters. Urologia. Published online October 7, 2021. doi: 10.1177/03915603211049888 [DOI] [PubMed] [Google Scholar]
- 44.Pereira SC, Oliveira PF, Oliveira SR, Pereira ML, Alves MG. Impact of environmental and lifestyle use of chromium on male fertility: focus on antioxidant activity and oxidative stress. Antioxidants (Basel). 2021;10(9):1365. doi: 10.3390/antiox10091365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paul M, Sohag MSU, Khan A, Barman RK, Wahed MII, Khan MRI. Pumpkin (Cucurbita maxima) seeds protect against formaldehyde-induced major organ damages. Heliyon. 2020;6(8):e04587. doi: 10.1016/j.heliyon.2020.e04587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ozen OA, Kus MA, Kus I, Alkoc OA, Songur A. Protective effects of melatonin against formaldehyde-induced oxidative damage and apoptosis in rat testes: an immunohistochemical and biochemical study. Syst Biol Reprod Med. 2008;54(4-5):169-176. doi: 10.1080/19396360802422402 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Associations of FEI With Semen Quality and Biochemistry Parameters of Seminal Plasma in Different Level of SOD Before and After Adjusting for Confounding Factors
eTable 2. Associations of FEI With Semen Quality and Biochemistry Parameters of Seminal Plasma in Different Level of MDA Before and After Adjusting for Confounding Factors
eTable 3. Associations of FEI With Semen Quality and Biochemistry Parameters of Seminal Plasma in Different Level of GSHPx Before and After Adjusting for Confounding Factors
eTable 4. Effect Estimates and 95% CIs of Semen Quality Parameters With a 1-Unit Increase in FEI With and Without Adjustment for Smoking and Alcohol Consumption
eTable 5. Effect Estimates and 95% CIs of Semen Quality Parameters Associated With a 1-Unit Increase in FEI in Each Subgroup
eFigure 1. Flowchart of Participant Recruitment
eFigure 2. Distribution of Sociodemographic Characteristics With and Without FA Exposure
eFigure 3. Percentage Changes in Semen Quality Associated With a 1-Unit Increase in FEI Before and After Adjusting for Confounding Factors
eFigure 4. Heatmap of the Associations Between FEI and Semen Quality in Different Groups of Antioxidant Defenses Before and After Adjusting for Confounding Factors
eFigure 5. Distribution of Semen Quality With and Without Occupational FA Exposure
eFigure 6. Significant Associations of FEI With Kinematic Sperm Parameters