Abstract
Objectives
To investigate differences in workplace exposure, demographic and clinical findings in engineered stone (ES) workers from a multinational consortium using the Engineered Stone Silicosis Investigators (ESSI) Global Silicosis Registry.
Methods
With ethics board approval in Israel, Spain, Australia and the USA, ES workers ages 18+ with a physician diagnosis of work-related silicosis were enrolled. Demographic, occupational, radiologic, pulmonary function and silica-related comorbidity data were compared cross-sectionally among countries using analysis of variance, Fisher’s exact tests and logistic regression.
Results
Among 169 ES workers with silicosis, most were men, with mean age 51.7 (±11.4) years. Mean work tenure in stone fabrication or masonry was 19.9 (±9.8) years. Different methods of case ascertainment explained some inter-country differences, for example, workers in Queensland, Australia with a state-based surveillance program were likely to be identified earlier and with shorter work tenure. Overall, 32.5% of workers had progressive massive fibrosis, the most severe form of dust-related pneumoconiosis, of whom 18.5% reported ≤10 years of work tenure. Lung function impairment including restriction, reduced diffusion capacity and hypoxaemia was common, as was autoimmunity.
Conclusions
Findings from a multinational registry represent a unique effort to compare demographic, exposure and clinical information from ES workers with silicosis, and suggest a substantial emerging population of workers worldwide with severe and irreversible silica-associated diseases. This younger worker population is at high risk for disease progression, multiple comorbidities and severe disability. The ESSI registry provides an ongoing framework for investigating epidemiological trends and developing prospective studies for prevention and treatment of these workers.
Keywords: Silicosis, Dust, Occupational Health, Cross-Sectional Studies, Respiratory Function Tests
Key messages.
What is already known about this subject?
Worker exposure to respirable crystalline silica (RCS) has likely increased worldwide with the increasing popularity of engineered stone (ES) countertops containing high levels of RCS. Outbreaks of silicosis have occurred globally.
What are the new findings?
The most severe form of silicosis, progressive massive fibrosis (PMF), is common among ES workers globally and is occurring in younger workers. Lung function impairment and comorbid autoimmune diseases are also prevalent among ES workers.
How might this impact policy or clinical practice in the foreseeable future?
The rapid progression of silicosis in our ES worker population suggests the need for greater investment in respiratory health surveillance of at-risk ES workers than exists currently. Additionally, the younger age of ES workers with PMF indicates an urgent need to more aggressively reduce RCS exposure, whether by greater attention to engineering controls or elimination of this high-silica product.
Introduction
Engineered stone (ES), also known as artificial, synthetic, or quartz stone, is a composite material often containing 90+% quartz bound with polymer resins and pigments for use in the production of countertop surfaces. In contrast to natural stone products (eg, marble or granite), which have lower silica content but must be mined from rock quarries, commercial ES has become increasingly favoured due to the lower production costs and numerous aesthetic and customisable options. A global surge in popularity of ES countertop surfaces over the last two decades has corresponded with recent outbreaks of severe silicosis among ES fabrication and masonry workers exposed to respirable crystalline silica (RCS) in several countries.1–7
Silicosis in ES workers has been diagnosed in younger workers (20–40 years), likely because of shorter disease latency (2–10 years) and rapid lung function decline compared with silicosis from other RCS exposures.8 9 In a US case series, 2 of 18 ES workers with silicosis died at ages 30 and 32.6 In contrast, latencies of 20–30 years have been reported in metal miners and pottery workers with silicosis.10 11 Less well understood is whether variability in clinical manifestations and severity among ES workers differs among countries due to differences in case ascertainment and/or dust control regulations. A seminal study from Israel identified a cohort of ES workers with severe silicosis requiring lung transplantation.2 In Australia, recognition of ES silicosis led to creation of medical surveillance programmes in several states that have identified early silicosis in asymptomatic ES workers.12 A report from Spain highlighted declining lung function and chest imaging abnormalities in ES workers despite removal from exposure.13 Globally, treatment options for severe progressive silicosis remain limited.
Besides pneumoconiosis, RCS exposure increases risk for autoimmune disorders14 but information about the prevalence of autoimmunity in ES workers is limited. Even less is known about international prevalence of other RCS-related diseases including emphysema, lung cancer, mycobacterial lung infection and kidney disease.
Recognising the need to address these knowledge gaps and observing concurrent cases, a group of respiratory physicians, physician scientists, occupational medicine specialists and epidemiologists met informally at an international pulmonary conference to discuss an international collaboration called the Engineered Stone Silicosis Investigators (ESSI) consortium. Our objectives are to investigate differences in workplace exposures, demographic characteristics, clinical findings, and social and regulatory policies that underpin the recognition and management of silicosis and associated co-morbidities in ES workers. Understanding country-specific differences will help inform optimal approaches to RCS exposure control and early disease detection. As demonstrated by previous global initiatives and health registries,15 international data sharing will help promote best practices for disease prevention and lay the foundation for well-designed research treatment trials for silicosis, particularly in its most severe and progressive form that affects ES workers worldwide. This study uses the ESSI registry database to describe cross-sectional demographic, exposure and clinical characteristics among ES workers with silicosis from four countries.
Methods
Study population
ES workers ages 18 and older involved with fabrication or masonry with a physician diagnosis of silicosis from workplace exposures to RCS were included in this study. Workers were excluded if the diagnosis of silicosis was not confirmed by chest imaging or histopathology, and/or if the patient did not have a known workplace exposure to ES dust.
ES workers were evaluated by participating physicians and institutions in four countries: Israel (Rabin Medical Center, Petach Tivka; Tel Aviv Sourasky Medical Center, Tel Aviv), Spain (Central University Hospital of Asturias, Oviedo, Asturias; Puerta del Mar University Hospital, Cádiz, Andalucía; Vall d’Hebron University Hospital, Barcelona, Catalonia), Australia (Pindara Hospital, Gold Coast, Queensland; The Wesley Hospital, Brisbane, Queensland; University of Queensland, Brisbane, Queensland) and the USA (National Jewish Health, Denver, Colorado; University of Washington, Seattle, Washington).
Case ascertainment by country
Cases of silicosis among US ES workers were reported by two occupational pulmonary specialists. In Israel, cases were reported by pulmonary physicians and via a research programme supported by the Israeli Ministry of Labour for biological monitoring of ES workers, including cases from lung transplant registries. Cases from Australia were reported by pulmonologists who evaluated ES workers referred through state-based medical surveillance programmes.16 Those from Spain were accessioned from lung disease clinics and hospital lung transplant databases.13 All cases were clinically evaluated between 2001 and 2021.
Data registry
The ESSI registry was established using a Research Electronic Data Capture (REDCap) database hosted at National Jewish Health in Denver, Colorado, USA.17 Data entry into REDCap began in early 2020, with entry completed through June 8, 2021 included in this cross-sectional analysis.
Demographics
Date of birth, sex and smoking status were recorded. We defined smokers as those reporting smoking more than 20 packs of cigarettes in a lifetime or more than one cigarette daily for 1 year.18 Estimated smoking pack-years were calculated as a product of years of smoking times average number of packs smoked daily.
Occupational history
We recorded years of work tenure and current or previous work status in a qualifying industry, including ES fabrication, cutting, shaping, finishing, laying, masonry and other ES-related industries. Work in other industries with RCS exposure was also recorded, including natural stone fabrication; cement, concrete and brick product manufacturing; foundry work; clay product manufacturing; glass/glass product manufacturing; dental technician duties; mining and sandblasting.
Radiology
Chest radiographic findings were recorded using the International Labour Office (ILO) International Classification of Radiographs of Pneumoconioses.19 The ILO system classifies lung parenchymal opacities by size, shape and profusion through direct comparison of the chest radiograph with standard radiographs. Most chest radiographs were classified by B readers certified by the US National Institute for Occupational Safety and Health, and a few were interpreted by experienced pulmonologists or radiologists. When available, findings from chest CT scans were documented, including images scored using the International Classification of High-Resolution CT for Occupational and Environmental Respiratory Disease (ICOERD).20
Simple silicosis was defined by the presence of small rounded or irregular opacities on chest radiograph (profusion ≥1/0) or those ≤1 centimeter in long axis diameter on chest CT. Progressive massive fibrosis (PMF), or complicated silicosis, was defined as the presence of large opacities on chest radiograph (ILO Category A, B or C), or nodules/masses >1 cm in longest axis on chest CT. Presence of enlarged mediastinal or hilar lymphadenopathy (calcified or non-calcified) and ground glass opacities was recorded. Those with mediastinal or hilar lymphadenopathy only (without parenchymal abnormalities) were classified as ‘lymph node silicosis’.21
Pulmonary function testing
We recorded spirometry measurements including forced expiratory volume in one second (FEV1), forced vital capacity (FVC) and the ratio of FEV1/FVC. Prebronchodilator and postbronchodilator spirometry values, total lung capacity (TLC) and diffusion capacity for carbon monoxide (DLCO) were recorded when available. American Thoracic Society (ATS)/European Respiratory Society (ERS) standards for spirometry, lung volume and DLCO measurements were used by all participating investigators.22–24 In cases with multiple pulmonary function tests, the test closest in time to the most recent chest CT was selected for analysis. Hypoxaemia was defined as need for supplemental oxygen or oxygen saturations <90% at rest or during a 6 min walk test.
Percent reference values and lower limits of normal for spirometry, TLC and DLCO were calculated using Global Lung Initiative reference values adjusting for age, sex, height and ethnicity.25–27 We defined restrictive, obstructive, mixed or normal ventilatory patterns using Pellegrino 2005 criteria.28
Autoimmunity and other silica-related clinical findings
We recorded available information on diagnosis of any autoimmune disease including systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, autoimmune myositis, mixed connective tissue disease, psoriasis and antineutrophil cytoplasmic antibody-mediated vasculitis. Workers with abnormal autoimmune serological testing without a definitive autoimmune diagnosis were also included. Other medical diagnoses including active pulmonary tuberculosis (TB) infection, latent tuberculosis infection (LTBI), nontuberculous mycobacterial (NTM) lung infection, primary lung cancer and chronic kidney disease were recorded.
Statistical analyses
Cross-country differences in demographics, chest imaging, pulmonary function and autoimmunity were assessed using one-way analysis of variance for continuous variables with post hoc pairwise comparisons using Tukey’s honest significance difference test, and Fisher’s exact tests for categorical variables. Pairwise Fisher’s exact tests were performed for individual country comparisons when the overall test was statistically significant (p<0.05). Adjusted analyses for the frequencies of different findings of interest were performed using logistic regression, with covariates of age and/or smoking pack-years included as appropriate a priori. The reference group for all comparisons was those with absence of the given finding. As a sensitivity analysis, country was added as a covariate to confirm observed associations were not due only to cross-country differences in case accessioning. Missing data were excluded from frequency calculations and corresponding comparative analyses. Data management was performed using R V.4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). Statistical analyses were conducted using SAS V.9.4 (SAS Institute).
Results
As of 8 June 2021, 197 workers were enrolled in the ESSI registry. Ten participants were excluded because they did not report having worked with ES, and 18 additional participants were excluded because imaging or pathology data were unavailable to confirm a diagnosis of silicosis. As shown in table 1, 169 ES workers with silicosis were eligible for analysis, including 14 in Australia, 125 in Israel, 20 in Spain and 10 in the USA. Workers were predominantly male (98.8%), with mean age 51.7 years (range 26–81) at time of entry into the registry. Mean work tenure was 19.9 years (range 3–48). Most (66.1%) were current or former smokers, reporting a mean of 20.8 pack-years. Notably, Australian workers were significantly younger than workers from any other country, and workers from Spain were significantly younger than workers from Israel. The USA was the only country with any enrolled female workers, both of whom performed ‘housekeeping duties’ (dry sweeping and dust clean-up) in a stone fabrication shop. Smoking prevalence was similar across countries, with more current or former smokers in Australia and with significantly longer smoking pack-years among workers from Israel. Additionally, workers in Australia had been employed in ES stone work for significantly less time than workers in Israel.
Table 1.
Demographic, smoking and occupational characteristics of engineered stone workers with silicosis from four countries
| Overall | Australia | Israel | Spain | USA | P value* | |
| N=169 | N=14 | N=125 | N=20 | N=10 | ||
| Demographics | ||||||
| Age, mean±SD | 51.7±11.4 | 34.1±8.3 | 54.2±10.4 | 47.5±8.2 | 52.9±8.5 | <0.0001 AI, AS, AU, IS |
| Male, n (%) | 167 (98.8) | 14 (100.0) | 125 (100.0) | 20 (100.0) | 8 (80.0) | 0.0032UI |
| Smoking | ||||||
| Current/former, n (%) | 111 (66.1) | 13 (92.9) | 82 (66.1) | 12 (60.0) | 4 (40.0) | 0.0390AU |
| Never, n (%) | 57 (33.9) | 1 (7.1) | 42 (33.9) | 8 (40.0) | 6 (60.0) | 0.0390 AU |
| Cigarette pack-years, mean±SD | 20.8±20.6 | 8.1±7.6 | 25.2±22.1 | 9.3±7.4 | 9.4±7.2 | 0.0034AI |
| Work tenure | ||||||
| Industry years worked†, mean±SD | 19.9±9.8 | 10.6±6.0 | 21.3±10.0 | 18.9±8.2 | 17.3±5.1 | 0.0008AI |
*One-way ANOVA for continuous variables and Fisher’s exact tests for categorical variables between all countries. Individual comparisons: A=Australia, I=Israel, S=Spain, U=USA.
†Years worked in industry include manufacture of engineered stone products, masonry and stone setting.
ANOVA, analysis of variance.
Spirometry and TLC measures were available for 128 workers, and DLCO for 137 workers. Table 2 shows the distribution of lung function abnormalities and hypoxaemia among ES workers by country. Overall, the most common findings were reduced DLCO (occurring in 63/137, 46%) and restrictive lung physiology (occurring in 25/128, 27%). Lung function abnormalities were significantly more common among ES workers from Israel, many identified through lung transplant registries. Resting or exertional hypoxaemia was reported in 39/166 (23.5%) for whom data were available, the majority (92%) from Israel.
Table 2.
Pulmonary function impairment in engineered stone workers with silicosis
| Overall | Australia | Israel | Spain | USA | P value* | |
| N=169 | N=14 | N=125 | N=20 | N=10 | ||
| Pulmonary function testing measurements, mean (SD)† | ||||||
| FEV1, % predicted (n=147) | 66.4±26.6 | 84.6±13.4 | 58.4±25.7 | 89.8±14.9 | 91.6±14.9 | <0.0001 IA, IS, US |
| FVC, % predicted (n=147) | 70.9±24.6 | 87.6±13.1 | 63.8±23.5 | 91.9±14.9 | 93.3±17.3 | <0.0001 IA, IS, US |
| FEV1/FVC, % (n=147) | 73.6±13.5 | 79.4±8.0 | 71.7±14.9 | 78.2±4.0 | 78.6±6.6 | 0.0474 |
| TLC, % predicted (n=129) | 84.9±23.3 | 87.5±15.8 | 83.0±23.9 | 93.3±4.6 | 99.3±22.0 | 0.1648 |
| DLCO, % predicted (n=137) | 75.0±27.8 | 86.1±14.2 | 68.1±26.4 | 93±20.2 | 108±28.1 | <0.0001 IU, IS |
| Abnormal pulmonary function testing, n (%)† | ||||||
| Restrictive‡ | 35/128 (27.3) | 2/11 (18.2) | 32/104 (30.8) | 0/3 (0.0) |
1/10 (10.0) | 0.3861 |
| Obstructive§ | 33/142 (23.2) | 1/14 (7.1) | 31/105 (29.5) | 0/13 (0.0) | 1/10 (10.0) | 0.0206IS |
| Mixed¶ | 12/128 (9.4) | 0/11 (0.0) | 12/104 (11.5) | 0/3 (0.0) | 0/10 (0.0) | 0.6816 |
| Normal** | 62/147 (42.2) | 11/14 (78.6) | 30/108 (27.8) | 13/15 (86.7) | 8/10 (80.0) | <0.0001 IA, IS, IU |
| Reduced TLC†† | 50/129 (38.0) | 2/11 (18.2) | 47/105 (44.8) | 0/3 (0.0) | 1/10 (10.0) | 0.0287IU |
| Reduced DLCO‡‡ | 63/137 (46.0) | 4/14 (28.6) | 56/101 (55.5) | 2/12 (16.7) | 1/10 (10.0) | 0.0016 IU, IS |
| Hypoxaemia | 39/166 (23.5) | 0/14 (0.0) | 36/122 (29.5) | 0/20 (0.0) | 3/10 (30.0) | 0.0009IA, IS, US |
*One-way ANOVA for continuous variables and Fisher’s exact test for cross-country comparison of categorical variables; individual comparisons: A=Australia, I=Israel, S=Spain, U=USA.
†Pulmonary function tests included measurements of FEV1, FVC, the ratio of FEV1/FVC, TLC, DLCO. Global lung Initiative reference values were used for FEV1, FVC, FEV1/FVC, DLCO and LLN for all parameters.
‡Restrictive defined as FEV1/FVC ratio ≥LLN, FVC <LLN and TLC <LLN.
§Obstructive defined as a FEV1/FVC ratio <LLN and FVC ≥LLN; or FEV1/FVC ratio ≥LLN, FVC <LLN and TLC ≥LLN; or FEV1/FVC ratio <LLN, FVC <LLN and TLC ≥LLN.
¶Mixed defined as FEV1/FVC ratio <LLN, FVC <LLN and TLC <LLN.
**Normal defined as FEV1/FVC ratio ≥LLN and FVC ≥LLN.
††Reduced TLC defined as a measured TLC value <LLN.
‡‡Reduced DLCO defined as a measured DLCO value <LLN.
ANOVA, analysis of variance; DLCO, diffusion capacity of lung for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; LLN, lower limits of normal; TLC, total lung capacity.
ILO classifications of chest radiographs were available for 30 workers, out of 51 who had chest radiographs available. Chest CT findings were available for 159 workers, including 28 workers with ICOERD classifications. As shown in table 3, PMF was diagnosed by chest imaging in 55 (32.5%) workers. Of those with PMF, 10 (18.5%) had worked in the ES industry for 10 years or less. Ground glass opacities were noted in 26 (15.4%) workers, most commonly in the USA and Australia. Mediastinal/hilar lymphadenopathy was prevalent (74.6%) in workers from all countries. Isolated lymphadenopathy was present in 3.6% overall, most commonly in Australian workers (14.3%) where medical surveillance for early disease detection has been implemented. Adjusting for smoking, younger workers were not more likely to have ground glass opacities (OR 0.98 per year; 95% CI 0.94 to 1.02; p=0.21). We found no significant cross-country differences in prevalence of abnormal chest imaging features. Representative chest imaging findings are shown from a US stonemason with 20 years of ES work tenure (figure 1). Chest imaging 3 years after initial diagnosis demonstrated progression of bilateral nodular and interstitial parenchymal abnormalities despite removal from workplace exposure to RCS.
Table 3.
Chest imaging abnormalities in engineered stone workers with silicosis
| Overall | Australia | Israel | Spain | USA | P value* | |
| N=169 | N=14 | N=125 | N=20 | N=10 | ||
| Abnormal chest imaging findings†, n (%) | ||||||
| Simple silicosis only‡ | 105 (62.1) | 7 (50.0) | 80 (64.0) | 10 (50.0) | 8 (80.0) | 0.3157 |
| Simple silicosis and complicated silicosis/PMF§ | 55 (32.5) | 5 (35.7) | 38 (30.4) | 10 (50.0) | 2 (20.0) | 0.2871 |
| Ground glass opacities | 26 (15.4) | 5 (35.7) | 16 (12.8) | 2 (10.0) | 3 (30.0) | 0.0608 |
| Mediastinal/hilar adenopathy | 126 (74.6) | 12 (85.7) | 94 (75.2) | 13 (65.0) | 7 (70.0) | 0.5649 |
| Lymph node silicosis only | 6 (3.6) | 2 (14.3) | 4 (3.2) | 0 (0.0) | 0 (0.0) | 0.1971 |
*Fisher’s exact test for cross-country comparison; individual comparisons.
†Three cases did not have findings consistent with silicosis on imaging, instead lung biopsy revealed findings consistent with silicosis. These cases are included in the overall analysis.
‡Simple silicosis defined as small ≤1 cm rounded or irregular opacities seen on chest CT, or ≥1/0 profusion small opacities on ILO B-read chest radiograph.
§Complicated silicosis/PMF defined as presence of lesions/masses >1 cm in diameter on chest CT or large opacities (category A/B/C) on ILO B-read chest radiograph.
ILO, International Labour Office; PMF, progressive massive fibrosis.
Figure 1.
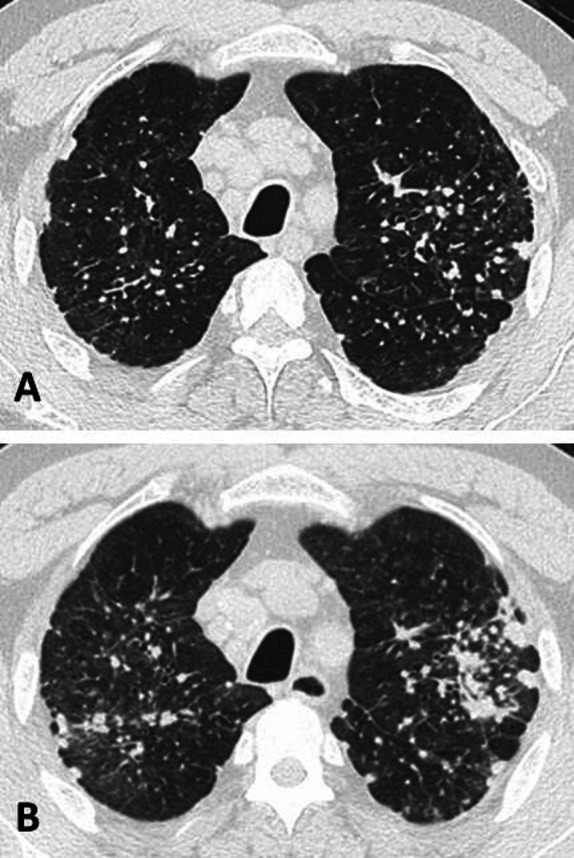
High-resolution chest CT image (A) from an engineered stone worker shows small rounded opacities in a perilymphatic distribution, interlobular septal thickening, mediastinal lymphadenopathy and areas of emphysema. Repeat chest CT scan 3 years later (B) shows increased ground glass nodularity and progression of bilateral conglomerate opacities despite removal from workplace exposure to respirable crystalline silica.
We evaluated the relationship between workers’ chest imaging findings and lung function abnormalities. Adjusting for smoking pack-years (table 4), odds of PMF increased 3.77, 2.45 and 2.24 times in workers with a mixed ventilatory pattern, reduced TLC or reduced DLCO, respectively, compared with workers without each given finding. Adjusting for smoking pack-years, those with reduced TLC were more likely to have mediastinal/hilar lymphadenopathy (OR=3.86, p=0.01) compared with those without reduced TLC, while those with obstruction had significantly reduced odds (OR=0.36, p=0.02) of lymphadenopathy compared with those without obstruction. In a sensitivity analysis (data not shown), country did not explain these associations or significantly change the results.
Table 4.
Odds of chest imaging findings associated with lung function abnormalities among engineered stone workers from four countries
| Lung function abnormality* | n | Complicated silicosis/PMF | Ground-glass opacities | Mediastinal/hilar lymphadenopathy | Ground-glass opacities or lymph node silicosis only | ||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||
| Unadjusted | |||||||||
| Restrictive pattern | 128 | 1.21 (0.53 to 2.77) |
0.65 | 1.17 (0.41 to 3.33) |
0.77 | 2.09 (0.73 to 5.99) |
0.17 | 0.97 (0.37 to 2.57) |
0.96 |
| Obstructive pattern | 142 | 0.85 (0.37 to 1.96) |
0.70 | 0.90 (0.31 to 2.65) |
0.85 |
0.39
(0.17 to 0.93) |
0.03 | 0.83 (0.31 to 2.25) |
0.72 |
| Mixed pattern | 128 |
3.52
(1.04 to 11.89) |
0.04 | 1.09 (0.22 to 5.39) |
0.92 | NE | NE | 0.77 (0.16 to 3.73) |
0.74 |
| Reduced TLC | 129 |
2.48
(1.16 to 5.31) |
0.02 | 1.36 (0.52 to 3.55) |
0.53 |
3.93
(1.39 to 11.12) |
0.01 | 0.98 (0.41 to 2.38) |
0.97 |
| Reduced DLCO | 137 |
2.49
(1.20 to 5.15) |
0.01 | 1.21 (0.49 to 3.02) |
0.68 | 1.75 (0.77 to 4.01) |
0.19 | 1.02 (0.45 to 2.35) |
0.96 |
| Adjusted† | |||||||||
| Restrictive pattern | 128 | 1.18 (0.51 to 2.71) |
0.70 | 1.18 (0.41 to 3.36) |
0.76 | 2.04 (0.71 to 5.87) |
0.19 | 0.98 (0.37 to 2.59) |
0.97 |
| Obstructive pattern | 142 | 0.77 (0.32 to 1.83) |
0.56 | 0.93 (0.32 to 2.77) |
0.90 |
0.36
(0.15 to 0.86) |
0.02 | 0.85 (0.31 to 2.33) |
0.76 |
| Mixed pattern | 128 |
3.77
(1.11 to 12.85) |
0.03 | 1.08 (0.22 to 5.36) |
0.93 | NE | NE | 0.76 (0.16 to 3.71) |
0.73 |
| Reduced TLC | 129 |
2.45
(1.14 to 5.27) |
0.02 | 1.37 (0.52 to 3.59) |
0.52 |
3.86
(1.36 to 10.96) |
0.01 | 0.99 (0.41 to 2.40) |
0.98 |
| Reduced DLCO | 137 |
2.24
(1.06 to 4.77) |
0.04 | 1.23 (0.48 to 3.17) |
0.66 | 1.59 (0.68 to 3.74) |
0.28 | 1.03 (0.44 to 2.44) |
0.95 |
All participants with mixed spirometry had mediastinal/hilar adenopathy.
Bolded values represent odd ratios with significance p<0.05.
*The reference group for all comparisons was those with absence of the specified lung function abnormality.
†Adjusted for smoking pack-years.
DLCO, diffusion capacity of lung for carbon monoxide; NE, not estimable; TLC, total lung capacity.
We also examined chest imaging and lung function abnormalities in ES workers based on years worked in the industry. While longer work tenure was not significantly associated with abnormal lung function (restrictive, obstructive, or mixed spirometry, reduced TLC, or reduced DLCO) after adjusting for smoking pack-years, the odds of abnormalities increased with each year of work (table 5). Adjusting for age, chest imaging findings of PMF were not associated with work tenure. In a sensitivity analysis (data not shown), country did not confound these analyses or affect overall findings.
Table 5.
Odds of chest imaging or lung function abnormalities per year worked in the stone industry among engineered stone workers from four countries
| Restrictive pattern n=128 | Obstructive pattern n=142 | Mixed pattern n=128 | Reduced TLC n=129 | Reduced DLCO n=137 | Complicated silicosis/PMF n=168 | ||
| Unadjusted | |||||||
| One additional year worked in industry | OR | 1.02 | 1.02 | 1.02 | 1.02 | 1.03 | 1.03 |
| (95% CI) | (0.98 to 1.06) | (0.98 to 1.06) | (0.96 to 1.08) | (0.99 to 1.06) | (0.99 to 1.06) | (0.99 to 1.06) | |
| P value | 0.34 | 0.32 | 0.55 | 0.22 | 0.17 | 0.14 | |
| Adjusted* | |||||||
| One additional year worked in industry | OR | 1.02 | 1.02 | 1.02 | 1.02 | 1.02 | 1.02 |
| (95% CI) | (0.98 to 1.06) | (0.98 to 1.06) | (0.96 to 1.08) | (0.99 to 1.06) | (0.98 to 1.06) | (0.98 to 1.07) | |
| P value | 0.37 | 0.44 | 0.52 | 0.24 | 0.34 | 0.27 | |
*Restrictive, obstructive, mixed pattern, reduced TLC and DLCO were adjusted for smoking pack-years. Complicated silicosis/PMF was adjusted for age.
DLCO, diffusion capacity of lung for carbon monoxide; PMF, progressive massive fibrosis; TLC, total lung capacity.
Among all ES workers with data available, 33/154 (21.4%) were diagnosed with an autoimmune disease or had abnormal autoimmune serology. Details are included in online supplemental table 1. Autoimmunity based on serological testing was particularly prevalent in Australian workers (7/14), perhaps reflecting more systematic testing of all ES workers. Rheumatoid arthritis was diagnosed in nine workers, systemic sclerosis in seven, and psoriasis/psoriatic arthritis in six. Controlling for age and smoking pack-years, we found no relationship between autoimmunity and ES work tenure (OR 1.02 per year worked; 95% CI 0.96 to 1.08; p=0.62).
oemed-2021-108190supp001.pdf (86.2KB, pdf)
Information was incomplete on several silica-related conditions. LTBI was reported in 11/150 (7.3%). Active TB was reported in 2/152 (1.3%), both cases from Israel. NTM lung infection was detected in 7/122 workers tested (4 Mycobacterium kansasii, 2 Mycobacterium avium, 1 Mycobacterium fortuitum), all in Israel. Lung cancer occurred in 2/151 (1.3%) workers, both from Israel where cases were older, had more advanced silicosis, and were more likely to have smoked. Chronic kidney disease was reported in 15/150 (10%) workers, 13 from Israel and 2 from the USA.
Discussion
Findings from this unique multinational registry represent the first reported effort to directly compare demographic, exposure, and clinical information from ES workers with silicosis from multiple countries. Notably, 32.5% of workers enrolled in the registry have PMF, the most severe form of mineral dust-related pneumoconiosis, 18.5% of whom reported 10 years or fewer of workplace exposure to ES dust. Further, the mean age of 51.7 years suggests that this relatively young worker population is at high risk for disease progression, multiple RCS-related comorbidities, and substantial loss in years of working life and overall life expectancy. Studies of silicosis in US workers from multiple industries suggest that age at first exposure to RCS is similar to that in ES workers.29 Our findings provide further evidence that ES-related silicosis occurs with shorter latency in younger workers who face severe disability.
In addition to PMF, the finding of ground glass opacities on chest imaging in over 15% of workers suggests that mineral dust-associated alveolar proteinosis may be a common feature of ES-related silicosis in this cohort. Silicoproteinosis is typically linked with acute forms of silicosis from high concentrations of RCS exposure.30 Prior to reports of CT abnormalities in ES workers,3 16 31 CT findings of silicoproteinosis were rarely described.31 Longitudinal follow-up of ground glass imaging abnormalities will be important to clarify associations with a more progressive disease phenotype. Future longitudinal analyses in this multi-national cohort are planned to identify patterns that predict a persistent fibrogenic response and disease progression.31
Not surprisingly, we found that PMF on chest imaging of ES workers was associated with reduced TLC (OR=2.45), reduced DLCO (OR=2.24) and a mixed spirometric pattern (OR=3.77) on pulmonary function testing. However, there was no significant association between PMF and either restrictive or obstructive spirometric impairment, suggesting that this common medical surveillance tool may, by itself, lack sensitivity in identifying ES workers with even more advanced silicosis. Widespread implementation of DLCO and lung volume measurements, along with standardised image classification protocols including the ILO or ICOERD classifications, may be important for effective medical surveillance in ES workers.
Limited information was available regarding workplace exposure risks. Moreover, estimates of total work tenure in ES are likely to be high since a number of participants worked with both natural and ES throughout their employment. After adjusting for smoking, we found that work tenure (by itself, a crude marker of workplace RCS exposure) was not significantly associated with odds of PMF or abnormal lung function. In contrast, a study of sandblasters found that silicosis was linked to both exposure concentration and duration.32 Our findings suggest that other exposure factors besides work tenure alone, including RCS concentration, particle size and mineralogical characteristics, and chemical composition of particulate matter retained in workers’ lungs, may be important in risk for disease from ES dust.33 34 Pavan et al hypothesise that enhanced free radical production from ES dust containing greater quantities of transition metal ions may augment the pulmonary fibrogenic response compared with quartz alone.35 Investigation of ES dust mineralogy using animal models and in situ techniques may provide additional insights into disease pathogenesis. Future analyses will explore differences in exposure risk factors among participating ESSI countries including workplace dust control strategies, policies for and adherence to silica regulations (eg, permissible exposure limits and medical surveillance requirements), specific job duties and use of respiratory protection.
Autoimmunity among ES workers with silicosis was considerably more common (21.4%) than the estimated baseline prevalence in Europe (3%),7 but the finding of autoimmunity in these workers was not associated with work tenure. Mechanisms of silica-related autoimmunity and ES dust immunogenicity are not well understood, though abnormalities in T-cell function, humoral immunity and immune-complex deposition have been implicated.36 Our findings suggest that screening and diagnostic evaluation of silica-associated conditions including autoimmunity, latent and active granulomatous lung infection, lung cancer and inflammatory kidney disease should be routinely included in medical surveillance guidelines and clinical management algorithms for ES workers.
Our study has several limitations. First, differences in clinical findings between countries likely are confounded by differences in case ascertainment, referral patterns and timing of clinical evaluation and country-specific policies on worker medical surveillance. These sources of selection and information bias are notable but unavoidable given the study design. Second, age at initial silicosis diagnosis was not available, therefore our findings probably overestimate the number of years required for PMF to occur. Third, regional differences in pulmonary function testing, chest imaging and autoimmunity testing methods may contribute to some variability. However, participating investigators used international ATS/ERS standards for lung function testing, and our analysis used the same prediction equations to compare measured lung function parameters for all countries. Similarly, chest radiographs were classified using the ILO standards and ICOERD standards for chest CT classification were analysed when available. Countries without access to those with expertise in use of standardised methods for imaging classification would greatly benefit from implementation of these tools, a focus of future registry efforts. Fourth, testing for autoimmunity, LTBI and chronic kidney disease was not available for all cases, resulting in underestimation of these comorbidities. Future ESSI collaborative efforts will focus on prospective collection of relevant data from all sites. Fifth, the workers enrolled into this registry are a small subset of the ES population with silicosis worldwide. We anticipate that greater recognition of the registry’s existence will encourage future collaborations aimed at preventing and treating this disabling disease. Finally, because information in the registry relies on individual providers’ clinical reports, there is limited detail from occupational histories on specific job duties, engineering controls, workplace housekeeping practices and use of respiratory protection. Clinicians vary widely in experience and time available for detailed occupational history-taking, despite the importance of these factors in understanding disease risk. Prospective use of a standardised occupational history questionnaire and multidisciplinary collaboration with occupational hygienists would enable better cross-country comparisons.
Efforts to eliminate ES dust-induced silicosis will require a multidisciplinary approach to improve early disease detection and control of workplace RCS exposure. For example, in response to a large outbreak of ES silicosis, Australia created a national taskforce to identify areas for harm reduction, with recommendations to stop imports of ES products if benchmarks are not reached within a 3-year period.37 Such an approach may be considered by other countries as well. Further analysis of cross-country differences in ES worker populations, workplace regulations, medical surveillance, occupational health and safety culture, cultural and language barriers to effective health and safety education, and national reporting systems for case ascertainment is planned to identify barriers to the protection of workers globally. Future investigation of histopathological findings and in situ mineralogical characteristics among cases with lung tissue will help inform pathogenesis of ES dust exposure. In the meantime, active outreach to other international collaborators and regular communication about national policy changes will enhance the ESSI registry as a multinational framework, similar to other clinical registries such as the Global Registry of Acute Coronary Events.15 38
Our international approach to data sharing may provide opportunities for rigorous evidence-based investigation of treatment for progressive ES silicosis. Whole lung lavage and immunosuppressive and antifibrotic medications have been proposed to slow disease progression, but studies of these interventions are limited by inadequate power, lack of control groups and absent consensus-based inclusion/exclusion criteria. Along with treatment considerations, longitudinal analyses are planned to investigate disease progression among ES worker cohorts stratified by country of origin to better understand the impact of prevention approaches on current and future ES workers worldwide.
Conclusions
Findings from this cross-sectional analysis of ES workers in four countries point to an emerging population of workers worldwide with severe, irreversible RCS-associated diseases. The international ESSI registry provides an ongoing framework for participating investigators, who together will track epidemiological trends and develop prospective studies for prevention and clinical care of these workers.
Footnotes
Contributors: JTH, LZ-B, LHTG, KSA, RAC and CSR planned the study. All authors were involved with conducting the study. JTH and LZ-B conducted statistical analyses and drafted the manuscript with critical input from LHTG, KSA, RAC and CSR. All authors reviewed and edited the final manuscript. RAC and CSR are guarantors for the research described in this publication.
Funding: Work conducted at National Jewish Health was partially supported by the Reuben M Cherniack fellowship award. Work conducted in Israel was partially supported by the Committee for Research and Prevention in Occupational Safety and Health (56/13).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Institutional review board approval (HS-3483) was obtained under exempt status for entry of deidentified data from all participating institutions.
References
- 1. Bartoli D, Banchi B, Di Benedetto F. Provisional results of the environmental and health survey conducted within the territory of USL 11 of Empoli in Tuscany among employees in the processing of quartz resin composite materials and review of the literature. Ital J Occup Environ Hyg 2012;3:133–80. [Google Scholar]
- 2. Grubstein A, Shtraichman O, Fireman E, et al. Radiological evaluation of artificial stone silicosis outbreak: emphasizing findings in lung transplant recipients. J Comput Assist Tomogr 2016;40:923–7. 10.1097/RCT.0000000000000454 [DOI] [PubMed] [Google Scholar]
- 3. Hoy RF, Baird T, Hammerschlag G, et al. Artificial stone-associated silicosis: a rapidly emerging occupational lung disease. Occup Environ Med 2018;75:3–5. 10.1136/oemed-2017-104428 [DOI] [PubMed] [Google Scholar]
- 4. Kramer MR, Blanc PD, Fireman E, et al. Artificial stone silicosis [corrected]: disease resurgence among artificial stone workers. Chest 2012;142:419–24. 10.1378/chest.11-1321 [DOI] [PubMed] [Google Scholar]
- 5. Pérez-Alonso A, Córdoba-Doña JA, Millares-Lorenzo JL, et al. Outbreak of silicosis in Spanish quartz conglomerate workers. Int J Occup Environ Health 2014;20:26–32. 10.1179/2049396713Y.0000000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rose C, Heinzerling A, Patel K, et al. Severe Silicosis in Engineered Stone Fabrication Workers - California, Colorado, Texas, and Washington, 2017-2019. MMWR Morb Mortal Wkly Rep 2019;68:813–8. 10.15585/mmwr.mm6838a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shtraichman O, Blanc PD, Ollech JE, et al. Outbreak of autoimmune disease in silicosis linked to artificial stone. Occup Med 2015;65:444–50. 10.1093/occmed/kqv073 [DOI] [PubMed] [Google Scholar]
- 8. Krefft S, Wolff J, Rose C. Silicosis: an update and guide for clinicians. Clin Chest Med 2020;41:709–22. 10.1016/j.ccm.2020.08.012 [DOI] [PubMed] [Google Scholar]
- 9. Leso V, Fontana L, Romano R, et al. Artificial stone associated silicosis: a systematic review. Int J Environ Res Public Health 2019;16:568. 10.3390/ijerph16040568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang H, Yang L, Zhang J, et al. Natural course of silicosis in dust-exposed workers. J Huazhong Univ Sci Technolog Med Sci 2006;26:257–60. 10.1007/BF02895832 [DOI] [PubMed] [Google Scholar]
- 11. Hnizdo E, Murray J, Sluis-Cremer GK, et al. Correlation between radiological and pathological diagnosis of silicosis: an autopsy population based study. Am J Ind Med 1993;24:427–45. 10.1002/ajim.4700240408 [DOI] [PubMed] [Google Scholar]
- 12. Edwards G. Accelerated Silicosis—An emerging epidemic associated with engineered stone. Int J Environ Res Public Health 2019;568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. León-Jiménez A, Hidalgo-Molina A, Conde-Sánchez Miguel Ángel, et al. Artificial stone silicosis: rapid progression following exposure cessation. Chest 2020;158:1060–8. 10.1016/j.chest.2020.03.026 [DOI] [PubMed] [Google Scholar]
- 14. Pollard KM. Silica, silicosis, and autoimmunity. Front Immunol 2016;7:97. 10.3389/fimmu.2016.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Antao VC, Muravov OI, Sapp J, et al. Considerations before establishing an environmental health registry. Am J Public Health 2015;105:1543–51. 10.2105/AJPH.2015.302642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edwards G, Knight R. Australia's current workplace epidemic: accelerated silicosis. Intern Med J 2019;49:26. [Google Scholar]
- 17. Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferris BG. Epidemiology standardization project (American thoracic Society). Am Rev Respir Dis 1978;118:1–120. [PubMed] [Google Scholar]
- 19. International Labour Office . Guidelines for the use of the ILO International classification of radiographs of Pneumoconioses. Geneva: International Labour Officce; Revised edition, 2011. [Google Scholar]
- 20. Kusaka Y, Hering K, Parker J. International classification of HRCT for occupational and environmental respiratory diseases, 2005. [Google Scholar]
- 21. Cox-Ganser JM, Burchfiel CM, Fekedulegn D, et al. Silicosis in lymph nodes: the Canary in the miner? J Occup Environ Med 2009;51:164–9. 10.1097/JOM.0b013e31818f6a0f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American thoracic Society and European respiratory Society technical statement. Am J Respir Crit Care Med 2019;200:e70–88. 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller MR, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–38. 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 24. Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J 2017;49:1600016. 10.1183/13993003.00016-2016 [DOI] [PubMed] [Google Scholar]
- 25. Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: global lung function initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J 2017;50:1700010. 10.1183/13993003.00010-2017 [DOI] [PubMed] [Google Scholar]
- 26. Hall GL, Filipow N, Ruppel G, et al. Official ERS technical standard: global lung function initiative reference values for static lung volumes in individuals of European ancestry. Eur Respir J 2021;57:2000289. 10.1183/13993003.00289-2020 [DOI] [PubMed] [Google Scholar]
- 27. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-Ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40:1324–43. 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948–68. 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 29. Reilly MJ, Timmer SJ, Rosenman KD. The burden of silicosis in Michigan: 1988-2016. Ann Am Thorac Soc 2018;15:1404–10. 10.1513/AnnalsATS.201802-117OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buechner HA, Ansari A. Acute silico-proteinosis. A new pathologic variant of acute silicosis in sandblasters, characterized by histologic features resembling alveolar proteinosis. Dis Chest 1969;55:274–8. 10.1378/chest.55.4.274 [DOI] [PubMed] [Google Scholar]
- 31. Marchiori E, Souza CA, Barbassa TG, et al. Silicoproteinosis: high-resolution CT findings in 13 patients. AJR Am J Roentgenol 2007;189:1402–6. 10.2214/AJR.07.2402 [DOI] [PubMed] [Google Scholar]
- 32. Vacek PM, Glenn RE, Rando RJ, et al. Exposure‒response relationships for silicosis and its progression in industrial sand workers. Scand J Work Environ Health 2019;45:280–8. 10.5271/sjweh.3786 [DOI] [PubMed] [Google Scholar]
- 33. León-Jiménez A, Mánuel JM, García-Rojo M, et al. Compositional and structural analysis of engineered stones and inorganic particles in silicotic nodules of exposed workers. Part Fibre Toxicol 2021;18:41. 10.1186/s12989-021-00434-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ophir N, Shai AB, Alkalay Y, et al. Artificial stone dust-induced functional and inflammatory abnormalities in exposed workers monitored quantitatively by biometrics. ERJ Open Res 2016;2:00086-2015–2015. 10.1183/23120541.00086-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pavan C, Polimeni M, Tomatis M, et al. Editor's highlight: abrasion of artificial stones as a new cause of an ancient disease. physicochemical features and cellular responses. Toxicol Sci 2016;153:4–17. 10.1093/toxsci/kfw101 [DOI] [PubMed] [Google Scholar]
- 36. Lee S, Hayashi H, Maeda M, et al. Environmental factors producing autoimmune dysregulation--chronic activation of T cells caused by silica exposure. Immunobiology 2012;217:743–8. 10.1016/j.imbio.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 37. National Dust Disease Taskforce . National dust disease Taskforce final report, 2021. [Google Scholar]
- 38. Fox KAA, Eagle KA, Gore JM, et al. The Global Registry of Acute Coronary Events, 1999 to 2009--GRACE. Heart 2010;96:1095–101. 10.1136/hrt.2009.190827 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
oemed-2021-108190supp001.pdf (86.2KB, pdf)
Data Availability Statement
Data are available on reasonable request.


