This cohort study assesses racial differences in presentation and outcome and identifies drivers for racial disparities among patients with mycosis fungoides and Sézary syndrome.
Key Points
Question
What are the clinical features and outcomes for Black patients with mycosis fungoides and Sézary syndrome (MF/SS)?
Findings
This cohort study of 566 patients with MF/SS found that Black patients had high-risk baseline clinical features and an increased risk of progression to a higher cancer stage compared with patients of other races and ethnicities, though no difference in survival. Patients with hypopigmented MF, which is highly associated with Black patients, had low-risk features and highly favorably long-term survival.
Meaning
Among Black patients, MF/SS may have highly varied clinical features and outcomes, with survival associated with age at diagnosis and MF subtype.
Abstract
Importance
Mycosis fungoides and Sézary syndrome (MF/SS) has an increased incidence in Black patients, but clinical characteristics, treatments, and outcomes have been poorly characterized.
Objective
To assess racial differences in presentation and outcome and identify drivers for racial disparities in MF/SS.
Design, Setting, and Participants
A retrospective cohort analysis was conducted of 566 patients with MF/SS diagnosed from 1990 to 2020 and seen at the Winship Cancer Institute of Emory University and Grady Memorial Hospital, both in Atlanta, Georgia. Self-reported race and ethnicity were obtained from patient medical records and analyzed as 2 groups: non-Hispanic Black (Black) and all other races and ethnicities, including Asian, Hispanic, White, and unknown/undeclared (non-Black).
Main Outcomes and Measures
Univariate and multivariable models and Kaplan-Meier assessments were analyzed for overall survival and time to next treatment. The primary outcome was to assess differences in overall survival by racial and ethnic group. The hypotheses were formulated prior to data collection.
Results
Of the 566 patients with MF/SS identified (mean [SD] age 55 [16.4] years; 270 (47.7%) female), 257 were Black and 309 were non-Black. Black race was associated with increased rates of progression to a higher TNMB stage (39.8% in Black patients vs 29.1% in non-Black patients; P < .001) but not survival. Black patients were younger and had increased female predominance, higher TNMB stage, higher tumor stage, nodal involvement, and higher lactate dehydrogenase level compared with non-Black patients with MF/SS. Hypopigmented MF (HMF) was found in 62 patients, who were mostly Black (n = 59). Hypopigmented MF was significantly associated with survival on univariate and multivariable models, with 10-year survival of 100% in patients with HMF compared with 51.8% in patients without HMF. Black race was only associated with inferior outcomes after excluding patients with HMF who were younger than 60 years (hazard ratio [HR], 1.61; 95% CI, 1.02-2.55; P = .04), but not in patients older than 60 years (HR, 1.20; 95% CI, 0.80-1.81; P = .37). On multivariate analysis, among the cohort without HMF who were younger than 60 years, Black race remained statistically significant when controlling for cancer stage and large-cell transformation (HR, 1.27; 95% CI, 1.08-2.87; P = .43).
Conclusions and Relevance
In this cohort study, Black patients with MF/SS showed distinct clinical presentations and patterns of progression with heterogeneous outcomes depending on age at presentation and presence of HMF.
Introduction
Black patients with mycosis fungoides and Sézary syndrome (MF/SS) have inferior survival and distinct clinical presentations. Black patients demonstrate increased incidence, increased female predominance, and a decade younger median age of diagnosis compared with White patients.1,2,3,4,5,6,7,8 However, studies assessing outcome differences have shown mixed results, with some demonstrating inferior survival among Black patients while others showed no difference. There have been limited studies comprehensively assessing racial differences in MF/SS despite reports that Black patients may also exhibit many high-risk features. Conversely, hypopigmented MF, a clinical and histopathologic subtype detected only in darker skin, is associated with a favorable prognosis.7,8 Registry studies are limited in the details provided in their databases. Data regarding MF subtypes, including hypopigmentation, large-cell transformation (LCT), accurate staging, and relevant treatment data, are not available. To determine whether there are differences in presentation, treatment, and outcomes, and to assess potential drivers of disparities, individual patient-level data are needed. In this cohort study, we sought to characterize clinical differences in presentation, treatment, and outcomes to identify drivers of disparities among Black patients with MF/SS.
Methods
Data Source
We performed a retrospective review of 566 patients with stage 1A to 4B MF/SS diagnosed between 1990 and 2020 using an existing internal cutaneous T-cell lymphoma database. The study protocol was approved by the Winship Cancer Institute of Emory University institutional review board (IRB00045798), and need for patient informed consent was waived owing to the retrospective nature of the database and use of deidentified data. Data were reported using Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Patient Population
The cutaneous T-cell lymphoma database includes patients seen at the Emory Healthcare Clifton campus (a quaternary-care academic facility and regional/national referral center) and Grady Memorial Hospital outpatient clinic (a tertiary-care, public facility), both in Atlanta, Georgia, from 1990 through 2020. A total of 566 patients with stage 1A to 4B MF/SS were analyzed. Patients were excluded if they did not have evidence of MF or SS by pathology report or internal review, or were seen for a single consultation visit.
We analyzed self-reported race and ethnicity in 2 groups: non-Hispanic Black (hereafter referred as Black) vs all other races and ethnicities, including Asian, Hispanic, White, and unknown/undeclared (herein referred as non-Black). The primary objective was to assess differences in overall survival (OS), which was defined as the date of diagnosis until death or loss to follow-up, where those alive were censored at the last follow-up. Clinical variables included demographics, disease characteristics, TNMB (tumor, node, metastasis, blood) staging, baseline laboratory values, and treatment patterns.9
Statistical Analysis
Descriptive analysis was performed for each variable and a comparison between Black and non-Black patients using analysis of variance for numerical covariates and χ2 test or Fisher exact test for categorical covariates. Kaplan-Meier curves for OS were generated. Univariate analysis between each covariate and OS was assessed using a Cox proportional hazards model. The multivariable Cox proportional hazard model was built by a backward variable selection procedure with α = .10 removal criteria. All analyses were computed using SAS, version 9.4 (SAS Institute Inc), and 2-sided P < .05 was considered statistically significant.
Results
Overall Patient Characteristics
Among 566 patients in the overall cohort, the mean (SD) age was 55 (16.4) years, 270 (47.7%) patients were female, 12 (2.1%) were Asian, 257 (45.4%) were Black, 4 (0.8%) were Hispanic, 284 (50.2%) were White, and 9 (1.6%) were unknown/undeclared. Of these, 177 patients (31.3%) progressed to a higher cancer stage. The median (range) survival of the overall population was 11.3 (0-19.9) years and follow-up was 6.2 (0-24.5) years.
Patient Characteristics by Race
Black patients presented a median 10 years younger with higher rates of female patients (Table 1). There were different distributions in stage, with Black patients less frequently diagnosed at stage 1A (Black, 50 of 257 patients [19.5%] vs non-Black, 106 of 309 patients [34.3%]; P < .001) and also less likely to remain at stage 1A, with only 25 of 257 (11.1%) Black patients vs 79 of 309 (28.4%) non-Black patients remaining at stage 1A for the duration of their disease (P < .001). Black patients had higher tumor and nodal stages at diagnosis (Table 1). Differences in nodal stages were largely driven by a higher rate of NX stage among Black patients. There was no difference in metastasis or blood stages. Black patients were also more likely to progress to a higher overall stage, which was largely driven by tumor and nodal progression in Black patients (Table 1). The median lactate dehydrogenase (LDH) level at diagnosis was higher in Black vs non-Black patients, and there was no difference in LCT, white blood cell count at diagnosis, or total lines of therapy.
Table 1. Patient Characteristics by Race and Ethnicity.
| Covariate | Level | Race and ethnicity, No. (%)a | P value | |
|---|---|---|---|---|
| Non-Black (n = 309) | Black (n = 257) | |||
| Age at diagnosis, median (range), y | NA | 62 (9-88) | 50 (9-95) | <.001 |
| Gender | Female | 123 (39.8) | 147 (57.2) | <.001 |
| Male | 186 (60.2) | 110 (42.8) | ||
| HMF | NA | 3 (1.0) | 59 (23.0) | <.001 |
| TNMB stage at diagnosis | 1A | 106 (34.3) | 50 (19.5) | <.001 |
| 1B | 97 (31.4) | 83 (32.3) | ||
| 2A | 18 (5.8) | 35 (13.6) | ||
| 2B | 35 (11.3) | 31 (12.1) | ||
| 3A-B | 19 (6.2) | 26 (10.1) | ||
| 4A1 | 17 (5.5) | 20 (7.8) | ||
| 4A2 | 12 (3.9) | 10 (3.9) | ||
| 4B | 5 (1.6) | 2 (0.8) | ||
| Tumor stage | T1 | 113 (37.1) | 37 (15.6) | <.001 |
| T2 | 108 (35.4) | 118 (49.6) | ||
| T3 | 40 (13.1) | 38 (16.0) | ||
| T4 | 44 (14.4) | 45 (18.9) | ||
| Nodal stage | N0 | 235 (79.7) | 152 (60.8) | <.001 |
| N1 | 23 (7.8) | 49 (19.6) | ||
| N2 | 12 (4.1) | 5 (1.9) | ||
| N3 | 10 (3.4) | 11 (4.4) | ||
| NX | 15 (5.1) | 33 (13.2) | ||
| Metastasis stage | M0 | 303 (98.4) | 251 (98.8) | .74 |
| M1 | 5 (1.6) | 3 (1.2) | ||
| Blood stage | B0 | 238 (83.8) | 213 (86.6) | .11 |
| B1 | 24 (8.5) | 10 (4.1) | ||
| B2 | 22 (7.8) | 23 (9.4) | ||
| TCR peripheral blood at diagnosis | Polyclonal | 117 (54.7) | 103 (52.8) | .12 |
| Clonal | 77 (36.0) | 61 (31.3) | ||
| Oligoclonal/indeterminate | 20 (9.4) | 31 (15.9) | ||
| WBC count at diagnosis, median (range) | NA | 8.8 (2.5-72.4) | 10.9 (2.8-390.0) | .36 |
| LDH level at diagnosis, median (range) | NA | 162 (64-511) | 191 (106-1082) | <.001 |
| Progressed to a higher stage | No | 197 (70.9) | 145 (60.2) | .01 |
| Yes | 81 (29.1) | 96 (39.8) | ||
| Large-cell transformation | Yes | 32 (11.2) | 38 (15.3) | .16 |
| Highest TNMB stage | 1A | 79 (28.4) | 25 (11.1) | <.001 |
| 1B | 62 (22.3) | 50 (22.1) | ||
| 2A | 11 (4.0) | 27 (12.0) | ||
| 2B | 46 (16.6) | 39 (17.3) | ||
| 3 | 22 (7.9) | 36 (15.9) | ||
| 4A | 40 (14.4) | 34 (15.0) | ||
| 4B | 18 (6.5) | 15 (6.6) | ||
| Highest tumor stage | 1 | 88 (31.9) | 34 (15.0) | <.001 |
| 2 | 70 (25.4) | 74 (32.7) | ||
| 3 | 63 (22.8) | 59 (26.1) | ||
| 4 | 55 (19.9) | 59 (26.1) | ||
| Highest nodal stage | 0 | 180 (64.5) | 121 (47.1) | .008 |
| 1 | 32 (11.5) | 51 (21.1) | ||
| 2 | 15 (5.4) | 18 (7.4) | ||
| 3 | 20 (7.2) | 18 (7.4) | ||
| 4 | 32 (11.5) | 34 (14.1) | ||
| Highest metastasis stage | 0 | 259 (92.5) | 224 (93.0) | .85 |
| 1 | 21 (7.5) | 17 (7.1) | ||
| Highest blood stage | 0 | 213 (78.0) | 179 (79.2) | .43 |
| 1 | 25 (9.2) | 14 (6.2) | ||
| 2 | 35 (12.8) | 33 (14.6) | ||
| Total lines of therapy | ≤2 | 146 (52.0) | 119 (48.6) | .17 |
| 3-5 | 75 (26.7) | 83 (33.9) | ||
| >5 | 60 (21.4) | 43 (17.6) | ||
Abbreviations: HMF, hypopigmented mycosis fungoides; LDH, lactate dehydrogenase; NA, not applicable; TCR, T-cell gene rearrangement; WBC, white blood cell.
Race and ethnicity were self-reported by patients and analyzed as 2 groups: non-Hispanic Black (Black) and all other races and ethnicities, including Asian, Hispanic, White, and unknown/undeclared (non-Black).
Overall Survival
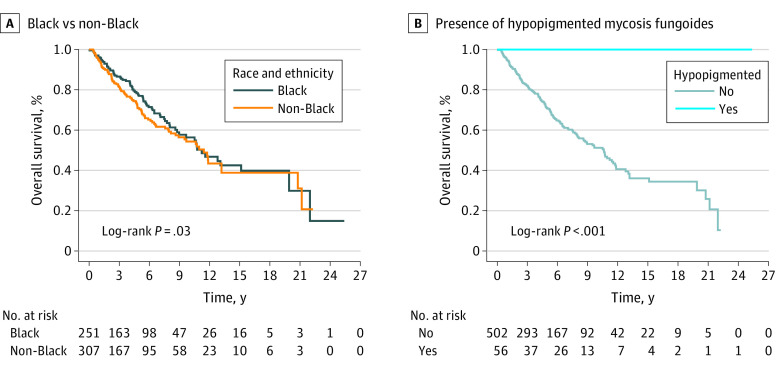
A total of 558 patients were analyzed for survival after 8 patients without survival data were excluded. Several factors were associated with inferior survival on univariate Cox proportional hazard model, including age older than 60 years; LCT; higher overall cancer stage or tumor, nodal, metastasis, or blood stage at diagnosis; progression to a higher cancer stage; clonal blood T-cell gene rearrangement; elevated white blood cell count; and elevated LDH (Table 2). Race and ethnicity were not associated with OS (Table 2 and Figure, A). Hypopigmented MF (HMF) was associated with statistically significantly improved survival (hazard ratio [HR], 0.02; 95% CI, 0.01-0.14; P < .001; Figure, B). On multivariable analysis including race and ethnicity; gender; age; tumor, nodal, metastasis, and blood stage; and T-cell gene rearrangement in the blood, age older than 60 years and higher tumor or nodal stage remained statistically significant for survival (Table 2).
Table 2. Cox Proportional Hazard Model for Overall Survival.
| Covariate | Level | No. | Overall survival, y | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | ||||
| Hazard ratio | Log rank | ||||
| Univariable model | |||||
| Racea | Black | 251 | 0.86 (0.64-1.16) | .34 | .34 |
| Non-Black | 307 | 1 [Reference] | 1 [Reference] | ||
| Age >60 y | Yes | 242 | 2.68 (1.98-3.63) | <.001 | <.001 |
| No | 316 | 1 [Reference] | 1 [Reference] | ||
| Gender | Female | 266 | 0.69 (0.51-0.92) | .01 | .01 |
| Male | 292 | 1 [Reference] | 1 [Reference] | ||
| Large-cell transformation | Yes | 69 | 1.83 (1.25-2.61) | .001 | .001 |
| No | 459 | 1 [Reference] | 1 [Reference] | ||
| Hypopigmented mycosis fungoides | Yes | 56 | 0.02 (0.01-0.14) | .006 | <.001 |
| No | 502 | 1 [Reference] | 1 [Reference] | ||
| TNMB stage at diagnosis | 1B | 177 | 2.15 (1.20-4.12) | .02 | <.001 |
| 2 | 119 | 4.78 (2.72-9.02) | <.001 | ||
| 3 | 45 | 4.73 (2.38-9.69) | <.001 | ||
| 4 | 66 | 13.23 (7.33-25.49) | <.001 | ||
| 1A | 151 | 1 [Reference] | 1 [Reference] | ||
| TNMB stage at diagnosis, range | 2A-4B | 177 | 4.09 (3.03-5.55) | <.001 | <.001 |
| 1A-1B | 381 | 1 [Reference] | 1 [Reference] | ||
| Tumor stage | T2 | 224 | 2.04 (1.20-3.69) | .01 | <.001 |
| T3 | 78 | 6.31 (3.63-11.59) | <.001 | ||
| T4 | 89 | 6.57 (3.79-12.03) | <.001 | ||
| T1 | 150 | 1 [Reference] | 1 [Reference] | ||
| Nodal stage | N1, N2, NX | 137 | 2.66 (1.93-3.65) | <.001 | <.001 |
| N3 | 21 | 6.75 (3.68-11.54) | <.001 | ||
| N0 | 379 | 1 [Reference] | 1 [Reference] | ||
| Metastasis stage | M1 | 8 | 6.12 (2.00-14.34) | <.001 | <.001 |
| M0 | 546 | 1 [Reference] | 1 [Reference] | ||
| Blood stage | B1 | 32 | 1.62 (0.88-2.74) | .09 | <.001 |
| B2 | 45 | 4.35 (2.89-6.37) | <.001 | ||
| B0 | 445 | 1 [Reference] | 1 [Reference] | ||
| Progressed to a higher stage | Yes | 171 | 1.76 (1.30-2.39) | <.001 | <.001 |
| No | 341 | 1 [Reference] | 1 [Reference] | ||
| Highest TNMB stage | 1B | 111 | 1.37 (0.56-3.76) | .51 | <.001 |
| 2A | 38 | 2.18 (0.72-6.65) | .16 | ||
| 2B | 85 | 5.44 (2.54-13.76) | <.001 | ||
| 3A-B | 57 | 6.30 (2.87-16.16) | <.001 | ||
| 4A | 74 | 11.35 (5.37-28.47) | <.001 | ||
| 4B | 33 | 14.40 (6.48-37.23) | <.001 | ||
| 1A | 104 | 1 [Reference] | 1 [Reference] | ||
| Total lines of therapy | 3-5 | 157 | 1.32 (0.93-1.89) | .12 | .29 |
| >5 | 102 | 1.20 (0.81-1.77) | .36 | ||
| ≤2 | 259 | 1 [Reference] | 1 [Reference] | ||
| TCR in the peripheral blood at diagnosis | Clonal | 135 | 2.03 (1.39-2.96) | <.001 | <.001 |
| Oligoclonal/indeterminate | 50 | 1.49 (0.80-2.58) | .18 | ||
| Negative | 219 | 1 [Reference] | 1 [Reference] | ||
| Highest tumor stage | T2 | 143 | 2.01 (0.94-4.87) | .09 | <.001 |
| T3 | 122 | 6.88 (3.45-15.96) | <.001 | ||
| T4 | 113 | 9.73 (4.88-22.60) | <.001 | ||
| T1 | 122 | 1 [Reference] | 1 [Reference] | ||
| Highest nodal stage | N1 | 83 | 3.45 (2.27-5.22) | <.001 | <.001 |
| N2 | 33 | 4.53 (2.69-7.42) | <.001 | ||
| N3 | 38 | 5.40 (3.30-8.67) | <.001 | ||
| NX | 66 | 3.54 (2.22-5.57) | <.001 | ||
| N0 | 293 | 1 [Reference] | 1 [Reference] | ||
| Highest metastasis stage | M1 | 38 | 3.54 (2.31-5.23) | <.001 | <.001 |
| M0 | 475 | 1 [Reference] | 1 [Reference] | ||
| Highest blood stage | B1 | 39 | 2.34 (1.41-3.68) | <.001 | <.001 |
| B2 | 68 | 3.84 (2.65-5.46) | <.001 | ||
| B0 | 390 | 1 [Reference] | 1 [Reference] | ||
| WBC count at diagnosis | NA | 382 | 1.04 (1.02-1.05) | <.001 | <.001 |
| LDH level at diagnosis | NA | 335 | 1.004 (1.002-1.009) | <.001 | <.001 |
| Multivariable model b , c | |||||
| Racea | Black | 0.84 (0.57-1.25) | .40 | .40 | |
| Non-Black | 1 [Reference] | 1 [Reference] | |||
| Age >60 y | Yes | 3.12 (2.08-4.68) | <.001 | <.001 | |
| No | 1 [Reference] | 1 [Reference] | |||
| Tumor stage | T2 | 2.67 (1.28-5.56) | .009 | <.001 | |
| T3 | 7.08 (3.22-15.54) | <.001 | |||
| T4 | 4.98 (2.22-11.18) | <.001 | |||
| T1 | 1 [Reference] | 1 [Reference] | |||
| Nodal stage | N1, N2, NX | 1.78 (1.15-2.75) | .01 | .04 | |
| N3 | 1.25 (0.55-2.84) | .60 | |||
| N0 | 1 [Reference] | 1 [Reference] | |||
| Metastasis stage | M1 | 2.79 (0.87-8.89) | .08 | .08 | |
| M0 | 1 [Reference] | 1 [Reference] | |||
| TCR blood at diagnosis | Clonal | 1.49 (0.98-2.26) | .07 | .08 | |
| Oligoclonal | 1.74 (0.95-3.19) | .07 | |||
| Nonclonal | 1 [Reference] | 1 [Reference] | |||
Abbreviations: LDH, lactate dehydrogenase; NA, not applicable; TCR, T-cell gene rearrangement; WBC, white blood cell.
Race and ethnicity were self-reported by patients and analyzed as 2 groups: non-Hispanic Black (Black) and all other races and ethnicities, including Asian, Hispanic, White, and unknown/undeclared (non-Black).
Number of observations in the original data set was 566. Number of observations used was 376.
Backward selection with an α level of removal of .10 was used. The following variables were removed from the model: blood stage, gender, large-cell transformation, and hypopigmented mycosis fungoides.
Figure. Kaplan-Meier Estimates of the Overall Population by Race and Ethnicity and Presence of Hypopigmented Mycosis Fungoides.
Race and ethnicity were self-reported by patients and analyzed as 2 groups: non-Hispanic Black (Black) and all other races and ethnicities, including Asian, Hispanic, White, and unknown/undeclared (non-Black).
Hypopigmented Mycosis Fungoides
Hypopigmented MF was present alone or concurrently with other MF subtypes in 62 patients (59 Black and 3 non-Black). Patients with HMF had a median 10-year survival of 100% vs 51.2% in patients who did not have HMF (eFigure in the Supplement). Hypopigmented MF was more common in patients younger than 60 years (272 of 504 [54.0%] patients without HMF vs 52 of 62 [83.9%] patients with HMF; P < .001) and female patients (227 of 504 [45.0%] patients without HMF vs 43 of 62 [69.4%] patients with HMF; P < .001). All patients with HMF presented with early-stage disease (62 of 62 [100%] patients with HMF vs 327 of 504 [64.9%] patients without HMF; P < .001). Extracutaneous involvement was considerably decreased in lymph nodes (N0 stage: 328 of 504 [67.9%] patients without HMF vs 59 of 62 [95.2%] patients with HMF; P < .001) and blood (396 of 504 [84.1%] patients without HMF vs 55 of 62 [93.2%] patients with HMF; P < .001), with no N3 or B2 cases. However, there were equivalent numbers of progression to a higher stage among patients with and without HMF, with 152 of 504 (42.7%) and 25 of 62 (33.1%) progressing in each group, respectively (P = .19). Similarly, there was no difference in LCT, which was noted in 4 of 62 patients with HMF (6.5%) and 66 of 504 without (13.9%).
Subgroup Analysis Excluding HMF, Stratified by Age
Given the potential for favorable prognostic effects of HMF to offset adverse prognostic effects of age in Black patients, the interaction between age and race independent of HMF was explored. Excluding patients with HMF, Black race was associated with inferior outcomes among patients younger than 60 years (HR, 1.61; 95% CI, 1.02-2.55; P = .04; eFigure in the Supplement). In this cohort, race remained statistically significant when controlling for cancer stage and LCT (Black race: HR, 1.27; 95% CI, 1.08-2.87; P = .04). However, among all ages and in patients older than 60 years without HMF, Black race was not statistically significantly associated with OS (HR, 1.20; 95% CI, 0.80-1.81; P = .37; eFigure in the Supplement).
Discussion
In the present cohort, Black patients presented with unique clinical features and greater rates of progression to a higher cancer stage but no difference in survival. Hypopigmented MF, found mostly in Black patients, was associated with improved survival. Hypopigmented MF is a clinical and histopathologic subgroup characterized by achromic lesions, a predilection for patients with darker skin, increased incidence in pediatric and juvenile populations, and a favorable prognosis.7,10 Increased incidence in darker skin phenotypes is likely related to improved detection. The favorable prognosis may be related to early detection, but some studies suggest that loss of the melanocytes is the result of an effective antitumor immune response.8 In the present cohort, HMF was indeed a very favorable characteristic predicting survival. Interestingly, we detected equivalent rates of progression to higher cancer stage in patients with HMF and without HMF, with some HMF subsequently developing LCT. However, this had no bearing on survival, as 100% of patients with HMF were alive at last follow-up. In contrast, younger Black patients with MF/SS without HMF at diagnosis demonstrated worse survival compared with non-Black patients.
This large cohort with detailed clinical annotation allowed us to detect additional differences among Black patients, including higher tumor and nodal stages, LDH level, and disparate patterns of progression. Nearly 40% of Black patients progressed to a higher cancer stage, with few (11%) remaining in stage 1A. Differences in progression were largely associated with progression in the nodal and skin compartments in Black patients, with 50% of Black patients developing abnormal lymph nodes during their treatment course.
Limitations
This study had some limitations, including missing data, referral bias, few other racial or ethnic groups, and lack of other surrogates of health care access. We also lacked central pathologic review and biologic correlates.
Conclusions
In this cohort study, Black patients with MF/SS showed distinct clinical presentations and patterns of progression with heterogeneous outcomes depending on age at presentation and presence of HMF.
eFigure. Overall Survival by Race and Age
References
- 1.Su C, Nguyen KA, Bai HX, et al. Racial disparity in mycosis fungoides: an analysis of 4495 cases from the US National Cancer Database. J Am Acad Dermatol. 2017;77(3):497-502.e2. doi: 10.1016/j.jaad.2017.04.1137 [DOI] [PubMed] [Google Scholar]
- 2.Kaufman AE, Patel K, Goyal K, et al. Mycosis fungoides: developments in incidence, treatment and survival. J Eur Acad Dermatol Venereol. 2020;34(10):2288-2294. doi: 10.1111/jdv.16325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nath SK, Yu JB, Wilson LD. Poorer prognosis of African-American patients with mycosis fungoides: an analysis of the SEER dataset, 1988 to 2008. Clin Lymphoma Myeloma Leuk. 2014;14(5):419-423. doi: 10.1016/j.clml.2013.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ai WZ, Keegan TH, Press DJ, et al. Outcomes after diagnosis of mycosis fungoides and Sézary syndrome before 30 years of age: a population-based study. JAMA Dermatol. 2014;150(7):709-715. doi: 10.1001/jamadermatol.2013.7747 [DOI] [PubMed] [Google Scholar]
- 5.Imam MH, Shenoy PJ, Flowers CR, Phillips A, Lechowicz MJ. Incidence and survival patterns of cutaneous T-cell lymphomas in the United States. Leuk Lymphoma. 2013;54(4):752-759. doi: 10.3109/10428194.2012.729831 [DOI] [PubMed] [Google Scholar]
- 6.Wilson LD, Hinds GA, Yu JB. Age, race, sex, stage, and incidence of cutaneous lymphoma. Clin Lymphoma Myeloma Leuk. 2012;12(5):291-296. doi: 10.1016/j.clml.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geller S, Lebowitz E, Pulitzer MP, et al. Outcomes and prognostic factors in African American and black patients with mycosis fungoides/Sézary syndrome: retrospective analysis of 157 patients from a referral cancer center. J Am Acad Dermatol. 2020;83(2):430-439. doi: 10.1016/j.jaad.2019.08.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martínez Villarreal A, Gantchev J, Lagacé F, et al. Hypopigmented mycosis fungoides: loss of pigmentation reflects antitumor immune response in young patients. Cancers (Basel). 2020;12(8):2007. doi: 10.3390/cancers12082007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—update 2017. Eur J Cancer. 2017;77:57-74. doi: 10.1016/j.ejca.2017.02.027 [DOI] [PubMed] [Google Scholar]
- 10.Virmani P, Levin L, Myskowski PL, et al. Clinical outcome and prognosis of young patients with mycosis fungoides. Pediatr Dermatol. 2017;34(5):547-553. doi: 10.1111/pde.13226 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Overall Survival by Race and Age