Abstract
Background:
Patients with autoimmune hepatitis (AIH) and primary or autoimmune sclerosing cholangitis are at nutritional risk; their body composition and has not been extensively studied. We aimed to describe their body composition and identify clinical links.
Methods:
Using magnetic resonance imaging (MRI), two reviewers segmented total psoas muscle area (tPMSA), visceral fat area (VFA) and subcutaneous fat area (mm2) and measured visceral and subcutaneous thickness (mm). Clinical, laboratory and quality of life (QoL; using PedsQL) data were collected. Sarcopenia was defined as tPMSA ≤5th percentile. Analysis of variance, Wilcoxon rank test and multivariable modelling were performed. A paediatric cohort with non-alcoholic fatty liver disease (NAFLD) was used as a comparator following propensity score matching.
Results:
Fifty-eight patients with autoimmune liver disease (AILD) (33 [57%] with AIH) were included: median age 16 years (interquartile range [IQR]: 13-18), 33 (57%) male. Median time from diagnosis to MRI was 15 months (IQR: 2-39 months). Two patients (3%) had a BMIz indicative of mild malnutrition. tPMSA was measurable in 52 subjects (90%). Of those, 25 (48%) had sarcopenia. Sarcopenic patients had a lower blood urea nitrogen compared to non-sarcopenic (median [IQR]: 9.5 [8.0, 12.0] vs 11 [10, 14] mg/dL; P = .023). There was no difference in corticosteroid use between groups. The VFA of sarcopenic patients was higher (3156 [2064, 7492]) vs 2084 [688, 3092]) mm2; P = .005). Patient-reported QoL negatively associated with VFA and general health negatively associated with VFA. Compared with NAFLD, the odds ratio for sarcopenia with AILD was 14.5 (95% confidence interval: 2.3-90.7).
Conclusion:
In autoimmune liver diseases, sarcopenia is highly prevalent, associated with increased visceral fat and QoL.
Keywords: autoimmune liver disease, sarcopenia, visceral fat
1 |. BACKGROUND
Changes in body composition, such as loss of muscle mass or function (sarcopenia) and change in fat mass and distribution, commonly occur in patients with chronic liver diseases.1 This finding is multifactorial and typically occurs due to inflammation, malabsorption, medication side effects, and decreased physical activity.2,3 Sarcopenia is seen in approximately 40%-70% of adult patients with cirrhosis.4–7 Paediatric data are more limited but consistent with adult findings.8–10 Sarcopenia, as well as subcutaneous and visceral adipose tissue mass alterations, can contribute to secondary cardiometabolic comorbidities, such as insulin resistance and dyslipidemia, which may become particularly challenging post-transplant.11,12 Furthermore, changes in body composition are associated with short- and long-term morbidity and mortality and may be linked to overall quality of life (QoL).10,13,14 Considering the risk of malnutrition posed by the presence of chronic liver diseases and the link between malnutrition-related changes in body composition and patient outcomes, it is important to understand the nutritional status of patients with chronic liver diseases.
Autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC), and autoimmune sclerosing cholangitis (ASC) are chronic liver diseases, wherein, similar to other chronic liver diseases, nutritional risk exists due to absorption and/or digestion impairment, altered eating behaviors, limited physical activity, increased inflammatory tone, as well as iatrogenic causes.15 Furthermore, autoimmune liver disease is commonly associated with inflammatory bowel disease (IBD), which has been shown to be an important risk factor for sarcopenia in children.16,17 In spite of that, the body composition of subjects with autoimmune liver disease remains to be investigated and it is unclear whether the nutritional tools used in clinical practice are adequate to detect patients at risk. These clinical questions are important to address as they may lead to changes in practice that could ultimately impact patient outcomes.
The objective of this study was therefore to describe the body composition of patients with AIH and ASC/PSC and to investigate whether it correlates with clinical markers. We hypothesize that sarcopenia is prevalent in autoimmune liver disease and is associated with nutritional status and health-related QoL.
2 |. PATIENTS AND METHODS
2.1 |. Patients
This was a substudy of a prospective cohort study that investigates magnetic resonance imaging (MRI) biomarkers in chronic liver diseases (NCT03 175471). In that study, patients aged less than 25 years with AIH/PSC/ASC and other chronic liver diseases consent to a research abdominal MRI examination. The following information is captured as part of the prospective study that has been active since January 2017: demographics, weight, height at time of imaging and clinical diagnoses, relevant laboratory investigations, as well as the responses to QoL questionnaires.
For the purposes of the current study, we included all patients who had undergone a research MRI if they had a known diagnosis of AIH, PSC or ASC (by histology or radiologic assessment for PSC), had available research abdominal MRI data from January 2017 to the initation of sub-study analysis in January 2020, and were receiving hepatology care at Cincinnati Children’s Hospital Medical Center (CCHMC). Exclusion criteria were liver disease other than AIH, PSC, ASC and MR images not including the L3/4 vertebral levels which were required for measurement of total psoas muscle area (tPMSA).
Demographics and clinical characteristics were obtained from the prospective cohort study. Specific data obtained included patient age at the time of imaging, duration of liver disease, diagnosis of IBD, and corticosteroid use (at time of MRI) and duration (cumulative since the disease onset). Laboratory data collected included serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), direct bilirubin, albumin, blood urea nitrogen (BUN), creatinine and international normalized ratio (INR), measured at the time of imaging. If multiple data (anthropometrics and labs) were available, values closest to the MRI were recorded, if within 6 months.
Given prior literature, prolonged corticosteroid exposure was defined as use for >3 consecutive months since diagnosis.18 Malnutrition was defined as BMI z-score <−1 in those <20 years of age and BMI <18 kg/m2 in those 20 years of age and older.19,20 The study protocol was approved by the institutional review board prior to the initiation of any study related activities.
2.2 |. MRI measurements
Magnetic resonance imaging examinations for this prospective cohort study include multiple sequences. For the current study the fat image from the axial multi-echo Dixon-based sequence (mDixon Quant®; Philips Healthcare, Best, the Netherlands) was used for all measurements. All measurements were performed at the L3/L4 (lumbar) intervertebral disk level with the axial image selected based on cross-referencing with coronal or sagittal plane images obtained as part of the same MRI examination.
Two reviewers (AAA and TY) independently obtained all body composition measurements utilizing Image J.21 The results were generated using the measurements of Reviewer 1. In cases where there was a >20% discrepancy between the measurements of Reviewer 1 and 2, these were reassessed by a third reviewer (Reviewer 3, ATT, board certified Paediatric Radiologist) whose measurements were used in these instances for the analyses. MR elastography measurements (liver stiffness, kPa) obtained using the study MRIs were collected as well.
2.2.1 |. Psoas muscle cross-sectional area
Psoas muscle area was measured using a geometric region of interest tool. tPMSA (mm2) was expressed as the sum of the left and right PMSA. tPMSA was subsequently corrected for height squared, expressed as tPMSA/m2 index (mm2/m2) for the purpose of study analyses. Additionally, tPMSA results were compared against norms previously published for children, using the tPMSA online tool (https://ahrc-apps.shinyapps.io/sarcopenia) published by Lurz et al22 Sarcopenia was defined as tPMSA<5th percentile.
2.2.2 |. Adipose tissue
Subcutaneous fat thickness (mm) was measured as the fat thickness from skin to surface of the outer (anterior) surface of the abdominis rectus muscles. Visceral fat thickness (mm) was measured as the fat thickness from the inner (posterior) surface of the rectus muscles to the anterior edge of the vertebral body.23 Visceral and subcutaneous fat area were measured in mm2 using the thresholding and geometric region of interest tools as described previously.23 In brief, each the total abdominal fat area, intraabdominal fat area, and retroperitonal fat area were measured. Retroperitoneal fat area was subtracted from intraabdominal fat area to obtain the visceral fat area. Visceral fat area measurements we compared against paediatric norms for males and females published by Harbaugh et al24 The results were grouped as <5th percentile, within 5-95th percentile or >95th percentile.
2.2.3 |. Quality of Life
Quality of Life was assessed via the Pediatric Quality of Life Inventory (PedsQL) 4.0SF 15 Generic Core Scales and the PedsQL General Well Being Scales for child and parent. The PedsQL4.0SF 15 Generic Core Scales are composed of the young children survey (age 5-7 years), children (age 8-12), teens (13-18 years) and young adult (18-25 years). There are four dimensions (physical functioning, emotional functioning, social functioning and school functioning) and a total of 15 questions. Participants answer questions on a scale of 0-5 (0: never a problem, 1: almost never a problem, 2: sometimes a problem, 3: often a problem, 4: almost always a problem). The PedsQL General Well Being Scale was completed for ages 8-25 years. There are two dimensions: the general well-being scale and general health. The survey includes seven questions. Similarly, parents/caregivers answer the questionnaires concerning the patients from the parents’ perspective. For assessment, each question is reverse scored (0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0). The Well Being Scales scores are transformed (0 = 0, 1 = 25, 2 = 50, 3 = 75, 4 = 100). The scores are then added and divided by the number of questions answered, giving a score between 0 and 100.
2.3 |. Comparator cohort
To determine whether the body composition findings were specific to autoimmune liver diseases, versus the presence of a chronic liver disease, we also obtained body composition data from patients with non-alcoholic fatty liver disease (NAFLD). These patients were included if they were younger than 20 years of age, had evidence of histologically confirmed NAFLD or presumed NAFLD (elevated liver enzymes and/or imaging evidence of hepatic steatosis with a negative work up for other causes of liver disease, such as AIH, Wilson’s disease, hemochromatosis, alpha 1 antitrypsin deficiency, viral hepatitis), as well as an abdominal MRI between April 1, 2009 and July 30, 2018, that allowed body composition measurements as described above, at the L3/4 level. Exclusion criteria were additional liver diseases, history of weight loss surgery or liver transplantation, as well as secondary causes of hepatic steatosis.
2.4 |. Statistical analysis
Descriptive statistics, frequency with percentage for categorical variables, means with standard deviations or medians with interquartile ranges (IQRs) for continuous variables, were used to summarize the demographic, clinical, laboratory, MRI characteristics and the body composition in the entire cohort as well as by the groups of interest. Exact Chi-square test or Wilcoxon rank-sum test was used for group differences based on variable types. Multivariable logistic regression was done to determine predictors of sarcopenia using prespecified variables. Multivariable linear regression models were built using backward model selections to study the relationship between predictors and QoL outcomes. Sarcopenia was forced in the final model regardless of its significance. Due to the considerations for the sample size, the number of selected variables was limited to be no more than five by varying the significance level for the variables to stay in the model, adjusted R2 were used for comparing models. The potential predictors were pre-specified by clinicians. After inspecting the data distribution through density plots, log-transformation was done for non-normally distributed variables. Krippendorff’s alpha coefficient, Spearman correlation coefficient, and intraclass correlation coefficient were used to determine agreement between Reviewers’ measurements. Statistical significance was set at a P-value of <.05. SAS software (version 9.4) was used for the analyses and R package (version 4.0.2) was used to determine the coefficients of agreement between the reviewers. Propensity score matching was used to compare the autoimmune liver disease and NAFLD cohorts.
3 |. RESULTS
Of the 71 patients with available imaging from the prospective MRI biomarkers study, 58 patients met inclusion/exclusion criteria for the current study (Figure S1). Of those, 25 (43%) were female, 55 (95%) were White, and 33 (57%) had AIH. Malnutrition based on BMI calculations was seen in 2 (3%) patients. The demographic and clinical characteristics of the cohort are summarized in Table 1.
TABLE 1.
Baseline characteristics of entire study cohort (n = 58)
| Variable | Total cohort (n = 58) | AIH (n = 33) | ASC/PSC (n = 25) |
|---|---|---|---|
| Sex, n (%) female | 25 (43%) | 15 (46%) | 10 (40%) |
|
| |||
| Race, n (%) White | 55 (95%) | 33 (100%) | 22 (88%) |
|
| |||
| Age at MRI, years | 16 (13, 18) | 16 (13, 18) | 16 (14, 17) |
|
| |||
| Duration of disease from diagnosis to MRI, months | 15 (2, 39) | 14 (2, 39) | 16 (2, 39) |
|
| |||
| IBD diagnosis, n (%) | 19 (33%) | 1 (3%)* | 18 (72%)* |
|
| |||
| Corticosteroid exposure, n (%) | |||
| Use at time of MRI | 28 (48%) | 18 (55%) | 10 (40%) |
|
| |||
| Total corticosteroid exposure since diagnosis, n (%) | |||
| ≥3 months | 35 (60%) | 19 (58%) | 16 (64%) |
| <3 months | 10 (17%) | 8 (24%) | 2 (8%) |
| None | 13 (22%) | 6 (18%) | 7 (28%) |
| Weight, kg at MRI | 65 (51, 75) | 67 (51, 75) | 63 (58, 71) |
| Height, cm at MRI | 168 (154, 176) | 165 (149, 171)* | 172 (162, 180)* |
| BMI, kg/m2 at MRI | 22 (20, 25) | 23 (20, 25) | 21 (19, 24) |
| BMI z score at MRI | 0.61 (−1, 1.4) | 0.7 (0.5, 1.4)* | 0.3 (−0.6, 1.0)* |
| BUN (mg/dL) | 11 (9, 12) | 11 (9, 12) | 10 (8, 12) |
| Creatinine (mg/dL) | 0.6 (0.5, 0.8) | 0.6 (0.5, 0.7) | 0.7 (0.6, 0.8) |
| AST (U/L) | 41 (27, 81) | 47 (34, 83) | 32 (24, 55) |
| ALT (U/L) | 65 (31, 91) | 68 (31, 94) | 56 (34, 86) |
| Direct bilirubin (mg/dL) | 0.2 (0.1, 0.3) | 0.2 (0.1, 0.3) | 0.1 (0.1, 0.3) |
| GGT (U/L) | 55 (17, 172) | 44 (15, 134) | 56 (27, 202) |
| Albumin (g/dL) | 3.8 (3.5, 4.1) | 3.9 (3.6, 4.2) | 3.7 (3.5, 4.1) |
| INR (s) | 1.1 (1.1, 1.2) | 1.2 (1.1, 1.2) | 1.1 (1.1, 1.1) |
Note: Continuous variables are expressed as median (IQR).
Abbreviations: AIH, autoimmune hepatitis; ALT, alanine aminotransferase; ASC, autoimmune sclerosing cholangitis; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; GGT, gamma glutamyl transferase; IBD, inflammatory bowel disease; INR, international normalized ratio; IQR, interquartile range; MRI, magnetic resonance imaging; PSC, primary sclerosing cholangitis.
P value < .05 between the groups.
Agreement between body composition measurements by the two reviewers was excellent as calculated by Krippendorff’s alpha coefficient, Spearman correlation coefficient, and intraclass correlation coefficient (all comparison coefficients were ≥.97).
Table 2 describes the results of the body composition measurements of the study cohort. Among patients with AIH and ASC/PSC, there were no differences in body composition.
TABLE 2.
Body composition results
| Variable | Total cohort (n = 58) | AIH (n = 33) | ASC/PSC (n = 25) | P-value | |
|---|---|---|---|---|---|
| L3/4 level | Subcutaneous fat area (mm2)a | 9099 (4984, 15 364) | 10 174 (6690, 17 964) | 6816 (4510, 13 003) | .156 |
| Visceral fat area (mm2)a | 2496 (848, 4156) | 2694 (1072, 4144) | 2309 (758, 4632) | .47 | |
| Subcutaneous fat thickness (mm)a | 15 (11, 26) | 17 (11, 28) | 14 (9, 26) | .183 | |
| Visceral fat thickness (mm)a | 65 (51, 81) | 63 (51, 84) | 66 (54, 76) | .9237 | |
| tPMSA index (mm2/mm)b | 876 (630, 1226) | 856 (631, 1286) | 876 (630, 1227) | .6397 |
Note: Variables are expressed as median (IQR).
Abbreviations: AIH, autoimmune hepatitis; ASC, autoimmune sclerosing cholangitis; IQR, interquartile range; PSC, primary sclerosing cholangitis; tPMSA, total psoas muscle surface area.
Fat depot measurements were possible in 54 patients (n = 30/33 AIH, n = 8/9 ASC and n = 16/16 PSC).
tPMSA measurements were possible in 52 patients (n = 28/33 AIH, n = 8/9 ASC and n = 16/16 PSC).
Total psoas muscle area measurements were possible in 52 patients (90%). The distribution of tPMSA measurements, expressed in percentiles for age and sex, in the AIH, PSC and ASC groups is shown in Figure 1. Of the 52 patients, 48% had sarcopenia (Table 3). Patients with sarcopenia had lower serum BUN levels (9.5 [8.0, 12.0] vs 11.0 [10.0, 14.0], respectively; P = .023) but otherwise had similar demographic and laboratory characteristics to patients without sarcopenia.
FIGURE 1.
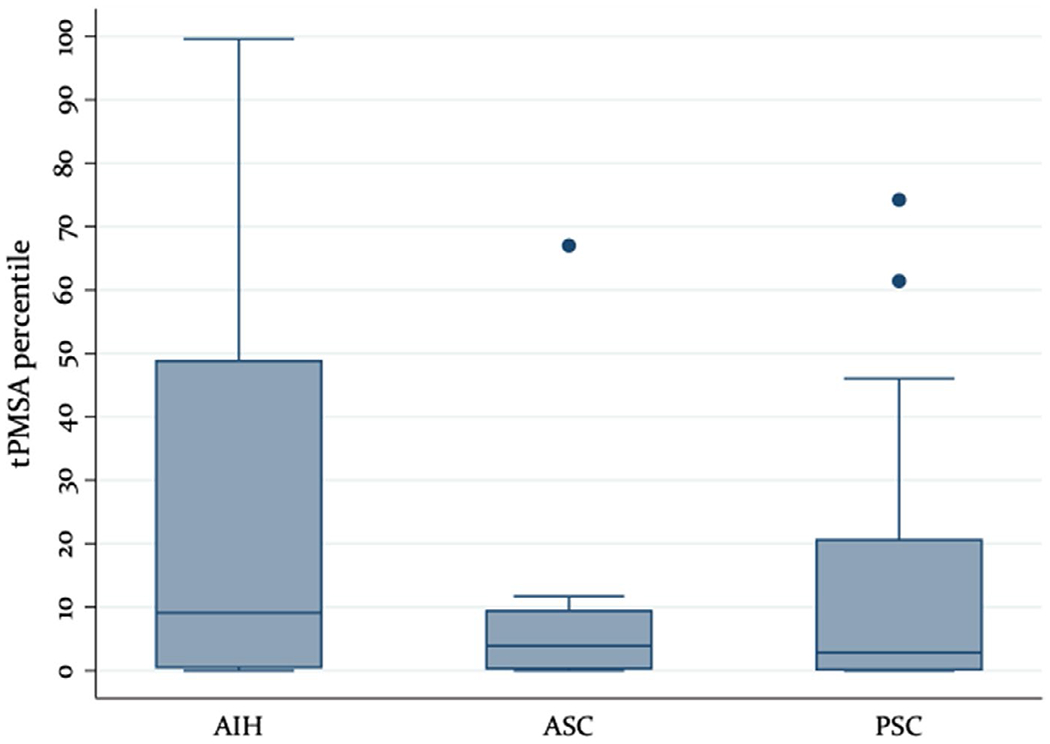
tPMSA distribution by diagnosis. tPMSA, total psoas muscle surface area
TABLE 3.
Comparison of sarcopenic and non-sarcopenic patients (n = 52)
| Variable | Sarcopenic, n = 25 | Non-sarcopenic, n = 27 | P value |
|---|---|---|---|
| Sex, n (%) female | 13 (52.0%) | 8 (29.6%) | .157 |
|
| |||
| Age at MRI | 16.0 (15.0, 17.0) | 14.0 (9.0, 18.0) | .242 |
|
| |||
| Duration of disease from diagnosis to MRI, months | 32.0 (2.0, 52.0) | 14.0 (2.0, 33.0) | .205 |
|
| |||
| Diagnosis, n (%) | |||
| AIH | 11 (44%) | 17 (63%) | .266 |
| ASC/PSC | 14 (56%) | 10 (37%) | |
| IBD present, n (%) | 10 (40%) | 9 (33%) | .774 |
| BUN (mg/dL) | 9.5 (8.0, 12.0) | 11.0 (10.0, 14.0) | .023 |
| Creatinine (mg/dL) | 0.7 (0.5, 0.8) | 0.6 (0.5, 0.8) | .467 |
| AST (U/L) | 34.0 (23.0, 69.0) | 37.0 (28.0, 83.0) | .458 |
| ALT (U/L) | 40.0 (26.0, 82.0) | 65.0 (43.0, 89.0) | .394 |
| Direct bilirubin (mg/dL) | 0.1 (0.1, 0.3) | 0.1 (0.1, 0.3) | .798 |
| GGT (U/L) | 54.0 (15.0, 141.0) | 40.0 (19.0, 174.0) | .956 |
| Albumin (g/dL) | 3.7 (3.5, 4.1) | 3.8 (3.6, 4.1) | .673 |
| INR (s) | 1.1 (1.0, 1.2) | 1.1 (1.1, 1.2) | .559 |
| L3/4 subcutaneous fat area (mm2) | 10 332 (5536, 18 992) | 8916 (4864, 13 880) | .360 |
| L3/4 subcutaneous fat thickness (mm) | 16 (11, 30) | 13 (10, 19) | .085 |
| L3/4 visceral fat area (mm2) | 3156 (2064, 7492) | 2084 (688, 3092) | .005 |
| L3/4 visceral fat thickness (mm) | 70 (55, 84) | 56 (49, 74) | .099 |
Note: N (%) for categorical variable; medians (IQRs) for numeric variables.
Abbreviations: AIH, autoimmune hepatitis; ALT, alanine aminotransferase; ASC, autoimmune sclerosing cholangitis; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; GGT, gamma glutamyl transferase; IBD, inflammatory bowel disease; INR, international normalized ratio; IQR, interquartile range; MRI, magnetic resonance imaging; PSC, primary sclerosing cholangitis.
Fat depots were possible to assess in 54 patients (93%). Of those, 8 (15%) had visceral fat area below the 5th percentile, whereas 5 (9%) were above the 95th percentile. The comparison of the fat depots of patients with and without sarcopenia is shown in Table 3. The visceral fat area of patients with sarcopenia was greater than that of their counterparts without sarcopenia (3156 [2064, 7492] vs 2084 [688, 3092] mm2, P = .005). There were no differences in the subcutaneous fat area or thickness between patients with and without sarcopenia.
There was no difference in the body composition measurements between patients who were on or off corticosteroids at the time of the MRI (Table S1). The proportion of patients with sarcopenia was also not different among those with no, limited (<3 months) or prolonged (>3 months) exposure to corticosteroids since diagnosis (P = .417). When assessing the effect of steroid exposure only on those with AIH, there was still no difference between the groups (P = .487).
Multivariable modelling was performed to determine predictors of sarcopenia (Table S2). Among the pre-specified potential covariates, including age at MRI, BMI z-score, underglying diagnosis, disease duration, L3/L4 visceral fat measures (area and/or thickness) and corticosteroid exposure, only L3/L4 visceral fat area (log-transformed) was significantly positively associated with sarcopenia (odds ratio [OR] with 95% confidence interval [CI]: 3.0 (1.1, 8.3), P = .03).
Concerning QoL, there were higher scores in parent reported general health for those without sarcopenia (median [IQR]: 75 [50,75]) than for those with sarcopenia (50 [50,75], P = .045), but otherwise no significant difference in QoL outcomes was seen (Table S3). In the multivariable models, when controlling for selected confounders, patient-reported QoL was negatively associated to visceral fat area (regression coefficient: −4.88, P = .048), and general health negatively correlated with visceral fat area (regression coefficient: −6.88, P = .044; Table S4). Lastly, parent-reported general health score was negatively correlated to sarcopenia (regression coefficient: −15.3, P = .027).
The NAFLD cohort included n = 53, predominantly Caucasian children with a median age of 14 years and a BMI z score of 2.5 (Table S5). When comparing patients with autoimmune liver diseases against those with NAFLD following propensity score matching adjusting for age, sex, race and BMI z-score, we found that the OR of sarcopenia in the context of autoimmune liver disease was 14.5 (95% CI 2.3-90.7).
4 |. DISCUSSION
In this study, we used cross-sectional imaging to investigate the body composition of children and young adults with AIH, PSC and AIH and correlated it with liver disease severity. In this cohort of 58 predominantly white patients (57% with AIH), with a median age of 16 years and at a median time of 15 months after diagnosis, 48% had sarcopenia and 15% had a visceral fat area <5th percentile and 9% >95th centile. Differences in body composition were not linked to expected exposures, such as corticosteroid use, or concomitant conditions previously associated with sarcopenia, such as IBD. Patients with sarcopenia had greater visceral fat area and difference in body composition were linked to QoL. Importantly, the comparison against a cohort of patients with NAFLD showed that sarcopenia is significantly more likely in the context of autoimmune liver disease, even though sarcopenia is thought to contribute to the pathogenesis of NAFLD.25 These findings suggest that sarcopenia may be implicated in the pathogenesis of autoimmune liver diseases.
Although there is no broadly accepted definition of sarcopenia in paediatrics, a tPMSA <5th percentile for age/sex suggests significant muscle mass loss. Surprisingly, in spite of essentially normal BMI z scores for the majority of our cohort, almost half of the patients studied were found to have a tPMSA below the 5th percentile. Although the tPMSA of those with AIH was variable, the distribution of the tPMSA of most patients with PSC and ASC clustered <15th percentile, suggesting a significant loss of muscle mass in these patients in particular (Figure 1). The radiologic evidence of sarcopenia in the study cohort was supported by laboratory data, as those with a tPMSA <5th percentile also had lower serum BUN levels. In this study, steroid use was not linked to body composition findings. It is known that long-term use of corticosteroids (>3 months) contributes to sarcopenia.18 It is possible that this link was not detected in our study either because other factors play a more important role than steroids (eg adipocytokines, physical activity, etc - not assessed in here) or due to type 2 error, as not all patients in our cohort (combined AIH, PSC, ASC) had been exposed to steroids. This remains to be investigated in larger cohorts.
In the only other study where to our knowledge the body composition of youth with AIH was assessed, bioelectrical impedance (BIA) and anthropometric measurements were obtained in a cohort of 37 children (83% female with a mean age of 14 years).3 The assessment was done at a median time of 18 months following diagnosis and 60% of patients were on corticosteroids at the time of BIA. In that study, 42% of female patients had excess body fat as determined by BIA.3 Our study found that 15% of patients were below the 5th percentile for visceral fat area, whereas 9% were over the 95th percentile. This indicates that the majority of patients were within normal range. This is likely due to that fact that there was a wide range of disease and corticosteroid exposure duration. Just over half (60%) of the patients had a history of steroid use for >3 months. Recurrent assessments of body composition throughout the natural history of AIH, PSC and ASC would allow for a more optimal understanding of the change in adiposity over time and may help assess the role, if any, of body composition in predicting patient outcomes.
Our study highlights the limitation of BMI (z-score for those younger than 20 years and specific BMI cutoffs for those older) in determining those at nutritional risk. Only 3% of the cohort met BMI criteria for mild malnutrition (BMI z-score <−1 or BMI <18.5 kg/m2 in adults), whereas 48% of the patients had a tPMSA<5th percentile and 15% had visceral fat <5th percentile. This is a known limitation of BMI, which is not meant to serve as a marker of body composition.26
We found that not only is the prevalence of sarcopenia staggering in patients with autoimmune liver disease, but it is associated with greater visceral adiposity. Although this association is not novel,27 it has not previously been reported in subjects with autoimmune liver diseases. The inverse proportionality between muscle mass and visceral fat occurs with metabolic dysregulation seen in the context of expanded visceral adipose tissue and is thought to drive an inflammatory tone which contributes to sarcopenia. Factors such as leptin and interleukins appear to play a role28–30 as leptin acts on both skeletal muscle and fat tissue for glucose regulation and fatty acid metabolism.30 The link between sarcopenia and visceral fat in patients with autoimmune liver diseases suggests that adipokines may be involved in the overall inflammatory process seen and furthermore may regulate appetite, to contributing to sarcopenia, which may ultimately even be affecting the QoL of these patients.28,31
In our study, visceral fat depot differences were negatively correlated to QoL measures and general health reported by the patient, whereas general health negatively correlated to sarcopenia as reported by a parent. For example, as shown in Table S4, sarcopenia was associated with a 15.3 point lower general health score in the parental report (on a scale from 0 to 100). This is significant, as it points to a possible functional deficit. Although this study was not designed to determine causation, one can postulate that since visceral fat depots and sarcopenia can limit the overall physical activity and endurance of an individual, it prevents them from engaging in activities that would improve their well-being and QoL. This impairment would also further feed the cycle of inactivity and sarcopenia, ultimately worsening overall patient outcomes. Given these findings, it would be important to determine whether improvements in body composition, and particularly sarcopenia, are associated with improvements in the QoL of patients with autoimmune liver diseases.
Strengths of our study include the use of MRI to describe body composition and its prospective design. Weaknesses of our study include the relatively small sample size (and the lack of previously published data on this topic to allow us to perform a sample size calculation), the variable time the MRI was obtained from diagnosis, and the tPSMA tool used to determine percentiles. The MRI examinations used here had been obtained at different time points since the liver disease diagnosis, which may have had an impact on the results of our study. Although cross-sectional imaging (MRI and computed tomography) is the reference standard for fat measurements, with MRI, one established limitation is the need for estimation of retroperitoneal area borders.32 This was accounted for in our study by the inclusion of two independent reviewers who in our study had excellent agreement between their measurements. For the interpretation of the tPMSA results, we used a tPMSA tool that has been developed to derive percentiles for patients up to 16 years of age. In our study we used the same tool for all patients, even if older than 16 years of age, as there is no other approach to interpret tPMSA data. Lastly, we did not include data on the socioeconomic status of patients, which may have had an impact on their body composition and this is something that should be studied in the future.
In conclusion, we demonstrated that subjects with AIH/PSC/AIH have significant alterations in their body composition, which are linked to overall QoL. Further studies investigating the body composition of a larger cohort of patients are necessary to validate our findings and to determine the natural history of these changes. Furthermore, studies investigating the pathophysiologic mechanisms of these alterations are needed. Lastly, the impact of these changes on short-and long-term morbidity and mortality is important to discern.
Supplementary Material
Key points.
Sarcopenia has not been previously assessed in pediatric autoimmune liver disease (AILD) and in this cohort was found to be highly prevalent.
Sarcopenia was associated with a higher visceral fat mass in youth with AILD.
Changes in body composition were linked to quality of life outcomes.
The odds of sarcopenia were significantly higher in youth with AILD versus those with non-alcoholic fatty liver disease.
Funding information
The study was funded by Center for Autoimmune Liver Disease (CALD) Center for Translational Fibrosis Research (CTFR) at Cincinnati Children’s Hospital Medical Center.
Abbreviations:
- AIH
autoimmune hepatitis
- ALT
alanine aminotransferase
- ASC
autoimmune sclerosing cholangitis
- AST
aspartate aminotransferase
- BIA
bioelectrical impedance
- BMI
body mass index
- BUN
blood urea nitrogen
- CI
confidence interval
- GGT
gamma glutamyl transferase
- IBD
inflammatory bowel diseas
- INR
international normalized ratio
- IQR
interquartile range
- MRI
magnetic resonance imaging
- NAFLD
non-alcoholic fatty liver disease
- OR
odds ratio
- PSC
primary sclerosing cholangitis
- QoL
quality of life
- tPMSA
total psoas muscle surface area
- VFA
visceral fat area
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest relevant to this article to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Houttu N, Kalliomäki M, Grönlund M-M, et al. Body composition in children with chronic inflammatory diseases: a systematic review. Clin Nutr. 2020;39:2647–2662. [DOI] [PubMed] [Google Scholar]
- 2.Gu DH, Kim MY, Seo YS, et al. Clinical usefulness of psoas muscle thickness for the diagnosis of sarcopenia in patients with liver cirrhosis. Clin Mol Hepatol. 2018;24:319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortez APB, de Morais MB, Speridião PDGL, et al. Food intake, growth and body composition of children and adolescents with autoimmune hepatitis. J Clin Gastroenterol. 2010;44:200–207. [DOI] [PubMed] [Google Scholar]
- 4.Montano-Loza AJ, Meza-Junco J, Prado CMM, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166–173.e1. [DOI] [PubMed] [Google Scholar]
- 5.Tandon P, Ney M, Irwin I, et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18:1209–1216. [DOI] [PubMed] [Google Scholar]
- 6.Ebadi M, Bhanji RA, Mazurak VC, et al. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol. 2019;54:845–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim G, Kang SH, Kim MY, et al. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PLoS One. 2017;12:e0186990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ooi PH, Thompson-Hodgetts S, Pritchard-Wiart L, et al. Pediatric sarcopenia: a paradigm in the overall definition of malnutrition in children? J Parenter Enteral Nutr. 2020;44:407–418. [DOI] [PubMed] [Google Scholar]
- 9.Chin SE, Shepherd RW, Thomas BJ, et al. The nature of malnutrition in children with end-stage liver disease awaiting orthotopic liver transplantation. Am J Clin Nutr. 1992;56:164–168. [DOI] [PubMed] [Google Scholar]
- 10.Lurz E, Patel H, Frimpong RG, et al. Sarcopenia in children with end stage liver disease. J Pediatr Gastroenterol Nutr. 2018;66:222–226. [DOI] [PubMed] [Google Scholar]
- 11.Ponziani FR, Gasbarrini A. Sarcopenia in patients with advanced liver disease. Curr Protein Pept Sci. 2018;19:681–691. [DOI] [PubMed] [Google Scholar]
- 12.Goossens GH. The metabolic phenotype in obesity: fat mass, body fat distribution, and adipose tissue function. Obes Facts. 2017;10:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalafateli M, Mantzoukis K, Choi Yau Y, et al. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J Cachexia Sarcopenia Muscle. 2017;8:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonati JG, Modeneze DM, Vilarta R, et al. Body composition and quality of life (QoL) of the elderly offered by the “University Third Age” (UTA) in Brazil. Arch Gerontol Geriatr. 2011;52:e31–e35. [DOI] [PubMed] [Google Scholar]
- 15.Maggiore G, Nastasio S, Sciveres M. Juvenile autoimmune hepatitis: spectrum of the disease. World J Hepatol. 2014;6:464–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies A, Nixon A, Muhammed R, et al. Reduced skeletal muscle protein balance in paediatric Crohn’s disease. Clin Nutr. 2020;39:1250–1257. [DOI] [PubMed] [Google Scholar]
- 17.Mager DR, Carroll MW, Wine E, et al. Vitamin D status and risk for sarcopenia in youth with inflammatory bowel diseases. Eur J Clin Nutr. 2018;72:623–626. [DOI] [PubMed] [Google Scholar]
- 18.Klein GL. The effect of glucocorticoids on bone and muscle. Osteoporos Sarcopenia. 2015;1:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouma S Diagnosing pediatric malnutrition. Nutr Clin Pract. 2017;32:52–67. [DOI] [PubMed] [Google Scholar]
- 20.Tamakoshi A, Yatsuya H, Lin Y, et al. BMI and all-cause mortality among Japanese older adults: findings from the Japan collaborative cohort study. Obesity (Silver Spring). 2010;18:362–369. [DOI] [PubMed] [Google Scholar]
- 21.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lurz E, Patel H, Lebovic G, et al. Paediatric reference values for total psoas muscle area. J Cachexia Sarcopenia Muscle. 2020;11:405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trout AT, Hunte DE, Mouzaki M, et al. Relationship between abdominal fat stores and liver fat, pancreatic fat, and metabolic comorbidities in a pediatric population with non-alcoholic fatty liver disease. Abdom Radiol (NY). 2019;44:3107–3114. [DOI] [PubMed] [Google Scholar]
- 24.Harbaugh CM, Zhang P, Henderson B, et al. Personalized medicine: enhancing our understanding of pediatric growth with analytic morphomics. J Pediatr Surg. 2017;52:837–842. [DOI] [PubMed] [Google Scholar]
- 25.Li AA, Kim D, Ahmed A. Association of sarcopenia and NAFLD: an overview. Clin Liver D/s (Hoboken). 2020;16:73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanderwall C, Randall Clark R, Eickhoff J, et al. BMI is a poor predictor of adiposity in young overweight and obese children. BMC Pediatr. 2017;17:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi KM. Sarcopenia and sarcopenic obesity. Korean J Intern Med. 2016;31:1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohara K, Ochi M, Tabara Y, et al. Leptin in sarcopenic visceral obesity: possible link between adipocytes and myocytes. PLoS One. 2011;6:e24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacraz G, Rakotoarivelo V, Labbé SM, et al. Deficiency of interleukin-15 confers resistance to obesity by diminishing inflammation and enhancing the thermogenic function of adipose tissues. PLoS One. 2016;11:e0162995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ceddia RB. Direct metabolic regulation in skeletal muscle and fat tissue by leptin: implications for glucose and fatty acids homeostasis. Int J Obes (Lond). 2005;29:1175–1183. [DOI] [PubMed] [Google Scholar]
- 31.van der Poorten D, Milner K-L, Hui J, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48:449–457. [DOI] [PubMed] [Google Scholar]
- 32.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


