This diagnostic study investigates the accuracy of targeted axillary dissection (radioactive iodine seed placement in the axilla with sentinel lymph node biopsy [RISAS]) after neoadjuvant chemotherapy in patients who have clinically node-positive breast cancer.
Key Points
Question
What is the diagnostic accuracy of targeted axillary dissection after neoadjuvant chemotherapy in patients who have clinically node-positive breast cancer?
Findings
In this multicenter diagnostic study including 212 patients, the false-negative rate of targeted axillary dissection (ie, radioactive iodine seed placement in the axilla with sentinel lymph node biopsy [RISAS] procedure) was 3.5%. The negative predictive value was 92.8%, meaning that residual disease was missed in only 1 of 14 patients with a pathologic complete response in the RISAS nodes.
Meaning
These findings suggest the potential for worldwide implementation of targeted axillary dissection to enable response-based management of the axilla and to guide adjuvant (systemic) treatment strategies.
Abstract
Importance
Several less-invasive staging procedures have been proposed to replace axillary lymph node dissection (ALND) after neoadjuvant chemotherapy (NAC) in patients with initially clinically node-positive (cN+) breast cancer, but these procedures may fail to detect residual disease. Owing to the lack of high-level evidence, it is not yet clear which procedure is most optimal to replace ALND.
Objective
To determine the diagnostic accuracy of radioactive iodine seed placement in the axilla with sentinel lymph node biopsy (RISAS), a targeted axillary dissection procedure.
Design, Setting, and Participants
This was a prospective, multicenter, noninferiority, diagnostic accuracy trial conducted from March 1, 2017, to December 31, 2019. Patients were included within 14 institutions (general, teaching, and academic) throughout the Netherlands. Patients with breast cancer clinical tumor categories 1 through 4 (cT1-4; tumor diameter <2 cm and up to >5 cm or extension to the chest wall or skin) and pathologically proven positive axillary lymph nodes (ie, clinical node categories cN1, metastases to movable ipsilateral level I and/or level II axillary nodes; cN2, metastases to fixed or matted ipsilateral level I and/or level II axillary nodes; cN3b, metastases to ipsilateral level I and/or level II axillary nodes with metastases to internal mammary nodes) who were treated with NAC were eligible for inclusion. Data were analyzed from July 2020 to December 2021.
Intervention
Pre-NAC, the marking of a pathologically confirmed positive axillary lymph node with radioactive iodine seed (MARI) procedure, was performed and after NAC, sentinel lymph node biopsy (SLNB) combined with excision of the marked lymph node (ie, RISAS procedure) was performed, followed by ALND.
Main Outcomes and Measures
The identification rate, false-negative rate (FNR), and negative predictive value (NPV) were calculated for all 3 procedures: RISAS, SLNB, and MARI. The noninferiority margin of the observed FNR was 6.25% for the RISAS procedure.
Results
A total of 212 patients (median [range] age, 52 [22-77] years) who had cN+ breast cancer underwent the RISAS procedure and ALND. The identification rate of the RISAS procedure was 98.2% (223 of 227). The identification rates of SLNB and MARI were 86.4% (197 of 228) and 94.1% (224 of 238), respectively. FNR of the RISAS procedure was 3.5% (5 of 144; 90% CI, 1.38-7.16), and NPV was 92.8% (64 of 69; 90% CI, 85.37-97.10), compared with an FNR of 17.9% (22 of 123; 90% CI, 12.4%-24.5%) and NPV of 72.8% (59 of 81; 90% CI, 63.5%-80.8%) for SLNB and an FNR of 7.0% (10 of 143; 90% CI, 3.8%-11.6%) and NPV of 86.3% (63 of 73; 90% CI, 77.9%-92.4%) for the MARI procedure. In a subgroup of 174 patients in whom SLNB and the MARI procedure were successful and ALND was performed, FNR of the RISAS procedure was 2.5% (3 of 118; 90% CI, 0.7%-6.4%), compared with 18.6% (22 of 118; 90% CI, 13.0%-25.5%) for SLNB (P < .001) and 6.8% (8 of 118; 90% CI, 3.4%-11.9%) for the MARI procedure (P = .03).
Conclusions and Relevance
Results of this diagnostic study suggest that the RISAS procedure was the most feasible and accurate less-invasive procedure for axillary staging after NAC in patients with cN+ breast cancer.
Introduction
Patients with clinically node-positive (cN+) breast cancer are often treated with neoadjuvant chemotherapy (NAC). As a result of NAC, approximately one-third of patients with cN+ breast cancer convert to a pathologic complete response (pCR) of the axilla.1 The axillary pCR rate depends on subtype and may be as high as 74% in ERBB2 (formerly HER2 or HER2/neu)–positive breast cancer.2,3,4 Until recently, patients with cN+ breast cancer were routinely treated with axillary lymph node dissection (ALND), irrespective of treatment response. However, patients with cN+ breast cancer who achieve axillary pCR are not expected to benefit from ALND.
Over the past years, several less-invasive staging procedures have been proposed to enable response-based management of the axilla in patients with cN+ breast cancer: sentinel lymph node biopsy (SLNB),5,6,7 excision of the pretreatment-marked positive lymph node (eg, marking axillary lymph node with radioactive iodine seed [MARI] procedure),8,9 and procedures combining SLNB and excision of the pretreatment-marked positive lymph node (eg, targeted axillary dissection).10,11 However, these procedures are of varying accuracy and may fail to detect chemotherapy-resistant residual axillary disease compared with ALND, although it is yet unclear whether this affects prognosis. Therefore, the preferred procedure to replace ALND should be the one with the lowest risk of missing residual axillary disease, ie, the number of false-negative results should be as small as possible.
In a meta-analysis1 including 17 studies on the diagnostic accuracy of SLNB in patients with cN+ breast cancer treated with NAC, the overall false-negative rate (FNR) was 17%. The negative predictive value (NPV) was 86% at best, which means residual axillary disease is missed in at least 1 in 6 patients with tumor-free SLNs. The MARI procedure was developed as an alternative to SLNB: the pathologically proven positive lymph node is marked with a radioactive iodine seed before NAC and excised after NAC. In a single-center prospective trial of 100 patients with cN+ breast cancer, the MARI procedure was associated with an FNR of 7% and an NPV of 83.3%.8 Thus, residual axillary disease may still be missed in cases of a tumor-free MARI node. Three trials10,12,13 evaluated the diagnostic accuracy of targeted axillary dissection: the FNR ranged from 2% to 4%, and the NPV ranged from 92% to 97%. These results are promising, but evidence is limited owing to their study designs and relatively small sample sizes, ranging from 35 to 85 patients.
This prospective, multicenter trial was designed to determine the diagnostic accuracy of the combination of SLNB and MARI, referred to as the radioactive iodine seed placement in the axilla with sentinel lymph node biopsy (RISAS) procedure, for axillary staging after NAC in patients with cN+ breast cancer. The study protocol has been published previously.14 It was hypothesized that the RISAS procedure would be noninferior to ALND for axillary staging and superior to the separate SLNB and MARI procedure.
Methods
Study Design
The RISAS trial was a single-group, prospective, multicenter validation trial. The primary objective was to determine the diagnostic accuracy of SLNB combined with the MARI procedure for axillary staging after NAC in patients with cN+ breast cancer. Secondary objectives included the accuracy of the SLNB and the MARI procedure separately. The medical ethics review committee of the Erasmus Medical Center (Rotterdam, the Netherlands) approved this study, and written informed consent was obtained from all patients. Fourteen institutions participated in this trial, of which 13 institutions actively accrued patients. The review boards of all participating centers approved trial participation. All participating institutions had prior experience with the use of iodine seeds for localization of breast lesions. The trial was registered on ClinicalTrials.gov15 and was funded by the Dutch Cancer Society. This study followed the Standards for Reporting of Diagnostic Accuracy (STARD) reporting guidelines.
Eligibility Criteria
Female patients 18 years or older were eligible for the study. In addition, patients with breast cancer clinical tumor categories 1 through 4 (cT1-4; tumor diameter <2 cm and up to >5 cm or extension to the chest wall or skin) and clinical node categories cN1 (metastases to movable ipsilateral level I and/or level II axillary nodes), cN2 (metastases to fixed or matted ipsilateral level I and/or level II axillary nodes), or cN3b (metastases to ipsilateral level I and/or level II axillary nodes with metastases to internal mammary nodes) and who were treated with NAC were eligible for inclusion. Nodal positivity had to be confirmed with either fine-needle aspiration cytology or core-needle biopsy before NAC. Patients with positive infraclavicular or supraclavicular lymph nodes, patients with (oligo)metastatic breast cancer, and patients with prior surgery or radiotherapy to the ipsilateral axilla (including SLNB before NAC) were excluded. Patient race and ethnicity data were not gathered for this study; it was not hypothesized that race or ethnicity would be associated with the diagnostic accuracy of the investigated procedure.
Neoadjuvant Chemotherapy
Recommendations for systemic therapy regimens were based on national guidelines. NAC generally included anthracycline- and/or taxane-based regimens. In patients with ERBB2-positive breast cancer, ERBB2-targeted therapy (trastuzumab with or without pertuzumab) was added to the chemotherapy regimen.
The RISAS Procedure
The RISAS procedure consisted of both the SLNB and the MARI procedure. Before NAC, patients underwent ultrasound-guided placement of a radioactive iodine seed within the pathologically proven positive lymph node. The seeds labeled with radioactive iodine I 125 (125I) had a maximum activity of 7.4 MBq and a half-life of 60 days. If multiple lymph nodes were suspicious, the lymph node with the most suspicious morphology on ultrasonography was marked. In 1 institution, a protocol deviation took place regarding iodine seed placement: in a small subset of patients, a clip instead of an iodine seed was placed before NAC followed by iodine seed placement after NAC. The study protocol recommended dual-tracer technique for SLNB, but this was not obligated. If a radioactive tracer was used, technetium Tc 99m nanocolloid was injected on the day of or on the day before the surgical procedure followed by lymphoscintigraphy. If blue dye was used, this was injected at the start of the operation and followed by massage of the injection site. The protocol did not require a minimum number of lymph nodes to be excised for the RISAS procedure. Excision of the lymph node containing the iodine seed was confirmed with a gamma probe and/or a specimen radiograph. All hot and/or blue lymph nodes were considered SLNs. Non-SLNs, such as palpable suspicious lymph nodes, were removed at the discretion of the surgeon. During the operation, the RISAS procedure was followed by ALND.
Histopathologic Evaluation
All lymph nodes were stained with hematoxylin and eosin and the pathology outcome was reported separately for the lymph node containing the iodine seed, the SLN(s), and the remaining lymph nodes of the ALND specimen. Axillary pCR was defined as the absence of residual disease, including the absence of isolated tumor cells and micrometastases. On-site use of immunohistochemistry (IHC) was not mandatory. Slides of the RISAS lymph nodes that were considered negative after on-site evaluation were centrally reviewed by a single pathologist (P.J.v.D.). In cases with less than 3 to 5 levels examined, additional sectioning up to the fifth level was done. IHC was performed on all levels (in case this was not performed on-site, including the additional levels).
Power and Sample Size Calculation
This trial was set up to determine if the RISAS procedure would be noninferior to the criterion standard, ALND. The null hypothesis of inferiority would be rejected at a significance level of 5% if the upper bound of the 2-sided 90% Clopper-Pearson CI of the observed FNR was below the noninferiority margin of 6.25%. This margin was considered clinically acceptable as it is far below the threshold of 10% that is generally considered for SLNB. For example, if 144 included patients had a positive ALND, the null hypothesis of inferiority could be rejected if the number of FN results was less than or equal to 4 (FNR 2.78%; 90% CI, 0.95%-6.24%). Assuming an FNR of 2%, a prevalence of a positive ALND of 64%, and a 10% dropout rate, a sample size of 248 patients was needed (with 84% power to reject the null hypothesis). An FNR of 6.25% (the noninferiority margin) corresponded to a NPV of 90%.
Statistical Analysis
The identification rate, the FNR, and the NPV were calculated for the RISAS procedure and for the SLNB and the MARI procedure separately. The identification rate was defined as the number of patients in whom the procedure was successful divided by the total number of patients in whom the procedure was attempted. The procedure was considered successful if at least 1 lymph node could be identified (an SLN and/or a MARI node). All SLN(s) and/or MARI nodes together were considered RISAS lymph nodes. If the surgeon identified a supposed SLN or MARI node, but no lymph node could be identified by the pathologist (eg, only subcutaneous tissue was found), the procedure was recorded as unsuccessful. The MARI node was considered to be an SLN if the surgeon documented that the MARI node was hot and/or blue or if the pathologist documented that the MARI node was blue. If the iodine seed appeared to be located in another lymph node than initially reported by the surgeon, the lymph node containing the iodine seed identified by the pathologist was recorded as the MARI node.
The FNR was defined as the number of FN results divided by the total number of patients with residual axillary disease, the sum of FN results plus true positive (TP) results = [FN / (FN + TP)]. The NPV was defined as the number of true negative (TN) results divided by the total number of patients with a negative test outcome = [TN / (TN + FN)]. An FN result occurred if the RISAS procedure incorrectly indicated axillary pCR (ie, the remaining lymph nodes of the ALND specimen did contain residual axillary disease).
The 1-sided McNemar exact test was used to test the null hypothesis that the FNR of the RISAS procedure was equal to the FNR of SLNB and of the MARI procedure. Statistical analysis was performed using the Statistical Package for the Social Sciences software, version 26 (IBM Corp). Data were analyzed from July 2020 to December 2021.
Results
A total of 252 patients provided informed consent from March 1, 2017, until December 31, 2019, of whom 227 patients underwent the RISAS procedure followed by completion ALND in 212 patients (median [range] age, 52 [22-77] years) (Figure 1A). The majority of patients had hormone receptor (HR)–positive/ERBB2-negative breast cancer (Table 1). The overall breast pCR (ypT0) rate was 30.5% (64 of 210), and the axillary pCR (ypN0) rate was 35.4% (75 of 212). Axillary pCR differed by subtype: 12.7% (13 of 102) for HR-positive/ERBB2-negative, 58.1% (25 of 43) for HR-positive/ERBB2-positive, 77.3% (17 of 22) for HR-negative/ERBB2-positive and 44.4% (20 of 45) for triple-negative breast cancer (P < .001).
Figure 1. Consolidated Standards of Reporting Trials (CONSORT) Diagram and Flowchart.
A, CONSORT diagram for the radioactive iodine seed placement in the axilla with sentinel lymph node biopsy (RISAS) procedure. B, Flowchart of patients who underwent sentinel lymph node biopsy (SLNB) and the marking of a pathologically confirmed positive axillary lymph node with radioactive iodine seed (MARI) procedure. In 3 of 9 patients with lymph node–negative (ypN0) status based on SLNB, residual disease was found in the MARI node. ALND indicates axillary lymph node dissection; cN+, clinically node positive; FN, false negative; NAC, neoadjuvant chemotherapy; TN, true negative; TP, true positive; ypN+, lymph node metastasis.
aAt the patients’ request, only the RISAS procedure without completion ALND was performed (this decision was always made before the surgical procedure took place).
bThese numbers represent all patients in whom SLNB was performed (ie, RISAS procedure in 227 patients and SLNB only [without MARI] in 1 patient for a total of 228 patients for SLNB) or in whom the MARI procedure was performed (ie, RISAS procedure in 227 patients and MARI only [without SLNB] in 11 patients for a total of 238 patients for MARI).
Table 1. Patient and Tumor Characteristics.
| Characteristic | No. (%) |
|---|---|
| All patients | 212 (100) |
| Age, median (range), y | 52 (22-77) |
| cT statusa | |
| 0 | 2 (0.9) |
| 1 | 26 (12.3) |
| 2 | 128 (60.4) |
| 3 | 49 (23.1) |
| 4 | 7 (3.3) |
| Multifocality | |
| No | 158 (74.5) |
| Yes | 54 (25.5) |
| cN status | |
| 1 | 154 (72.6) |
| 2 | 44 (20.8) |
| 3b | 14 (6.6) |
| Stage | |
| II | 155 (73.1) |
| III | 157 (26.9) |
| Subtype | |
| HR+/ERBB2−b | 102 (48.1) |
| HR+/ERBB2+ | 43 (20.3) |
| HR−/ERBB2+ | 22 (10.4) |
| Triple negative | 45 (21.2) |
| Type of breast surgery | |
| Lumpectomy | 119 (56.1) |
| Mastectomy | 93 (43.9) |
Abbreviations: cN, clinical node status; cT, clinical tumor status; HR, hormone receptor.
cT status: 0, no evidence of primary tumor; 1, tumor is less than or equal to 2 cm in diameter; 2, tumor is greater than 2 cm but less than 5 cm in diameter; 3, tumor is greater than 5 cm in diameter; 4, tumor has extended to the chest wall or skin.
Formerly HER2 or HER2/neu.
RISAS Procedure
The RISAS procedure was successful in 223 of 227 patients (ie, at least 1 SLN and/or MARI node was identified), which resulted in an identification rate of 98.2%. The ALND specimen contained residual axillary disease in 2 of the 4 patients (50%) in whom the RISAS procedure was unsuccessful. A mean (SD) of 1.8 (1.1) lymph nodes was removed with the RISAS procedure (median [range], 2 [1-8] lymph nodes). In 35 of 223 patients (15.7%), either only the SLNB or only the MARI procedure was successful. In 188 of 223 patients (84.3%), both the SLNB and the MARI procedure were successful: the MARI node was also an SLN in 134 of 188 patients (71.3%). Residual axillary disease was located in either the SLN(s) or the MARI node in 19% of patients.
In 208 of 223 patients (93.3%) in whom the RISAS procedure was successful, completion ALND was performed, and these patients were included in the accuracy analysis (Figure 1A). Based on the on-site pathology evaluation of the RISAS lymph nodes, 73 patients had axillary pCR, 7 patients had an FN RISAS result, and 128 patients had residual axillary disease. Central pathology review was performed on the RISAS slides of the 80 patients with on-site axillary pCR in the RISAS lymph nodes. Two patients did not have tissue available for additional sectioning. Central review revealed residual disease in 11 patients (based on revision of existing slides, n = 4; based on review of slides from additional sectioning, n = 7 [including 2 patients who initially had an FN RISAS result]). The residual disease consisted of macrometastasis in 1 patient (Figure 2) and isolated tumor cells and/or micrometastasis in the remaining patients. In all but 1 patient in whom central pathology review revealed residual disease, the lymph nodes showed signs of regression.
Figure 2. Digital Hematoxylin and Eosin–Stained Tissue Sections From a Radioactive Iodine Seed Placement in the Axilla With Sentinel Lymph Node Biopsy (RISAS) Lymph Node That Underwent Central Pathology Review.
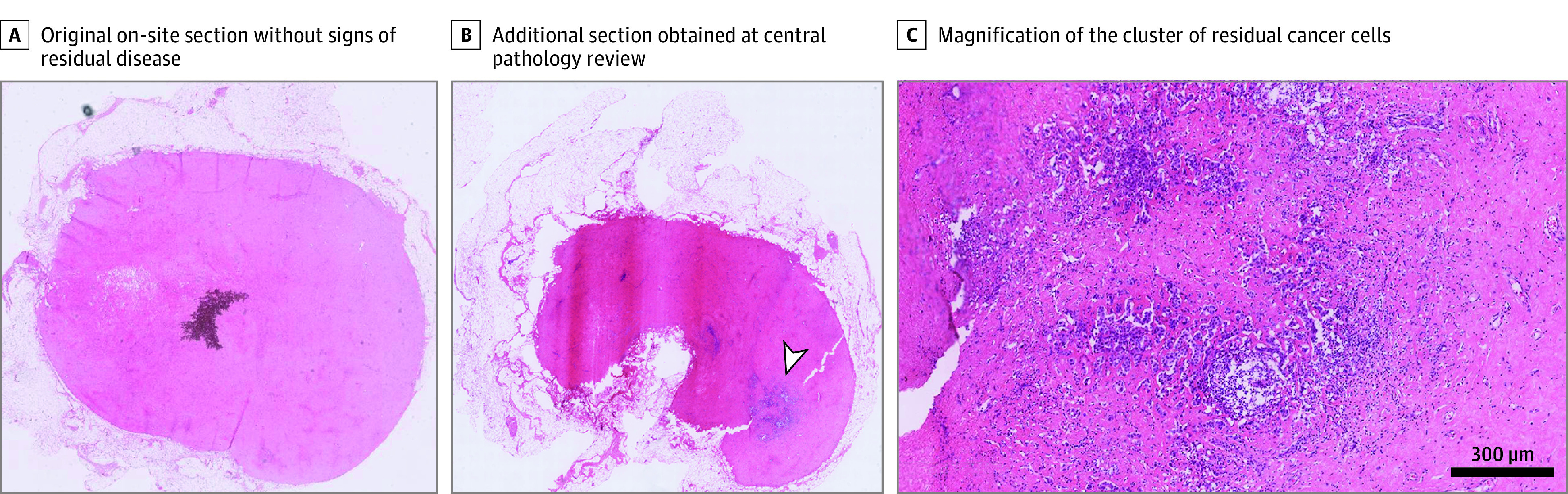
A, The original on-site section without signs of residual disease. B, An additional section obtained at central pathology review with a large cluster (>2 mm) of residual cancer cells (arrowhead). C, Magnification of the cluster of residual cancer cells from panel (B).
In summary, 64 patients had a TN result, 5 patients had an FN result, and 139 patients had a TP result after central pathology review. This yielded an FNR of 3.5% (5 of 144; 90% CI, 1.38-7.16) and an NPV of 92.8% (64 of 69; 90% CI, 85.37-97.10) (Table 2). The residual disease in the completion ALND consisted of macrometastasis in 4 patients (3 patients with 1 macrometastatic lymph node and 1 patient with 2 macrometastatic lymph nodes) and micrometastasis in 1 patient (in 2 lymph nodes). All FN results occurred in different institutions, and 4 of 5 FN results (80%) occurred within the first 10 included patients of the involved institutions. Table 3 shows characteristics of patients with an FN result. In 81 of 139 patients (58.3%) with positive RISAS lymph nodes, the completion ALND specimen contained additional positive nodes.
Table 2. Identification Rate and Accuracy of the Radioactive Iodine Seed Placement in the Axilla With Sentinel Lymph Node Biopsy (RISAS) Procedure, Sentinel Lymph Node Biopsy (SLNB), and the Marking of a Pathologically Confirmed Positive Axillary Lymph Node With Radioactive Iodine Seed (MARI) Procedurea .
| Characteristic | No./total No. (%) | ||
|---|---|---|---|
| RISAS | SLNB | MARI | |
| Whole cohort b | |||
| Identification rate | 223/227 (98.2) | 197/228 (86.4) | 224/238 (94.1) |
| FNR | 5/144 (3.5) | 22/123 (17.9) | 10/143 (7.0) |
| NPV | 64/69 (92.8) | 59/81 (72.8) | 63/73 (86.3) |
| Risk of missing residual disease when staging procedure indicates axillary pCR (1 in No. of patients)c | 1 in 13.8 (69/5) | 1 in 3.7 (81/22) | 1 in 7.3 (73/10) |
| Subgroup analysis | |||
| Identification rate | 174/174 (100) | 174/174 (100) | 174/174 (100) |
| FNRd | 3/118 (2.5) | 22/118 (18.6) | 8/118 (6.8) |
| NPV | 56/59 (94.9) | 56/78 (71.8) | 56/64 (87.5) |
| Risk of missing residual disease when staging procedure indicates axillary pCR (1 in No. of patients)c | 1 in 19.7 (59/3) | 1 in 3.6 (78/22) | 1 in 8.0 (64/8) |
Abbreviations: FNR, false-negative rate; NPV, negative predictive value; pCR, pathologic complete response.
Provided separately based on analysis of the whole cohort and based on analysis of a subgroup of patients within whom both the SLNB and MARI procedures were successful.
Accuracy analysis for the RISAS procedure was based on 208 patients, for SLNB was based on 182 patients, and for the MARI procedure was based on 206 patients.
Based on NPV rates of the different staging procedures.
There was a statistically significant difference in FNR between the RISAS procedure and SLNB (P < .001) and between the RISAS and MARI procedures (P = .03).
Table 3. Characteristics of Patients With a False Negative Radioactive Iodine Seed Placement in the Axilla With Sentinel Lymph Node Biopsy (RISAS) Result (n = 5).
| Age, y | Subtype | Clinical tumor status | Suspicious lymph node pre-NAC | Breast pCR |
|---|---|---|---|---|
| 68 | HR+/ERBB2−a | T2 | <4 | No |
| 63 | HR+/ERBB2− | T2 | ≥4 | No |
| 58 | HR+/ERBB2+ | T3 | ≥4 | Yes |
| 48 | Triple negative | T2 | ≥4 | No |
| 51 | Triple negative | T3 | ≥4 | Yes |
Abbreviations: NAC, neoadjuvant chemotherapy; pCR, pathologic complete response.
Formerly HER2 or HER2/neu.
The number of FN results was 2 for patients with cN1, 3 for patients with cN2, and 0 for patients with cN3b status. Owing to the small number of patients in these subgroups, FNR and NPV were not provided separately dependent on cN status.
SLNB
The SLNB was successful in 197 of 228 patients (86.4%). Sampling was performed with Tc 99m nanocolloid with or without blue dye in 215 of 228 (94.3%) patients (identification rate, 88.4% [190 of 215]). In the remaining 13 patients, sampling was performed with blue dye only (identification rate, 53.8% [7 of 13]). A total of 182 patients in whom SLNB was successful and completion ALND was performed were included in the accuracy analysis (Figure 1B). In 22 patients, no residual axillary disease was found in the SLN(s), whereas residual axillary disease was found in the nodes of the MARI procedure and/or completion ALND. This yielded an FNR of 17.9% (22 of 123; 90% 12.4%-24.5%) and an NPV of 72.8% (59 of 81; 90% 63.5%-80.4%) (Table 2).
One patient had lymph drainage to the contralateral axilla on lymphoscintigraphy and underwent bilateral axillary surgery; the contralateral SLN was tumor free, and the ipsilateral MARI node (which also appeared to be a hot node) contained a macrometastasis. In 17 of the 20 lymph nodes (85%) in the ipsilateral completion ALND specimen, macrometastases were found. One patient sustained an anaphylactic shock attributable to blue dye. A surgical procedure was performed successfully 2 weeks later, and the patient fully recovered from the adverse event.
MARI Procedure
In 224 of 238 patients (94.1%) the MARI procedure was successful. A total of 206 patients in whom the MARI procedure was successful and completion ALND was performed were included in the accuracy analysis (Figure 1B). In 10 patients, no residual axillary disease was found in the MARI lymph node, whereas residual axillary disease was found in the nodes of the SLNB and/or completion ALND. This yielded an FNR of 7.0% (10 of 143; 90% CI, 3.8%-11.6%) and an NPV of 86.3% (63 of 73; 90% CI, 77.9%-92.4%) (Table 2).
Comparison of Accuracy of the RISAS Procedure With SLNB and the MARI Procedure
In 174 patients, both the SLNB and MARI procedure were successful and a completion ALND was performed. For this subgroup of patients, 56 patients (32.2%) had axillary pCR, and 118 patients (67.8%) had residual axillary disease. The FNR was 2.5% (3 of 118; 90% CI, 0.7%-6.4%) for the RISAS procedure, compared with 18.6% (22 of 118; 90% CI, 13.0%-25.5%; P < .001) for SLNB and 6.8% (8 of 118; 90% CI, 3.4%-11.9%; P = .03) for MARI (Table 2). The NPV was 94.9% (56 of 59) for the RISAS procedure, 71.8% (56 of 78) for SLNB, and 87.5% (56 of 64) for MARI.
Discussion
To our knowledge, this was the first prospective validation trial with a multicenter design, rather than a registry study, of patients with cN+ breast cancer who were treated with NAC. Results demonstrated that the RISAS procedure, a combination of SLNB and the MARI procedure, was associated with an FNR of 3.5% and an NPV of 92.8%. The upper bound of the confidence interval (2-sided 90% CI, 1.38%-7.16%) slightly exceeded the prespecified noninferiority margin of 6.25%, yet the difference was small, and therefore, it is expected that ALND added little to the detection of residual disease compared with the RISAS procedure. The RISAS procedure was associated with superior diagnostic accuracy compared with SLNB and MARI (FNR of 17.9% and 7% and NPV of 72.8% and 86.3%, respectively). Moreover, the RISAS procedure was associated with the highest identification rate of 98%; thus, only 2% of patients needed ALND to determine axillary treatment response. The results of the current trial corroborate results from previous studies, and the evidence is mounting that RISAS (ie, a targeted axillary dissection procedure) may be the preferred procedure to replace ALND for axillary staging.
In the current trial, results suggest that the RISAS procedure had 2 advantages over sole performance of either SLNB or MARI. First, the RISAS procedure was associated with an excellent identification rate of 98.2%. If only SLNB had been performed, approximately 15% of patients would have had no nodes identified and would have to undergo ALND to determine the axillary treatment response. The MARI procedure also had a lower identification rate than that of the RISAS procedure (94.1%). Second, the RISAS procedure was associated with improved accuracy, with a significantly lower FNR compared with SLNB and the MARI procedure. This was not only explained by the better identification rate; the MARI node may be tumor free, whereas the SLN harvests residual disease and vice versa. Reasons to explain this finding may include blockage of lymph drainage by residual tumor or a heterogeneous tumor response among axillary lymph nodes. The improved accuracy of procedures like RISAS is probably not simply attributable to the excision of more lymph nodes. The median number of lymph nodes excised with the RISAS procedure was only 2. Therefore, when SLNB is combined with MARI procedure, it is not necessary to harvest a minimum of 3 SLNs, in contrast to the separate SLNB.16
Axillary staging should not only serve to prevent unnecessary ALND in patients with an axillary pCR but also to accurately detect residual disease to guide adjuvant treatment decisions. The Trastuzumab Emtansine for Residual Invasive ERBB2-Positive Breast Cancer (KATHERINE)17 and Adjuvant Capecitabine for ERBB2-Negative Breast Cancer After Preoperative Chemotherapy (CREATE-X)18 trials showed that patients with residual disease may benefit from additional adjuvant systemic therapy (trastuzumab emtansine for ERBB2-positive and capecitabine for ERBB2-negative breast cancer) in terms of decreased risk of recurrence. Consequently, accurate assessment of treatment response is pivotal. Because the RISAS procedure was associated with the lowest FNR, it carries the lowest risk of missing residual disease (and thus the lowest risk of missing out on adjuvant systemic therapy).
It is unknown whether omission of ALND affects oncologic safety in terms of disease-free and overall survival. Several randomized controlled trials are currently assessing these end points, both for patients with axillary pCR (Axillary Management in Breast Cancer Patients With Needle Biopsy–Proven Nodal Metastases After Neoadjuvant Chemotherapy [ATNEC])19 and B51/RTOG130420 trials) and for patients with residual disease (Alliance 01120221 and Tailored Axillary Surgery With or Without Axillary Lymph Node Dissection Followed by Radiotherapy in Patients With Clinically Node-Positive Breast Cancer [TAXIS]22 trials). In the Netherlands, a prospective registry for patients with cN+ breast cancer treated with NAC is currently recruiting patients (the Minimal vs Maximal Invasive Axillary Staging and Treatment After Neoadjuvant Systemic Therapy in Node-Positive Breast Cancer [MINIMAX]23 trial). With the heterogenous application of less and more invasive axillary management strategies in daily practice,24 this nationwide cohort allows for comparison between the different strategies in terms of survival as well as quality of life. Because ALND is already increasingly being omitted worldwide in patients with cN+ breast cancer treated with NAC,25,26,27 these data are highly anticipated.
Limitations
This trial had a few limitations. Regarding central pathology review, the use of IHC on RISAS lymph nodes decreased the number of FN results, without substantially decreasing the number of TN results. However, in some patients, on-site IHC was performed, and therefore, the full effect of IHC cannot be determined within this trial. The participating institutions of the current trial had ample experience with iodine seed localization of breast lesions but little experience with localization of lymph nodes. Although these procedures are basically similar, institutions with vast experience in localizing lymph nodes may obtain better results (particularly because most of the FN results occurred within the first 10 included patients of the involved institutions). Nevertheless, the results of the MARI procedure within this trial correspond to data from the MARI trial itself, which reported an identification rate of 97% and FNR of 7%.8 Regarding SLNB, the identification rate and accuracy of SLNB might have been better if dual-tracer technique was performed in all patients. Again, the findings of our trial correspond to data of a meta-analysis including 17 trials on SLNB, which reported an overall identification rate of 89% (range, 87%-92%) and overall FNR of 17% (range, 14%-20%).1 Moreover, this study demonstrated that the RISAS procedure is clinically feasible in a multicenter setting, which supports implementation of this procedure in daily practice.
Conclusions
Results of this diagnostic study suggest that combining SLNB with excision of the marked lymph node may be the most accurate less-invasive procedure available for axillary staging after NAC in patients with cN+ breast cancer. Therefore, if less-invasive axillary staging is considered, the RISAS procedure (or a similar type of targeted axillary dissection), is clinically feasible in a multicenter setting, which supports implementation of this procedure in daily practice.
References
- 1.Simons JM, van Nijnatten TJA, van der Pol CC, Luiten EJT, Koppert LB, Smidt ML. Diagnostic accuracy of different surgical procedures for axillary staging after neoadjuvant systemic therapy in node-positive breast cancer: a systematic review and meta-analysis. Ann Surg. 2019;269(3):432-442. doi: 10.1097/SLA.0000000000003075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dominici LS, Negron Gonzalez VM, Buzdar AU, et al. Cytologically proven axillary lymph node metastases are eradicated in patients receiving preoperative chemotherapy with concurrent trastuzumab for HER2-positive breast cancer. Cancer. 2010;116(12):2884-2889. doi: 10.1002/cncr.25152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mougalian SS, Hernandez M, Lei X, et al. Ten-year outcomes of patients with breast cancer with cytologically confirmed axillary lymph node metastases and pathologic complete response after primary systemic chemotherapy. JAMA Oncol. 2016;2(4):508-516. doi: 10.1001/jamaoncol.2015.4935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samiei S, Simons JM, Engelen SME, Beets-Tan RGH, Classe JM, Smidt ML; EUBREAST Group . Axillary pathologic complete response after neoadjuvant systemic therapy by breast cancer subtype in patients with initially clinically node-positive disease: a systematic review and meta-analysis. JAMA Surg. 2021;156(6):e210891. doi: 10.1001/jamasurg.2021.0891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258-264. doi: 10.1200/JCO.2014.55.7827 [DOI] [PubMed] [Google Scholar]
- 6.Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609-618. doi: 10.1016/S1470-2045(13)70166-9 [DOI] [PubMed] [Google Scholar]
- 7.Boughey JC, Suman VJ, Mittendorf EA, et al. ; Alliance for Clinical Trials in Oncology . Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455-1461. doi: 10.1001/jama.2013.278932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donker M, Straver ME, Wesseling J, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015;261(2):378-382. doi: 10.1097/SLA.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 9.Straver ME, Loo CE, Alderliesten T, Rutgers EJ, Vrancken Peeters MT. Marking the axilla with radioactive iodine seeds (MARI procedure) may reduce the need for axillary dissection after neoadjuvant chemotherapy for breast cancer. Br J Surg. 2010;97(8):1226-1231. doi: 10.1002/bjs.7073 [DOI] [PubMed] [Google Scholar]
- 10.Caudle AS, Yang WT, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34(10):1072-1078. doi: 10.1200/JCO.2015.64.0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (alliance). Ann Surg. 2016;263(4):802-807. doi: 10.1097/SLA.0000000000001375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siso C, de Torres J, Esgueva-Colmenarejo A, et al. Intraoperative Ultrasound-Guided Excision of Axillary Clip in Patients with Node-Positive Breast Cancer Treated With Neoadjuvant Therapy (ILINA trial): a new tool to guide the excision of the clipped node after neoadjuvant treatment. Ann Surg Oncol. 2018;25(3):784-791. doi: 10.1245/s10434-017-6270-z [DOI] [PubMed] [Google Scholar]
- 13.Kuemmel S, Heil J, Rueland A, et al. A prospective, multicenter registry study to evaluate the clinical feasibility of targeted axillary dissection (TAD) in node-positive breast cancer patients. Ann Surg. 2020;(Nov). doi: 10.1097/SLA.0000000000004572 [DOI] [PubMed] [Google Scholar]
- 14.van Nijnatten TJA, Simons JM, Smidt ML, et al. A novel less-invasive approach for axillary staging after neoadjuvant chemotherapy in patients with axillary node-positive breast cancer by combining Radioactive Iodine Seed Localization in the Axilla With the Sentinel Node Procedure (RISAS): a Dutch prospective multicenter validation study. Clin Breast Cancer. 2017;17(5):399-402. doi: 10.1016/j.clbc.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 15.RISAS procedure in node-positive breast cancer following NAC (RISAS). ClinicalTrials.gov identifier: NCT02800317. Updated February 10, 2022. Accessed Month Day, Year. https://clinicaltrials.gov/ct2/show/NCT02800317
- 16.Montagna G, Mamtani A, Knezevic A, Brogi E, Barrio AV, Morrow M. Selecting node-positive patients for axillary downstaging with neoadjuvant chemotherapy. Ann Surg Oncol. 2020;27(11):4515-4522. doi: 10.1245/s10434-020-08650-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Minckwitz G, Huang CS, Mano MS, et al. KATHERINE Investigators. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617-628. doi: 10.1056/NEJMoa1814017 [DOI] [PubMed] [Google Scholar]
- 18.Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147-2159. doi: 10.1056/NEJMoa1612645 [DOI] [PubMed] [Google Scholar]
- 19.Axillary management in breast cancer patients with needle biopsy proven nodal metastases after neoadjuvant chemotherapy (ATNEC). ClinicalTrials.gov identifier: NCT04109079. Updated June 1, 2022. Accessed July 3, 2021. https://clinicaltrials.gov/ct2/show/NCT04109079
- 20.Standard or comprehensive radiation therapy in treating patients with early-stage breast cancer previously treated with chemotherapy and surgery. ClinicalTrials.gov identifier: NCT01872975. Updated May 9, 2022. Accessed July 3, 2021. https://clinicaltrials.gov/ct2/show/NCT01872975
- 21.Comparison of axillary lymph node dissection with axillary radiation for patients with node-positive breast cancer treated with chemotherapy. ClinicalTrials.gov identifier: NCT01901094. Updated August 3, 2022. Accessed July 3, 2021. https://clinicaltrials.gov/ct2/show/NCT01901094
- 22.Tailored axillary surgery with or without axillary lymph node dissection followed by radiotherapy in clinically node-positive breast cancer (TAXIS). ClinicalTrials.gov identifier: NCT 03513614. Updated November 23, 2021. Accessed July 3, 2021. https://clinicaltrials.gov/ct2/show/NCT03513614
- 23.de Wild SR, Simons JM, Vrancken Peeters MTFD, Smidt ML, Koppert LB; MINIMAX Group . MINImal vs MAXimal Invasive Axillary Staging and Treatment After Neoadjuvant Systemic Therapy in Node Positive Breast Cancer: protocol of a Dutch multicenter registry study (MINIMAX). Clin Breast Cancer. 2022;22(1):e59-e64. doi: 10.1016/j.clbc.2021.07.011 [DOI] [PubMed] [Google Scholar]
- 24.Simons JM, Koppert LB, Luiten EJT, et al. De-escalation of axillary surgery in breast cancer patients treated in the neoadjuvant setting: a Dutch population-based study. Breast Cancer Res Treat. 2020;180(3):725-733. doi: 10.1007/s10549-020-05589-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Hilli Z, Hoskin TL, Day CN, Habermann EB, Boughey JC. Impact of neoadjuvant chemotherapy on nodal disease and nodal surgery by tumor subtype. Ann Surg Oncol. 2018;25(2):482-493. doi: 10.1245/s10434-017-6263-y [DOI] [PubMed] [Google Scholar]
- 26.Ollila DW, Cirrincione CT, Berry DA, et al. Axillary management of stage II/III breast cancer in patients treated with neoadjuvant systemic therapy: results of CALGB 40601 (HER2-positive) and CALGB 40603 (triple-negative). J Am Coll Surg. 2017;224(4):688-694. doi: 10.1016/j.jamcollsurg.2016.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen TT, Hoskin TL, Day CN, et al. Decreasing use of axillary dissection in node-positive breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol. 2018;25(9):2596-2602. doi: 10.1245/s10434-018-6637-9 [DOI] [PubMed] [Google Scholar]



