Abstract
Introduction
The growing number of women diagnosed with breast cancer (BCa) together with high survival has resulted in an increasing population of survivors at risk of subsequent primary cancers. This study aimed to estimate the long-term risk and survival of third primary cancers (TPCs) among females with a first primary BCa.
Methods
Breast first primary cancers (FPCs) from the Portuguese North Region Cancer Registry, diagnosed between 2000 and 2010 (n = 15,981), were followed for a TPC (December 31, 2015) and death from any cause (June 30, 2021). The cumulative incidence of and mortality among TPCs were estimated. To compare survival, female patients with a TPC were matched (1:1, by age group, years between FPC and second primary cancer [SPC] diagnosis, and SPC location) to FPC + SPC patients without a TPC.
Results
Overall, 67 (0.4% of FPCs and 5.4% of SPCs) TPCs were diagnosed. The most common TPC sites were digestive, breast, and female genital organs. Among all FPCs, the 15-year cumulative incidence (95% confidence interval [CI]) of a TPC was 0.69% (0.47–0.90%) and among SPCs, 7.21% (4.99–9.43%). The 15-year cumulative mortality of TPCs and matched patients was 70.0% and 51.5%, respectively. For TPCs, compared to matched SPC only patients, the age-adjusted hazard ratio (95% CI) for death was 2.86 (1.61–5.07).
Discussion/Conclusion
The most common TPC sites were digestive, breast, and female genital organs, with a 15-year cumulative incidence of 0.69% among FPCs. TPCs had a worse long-term survival compared to patients with an SPC only.
Keywords: Epidemiology, Mortality, Multiple primary neoplasms, Registries, Breast neoplasms
Introduction
Breast cancer (BCa) is the most common malignancy diagnosed worldwide, accounting for 7.8 million women alive who were diagnosed with BCa in the past five years, and the second leading cause of death from cancer [1]. The survival of patients with BCa has increased in the last decades [2] due to improved access to early diagnosis [3] and effective treatments, including new adjuvant drugs and less aggressive surgeries [4], leading to a growing number of BCa survivors. These patients have been shown to be at increased risk for subsequent primary cancers at several sites due to shared etiology, including genetic susceptibility and environmental factors, as well as treatment effects [5, 6]. Previous studies have described the epidemiological and clinical characteristics of BCa patients with second primary cancers (SPCs) [7, 8, 9], as well as the occurrence of third primary cancers (TPCs) of nonbreast origin among women with bilateral BCa [10]. Nevertheless, there is a lack of published data addressing the risk and survival of BCa patients with higher order primary cancers. Therefore, considering that BCa is the most frequent malignancy in Northern Portugal [11] and responsible for the highest proportion of SPCs among females [12], this study aimed to estimate the long-term risk and survival of TPCs among patients with a breast first primary cancer (FPC).
Methods
A population-based cohort of female breast (International Statistical Classification of Diseases and Related Health Problems 10th Revision C50 [13]) FPCs (n = 15,981) from the North Region Cancer Registry of Portugal (RORENO) diagnosed in 2000–2010 was followed for the diagnosis of multiple primary cancers (MPCs; December 31, 2015) and vital status (June 30, 2021). MPCs were defined as proposed by the International Association of Cancer Registries and International Agency for Research on Cancer [14]. These were considered SPCs (n = 1,229), TPCs (n = 67), and fourth or higher order primary cancers (n = 4) when two, three, or more than three primary cancers, respectively, were diagnosed in the same individual.
Cumulative incidence and corresponding 95% confidence intervals (CIs) for the occurrence of TPCs considering the date of diagnosis of the breast FPC and of the SPC were estimated separately, accounting for the competing event of death [15]. The maximum possible follow-up time from FPC to TPC and from SPC to TPC was 15 years.
A matched-sample analysis was conducted to estimate the survival of TPCs; patients diagnosed with more than three primary cancers (n = 4) were excluded. Females with TPCs (SPC + TPC) were matched 1:1 to females with an SPC but without a TPC (SPC only) who were alive when the corresponding TPC was diagnosed, by age-group (<45, 45–54, 55–64, 65–74, and ≥75 for 39 matches; <45, 45–64, and ≥65 for 21 matches; <65 and ≥65 for three matches), number of years between FPC and SPC diagnoses (single year [from 1 to 15 years] for 49 matches; first-year and then two-year groups [from 2–3 years to 14–15 years] for four matches; and three-year groups [from 1–3 years to 13–15 years] for 10 matches), and SPC location (SPC site for 56 matches; SPC group: digestive organs [C15–C26]; respiratory and intrathoracic organs [C30–C39]; skin melanoma [C43]; breast [C50]; female genital organs [C51–C58]; urinary tract [C64–C68]; eye [C69]; brain and other parts of central nervous system [C70–C72]; lymphoid, hematopoietic, and related tissue [C81–C96]; uncertain or unknown behavior [D37–D48] [13] for seven matches).
Survival time for patients with a TPC was considered as the time between TPC diagnosis and death from any cause or end of study follow-up (June 30, 2021), whichever occurred first. For SPC only patients, survival time was considered as the time between SPC diagnosis and death or end of study follow-up (June 30, 2021), whichever occurred first, minus the time between the SPC diagnosis and the TPC diagnosis of the matched patients with TPC. The maximum possible vital status follow-up time for SPC only patients (from SPC diagnosis) was 21 years and for SPC + TPC patients (from TPC diagnosis) was 18 years.
Cox proportional hazards regression analyses were used to compute hazard ratios for all-cause mortality adjusted for age (continuous) with the corresponding 95% CI. The proportional hazards assumption was evaluated using Schoenfeld residuals. The observed cumulative all-cause mortality was estimated using 1 − Kaplan-Meier [16]. Statistical analyses were conducted using Stata®, version 15.1 (StataCorp., College Station, TX, USA).
Results
During follow-up to the end of 2015, 1,229 patients developed a subsequent primary cancer (7.7% of breast FPCs) and 67 patients a TPC (0.4% of breast FPCs and 5.4% of FPC + SPC; Table 1). A total of 56 contralateral BCas were diagnosed among patients without a TPC (n = 1,162), while one contralateral second primary BCa was observed in patients with a TPC (n = 67). The median (percentile 25–percentile 75 [P25–P75]) age at breast FPC diagnosis was 57 (47–68) years. For patients diagnosed with an SPC only, the median (P25–P75) time between the FPC and the SPC was 4.0 (1.2–7.3) years; the corresponding time for patients with a TPC was 3.6 (1.0–6.4) years. The median (P25–P75) time between an SPC and a TPC was 0.6 (0.2–1.9) years.
Table 1.
Characteristics of female breast FPC patients with and without a subsequent primary cancer
| N (%) | Total 15,981 (100.0) | Patients without a subsequent primary 14,752 (92.3) | Patients with a subsequent primary 1,229 (7.7) |
|
|---|---|---|---|---|
| Patients without a TPC 1,162 (93.2) | Patients with a TPC 67 (5.4 of SPCs; 0.4 of FPCs) | |||
| Age at diagnosis of BCa (median [P25–P75]), years | 57 (47–68) | 56 (47–68) | 60 (50–70) | 62 (54–74) |
| <45 | 3,032 (19.0) | 2,879 (19.5) | 146 (12.6) | 7 (10.4) |
| 45–54 | 4,162 (26.0) | 3,874 (26.3) | 278 (23.9) | 10 (14.9) |
| 55–64 | 3,565 (22.3) | 3,267 (22.1) | 276 (23.7) | 22 (32.8) |
| 65–74 | 2,274 (14.2) | 2,639 (17.9) | 293 (25.2) | 13 (19.4) |
| ≥75 | 2,274 (14.2) | 2,090 (14.2) | 169 (14.5) | 15 (22.4) |
| BCa laterality | ||||
| Right | 5,839 (36.5) | 5,392 (36.5) | 419 (36.1) | 28 (41.8) |
| Left | 6,490 (40.6) | 5,980 (40.5) | 484 (41.6) | 26 (38.8) |
| Bilateral | 57 (0.4) | 0 (0.0) | 56 (4.8) | 1 (1.5) |
| Unknown/missing | 3,595 (22.5) | 3,380 (22.9) | 203 (17.5) | 12 (17.9) |
| Time to diagnosis (median [P25–P75]), years | − | − | 4.0 (1.2–7.3) FPC to SPC | 3.6 (1.0–6.4) FPC to SPC 0.6 (0.2–1.9) SPC to TPC |
May not add to 15,981 due to missing data. BCa, breast cancer; FPC, first primary cancer; P25, percentile 25; P75, percentile 75; SPC, second primary cancer; TPC, third primary cancer.
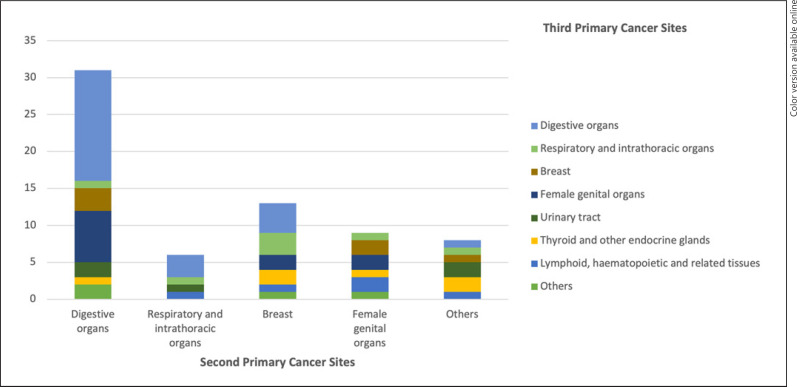
Among the 67 females diagnosed with a TPC, over one-third of TPCs (34.3%, n = 23) occurred in digestive organs, and nearly half (46.3%, n = 31) of TPCs occurred in patients with a previous digestive SPC (Fig. 1; online suppl. Table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000522057). Breast and female genital organs were also common TPC sites, occurring in 6 (8.9%) and 11 (16.4%) females, respectively, as well as an SPC among females diagnosed with a TPC, accounting for 19.4% (n = 13) and 13.4% (n = 9) of cases, respectively.
Fig. 1.
Distribution of TPCsa among SPCsb in female breast FPC patients. FPC, first primary cancer; ICD-10, International Statistical Classification of Diseases and Related Health Problems 10th Revision; SPC, second primary cancer; TPC, third primary cancer. a Digestive organs (C15–C26); respiratory and intrathoracic organs (C30–C39); breast (C50); female genital organs (C51–C58); urinary tract (C64–C68); thyroid and other endocrine glands (C73–C75); lymphoid, hematopoietic, and related tissues (C81–C96); and others: lip, oral cavity and pharynx (C1–C14), skin melanoma (C43), mesothelial and soft tissue (C45–C49), ill-defined, secondary, or unspecified sites (C76–C80) defined according to the ICD-10 [13]. b Digestive organs (C15–C26), respiratory and intrathoracic organs (C30–C39), breast (C50), female genital organs (C51–C58), others: skin melanoma (C43), urinary tract (C64–C68), eye (C69), brain and other parts of the central nervous system (C70–C72), lymphoid, hematopoietic, and related tissue (C81–C96), uncertain or unknown behavior (D37–D48) defined according to the ICD-10 [13].
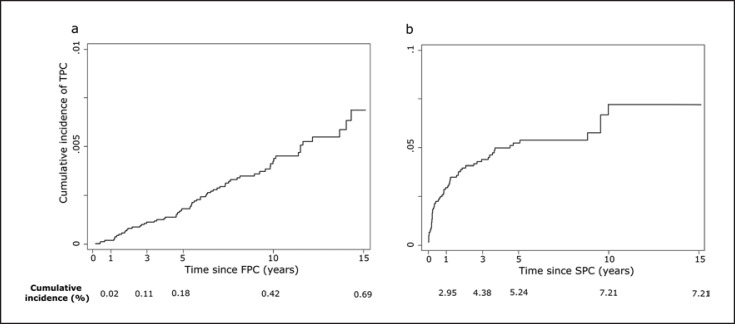
The 10- and 15-year cumulative incidences (95% CI) of a TPC were 0.42% (0.31–0.54%) and 0.69% (0.47–0.90%), respectively, among patients with a breast FPC (Fig. 2a). Among patients with an SPC following a breast FPC, the 10-year cumulative incidence (95% CI) of a TPC was 7.21% (4.99–9.43%; Fig. 2b).
Fig. 2.
Cumulative incidence of TPCs, among female breast FPCs (a) and SPCs (b) following a breast FPC, considering the competing event of death. FPC, first primary cancer; SPC, second primary cancer; TPC, third primary cancer. Note that a different scale is used for the two graphs.
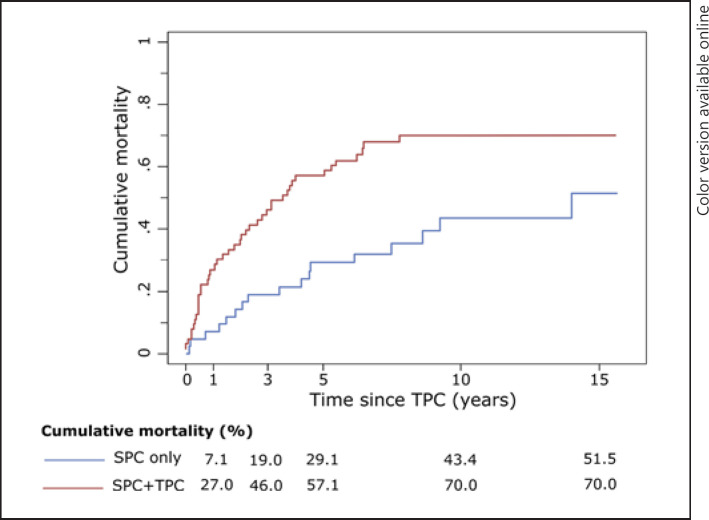
The five-year cumulative all-cause mortality (95% CI) of the SPC + TPC patients and matched SPC-only patients was 57.1% (47.6–66.6%) and 29.1% (19.5–38.7%), respectively, whereas the corresponding risk of mortality at 10 years was 70.0% (60.0–80.0%) and 43.4% (33.3–53.5%; Fig. 3). For SPC + TPC patients, compared to matched SPC only patients, the crude and age-adjusted hazard ratios (95% CI) for death were 2.43 (1.39–4.27) and 2.86 (1.61–5.07), respectively.
Fig. 3.
Observed cumulative all-cause mortalitya of female breast FPCs with an SPC, and with and without a TPC. FPC, first primary cancer; SPC, second primary cancer; TPC, third primary cancer. a Calculated using 1 − Kaplan-Meier [16].
Discussion/Conclusion
Overall, the most common TPCs occurred in the digestive system, as well as breast and female genital organs, with a relatively low 15-year cumulative incidence (0.69%) among all breast FPC patients. SPC + TPC patients had a higher probability of all-cause mortality over 15 years compared to patients with an SPC only, highlighting the contribution of a TPC to the mortality of these patients.
Few studies have been conducted on the frequency of MPCs with no population-based studies focusing on the risk and survival of TPCs among BCa survivors, regardless of the SPC site [10]. Generally, the proportion of TPCs is small, ranging from 0.0% to 2.0% of all cancer diagnoses [17]. In Italy, among cancers (all sites) diagnosed between 1976 and 2010, and followed to the end of 2012, 0.3% of patients were diagnosed with a TPC [18]. A previous study conducted in the Netherlands found nearly 7% nonbreast TPCs occurring among 8,752 women diagnosed with bilateral BCa between 1989 and 2008, with a median time of 2.4 years between the first and the second BCa [10]. We estimated a proportion of 0.4% TPCs among breast FPCs, which falls within the previously reported range of TPCs [17].
In the current study, we found that TPCs were more often diagnosed in the digestive system, breast, and female genital organs. This may be because the factors, including host characteristics and environmental exposures, that contribute to the occurrence of FPCs also increase the risk of developing a subsequent primary cancer [6]. Previous studies have shown that caloric excess, obesity, physical inactivity, and reproductive factors may contribute to a clustering of hormone-dependent tumors, including breast, uterine, ovary, and colon tumors [5, 6, 19, 20]. Different hormone receptor statuses are associated with second female genital cancers among BCa survivors, with an increased risk being observed among hormone receptor-negative BCa survivors [21, 22]. Moreover, although cancer hereditary syndromes likely account for only a small proportion of MPCs [6], previous studies have shown that BCa survivors with inherited mutations of BRCA1/2, PTEN, or PT53 have an increased risk of developing several subsequent cancers, including cancers of the pancreas, contralateral breast, female genital organs, thyroid, and connective tissues [7, 23, 24, 25].
The survival of BCa patients who develop a TPC has been seldom described. However, previous studies have found a worse long-term mortality for women with two cancers compared to those with a BCa only [8, 10]. Similarly, the estimates provided here highlight that BCa patients diagnosed with a TPC have a higher mortality. Consequently, the occurrence of MPCs should be considered by clinicians when establishing a prognosis and treatment plan for these patients.
This study is based on data obtained from RORENO, which is representative of BCa survivors from Northern Portugal; however, extrapolating these results to all Portuguese BCa survivors should be done cautiously since there is an uneven distribution of BCa incidence and mortality within the country [26, 27]. Nevertheless, the sample is population-based, and the results may be generalized to settings where the overall patterns of cancer incidence and mortality, and access to health care are not markedly different. Furthermore, an evaluation considering specific SPC or TPC combinations was not possible due to the small number of TPCs observed, and as such, only a descriptive analysis is provided. The current study is also limited by the information available in the registry; thus, we were unable to account for factors such as family history and mutation status, which have been shown to be associated with MPCs [7, 23, 24, 25]. Nevertheless, the results of the current study are useful and exhaustive since the incidence and survival of TPCs among female breast FPCs have not been widely reported in previous population-based studies.
The cumulative incidence of TPCs among all breast FPC patients was relatively low with an estimate of 0.69% over a maximum 15-year follow-up period, which were more often observed in the breast, digestive, and female genital organs. Further, breast FPC patients who develop a TPC have a clearly worse survival compared to those with an FPC and SPC only. Considering the increasing number of patients with BCa at risk of developing MPCs, this study highlights the need for additional research to further quantify the burden and survival of TPCs among these patients. This is necessary to adequately determine a comprehensive and personalized treatment approach that considers previous treatments and associated toxicities, and in some cases, may require a more aggressive approach, as well as define surveillance strategies and manage patients' expectations regarding the cancer survivorship burden. Taken together, these may potentially lead to improvements in survival and quality of life among patients with multiple cancers.
Statement of Ethics
The study was approved by the Ethics Committee of the Portuguese Institute of Oncology of Porto (Ref. CES IPO: 332/020), and the analyses were performed according to RORENO guidelines for pseudo anonymization of information used. All procedures performed were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was granted as exemption from requiring written informed consent by the Ethics Committee of the Portuguese Institute of Oncology of Porto (Ref. CES IPO: 332/020).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by national funds from the Foundation for Science and Technology − FCT (Portuguese Ministry of Science, Technology and Higher Education), under the Unidade de Investigação em Epidemiologia − Instituto de Saúde Pública da Universidade do Porto (EPIUnit; UIDB/04750/2020). S.M. was funded by FEDER through the Operational Program Competitiveness and Internationalization and national funding from FCT under the scope of the project “NEON-PC − Neuro-oncological complications of prostate cancer: longitudinal study of cognitive decline” (POCI-01-0145-FEDER-032358; ref. PTDC/SAU-EPI/32358/2017) and received funding from the EPIUnit − Junior Research − Prog Financing (UIDP/04750/2020). The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author Contributions
S.M. was responsible for formal analysis, investigation, methodology, and writing − original draft. S.M. and N.L. conceptualized and visualized the study. N.L. supervised the study. All the authors were responsible for data curation and writing − review and editing.
Data Availability Statement
The data that support the findings of this study are available from RORENO, but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of RORENO.
Supplementary Material
Supplementary data
References
- 1.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer; 2020. [Google Scholar]
- 2.Crocetti E, Roche L, Buzzoni C, di Costanzo F, Molinié F, Caldarella A. Trends in net survival from breast cancer in six European Latin countries: results from the SUDCAN population-based study. Eur J Cancer Prev. 2017 Jan;26:S85–91. doi: 10.1097/CEJ.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 3.Independent UK Panel on Breast Cancer Screening The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380((9855)):1778–86. doi: 10.1016/S0140-6736(12)61611-0. [DOI] [PubMed] [Google Scholar]
- 4.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005 Dec 17;366((9503)):2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 5.Curtis R, Freedman D, Ron E, Ries L, Hacker D, Edwards B, et al. New malignancies among cancer survivors: SEER cancer registries, 1973–2000. Bethesda, MD: 2006. [Google Scholar]
- 6.Travis LB, Demark Wahnefried W, Allan JM, Wood ME, Ng AK. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol. 2013 May;10((5)):289–301. doi: 10.1038/nrclinonc.2013.41. [DOI] [PubMed] [Google Scholar]
- 7.Molina-Montes E, Pérez-Nevot B, Pollán M, Sánchez-Cantalejo E, Espín J, Sánchez MJ. Cumulative risk of second primary contralateral breast cancer in BRCA1/BRCA2 mutation carriers with a first breast cancer: a systematic review and meta-analysis. Breast. 2014 Dec;23((6)):721–42. doi: 10.1016/j.breast.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Molina-Montes E, Requena M, Sánchez-Cantalejo E, Fernández MF, Arroyo-Morales M, Espín J, et al. Risk of second cancers cancer after a first primary breast cancer: a systematic review and meta-analysis. Gynecol Oncol. 2015 Jan;136((1)):158–71. doi: 10.1016/j.ygyno.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Pan B, Xu Y, Zhou YD, Yao R, Wu HW, Zhu QL, et al. The prognostic comparison among unilateral, bilateral, synchronous bilateral, and metachronous bilateral breast cancer: a meta-analysis of studies from recent decade (2008–2018) Cancer Med. 2019;8((6)):2908–18. doi: 10.1002/cam4.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwast ABG, Liu L, Roukema JA, Voogd AC, Jobsen JJ, Coebergh JW, et al. Increased risks of third primary cancers of non-breast origin among women with bilateral breast cancer. Br J Cancer. 2012;107((3)):549–55. doi: 10.1038/bjc.2012.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Registo Oncológico Regional do Norte . Registo Oncológico Regional do Norte 2015 [North Region Cancer Registry 2015] Porto, Portugal: Instituto Português de Oncologia do Porto Francisco Gentil - EPE; 2021. [Google Scholar]
- 12.Pacheco-Figueiredo L, Antunes L, Bento MJ, Lunet N. Evaluation of the frequency of and survival from second primary cancers in North Portugal: a population-based study. Eur J Cancer Prev. 2013 Nov;22((6)):599–606. doi: 10.1097/CEJ.0b013e32835f3bbc. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization . International statistical classification of diseases and related health problems 10th revision. Geneva: World Health Organization; 1992. [Google Scholar]
- 14.Working Group Report International rules for multiple primary cancers (ICD-0 third edition) Eur J Cancer Prev. 2005 Aug;14((4)):307–8. doi: 10.1097/00008469-200508000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Kalbfleisch J, Prentice R. The statistical analysis of failure time data. Second edition. New York: John Wiley & Sons; 2002. [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53((282)):457–81. [Google Scholar]
- 17.Rosso S, De Angelis R, Ciccolallo L, Carrani E, Soerjomataram I, Grande E, et al. Multiple tumours in survival estimates. Eur J Cancer. 2009 Apr;45((6)):1080–94. doi: 10.1016/j.ejca.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 18.AIRTUM Working Group Italian cancer figures, report 2013: multiple tumours. Epidemiol Prev. 2013 Jul–Oct;37((4–5 Suppl 1)):1–152. [PubMed] [Google Scholar]
- 19.Druesne-Pecollo N, Touvier M, Barrandon E, Chan DS, Norat T, Zelek L, et al. Excess body weight and second primary cancer risk after breast cancer: a systematic review and meta-analysis of prospective studies. Breast Cancer Res Treat. 2012 Oct;135((3)):647–54. doi: 10.1007/s10549-012-2187-1. [DOI] [PubMed] [Google Scholar]
- 20.Ricceri F, Fasanelli F, Giraudo MT, Sieri S, Tumino R, Mattiello A, et al. Risk of second primary malignancies in women with breast cancer: results from the European Prospective Investigation into Cancer and nutrition (EPIC) Int J Cancer. 2015 Aug 15;137((4)):940–8. doi: 10.1002/ijc.29462. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Jiang W, Mao K, An Y, Su F, Kim BYS, et al. Elevated risks of subsequent endometrial cancer development among breast cancer survivors with different hormone receptor status: a SEER analysis. Breast Cancer Res Treat. 2015;150((2)):439–45. doi: 10.1007/s10549-015-3315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Wu Q, Song J, Zhang Y, Zhu S, Sun S. Risk of second primary female genital malignancies in women with breast cancer: a SEER analysis. Horm Cancer. 2018;9((3)):197–204. doi: 10.1007/s12672-018-0330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson D, Easton DF. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002 Sep 18;94((18)):1358–65. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 24.Metcalfe KA, Lynch HT, Ghadirian P, Tung N, Olivotto IA, Foulkes WD, et al. The risk of ovarian cancer after breast cancer in BRCA1 and BRCA2 carriers. Gynecol Oncol. 2005 Jan;96((1)):222–6. doi: 10.1016/j.ygyno.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 25.Travis LB, Rabkin CS, Brown LM, Allan JM, Alter BP, Ambrosone CB, et al. Cancer survivorship: genetic susceptibility and second primary cancers − research strategies and recommendations. J Natl Cancer Inst. 2006 Jan 4;98((1)):15–25. doi: 10.1093/jnci/djj001. [DOI] [PubMed] [Google Scholar]
- 26.Registo Oncológico Regional do Norte . Registo Oncológico Nacional 2010 [National Cancer Registry 2010] Porto, Portugal: Instituto Português de Oncologia do Porto Francisco Gentil - EPE; 2016. [Google Scholar]
- 27.Gomes IA, Nunes C. Analysis of the breast cancer mortality rate in Portugal over a decade: spatiotemporal clustering analysis. Acta Med Port. 2020 May 4;33((5)):305–10. doi: 10.20344/amp.11749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
The data that support the findings of this study are available from RORENO, but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of RORENO.