Abstract
Objective
In this study, we investigated the feasibility and efficacy of immediate breast augmentation with autologous fat grafting after removal of polyacrylamide hydrogel (PAAG) and fibrotic capsule.
Methods
A retrospective study was conducted on 162 female patients who underwent removal of breast filler PAAG and the fibrotic capsule which produced after injection of PAAG via areola omega-shaped incision. Then autologous fat grafting was immediately performed evenly and radially around the areola via the same incision into different layers (subcutaneous, submammary tissue, pectoralis major intramuscular, and inferior pectoralis major space) except the empty cavity. The cavity left by removal of PAAG and fibrous capsule was closed with negative pressure drainage tube and slight external pressure.
Results
All patients recovered well without severe complications. The average score of postoperative satisfaction with physical well-being: chest was 99.83 (total score: 100) compared with the average satisfaction score of 71.69 (total score: 100) preoperatively by means of BREAST-Q™ evaluation (p < 0.01). All patients were satisfied with their postoperative breast shape.
Conclusions
Removing as much as possible is critical for patients who underwent the PAAG injection. Our experience in immediate breast augmentation with autologous fat grafting after removal of PAAG and fibrotic capsule proved useful and effective to maintain the balance between removing the PAAG as much as possible and retaining soft tissue to reshape breasts.
Level of Evidence
IV.
Keywords: Polyacrylamide hydrogel, Autologous fat grafting, Fibrotic capsule
Introduction
Polyacrylamide hydrogel (PAAG), which is also called Interfall (produced in Ukraine) or Amazingel (produced in China), has been once all the rage for breast augmentation among female in China since it was approved by the China Food and Drug Administration (CFDA) for its broader clinical usage in 1999 [1]. By the time the approval was withdrawn in 2006, 300,000 or more female injected with PAAG in breast augmentation were complaining about the complications associated with PAAG, such as infection, pain, hematoma, multiple induration and lumps, breastfeeding difficulties, breast autoinflation, displacement of the injected material, as well as non-negligible psychological problems [2, 3, 4, 5]. Delayed complications have also been reported recently, including a long-lasting autoimmune reaction with general complications [6, 7]. The only way out for patients with these complications is to remove the PAAG as much as possible. In order to maintain the desired breast shape, most patients prefer reconstructive surgery after removing PAAG to improve breast shape and regain self-confidence and quality of life. Breast reconstruction includes the main procedure of autologous fat grafting [8], silicone prosthesis implantation [8, 9, 10], periareolar mammoplasty with the tissue folding technique [11], and other reconstructive methods as well. The injection breast augmentation is defined as the breast augmentation material directly injected into the breasts to achieve immediate breast enlargement. It has been more favored by females in recent years because it is a simple operation with fast recovery, no added scars, and relatively minor trauma. Patients prefer autologous tissue rather than foreign materials, and at the same time body shape remodeling can be achieved through liposuction of specific fat areas. In this study, we retrospectively reviewed 162 patients in the past decade who underwent removal of breast filler PAAG and the fibrotic capsule which produced after injection of PAAG and meanwhile autologous fat grafting was performed immediately.
Patients and Methods
Patients
A total of 200 female patients were observed and at last 162 female patients were enrolled in this study. Two cases were excluded from this study because of highly suspected malignancy in preoperative examination and later confirmed by specialist hospitals. Three cases were excluded because of skin ulceration and tissue ulceration. Several other patients did not meet the inclusion criteria including uncontrolled severe psychological problems and other operative contraindicated reasons of patients themselves.162 female patients (aged from 41 to 67 years, averaging 48.3) had a history of PAAG injection breast augmentation and had suffered from some sort of PAAG-associated complications. They had PAAG and fibrotic capsule removed and had immediately augmentation mammoplasty with autologous fat grafting under general anesthesia or intravenous anesthesia between October 2012 and April 2021 at Jingmei Cosmetic Surgery Clinic. The study was approved by the Ethics Committee of Jingmei Cosmetic Surgery Clinic (No. 110-002), and written informed consent was obtained from all of the patients for use of their data and portrait rights (surgical areas with no face exposure). Demographic data including patient age, gender, surgical history, breast filler, medications, body mass index, pregnancy situation, and allergies were recorded. Patients those who were pregnant, had a pacemaker installed, had breast cancer without treatment or under treatment, mental illness, had ulceration or infection in the surgical area, and had a history of smoking within 3 months were excluded in this study as a standard procedure. All patients needed to have adequate body fat. And all of them were interviewed prior to the operation to confirm the purpose, potential risks, surgical procedure, duration of fat absorption, and complications of autologous fat grafting. The American Society of Anesthesia (ASA) Class was assessed. Physical examination was carried out to observe the symmetry of the breast and areola, skin conditions, breast shape and softness, and whether there was any abnormal mass or not. The doctor and the patient needed to agree preoperatively on the approximate volume of the breasts after removing as much PAAG and PAAG fibrotic capsule as possible, the rough volume of autologous fat may be injected, and the donor site of fat. Preoperative (before operation), intraoperative (immediately after PAAG and fibrotic capsule were taken), and postoperative (at least 6 months after fat grafting) photos were taken in standard postures. Preoperative magnetic resonance imaging (MRI) or B-mode ultrasound of the breasts was performed to exclude breast mass [12]. A complete blood count and blood coagulation index were tested conventionally. The surgical procedures were performed by our surgical team at the Department of Cosmetic Surgery, Jingmei Cosmetic Surgery Clinic. The duration of the operation, the volume of liposuction at donor site, and the volume of fat for breast recipient areas were recorded. BREAST-QTM-Augmentation Module version 2.0 was used to assess the preoperative satisfaction with breast and satisfaction with outcome.
Methods
Removal of PAAG and Fibrotic Capsule
The modified semi-periareola incision (an incision in the areola shaped like an “Ω”/omega-shaped) approach was routinely used in all patients according to internal guidelines. PAAG was removed as much as possible after bilateral breast areas were infiltrated with tumescent fluid. The inflammatory fibrotic capsule dotted with nodules formed in the mammary gland after injection of PAAG should be removed completely [13, 14] (Fig. 1), with enough care taken to protect the ducts and preserve as much normal breast tissue as possible. During the procedure of capsule resection, electric coagulation and electric knife equipment were not used to avoid high temperature damage. The tools we chose for removal were the simplest surgical scissors and surgical blades. This procedure was also routinely performed according to internal guidelines. The inflammatory fibrotic capsule dotted with nodules formed in the mammary gland after injection of PAAG should be removed completely. After the fibrotic capsule was removed, the dead cavity was continuously rinsed with sterile normal saline, and a continuous negative pressure suction tube was placed in the cavity through a small concealed needle eye in the axillary front. The negative pressure drainage tube continued to work until the drainage flow was less than 20 mL per day.
Fig. 1.
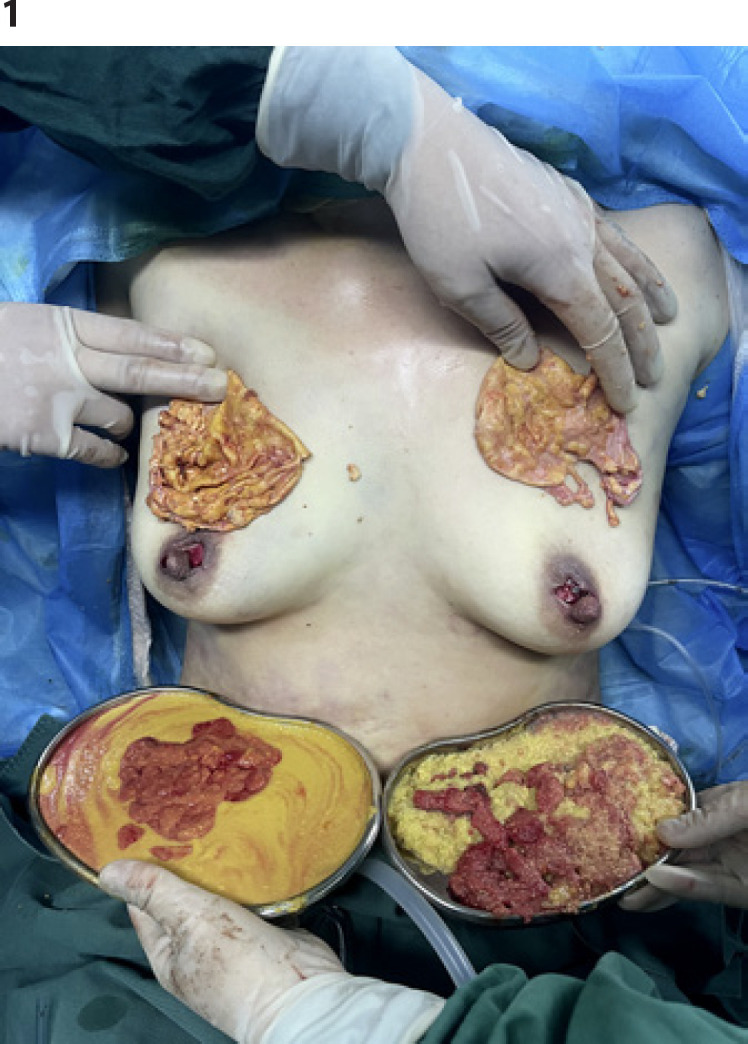
Gross pathological specimen demonstrating bilateral extensive fibrotic capsules and porridge-like consistency of PAAG.
Autologous Fat Grafting Technique
At the same time, liposuction at the donor site was performed in accordance with the surgical technique of fat grafting dscribed by Coleman [15]. The donor site we usually choose is abdomen and/or thigh, depending on each patient's fat distribution. A 2.5–3-mm diameter aspiration cannula was used with a liposuction aspirator under a low negative pressure (300–375 mm Hg) to harvest grafted fat. After centrifugation (1,000 g 3 min) and refinement, the grafted fat was collected in an empty sterile saline plastic bag. It was connected to the infusion tube through a special interface. One end of the infusion tube was connected to a four-way device/three-way device, and the other ends of the three-way/four-way device were, respectively, connected to a 1-mL syringe and a thread grease needle. The extra end was closed or used for standby use like adding drugs (Fig. 2). Each saline plastic bag can independently store as much fat as needed for each breast and can be stored in icy water. Then the fat was layered evenly and radially around the areola into different layers from the chest wall to the skin (subcutaneous, submammary tissue, pectoralis major intramuscular, and inferior pectoralis major space), except the residual dead cavity left by removal of PAAG and fibrotic capsule. Finally, the areola incision was intermittent subcutaneous suture with 5-0 absorbable suture and skin intermittent suture with 7-0 monofilament nylon thread. The breasts were compressed by a chest band with slight pressure, and the donor areas were compressed by an elastic garment for 1 to 3 months.
Fig. 2.
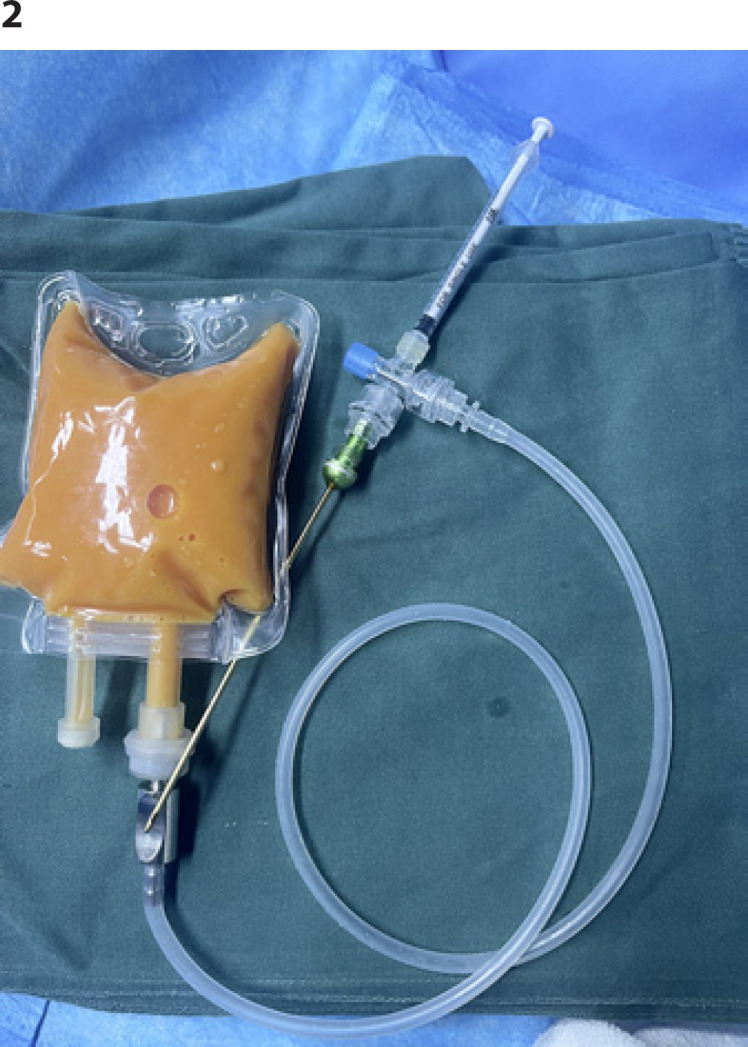
Granular fat storage and injection device.
Breast Volume Measurement and BREAST-QTM Assessment
All patients underwent breast volume measurement at three time points: before surgery, PAAG and fibrotic capsule just taken intraoperatively, at least 6 months after fat grafting (Fig. 3) with the use of the Crisalix 3D Imaging platform (© 2021 Crisalix S.A., Lausanne, Switzerland) (Fig. 4). Three standard photographs of each patient's breasts were taken anteriorly, 45° to the left, and 45° to the right with an ordinary digital camera. And the distance between bilateral nipple was measured at each time point. The Breast-QTM is a reliable and objective evaluation tool for breast-related surgery, mainly including satisfaction with breast appearance, psychological well-being, sexual well-being, physical well-being, and so on [16]. In this study, we mainly focused on the postoperative satisfaction with the breast appearance.
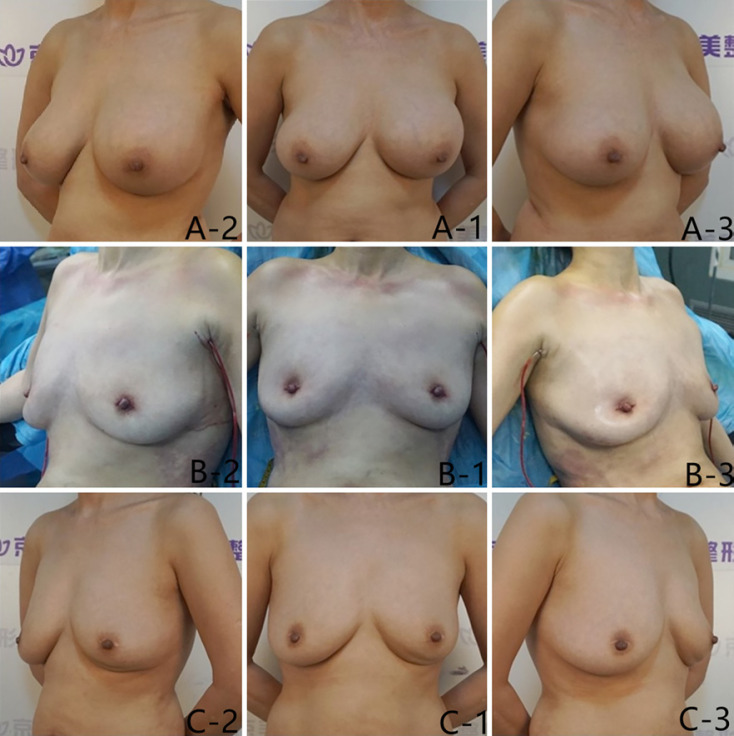
Fig. 3.
A 48-year-old female.A-1,A-2,A-3Front view, 45° to the right view, and 45° to the left view of breasts injected with PAAG for 14 years before surgery.B-1,B-2,B-3Front view, 45° to the right view, and 45° to the left view of breasts immediately after PAAG and fibrotic capsule were taken.C-1,C-2,C-3Front view, 45° to the right view, and 45° to the left view of breasts 24 months after fat grafting.
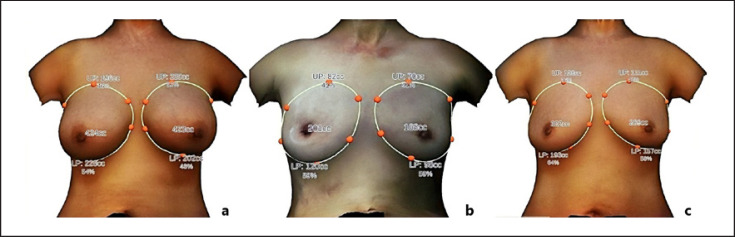
Fig. 4.
A 48-year-old female.aAnteroposterior 3D breast volume measurement with PAAG injected for 14 years before surgery.bAnteroposterior 3D breast volume measurement immediately after PAAG and fibrotic capsule were taken.cAnteroposterior 3D breast volume measurement 24 months after fat grafting.
Results
Patient Profile
A total of 162 female patients were enrolled in our study, and the average age of the cohort was 48.3 (range 41–67) years. The average body mass index was 21.59 (range 16.92–28.83). Pain and all other symptoms belonged to the PAAG injection were the cause for the operation. The average time of postoperative follow-up was 17.6 months (6–30 months). All the patients were categorized into ASA Class I and Class II (Table 1). 146 patients (90.12%) complained about pain (Fig. 5). 16 patients (9.88%) had displacement of the injected material and distortion of the breast. 74 patients (45.68%) complained of induration and lumps in breast areas. Abnormal breast enlargement occurred in 23 patients (14.20%). Bilateral breast asymmetry was found in 89 patients (54.94%). Fifty-seven patients (35.19%) suffered from anxiety, depression, and other psychological problems. All the patients underwent immediate breast augmentation with autologous fat grafting after removal of PAAG and fibrotic capsule. The 162 patients received an average of 1.6 sessions of fat injections (range 1–3). The average injection volume of fat tissue per breast was 310 mL (range 220–450 mL).
Table 1.
Patients' data
| N | Total |
|---|---|
| Female | 162 |
| Age, years | |
| Range | 41–67 |
| Mean | 48.3 |
| BMI, kg/m2 | |
| Range | 16.92–27.83 |
| Mean | 21.59 |
| ASA | |
| Class I | 94 |
| Class II | 68 |
| PAAG complications | |
| Pain | 146 |
| Displacement | 16 |
| Induration and lumpy | 74 |
| Abnormal breast enlargement | 23 |
| Asymmetry | 89 |
| Psychological problems (anxiety, depression, etc) | 57 |
| Volume of fat injected, mL/unilatral | |
| Mean | 310 |
| Range | 220–450 |
| Sessions of fat injections | |
| Mean | 1.6 |
| Range | 1–3 |
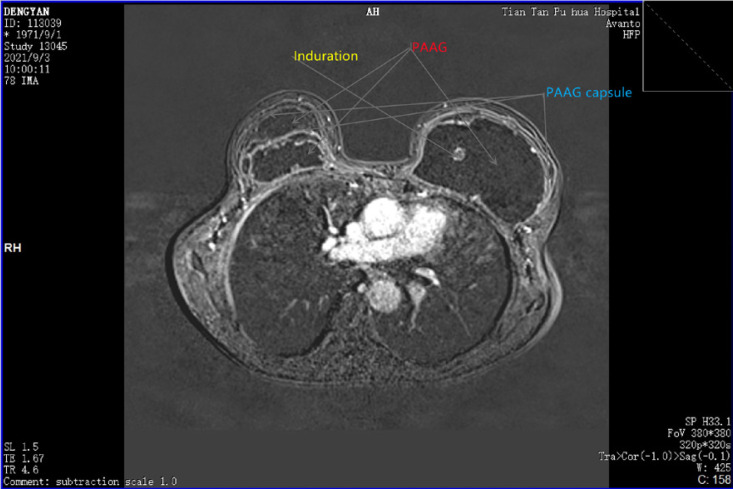
Fig. 5.
MRI image of a 40-year-old female who underwent breast augmentation with PAAG for 16 years. The marks in the image were PAAG, fibrotic capsule, and induration, respectively.
Surgical Efficacy and BREAST-QTM Scale
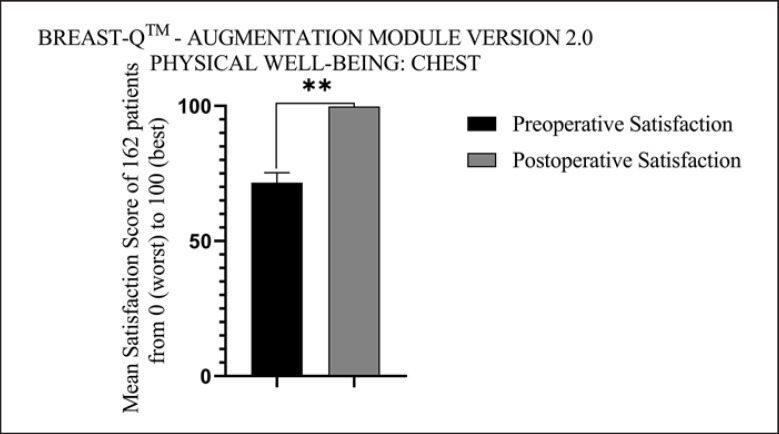
Of all the 162 female patients evaluated for enrollment in the study, there were no complications such as fat liquefaction, infection, and fat embolism. The postoperative results showed no defects in the breast shape, no visible malformation in the body by naked eyes. The removed inflammatory fibrotic capsule dotted with nodules formed in the mammary gland was sent for pathological examination and HE-stain showing accumulation and effusion of inflammatory cells, gathering of phagocyte and nodules of giant cells in the fibrotic capsule (Fig. 6). In clinical practice, the surgical efficacy was evaluated according to the subjective statement of the patients and the postoperative satisfaction with the breasts evaluated with BREAST-QTM − Augmentation Module (postoperative) version 2.0. The average score of postoperative satisfaction with the breasts was 78.36 (total score: 100, range: 64–91). The most satisfying thing for patients was that they could sleep better without worrying about complications associated with PAAG in their bodies, and their intimate partner would not find out they have had breast augmentation. The average score of postoperative satisfaction with physical well-being: chest was 99.83 (total score: 100) compared with the average satisfaction score of 71.69 (total score: 100) preoperatively; there was a significant statistical difference (p < 0.01) (Fig. 7). Their symptoms of pain and all other discomfort belonged to the PAAG injection were partially or totally relieved after surgery according to postoperative results at follow-up.
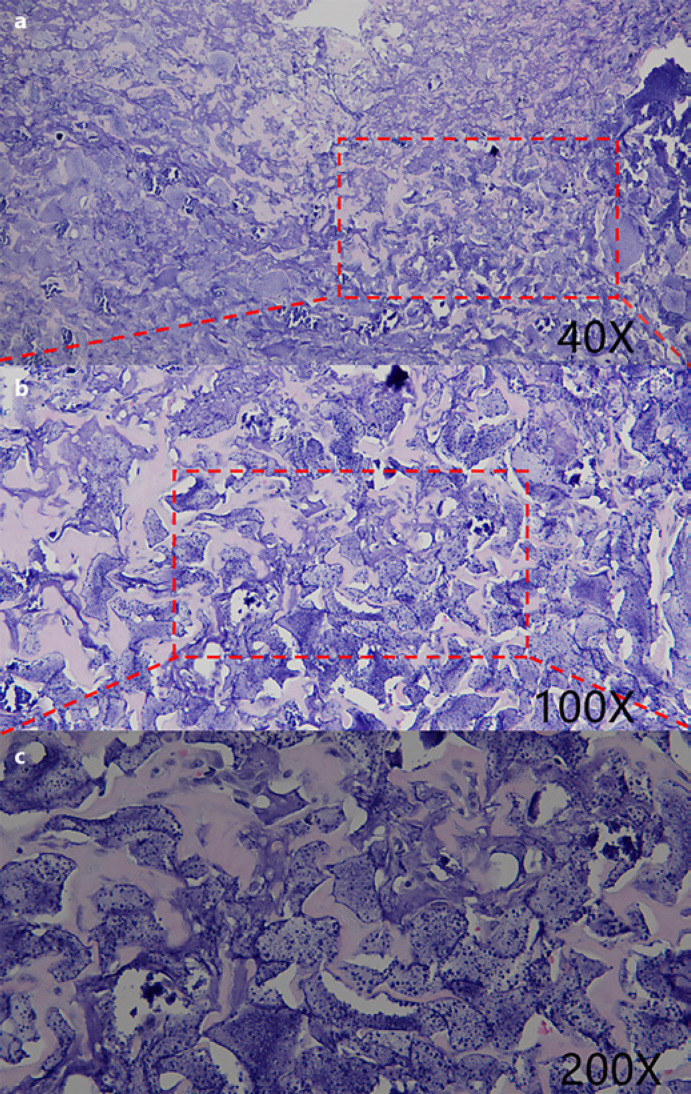
Fig. 6.
HE-stain showing accumulation and effusion of inflammatory cells, gathering of phagocyte and nodules of giant cells in the removed fibrotic capsule (×40 magnification,a; ×100 magnification,b; ×200 magnification,c).
Fig. 7.
Patients' satisfaction with physical well-being: chest.
Discussion
Studies have shown that polyacrylamide is harmless to the body, but the acrylamide monomer released from polyacrylamide contains toxins [17]. The toxicity of acrylamide mainly includes neurotoxicity, reproductive toxicity, genetic toxicity, immunotoxicity, and potential carcinogenicity [18, 19, 20]. Residues of acrylamide monomer are inevitably present during production processes of cross-linking. Besides, when PAAG is injected into local tissue of the body, it is inevitable to contact with body fluids and various bio-enzymes, which may produce degradation in vivo. So, the only way to cure these victims caused by the misuse of PAAG in China for nearly a decade is to remove PAAG as thoroughly as possible. Yet, removing PAAG as much as possible can lead to breast size reduction, deformation, asymmetry, and other marked change in breast shape (e.g., a change in nipple orientation due to the change in breast volume). Patients are not willing to accept the marked change in breasts. So, most of the patients choose augmentation surgery immediately after PAAG and fibrotic capsule were removed. Both silicone prosthesis implant and autologous fat grafting augmentation mammoplasty are available, but prosthesis implantation cannot fine-tune the shape of the breast and accurately adjust the volume of the damaged breast. So, autologous fat grafting has been more popular for immediate repair in recent years. In the past, doctors recommended a second stage augmentation mammoplasty after a period of time when the dead cavity caused by the removal of PAAG was closed. The dead cavity is difficult to close unless the fibrotic capsule is removed completely and the remaining normal tissue is closely contacted by negative pressure attraction. Therefore, it is not enough to remove PAAG alone; the inflammatory fibrotic capsule should be removed as well.
Because PAAG injections have been performed predominantly in private clinics in the past, the real volume of PAAG injections remains unclear [21]. Moreover, it is difficult to calculate the volume of fat needed to be injected immediately after removing PAAG and capsule. The online 3D software system Crisalix (© 2021 Crisalix S.A.) can accurately calculate the volume of each breast. In this study, we used the Crisalix 3D Imaging platform immediately to calculate the volume difference through preoperative and intraoperative photo collection (Fig. 3). This method of volume calculation is very simple and fast. If MRI is used to calculate fat volume, it is necessary to take MRI photos during the operation. Intraoperative MRI shooting is very inconvenient and costs time. Besides, using MRI to calculate volume is relatively cumbersome. There are also several reports that special 3D photo cameras are used to collect volume data, but 3D cameras are far more expensive and complicated than our Crisalix 3D Imaging platform. The online 3D software system Crisalix is a simple, convenient, and practical system for the volume calculation in breast augmentation surgery.
We would like to state the bias that we excluded patients with postoperative infection and/or ulceration in the surgical area and other complication in our study. Removal of PAAG thoroughly and the wound complete debridement are the first and most important procedures for those with PAAG infection and/or skin ulceration. Fat grafting should be delayed in those patients until the infection is fully under control. This is a well-known principle of plastic surgery. So, the main conclusion of the study is that only in our large group of patients without any major complication, the satisfaction rate is high. To achieve a definitive conclusion, we need to enlarge the sample to those patients with postoperative infection and other complication. Broadening surgical indications is a big challenge for surgeons and it should be treated with enough caution. Perhaps in the future, the application of anti-inflammatory factors and immunomodulatory therapy can fulfill this temporarily unattainable challenge. Nevertheless, if successful, it will be a milestone that changed the history of surgery.
Conclusions
Fat injection has advantages in breast shape, sense of touch, movement, and symmetry and is a good solution to the lack of breast volume after PAAG removal. To remove as much as possible is critical for patients who underwent PAAG injection. Our experience in immediate breast augmentation with autologous fat grafting after removal of PAAG and fibrotic capsule proved useful and effective to maintain the balance between removing the PAAG and capsule as much as possible and retaining soft tissue to reshape breasts.
Statement of Ethics
Written informed consent was obtained from all of the patients for use of their data and portrait rights (surgical areas with no face exposure) in accordance with the ethical standards of the Ethics Committee of Jingmei Cosmetic Surgery Clinic, approval number: 110-002.
Conflict of Interest Statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding Sources
The authors received no financial support for the research, authorship, and publication of this article.
Author Contributions
Qiuni Gao, Peiming Zhai; Jun Qi, Zhenyu Yang, Yuling Hu, Xihang Yuan, Chengsheng Liu, and Zuoliang Qi performed the research. Qiuni Gao, Chengsheng Liu, and Zuoliang Qi designed this study. Peiming Zhai and Jun Qi analyzed the data. Zhenyu Yang, Yuling Hu, and Xihang Yuan followed up the patients and wrote this paper. Zuoliang Qi provided advice and critical discussions on the project. All of the authors read and approved the final version of this paper and participated in reviewing and editing the manuscript and approved the final version.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1.Christensen LH, Breiting VB, Aasted A, Jørgensen A, Kebuladze I. Long-term effects of polyacrylamide hydrogel on human breast tissue. Plast Reconstr Surg. 2003;111((6)):1883–90. doi: 10.1097/01.PRS.0000056873.87165.5A. [DOI] [PubMed] [Google Scholar]
- 2.Amin SP, Marmur ES, Goldberg DJ. Complications from injectable polyacrylamide gel, a new nonbiodegradable soft tissue filler. Dermatol Surg. 2004;30:1507–9. doi: 10.1111/j.1524-4725.2004.30551.x. [DOI] [PubMed] [Google Scholar]
- 3.Ono S, Ogawa R, Hyakusoku H. Complications after polyacrylamide hydrogel injection for soft-tissue augmentation. Plast Reconstr Surg. 2010 Oct;126((4)):1349–57. doi: 10.1097/PRS.0b013e3181ead122. [DOI] [PubMed] [Google Scholar]
- 4.Jin R, Luo X, Wang X, Ma J, Liu F, Yang Q, et al. Complications and treatment strategy after breast augmentation by polyacrylamide hydrogel injection: summary of 10-year clinical experience. Aesthetic Plast Surg. 2018 Apr;42((2)):402–9. doi: 10.1007/s00266-017-1006-9. [DOI] [PubMed] [Google Scholar]
- 5.Unukovych D, Khrapach V, Wickman M, Liljegren A, Mishalov V, Patlazhan G, et al. Polyacrylamide gel injections for breast augmentation: management of complications in 106 patients, a multicenter study. World J Surg. 2012 Apr;36((4)):695–701. doi: 10.1007/s00268-011-1273-6. [DOI] [PubMed] [Google Scholar]
- 6.DeLuca M, Shapiro A, Banayan E, Zielinski G, Karanetz I, Asarian A, et al. Complications 18 years after polyacrylamide hydrogel augmentation mammoplasty: a case report and histopathological analysis. J Surg Case Rep. 2021 Jun 22;2021((6)):rjab276. doi: 10.1093/jscr/rjab276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woźniak-Roszkowska E, Maślińska M, Gierej P, Noszczyk B. Autoimmune syndrome induced by adjuvants after breast enhancement with polyacrylamide hydrogel: a study in Poland. Rheumatol Int. 2020 Nov;40((11)):1851–6. doi: 10.1007/s00296-020-04605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian B, Xiong L, Guo K, Wang R, Yang J, Wang Z, et al. Comprehensive management of breast augmentation with polyacrylamide hydrogel injection based on 15 years of experience: a report on 325 cases. Ann Transl Med. 2020 Apr;8((7)):475. doi: 10.21037/atm.2020.03.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen B, Song H. Management of breast deformity after removal of injectable polyacrylamide hydrogel: retrospective study of 200 cases for 7 years. Aesthetic Plast Surg. 2016 Aug;40((4)):482–91. doi: 10.1007/s00266-016-0646-5. [DOI] [PubMed] [Google Scholar]
- 10.Luo SK, Chen GP, Sun ZS, Cheng NX. Our strategy in complication management of augmentation mammaplasty with polyacrylamide hydrogel injection in 235 patients. J Plast Reconstr Aesthet Surg. 2011;64((6)):731–7. doi: 10.1016/j.bjps.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Leng B, Ren J, Yang Z, Wei J, Jiang G. Breast reconstruction with silicone prosthesis immediately following polyacrylamide gel removal. J Coll Physicians Surg Pak. 2019 Nov;29((11)):1092–5. doi: 10.29271/jcpsp.2019.11.1092. [DOI] [PubMed] [Google Scholar]
- 12.Cai Y, Liu B, Liao M, He L, Zhu C. Application of periareolar mammaplasty with the tissue folding technique in breast reshaping following polyacrylamide hydrogel removal. Breast Care. 2020 Apr;15((2)):157–62. doi: 10.1159/000500879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unukovych D, Khrapach V, Wickman M, Liljegren A, Mishalov V, Patlazhan G, et al. Polyacrylamide gel injections for breast augmentation: management of complications in 106 patients, a multicenter study. World J Surg. 2012 Apr;36((4)):695–701. doi: 10.1007/s00268-011-1273-6. [DOI] [PubMed] [Google Scholar]
- 14.Yu L, Wang J, Zhang B, Zheng DN, Zhu C. Treatment of breast injection with polyacrylamide hydrogel with infiltrated fascia capsule removal: report on 104 cases. Aesthetic Plast Surg. 2012 Oct;36((5)):1120–7. doi: 10.1007/s00266-012-9928-8. [DOI] [PubMed] [Google Scholar]
- 15.Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: safety and efficacy. Plast Reconstr Surg. 2007;119((3)):775–85. doi: 10.1097/01.prs.0000252001.59162.c9. discussion 786–7. [DOI] [PubMed] [Google Scholar]
- 16.Zhang F, Wang X, Guo H. Different types of breast deformity induced by two types of polyacrylamide hydrogel and corresponding treatment. Aesthetic Plast Surg. 2020 Jun;44((3)):726–34. doi: 10.1007/s00266-020-01626-0. [DOI] [PubMed] [Google Scholar]
- 17.Xu C, Cao M, Bao B, Li H, Yin D. Tissue degeneration 7 years after breast augmentation with injected polyacrylamide hydrogel (PAAG) Aesthetic Plast Surg. 2012 Feb;36((1)):160–2. doi: 10.1007/s00266-011-9779-8. [DOI] [PubMed] [Google Scholar]
- 18.Yoshioka T, Hamamura M, Yoshimura S, Okazaki Y, Yamaguchi Y, Sunaga M, et al. Evaluation of acrylamide and 3,3′-iminodipropionitrile-induced neurotoxicity in a rat 28-day oral toxicity study: collaborative project for standardization of test procedures and evaluation of neurotoxicity. J Toxicol Pathol. 2001;14:279–87. [Google Scholar]
- 19.Smith EA, Oehme FW. Acrylamide and polyacrylamide: a review of production, use, environmental fate and neurotoxicity. Rev Environ Health. 1991 Oct–Dec;9((4)):215–28. doi: 10.1515/reveh.1991.9.4.215. [DOI] [PubMed] [Google Scholar]
- 20.Bourke AG, Jose C. Recurrent complications of PAAG implants during lactation. BMJ Case Rep. 2018 Nov 5;2018:bcr2017219688. doi: 10.1136/bcr-2017-219688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patlazhan G, Unukovych D, Pshenisnov K. Breast reconstruction and treatment algorithm for patients with complications after polyacrylamide gel injections: a 10-year experience. Aesthetic Plast Surg. 2013 Apr;37((2)):312–20. doi: 10.1007/s00266-012-0045-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.