Abstract
Devastating and persisting traumatic memories are a central symptom of post-traumatic stress disorder (PTSD). Sleep problems are highly co-occurrent with PTSD and intertwined with its etiology. Notably, sleep hosts memory consolidation processes, supported by sleep spindles (11–16 Hz). Here we assess the hypothesis that intrusive memory symptoms in PTSD may arise from excessive memory consolidation, reflected in exaggerated spindling. We use a newly developed spindle detection method, entailing minimal assumptions regarding spindle phenotype, to assess spindle activity in PTSD patients and traumatized controls. Our results show increased spindle activity in PTSD, which positively correlates with daytime intrusive memory symptoms. Together, these findings provide a putative mechanism through which the profound sleep disturbance in PTSD may contribute to memory problems. Due to its uniform and unbiased approach, the new, minimal assumption spindle analysis seems a promising tool to detect aberrant spindling in psychiatric disorders.
Keywords: post-traumatic stress disorder (PTSD), sleep, sleep spindles, sleep-dependent memory consolidation, intrusive memory symptoms, minimal assumption spindle detection
Statement of Significance.
Post-traumatic stress disorder (PTSD) is characterized by intrusive traumatic memories and frequent sleep problems. Sleep hosts memory consolidation processes supported by sleep spindles (11–16 Hz). Here we assess the hypothesis that intrusive memory symptoms in PTSD may arise from excessive memory consolidation, reflected in exaggerated spindling. We use a newly developed spindle detection method, entailing minimal assumptions regarding spindle phenotype. Our results show increased spindle activity in PTSD, which positively correlates with daytime intrusive memory symptoms. Together, these findings provide a putative mechanism through which profound sleep disturbance in PTSD may contribute to memory problems. Due to its uniform and unbiased approach, the new, minimal assumption spindle analysis seems a promising tool to detect aberrant spindling in psychiatric disorders.
Introduction
The majority of individuals (over 70%) experience a traumatic event in their lifetime, leading to a lifetime prevalence of post-traumatic stress disorder (PTSD) in about 7% of the general population [1, 2]. A characteristic symptom of PTSD is involuntary, distressing recall of the traumatic event, which can occur in the form of intrusive memories, flashbacks, or nightmares. Besides, these memory disturbances, PTSD patients suffer from severe sleep problems [3], like insomnia, nightmares, distressed awakenings, nocturnal panic attacks, and sleep terrors [4]. These sleep problems, which occur in 70%–90% of patients [5] and often predate the trauma [6], are thought to play an important role in PTSD genesis and maintenance [5, 7, 8]. Indeed, sleep problems, either predating or following trauma, are a strong predictor for the development of PTSD [9, 10]. They are also a frequent residual symptom after successful psychopathology-oriented treatment [11] and then constitute a strong predictor for relapse.
Interestingly, sleep importantly supports memory consolidation [12] through sleep spindles [13–16]: waxing and waning oscillations in the sigma frequency band (11–16 Hz), generated by thalamocortical feedback loops. Sleep spindles have been shown to reflect the reprocessing of individual memory traces [13] and have been implicated in memory consolidation by a large body of evidence [17–19], including some recent studies regarding emotional memory ([15, 20]). Sleep spindles are temporally coupled with sharp-wave ripples, marking hippocampal neuronal replay, and synchronize across different cortical areas through the coordinating action of slow oscillations [21]. This spatiotemporal coupling appears particularly relevant for the consolidation of episodic memories ([19, 22]).
In a recent study [23], we observed increased sigma power during NREM sleep in PTSD. As sigma power is highly correlated to sleep spindle activity [24], this might reflect altered spindling in PTSD. The sigma power increase occurred against the backdrop of a strong loss of slow oscillation power and shift toward higher-frequency activity, peaking over frontal, especially right-frontal, areas. This desynchronization of activity marks a local loss of sleep depth and suggests increased information processing in prefrontal association cortices, which are normally deactivated during sleep [25]. Of note, markers of (frontal) cortical desynchronization during PTSD sleep have been observed previously [26–28] and have been related to a state of chronic distress and hyperarousal persisting during sleep ([8, 29–33]). Furthermore, some studies suggest that desynchronization may accompany states of stress [34] and emotional distress [35, 36] during sleep, also in the absence of PTSD.
In view of the above, we propose—as a working hypothesis—that the role of sleep disturbance in the pathogenesis of PTSD involves aberrant memory consolidation. As a step towards testing this hypothesis, we aim to assess spindle activity in PTSD, as a neural marker that directly reflects memory reactivation and consolidation. We, furthermore, aim to assess whether spindle activity is associated with intrusive memory complaints.
Several methods to detect discrete spindles have previously been published [19, 37]. However, an acknowledged pitfall [38–41] in these methods is the use of nonuniform, a priori criteria for the definition of sleep spindles. These criteria lack a physiological base and often include high amplitude and duration thresholds, leading to arbitrary cutoffs in the analyzed spindle activity. The use of such methods in quantitative research has several important drawbacks: for one, spindle activity below and above the detection thresholds is not taken into account in the analysis. Second, arbitrary choices with respect to the aforementioned criteria and their calculation can lead to large differences in results, and thus in difficulties comparing studies. These drawbacks may particularly hamper spindle analyses in neuropsychiatric disorders, since standard assumptions about spindle morphology may not apply and might even prevent the detection of deviant physiology, representative of the disorder.
To analyze sleep spindles in an unbiased manner, a new, minimal assumptions, spindle analysis (MASA) method was developed. This method defines spindle fluctuations in terms of waxing and waning dynamics in the sigma band. The waxing and waning characteristic is an intrinsic property of sleep spindles, reflecting the progressive recruitment of thalamocortical neurons into the spindle dynamic (waxing phase) followed by its gradual termination (waning phase), based on neural mechanisms that are well understood [42–44]. The core of the MASA method, hence, entails the detection of individual sigma band wax and wane patterns of virtually any size. Based on EEG alone, the source of individual EEG patterns cannot be distinguished. As such, MASA considers adjacent spindle fluctuations independently, even if in some cases they may be generated by the same spindle dynamic. Following the detection of discrete spindle fluctuations, multiple amplitude and duration parameters are calculated for each event that, together, provide an estimate of the shape and size of each spindle fluctuation. MASA also produces a count of detected spindle fluctuations. Overall, the method delivers rich and comprehensive data about spindle activity.
Here we use MASA to assess spindle activity in a previously acquired data set that includes sleep EEGs of police officers and war veterans with and without PTSD [23]. Next, we assess whether changes in spindle activity in PTSD are associated to intrusive memory complaints. To understand how potential changes at the level of discrete spindle fluctuations relate to spectral EEG measures absolute sigma power is also assessed.
Methods
Participants
The participants consisted of patients with chronic PTSD (n = 14), according to the Clinician-Administered PTSD Scale (CAPS) for DSM-IV [45], and trauma-exposed controls (n = 14). Trauma-exposed controls had experienced a traumatic event, as defined by DSM-IV, but did not meet de diagnostic criteria for PTSD. All CAPS interviews were performed by a trained clinician. Participants with PTSD were recruited at ARQ Centrum‘45, a Dutch national center for the diagnosis and treatment of PTSD. Trauma-exposed controls were recruited used through police departments and veterans’ centers. The two groups were matched on age, gender, profession, and educational level (Table 1). No differences in alcohol-related disorders and use were found between groups. Participants were asked to refrain from medication use prior to the experiment, however, for six of the PTSD patients medication could not be interrupted. Five of these patients used serotonin reuptake inhibitors (Paroxetine, Venlafaxine, Sertraline, Citalopram) and one used a benzodiazepine (Temazepam). Participants were excluded in case of acute suicidality, presence of a psychotic, or bipolar disorder, depression with psychotic features, excessive substance related disorder over the past 3 months before inclusion, history of neurological or sleep disorders (prior to PTSD onset), a habitual sleep pattern with less than 6 hours of sleep per night, or a sleep window outside 10 PM till 10 AM. The study protocol was approved by the Medical Ethical Committee of the Amsterdam Medical Center (AMC) and all participants gave written informed consent.
Table 1.
Sociodemographic and clinical characteristics of PTSD patients and trauma-exposed controls
| PTSD | CTRL | |
|---|---|---|
| Professional background (n, %) | ||
| Police | 12(75%) | 10(71%) |
| Veteran | 4(25%) | 4(29%) |
| Mean age(SD) | 45.6(7.9) | 44.4(8.7) |
| Gender(n,%) | ||
| Male | 15(94%) | 13(93%) |
| Female | 1(6%) | 1(7%) |
| Educational level(n,%) | ||
| Lower vocational education | 3(19%) | 0(0%) |
| Middle vocational education | 10(63%) | 11(79%) |
| Higher vocational education | 3(19%) | 3(21%) |
| Clinical characteristics | ||
| CAPS score(mean,SD) | 82.8(11.6) | 5.3(4.7) |
| Medication (n, %) | ||
| Unmedicated | 8 (57.1%) | 14 (0%) |
| Serotonin Reuptake Inhibitors (SSRI) | 5 (35.7%) | 0 (0%) |
| Benzodiazepine | 1 (7.1%) | 0 (0%) |
Polysomnography
For polysomnographic recordings, participants were given the opportunity to sleep undisturbed for nine hours during a lights-off period, starting between 11 PM and 12 PM, depending on habitual sleep times. The polysomnographic recordings took place at the in-patient clinic of ARQ Centrum’45, a Dutch national center for the diagnosis and treatment of PTSD. Participants slept at the department twice, in the context of a broad diagnostic assessment before treatment onset. Data for the current study were recorded on one of the two nights, with the order of the nights counterbalanced over participants and disease status (PTSD, trauma-control) through semi randomization. Polysomnography included an EEG recording (F3, F4, C4, O2, referenced to the average of the mastoids (M1, M2), two EOG electrodes monitoring eye-movements, and two electrodes for submental EMG (for the full montage see [23]). All signals were recorded with an ambulatory 16-channel Porti amplifiers (TMS-i) and Galaxy sleep analysis software (PHI international), sampled at a rate of 512Hz. Sleep stages were scored visually, according to the standard AASM criteria [46], in duplicate, by two highly experienced professionals (a sleep scientist/lab technician and a sleep scientist/clinician).
Sigma power
In a previously published study using this data set [23], we showed that NREM sleep of patients with PTSD showed a substantial loss of slow oscillation power, while higher-frequency activity, including sigma band activity, was increased compared with controls, especially over right-frontal areas. Importantly, however, the study was based on relative power measures, which—given the power-law distribution of EEG signals (that is, power tends to fall off with increasing frequency following a power-law function) means that changes in higher frequencies can partially reflect changes in lower frequencies. Thus, we here analyzed right-frontal absolute sigma power, which is independent of power in other frequencies. The sigma frequency content of the EEG (11–16 Hz) was analyzed using fast Fourier transform-based spectral analysis (4-second time windows with 50% overlap, 0.25 Hz bin size; Hamming window) on right-frontal electrode, F4, for N2 sleep. First, through visual inspection of the data, EEG epochs containing artifacts were removed. Next, for each frequency bin in the sigma band, the power per 30-second epoch was computed and summated over all epochs. Finally, absolute power bins were merged across frequencies, and power was divided by time in N2 for each participant.
Minimal assumptions spindle analysis
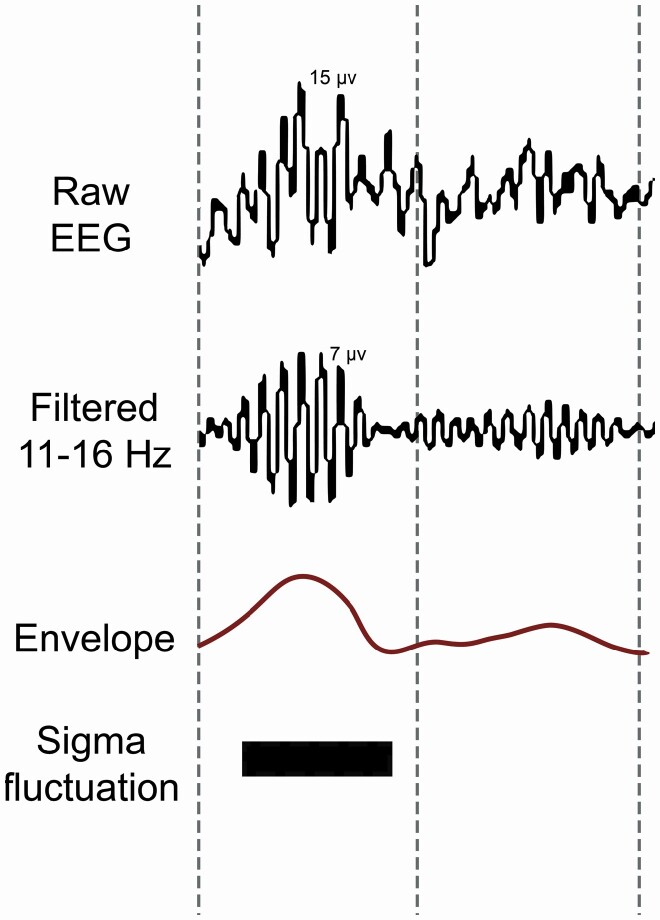
Automatized detection of discrete spindle events and computation of spindle event parameters, used a minimal assumptions spindle analysis algorithm (module within Galaxy sleep analysis software, see Figure 1), provided by PHI international, Amsterdam, The Netherlands. Specifically, sleep EEG data of electrode F4 (referenced to the average of the mastoids), were filtered in the sigma range (11–16 Hz) using a Finite Impulse Response (FIR) filter built into the algorithm (bandwidth 0.5 Hz). A FIR filter was chosen for its linear phase response and nonrecursive characteristics, to minimize filtering artifacts (which otherwise get introduced at the frequency boundaries of the filter’s bandwidth). With a moving window of 0.2 seconds, shifting per sample, the standard deviation of the filtered signal was calculated, resulting in a spindle envelope with a sample rate of 512 Hz. The size of the moving window was chosen to create a spindle envelope that follows the waxing and waning of sigma frequency oscillations faithfully, without following individual spindle oscillations at the lowest frequency in the filtered frequency range (11 Hz). The resulting envelope thus represents the burst-like shape of the signal. To convert the spindle envelope of the filtered signal to µV, the envelope was multiplied by √2. To define peaks and troughs, the first derivative (slope) of the envelope was calculated, after first down-sampling the data to 5 Hz (achieved by taking the root mean square of the envelope data over sequential stretches of 0.2 seconds). A peak was defined if the slope changed from positive to negative, a trough vice versa. A hysteresis of 0.2 µV was used to neglect low amplitude background noise in the envelope, while still including a broad range of spindle fluctuations for analyses. Next discrete spindle fluctuations were defined as the stretch of data from one trough to the next. In the current version of MASA, we did not apply a duration threshold, nor a temporal proximity threshold to merge adjacent spindle fluctuations, meaning that visual spindles may be split up into various detected spindle fluctuations. All resulting spindle fluctuations with amplitude between 5 and 35 µV were analyzed. This amplitude window was chosen (to some extent arbitrarily) to exclude fluctuations that might reflect background noise (<5 µV), as well as those that, given an amplitude > 35 µV, might also incorporate noise (sources other than spindle dynamics).
Figure 1.
Spindle detection method and definition of spindle parameters. The EEG signal was filtered into spindle frequency (11–16 Hz). With a moving window, the standard deviation of the filtered signal was calculated, resulting in a spindle envelope that follows the burst-like shape of the signal, from which spindle fluctuations are detected.
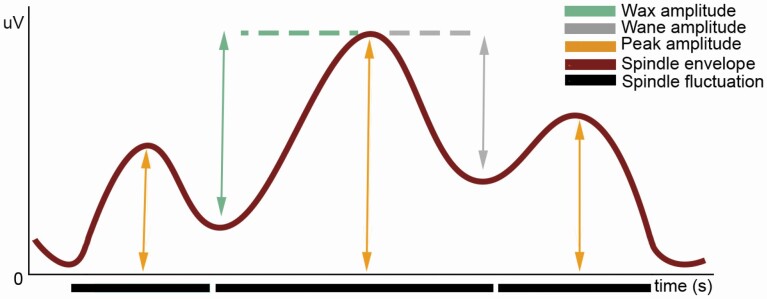
For each spindle fluctuation, four parameters were calculated: duration, peak amplitude (absolute maximal amplitude), waxing amplitude (difference between the absolute level of the peak and the absolute level of the trough prior to the peak), and waning amplitude (difference between the absolute level of the peak and the absolute level of the trough after the peak) (see Figure 2).
Figure 2.
Spindle fluctuation characteristics. From the spindle envelope, the following amplitude parameters are calculated: peak amplitude (absolute maximal amplitude), waxing amplitude (difference between the absolute level of the peak and the absolute level of the trough prior to the peak), and waning amplitude (difference between the absolute level of the peak and the absolute level of the trough after the peak).
Spindle index
In order to correlate spindle activity to clinical measures reflecting memory intrusions, we developed an index that captured the deviations of the PTSD spindle envelope into a single measure. To this purpose, we only considered spindle fluctuations that were significantly different between groups. These parameters were intercorrelated to assess the shared information content. The correlation matrix of the spindle parameters that were changed in the PTSD group (peak, wax, and wane amplitude) showed that wax and wane amplitude were highly intercorrelated (r = 0.989, p < 0.01). Thus, these two variables showed a similar information content and were subsequently averaged into a single variable for the creation of the spindle index. Wax and wane amplitude were each less correlated with peak amplitude (wax amplitude vs peak amplitude: r = −0.651, p < 0.01; wane amplitude vs peak amplitude: r = −0.660, p < 0.01). Therefore, a Spindle Amplitude Index (SAI) was calculated as peak amplitude divided by the average of the wax amplitude and wane amplitude.
Statistical analyses
As the spindle power and spindle parameter data were left normally and nonnormally distributed, statistical analyses were performed using nonparametric methods and all averages in the results are given as mean ranks. For nonparametric correlation analyses Spearman’s Rank test was used.
Results
Sigma power
Absolute right-frontal sigma power in N2 was compared between PTSD and controls using a two-tailed Mann–Whitney test. Right-frontal sigma power was increased in PTSD patients relative to controls by 144% (PTSD = 17.36, CTRL = 10.38, U = 44, p = 0.02, see Supplementary Information S2 for a table including all sleep macro architecture and sigma power). To control for possible medication effects on right-frontal sigma power, a Quade’s ANCOVA was conducted in which medication intake (yes/no) was added as a covariate. In this analysis, the increase in right-frontal sigma power in PTSD patients remained significant (F(1,27) = 4.323, p = 0.048), suggesting a PTSD-related sigma power increase independent of medication effects.
Spindle parameters
As a first analysis we assessed whether the density of spindle fluctuations (number of fluctuations/minute) differed between the PTSD and control participants. This was not the case (PTSD = 15.71, CTRL = 13.29, U = 81, p = 0.454). Given the nature of our method, visually detected spindles may sometimes hold several spindle fluctuations detected with MASA. Therefore, we repeated the analysis after merging fluctuations that were directly adjacent, again finding no difference between groups (PTSD = 14.6, CTRL = 14.4, U = 98, p = 0.945). Next, averages for each spindle fluctuation parameter were calculated over the entire distribution and compared between PTSD patients and controls, using a Mann–Whitney U-test. These tests revealed higher mean peak amplitude in PTSD participants (17.93) compared to controls (11.07; U = 50, p = 0.027). In contrast, local fluctuations between peaks and troughs in the spindle envelope were shallower in PTSD relative to controls (waxing amplitude: PTSD = 10.14, CTRL = 18.86, U = 37, p = 0.005; waning amplitude: PTSD = 10.14, CTRL = 18.86, U = 37, p = 0.004). No statistically significant difference in the duration of these local envelope fluctuations was found (PTSD = 12.00, CTRL = 17.00, p = 0.108). The combined evidence points to an inflated spindle envelope in PTSD patients that rarely drops to lower amplitude levels. This, in turn, suggests increased spindle activity in PTSD compared to controls.
To control for medication effects on these spindle parameters, Quade’s ANCOVAs were conducted, with medication intake (yes/no) as a covariate. For peak amplitude, the differences between groups reduced to trend-level significance (F(1,28) = 3.233, p = 0.084) after controlling for medication intake. The analyses suggest a minor influence of medication on peak amplitude. Differences between groups for local trough to peak fluctuations were unaffected by medication effects (F(1,28) = 6.940, p = 0.014).
In a subsequent analysis step, we examined, for each spindle parameter, whether the density (spindle fluctuations per minute) distribution was similar between the PTSD patient and control group, using a two-sample Kolmogorov-Smirnov test. This assessed whether differences between groups might be specific to a particular part of the distribution (e.g. to the smallest or the largest spindles). No differences were found (all ps > 0.1, see Supplementary Information S1).
To illustrate the higher sensitivity a MASA approach may have compared to a more traditional “tresholded approach,” we repeated the analyses on the spindle parameters using a 12.5 µV amplitude threshold, to mimic more classical spindle detection approaches. The results of these analyses and a comparative evaluation of the two approaches can be found in Supplementary Information S3.
Spindle activity & intrusive memory symptoms
To analyze whether changes in spindle activity were associated with PTSD intrusive memory complaints, spindle activity, indexed by the SAI (see methods) was correlated to the intrusive memory symptom score of the diagnostic tool for PTSD (total score of items under criterion B in CAPS-IV) using Spearman’s rank correlation coefficient test. This revealed a positive correlation (rs = 0.383, p = 0.045), indicating a link between increased spindle activity and memory intrusions of the traumatic event.
Next, we explored whether this correlation was present for all types of intrusive memory symptoms, by correlating the SAI to individual scores on the B items of the CAPS-IV. SAI was significantly correlated to memory intrusions (CAPS-B1 rs = 0.477, p = 0.010), reexperiencing (flashbacks of) the traumatic event (CAPS-B3 rs = 0.406, p = 0.032), emotional and physical responses to reminders of the trauma (respectively, CAPS-B4 rs = 0.412, p = 0.029, CAPS-B5 rs = 0.419, p = 0.026). The SAI showed only trend-level correlation to the frequency of trauma-related nightmares (CAPS-B2F rs = 0.322, p = 0.085) and no notable correlation to nightmare intensity (CAPS-B2I, p = 0.251). Together, these findings demonstrate that increased spindle activity is linked to intrusive memory symptoms during wake; the relationship between increased spindle activity and nightmares warrants further research.
Discussion
Using a minimal assumption spindle analysis (MASA), this study revealed increased spindle activity in PTSD patients compared to trauma-exposed controls. The increase was reflected in inflation of the spindle envelope. That is, spindle fluctuations had, on average, a higher peak amplitude and the throughs between peaks dropped less than in controls. In line with increased spindle activity, absolute sigma power was also increased. Furthermore, inflation of the spindle envelope was associated with more daytime, intrusive trauma memories.
A large body of evidence supports the relation between spindle activity and memory performance [15, 17, 47–49]. According to this literature, spindles reflect the reactivation and consolidation of cortical memory traces, enhancing post-sleep memory for the pertaining information. Spindles are largely local phenomena [50], reflecting reprocessing of the type of information encoded in a particular cortical area [13]. The increase in PTSD spindle activity in this study was found at a right-frontal location. Functionally, the right prefrontal cortex appears to have a role in processing of emotional and self-relevant information [32, 33, 35, 36, 51]. Speculatively, the observed increase in PTSD spindle activity in this area might reflect the excessive reprocessing and strengthening of trauma-related memories during sleep. Supporting this notion, spindle activity was positively correlated with daytime intrusive memory symptoms in PTSD patients. We found only a trend-level relationship between spindle activity and (frequency of) nighttime intrusive memory symptoms in PTSD, in the form of nightmares. Given the small sample size of our study and the relatively sparse incidence of nightmares this relation should be interpreted carefully.
The current study only assessed spindle activity at a right-frontal locus, based on findings of our previous study on the same dataset [23] showing spectral power alterations, including a sigma band increase, being maximal in this region. A spatially differentiated study on spindle activity may render important additional information. However, such a study would preferably differentiate between fast and slow spindles, which have a different EEG topology [52]. To do this based on a minimal assumption spindle detection method warrants further development.
Besides alterations in sleep spindling, SO dynamics are strongly impaired in PTSD [23]. SO’s synchronize sleep spindles and higher-frequency activity within and across cortical regions [21]. This is thought to support long-range communication across the brain, including the hippocampo-cortical cross-talk underlying system-level consolidation of episodic memories [21, 53]. Accordingly, several studies have found positive correlations between SO-spindle synchronization and memory consolidation ([54, 55]). The lack of coordinating SO activity (in PTSD) may undermine these system-level consolidation processes. As a result, event memory traces might be strengthened in their original hippocampus-dependent form, with preservation of episodic detail and emotional tone, rather than being integrated into memory networks as more general memories.
It might here be noted that the alterations in sleep spindles and slow oscillations discussed above are likely interlinked with other observed changes in PTSD sleep physiology, including changes in REM sleep’s spectral topology [13], neuroendocrine [27] and autonomic regulation [56]. These changes, which to some extent may reflect a state of hyperarousal during sleep, are all likely to affect information processing, as well as the normal play out of general recovery processes, during sleep.
A particular strength of this study was the use of a novel spindle detection method, involving minimal assumptions regarding spindle morphology. This method holds several benefits over traditional spindle detection methods using high, arbitrary thresholds. For one, criteria that base themselves on the norm, by definition neglect phenomenology outside of the norm, thus underestimating or altogether failing to detect abnormalities in nonnorm groups or conditions. Compounding the problem, arbitrary threshold, and baseline choices in traditional spindle detection methods can vastly affect analysis results, undermining findings’ robustness and comparability between studies. MASA could increase sensitivity, reproducibility, and comparability in spindle research, while combating arbitrarily chosen spindle detection threshold parameters, as urged by various experts in the field [15, 24].
Our study also has limitations, including a limited sample size. While the tight matching of patient and trauma-controls on a broad range of sociodemographic variables aids the power to detect PTSD-related differences, the uniform participant sample (treatment-seeking, mostly male, police officers, and veterans, with severe, chronic PTSD) warrants caution in extrapolating to other PTSD populations.
In summary, increased sleep spindle activity may provide a mechanism through which the profound sleep disturbances in PTSD contribute to intrusive memory symptoms. Besides their fundamental interest, these findings emphasize the need to take sleep-related symptoms into account in PTSD treatment strategies, both as a treatment target and as an outcome measure. While it might be tempting to consider treatments targeted at sleep spindle disruption, we would rather advocate treatments aimed at restoring healthy sleep, including restoration of sleep continuity and sleep depth and, therewith, normalizing spindling as well as other sleep physiological parameters.
This study constitutes a first effort to develop a minimal assumption approach to quantifying spindle activity in the EEG and illustrate its potential in a clinical study. Furthermore, method development and application of this approach to other datasets could strengthen the method’s potential. Applying minimal assumptions for spindle detection, may have powerful applications in psychiatric research due to a broad, uniform detection range, which is permissive towards pathology-associated physiological aberrancies, while increasing comparability between studies.
Supplementary Material
Acknowledgments
We thank the staff of ARQ Centrum‘45 for their involvement in patient recruitment and varied practical support that has been crucial to the realization of this study. We thank Anand Kumar from PHI International for his contribution and help regarding the spindle detection in this project.
Contributor Information
Anna C van der Heijden, Department of Psychology, Brain & Cognition, University of Amsterdam, Amsterdam, The Netherlands; Department of Psychiatry, Department of Anatomy and Neuroscience, Amsterdam UMC location Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Amsterdam Neuroscience, Mood Anxiety Psychosis Stress Sleep, Amsterdam, The Netherlands; GGZ inGeest Specialized Mental Health Care, Amsterdam, The Netherlands.
Winni F Hofman, Department of Psychology, Brain & Cognition, University of Amsterdam, Amsterdam, The Netherlands.
Marieke de Boer, Department of Psychology, Brain & Cognition, University of Amsterdam, Amsterdam, The Netherlands.
Mirjam J Nijdam, ARQ Centrum‘45, Oegstgeest, The Netherlands; ARQ National Psychotrauma Centre, Diemen, The Netherlands; Department of Psychiatry, Amsterdam UMC location AMC, Amsterdam, The Netherlands.
Hein J F van Marle, Department of Psychiatry, Department of Anatomy and Neuroscience, Amsterdam UMC location Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Amsterdam Neuroscience, Mood Anxiety Psychosis Stress Sleep, Amsterdam, The Netherlands; GGZ inGeest Specialized Mental Health Care, Amsterdam, The Netherlands.
Ruud A Jongedijk, ARQ Centrum‘45, Oegstgeest, The Netherlands; ARQ National Psychotrauma Centre, Diemen, The Netherlands.
Miranda Olff, Amsterdam Neuroscience, Mood Anxiety Psychosis Stress Sleep, Amsterdam, The Netherlands; ARQ National Psychotrauma Centre, Diemen, The Netherlands; Department of Psychiatry, Amsterdam UMC location AMC, Amsterdam, The Netherlands; Amsterdam Public Health Research Institute, Mental Health, Amsterdam, The Netherlands and.
Lucia M Talamini, Department of Psychology, Brain & Cognition, University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Brain and Cognition, University of Amsterdam, Amsterdam, The Netherlands.
Funding
None declared.
Disclosure Statement
None declared.
Data Availability
The data underlying this article cannot be shared publicly due to ethical and privacy regulations. Data available on request.
Preprint repositories: https://biorxiv.org/cgi/content/short/2021.07.29.453342v1
References
- 1. Kessler RC, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Gen Hosp Psychiatry. 1995;52:1048–1060. [DOI] [PubMed] [Google Scholar]
- 2. De Vries GJ, et al. The lifetime prevalence of traumatic events and posttraumatic stress disorder in the Netherlands. J Trauma Stress. 2009;22(4):259–267. [DOI] [PubMed] [Google Scholar]
- 3. Lewis C, et al. Sleep disturbance in post-traumatic stress disorder (PTSD): a systematic review and meta-analysis of actigraphy studies. Eur J Psychotraumatol. 2020;11(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spoormaker VI, et al. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12(3):169–184. [DOI] [PubMed] [Google Scholar]
- 6. Babson KA, et al. Temporal relations between sleep problems and both traumatic event exposure and PTSD: a critical review of the empirical literature. J Anxiety Disord. 2010;24(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pace-Schott EF, et al. Sleep and REM sleep disturbance in the pathophysiology of PTSD: the role of extinction memory. Biol Mood Anxiety Disord. 2015;5(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sopp MR, et al. Differential effects of sleep on explicit and implicit memory for potential trauma reminders: findings from an analogue study. Eur J Psychotraumatol. 2019;10(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koren D, et al. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159(5):855–857. [DOI] [PubMed] [Google Scholar]
- 10. Bryant RA, et al. Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep. 2010;33(1):69–74. doi: 10.1093/sleep/33.1.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smid GE, et al. Predictors of outcome and residual symptoms following trauma-focused psychotherapy in police officers with posttraumatic stress disorder. J Trauma Stress. 2018;31(5):764–774. [DOI] [PubMed] [Google Scholar]
- 12. Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437(7063):1272–1278. [DOI] [PubMed] [Google Scholar]
- 13. Cox R, et al. Local sleep spindle modulations in relation to specific memory cues. Neuroimage. 2014;99:103–110. [DOI] [PubMed] [Google Scholar]
- 14. Fogel SM, et al. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35(5):1154–1165. [DOI] [PubMed] [Google Scholar]
- 15. Mednick SC, et al. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci. 2013;33(10):4494–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaestner EJ, et al. Pharmacologically increasing sleep spindles enhances recognition for negative and high-arousal memories. J Cogn Neurosci. 2013;25(10):1597–1610. doi: 10.1162/jocn_a_00433 [DOI] [PubMed] [Google Scholar]
- 17. Schabus M, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2005;27(8):1479–1485. doi: 10.1093/sleep/27.7.1479 [DOI] [PubMed] [Google Scholar]
- 18. Petzka M, et al. Sleep spindles track cortical learning patterns for memory consolidation. Curr Biol. 2021;32(11):2349–2356. doi: 10.1101/2021.09.01.458569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cox R, et al. Involvement of spindles in memory consolidation is slow wave sleep-specific. Learn Mem. 2012;19(7):264–267. [DOI] [PubMed] [Google Scholar]
- 20. Cairney SA, et al. Targeted memory reactivation during slow wave sleep facilitates emotional memory consolidation. Sleep. 2014;37(4):701–707. doi: 10.5665/sleep.3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cox R, van Driel J, de Boer M, Talamini LM. Systems/circuits slow oscillations during sleep coordinate Interregional communication in cortical networks. J Neurosci. 2014;34(50):16890–16901. doi: 10.1523/JNEUROSCI.1953-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Staresina BP, et al. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat Neurosci. 2015;18(11):1679–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Boer MD, et al. The spectral fingerprint of sleep problems in posttraumatic stress disorder. Sleep. 2020;43(4). doi: 10.1093/sleep/zsz269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Warby SC, et al. Sleep-spindle detection: crowdsourcing and evaluating performance of experts, non-experts and automated methods. Nat Methods. 2014;11(4):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muzur A, et al. The prefrontal cortex in sleep. Trends Cogn Sci. 2002;6(11):475–481. [DOI] [PubMed] [Google Scholar]
- 26. Cowdin N, et al. Theta frequency activity during rapid eye movement (REM) sleep is greater in people with resilience versus PTSD. Exp Brain Res. 2014;232(5):1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang C, et al. An attempt to identify reproducible high-density EEG markers of PTSD during sleep. Sleep. 2020;43(1). doi: 10.1093/sleep/zsz207 [DOI] [PubMed] [Google Scholar]
- 28. Woodward SH, et al. PTSD-related hyperarousal assessed during sleep. Physiol Behav. 2000;70(1):197–203. [DOI] [PubMed] [Google Scholar]
- 29. Inslicht SS, et al. Sleep and hypothalamic pituitary adrenal axis responses to metyrapone in posttraumatic stress disorder. Psychoneuroendocrinology. 2018;88:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ross JA, et al. The role of catecholamines in modulating responses to stress: Sex-specific patterns, implications, and therapeutic potential for post-traumatic stress disorder and opiate withdrawal. Eur J Neurosci. 2020;52(1):2429–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Southwick SM, et al. Neurotransmitter alterations in PTSD: catecholamines and serotonin. Semin Clin Neuropsychiatry. 1999;4(4):242–248. [DOI] [PubMed] [Google Scholar]
- 32. Kao CY, et al. Norepinephrine and corticosterone in the medial prefrontal cortex and hippocampus predict PTSD-like symptoms in mice. Eur J Neurosci. 2015;41(9):1139–1148. [DOI] [PubMed] [Google Scholar]
- 33. Mizoguchi K. Stress and prefrontal cortical dysfunction in the rat. Prefrontal Cortex From Synaptic Plast to Cogn. 2004;153–174. [Google Scholar]
- 34. Gemignani A, et al. How stressful are 105 days of isolation? Sleep EEG patterns and tonic cortisol in healthy volunteers simulating manned flight to Mars. Int J Psychophysiol. 2014;93(2):211–219. [DOI] [PubMed] [Google Scholar]
- 35. Flo E, et al. Transient changes in frontal alpha asymmetry as a measure of emotional and physical distress during sleep. Brain Res. 2011;1367:234–249. [DOI] [PubMed] [Google Scholar]
- 36. Sikka P, et al. EEG frontal alpha asymmetry and dream affect: alpha oscillations over the right frontal cortex during rem sleep and presleep wakefulness predict anger in REM sleep dreams. J Neurosci. 2019;39(24):4775–4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schimicek P, et al. Automatic sleep-spindle detection procedure: aspects of reliability and validity. Clin Electroencephalogr. 2016;25(1):26–29. [DOI] [PubMed] [Google Scholar]
- 38. Huupponen E, et al. Optimization of sigma amplitude threshold in sleep spindle detection. J Sleep Res. 2000;9(4):327–334. [DOI] [PubMed] [Google Scholar]
- 39. Manoach DS, et al. Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource. Nat Commun. 2017;8(May):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. He J, et al. Effect of conditioned stimulus exposure during slow wave sleep on fear memory extinction in humans. Sleep. 2015;38(3):423–431. doi: 10.5665/sleep.4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang D, et al. A robust two-stage sleep spindle detection approach using single-channel EEG You may also like Spindler: a framework for parametric analysis and detection of spindles in EEG with application to sleep spindles. J Neural Eng. 2021;18:1–13. [DOI] [PubMed] [Google Scholar]
- 42. Bonjean M, et al. Corticothalamic feedback controls sleep spindle duration in vivo. J Neurosci. 2011;31(25):9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Steriade M, et al. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–685. [DOI] [PubMed] [Google Scholar]
- 44. Steriade M. The corticothalamic system in sleep. Front Biosci. 2003;8:878–899. [DOI] [PubMed] [Google Scholar]
- 45. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (IV). 1994. [Google Scholar]
- 46. Iber C. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Am Acad Sleep Med. [Google Scholar]
- 47. Fernandez LMJ, et al. Sleep spindles: mechanisms and functions. Physiol Rev. 2020;100(2):805–868. [DOI] [PubMed] [Google Scholar]
- 48. van der Helm, et al. Sleep-dependent facilitation of episodic memory details. PLoS One. 2011;6(11):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rauchs G, et al. Is there a link between sleep changes and memory in Alzheimer’s disease? Neuroreport. 2008;19(11):1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Piantoni G, et al. Spatiotemporal characteristics of sleep spindles depend on cortical location. Neuroimage. 2017;146(June 2016):236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. del Giudice R, et al. Oscillatory brain responses to own names uttered by unfamiliar and familiar voices. Brain Res. 2014;1591:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zeitlhofer J, et al. Topographic distribution of sleep spindles in young healthy subjects. J Sleep Res. 1997;6(3): 149–155. [DOI] [PubMed] [Google Scholar]
- 53. Mölle M, et al. Slow oscillations orchestrating fast oscillations and memory consolidation. Prog Brain Res. 2011;193:93–110. [DOI] [PubMed] [Google Scholar]
- 54. Lendner J, et al. Bidirectional prefrontal-hippocampal dynamics organize information transfer during sleep in humans. Nat Commun. 2019;10(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Muehlroth BE, et al. Precise slow oscillation–spindle coupling promotes memory consolidation in younger and older adults. Sci Rep. 2019;9(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Van Liempt S, et al. Sympathetic activity and hypothalamo-pituitary-adrenal axis activity during sleep in post-traumatic stress disorder: a study assessing polysomnography with simultaneous blood sampling. Psychoneuroendocrinology. 2013;38(1):155–165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to ethical and privacy regulations. Data available on request.
Preprint repositories: https://biorxiv.org/cgi/content/short/2021.07.29.453342v1