Immunomodulation and immunity from vaccination and natural infection have reduced mortality from coronavirus disease 2019 (COVID-19). However, there are ongoing concerns regarding emerging variants and residual pulmonary sequelae in survivors, given that the lungs are the principal site for the triumvirate of infection, inflammation and injury. The initial waves of acute, severe COVID-19 were profoundly inflammatory, usually manifest as organising pneumonia ± acute respiratory distress syndrome (ARDS). The extent of the fibrogenic potential of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and the modifiability of the pathogenic processes and disease course are unclear. Interestingly patients can develop ‘post-COVID interstitial lung disease’ (PC-ILD) irrespective of having ARDS during the acute phase. Here, we describe our evolving understanding of PC-ILD and the critical need to identify risk factors, including within the critical care setting. We also discuss immunopathomechanisms that may facilitate early intervention to prevent, slow or arrest progression of lung damage e.g. with immunomodulatory and/or anti-fibrotic agents.
Post-COVID ILD (PC-ILD): a new disease entity
Approximately 1-in-7 hospitalised patients with COVID-19 in the United Kingdom (UK) required critical care admission, the majority for management of ARDS [1]. The onset of COVID-ARDS in the second week post-symptom-onset (at declining viral loads) and response to immunomodulation, suggests pathogenic immune dysregulation. Survivors often required protracted mechanical ventilation, with associated risk of developing ventilator-induced lung injury (VILI). It is well-described that a sub-group of patients with all-cause ARDS can develop persistent and non-progressive pulmonary fibrosis with enduring physiological and radiological abnormalities and considerable impact on quality of life [2]. Risk factors for ARDS-related fibrosis include increasing age, acute illness severity and duration of mechanical ventilation [2]. Lung protective pulmonary strategies have reduced the incidence of ARDS-related fibrosis. It is unclear whether similar approaches might apply to PC-ILD, and whether COVID-ARDS and PC-ILD overlap or represent two poles of a pathogenic continuum. Notably, PC-ILD may occur without prior ARDS or mechanical ventilation [3].
Pulmonary fibrosis may complicate viral pneumonitis [4]. Given the unprecedented scale of the COVID-19 pandemic, even a low event rate may have significant population-level impact (morbidity, late mortality). Most descriptions of post-COVID syndromes stipulate symptom duration for > 3 months [5]. Clinically significant ILD refers to > 10% lung parenchymal changes on chest computed tomography (CT). A consensus definition and the true burden of PC-ILD have not yet been determined. PC-ILD should be considered in patients with persistent respiratory symptoms (e.g. cough and dyspnea) 3 months post-COVID-19 symptom-onset and patients with > 10% CT changes should be monitored. Accumulating data suggest that while the majority of scans show improvement, at 12 months the prevalence of (non-progressive) fibrosis is ~ 10% in hospitalised patients, particularly in severe disease and older age [6]. In a meta-analysis of 46 studies, within a 12-month follow-up period, inflammatory radiological sequelae (ground glass, consolidation) reduced at a faster rate than fibrosis-like changes including reticulation, honeycombing and traction bronchiectasis [4]. This supports a hypothesis of resolving inflammatory and persisting fibrotic-like changes.
Whether PC-ILD should be dichotomised into binary “inflammatory” and “fibrotic” categories based on radiological patterns remains contentious, given the ambiguity of some features (e.g. irregular lines), absence of histological correlates, uncertain trajectories and likelihood of reversibility. Persistent ground-glass changes may indicate fine/immature fibrosis rather than inflammation, and fibrotic-like changes may be capable of regression and remodelling, albeit at a slower rate. Idiopathic pulmonary fibrosis (IPF) is the archetypal fibrotic ILD (f-ILD), but most existing ILD syndromes are thought to reside on an overlapping fibroinflammatory spectrum, e.g. hypersensitivity pneumonitis, an exaggerated aeroantigen-induced immune response and rheumatic-associated ILD. This arbitrary stratification into predominant fibrotic or inflammatory phenotypes may have therapeutic and prognostic implications (although targeted treatments may be used concurrently), with potential extrapolation to PC-ILD.
Aetiopathogenesis of post-COVID ILD
Mechanistic hypotheses of post-COVID syndromes, potentially applicable to PC-ILD, include direct tissue damage/injury and autoimmunity (exposure of neoantigens, break of tolerance and generation of (currently unidentified) antibodies. In the absence of pre-COVID imaging or serology, it is unclear whether PC-ILD (i) unmasks and accelerates pre-existing undiagnosed or asymptomatic ILD, (ii) serves as a provocation challenge triggering de novo classifiable ILD, or (iii) whether any detected antibodies (e.g. anti-synthetase) represent a virally induced epiphenomenon, rather than being pathogenic [7]. Other hypotheses include viral persistence and immune evasion with sustained immunostimulation and microthrombi, although predominant microvasculopathy is likely when CT scans are normal [8].
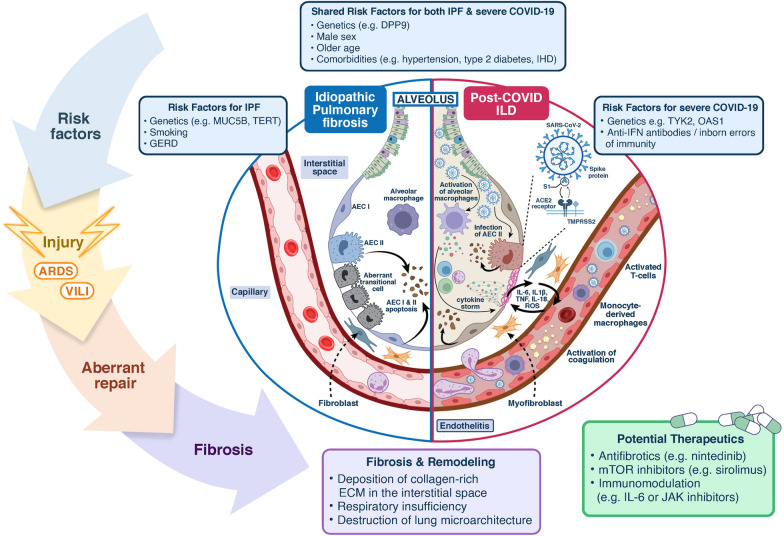
PC-ILD probably results from a complex interplay between immunogenetic susceptibility, injury (epithelial and endothelial from direct viral infection and mechanical ventilation), ARDS and/or VILI, macrophage infiltration and activation, hyperinflammation and hypercoagulability on a background of cellular senescence and stem cell exhaustion. This creates a profibrotic milieu (niche) and triggers a fibroproliferative cascade. An aberrant wound healing response with recruitment and differentiation of fibroblasts into myofibroblasts results in deposition of excessive extracellular matrix in the interstitial space, impaired gas exchange and destruction of lung microarchitecture culminating in fibrosis (Fig. 1).
Fig. 1.
Aetiopathogenesis of ILD idiopathic pulmonary fibrosis (IPF) and post-COVID ILD. In an immunogenetically at-risk individual, repetitive tissue injury may trigger a fibroproliferative cascade with an aberrant wound healing response, fibroblast proliferation and differentiation of TGF-β into myofibroblasts with deposition of collagen-rich ECM in the interstitial space. This culminates in destruction of lung microarchitecture and fibrosis. A In IPF, resident cell populations are altered with loss of AEC I and a new subpopulation of ‘aberrant transitional cells’. In both IPF (A) and Post-COVID ILD (B), AEC type I and II undergo apoptosis. B In Post-COVID ILD, SARS-CoV-2 can directly infect AEC II, via the ACE2 receptor and the TMPRSS2 co-factor. ARDS and VILI may also contribute to injury. Macrophage activation and recruitment of immune cells results in sustained production of pro-inflammatory cytokines (e.g. “localised cytokine storm” IL-6, IL-1β, IL-18, TNF) with systemic impact and local release and action of ROS). Alveolar epithelial cell and endothelial cell damage triggers activation of fibroblasts which deposit collagen. Endothelilitis with expanded populations of activated T cells and hypercoagulability also increases the risk of thrombosis. Potential therapeutic options being explored in clinical trials for Post-COVID ILD include antifibrotics e.g. nintedinib (already licensed for IPF), mTOR inhibitors (e.g. sirolimus) and immunomodulation e.g. Janus Kinase (JAK) or IL-6 inhibitors. GERD Gastro-(o)esophageal reflux disease; IHD ischaemic heart disease; TGF-β transforming growth factor beta; ECM extracellular matrix; IPF idiopathic pulmonary fibrosis; ARDS acute respiratory distress syndrome; VILI ventilator associated lung injury; AEC I alveolar epithelial type I cells; AEC II alveolar epithelial type II cells; ACE2 angiotensin converting enzyme 2; TMPRSS2 transmembrane protease serine 2; TNF tumour necrosis factor; IL interleukin; ROS reactive oxygen species
Although IPF is a progressive, irreversible f-ILD and available evidence (pending longer follow-up data) suggests that PC-ILD is non-progressive with reversible components, there are shared epidemiological and molecular features suggesting a common fibrotic pathway. Viruses can trigger acute exacerbations of IPF and progressive fibrosis. Risk factors for both severe COVID-19 and IPF include increasing age, male sex and co-morbidities, smoking and genetic polymorphisms (e.g. DPP9) [9]. Intriguingly, the strongest associated IPF variant (MUC5B), appears to be negatively associated with COVID-19 severity [10] similar to the survival benefit observed in IPF [11], although this apparent protective effect may be confounded by shielding behaviours [12]. There are also molecular similarities to IPF. Emerging data at single-cell resolution of bronchoalveolar lavage (BAL) fluid from acute COVID-19 patients suggest that SARS-CoV-2 (unlike influenza A) induces a profibrotic transcriptome and proteome in CD163-expressing monocyte-derived macrophages in the lung (comparable to IPF), with pronounced fibroproliferative ARDS [13]. The BAL proteomic immune landscape signature at 3–6 months’ follow-up differs according to radiological pattern [3]. Increased BAL cytotoxic T cells associate with epithelial damage and airways disease, while myeloid and B cell numbers correlate with the degree of chest CT abnormality [3]. Peripheral blood mononuclear cell signatures in COVID-19 lung disease and IPF have similar transcriptional signatures and prognostic value [14].
Future directions
The prevalence, severity and impact on healthcare systems of PC-ILD are currently unknown. The influence of viral load, new variants (with varying lung tropism), vaccination, ventilatory strategies, systemic hyperinflammation and immunomodulation [15] (e.g. corticosteroids or cytokine blockade (e.g. interleukin-6 antagonism) in the acute phase of PC-ILD are unknown. The role of immunomodulatory or anti-fibrotic therapies in PC-ILD is currently unresolved. It is tempting to speculate that immunomodulation (used for autoimmune ILD) may have a role in accelerating reversal of “inflammatory” changes, and anti-fibrotics (e.g. nintedanib used in progressive-fibrosing ILD) may attenuate “fibrotic-like” changes. However thus far PC-ILD appears to be non-progressive and clinical trial outcome data are eagerly awaited (Fig. 1).
Systematic, longitudinal data capture and deep phenotyping of patients with both persisting and resolving respiratory symptoms post-COVID are critical to unpick the aetiopathogenesis of PC-ILD. This should include clinical, physiological and radiological correlates with paired bio-sampling of both the lung microenvironment (e.g. BAL) and peripheral blood compartments to enable comparison to established f-ILDs. Serial CTs (ideally with in- or pre-hospital baselines) quantifying the extent and character of parenchymal change are needed to understand the relationship, reciprocity and evolution of radiological regions of inflammation and fibrosis. Understanding PC-ILD has global public health implications, but is also a unique opportunity to better understand molecular and cellular mechanisms of initiation, propagation and resolution of lung fibrosis. This may uncover novel therapeutic targets of wider relevance for multi-organ fibrotic diseases.
Acknowledgements
Rachel C Chambers and Joanna C Porter, University College London (UCL) Respiratory, for helpful discussions.
Author contributions
PM idea conception and drafted the manuscript. All authors finalised and approved the final version.
Declarations
Conflicts of interest
PM is a Medical Research Council (MRC)-GlaxoSmithKline EMINENT clinical training fellow with project funding outside of the submitted work; PM reports consultancy fees from SOBI, Abbvie, UCB, EUSA Pharma, Boehringer Ingelheim and Lilly outside of the submitted work. IOR reports funding for an investigator initiated clinical trial from Genentech on behalf of the TRAIL network in addition to participation in advisory boards of Boehringer Ingelheim and Genentech/Roche. MS reports grants outside of this project from Apollo Therapeutics, the UCL Technology Fund, the Wellcome Trust, MRC, Rosetrees Trust and NIHR. Others (personal or to institution) from Abbott, Biomerieux, Biotest, Deltex Medical, Enlivex, Fresenius, Hemotune, NewB, Paion, Radiometer, Roche Diagnostics, Safeguard Biosystems and Spiden outside of this project.
Ethical declarations
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Drake TM, Riad AM, Fairfield CJ, Egan C, Knight SR, Pius R, et al. Characterisation of in-hospital complications associated with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol UK: a prospective, multicentre cohort study. Lancet. 2021;398(10296):223–237. doi: 10.1016/S0140-6736(21)00799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall R, Bellingan G, Laurent G. The acute respiratory distress syndrome: fibrosis in the fast lane. Thorax. 1998;53(10):815. doi: 10.1136/thx.53.10.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vijayakumar B, Boustani K, Ogger PP, Papadaki A, Tonkin J, Orton CM, et al. Immuno-proteomic profiling reveals aberrant immune cell regulation in the airways of individuals with ongoing post-COVID-19 respiratory disease. Immunity. 2022;55(3):542–56.e5. doi: 10.1016/j.immuni.2022.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabbri L, Moss S, Khan FA, Chi W, Xia J, Robinson K et al (2022) Parenchymal lung abnormalities following hospitalisation for COVID-19 and viral pneumonitis: a systematic review and meta-analysis. Thorax. https://thorax.bmj.com/content/early/2022/03/24/thoraxjnl-2021-218275 [DOI] [PubMed]
- 5.Munblit D, O'Hara ME, Akrami A, Perego E, Olliaro P, Needham DM. Long COVID: aiming for a consensus. Lancet Respir Med. 2022;10(7):632–634. doi: 10.1016/S2213-2600(22)00135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorent N, Vande Weygaerde Y, Claeys E, Guler Caamano Fajardo I, De Vos N, De Wever W, et al. Prospective longitudinal evaluation of hospitalised COVID-19 survivors 3 and 12 months after discharge. ERJ Open Res. 2022;8(2):00004–2022. doi: 10.1183/23120541.00004-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight JS, Caricchio R, Casanova JL, Combes AJ, Diamond B, Fox SE, et al. The intersection of COVID-19 and autoimmunity. J Clin Investig. 2021;131(24):e154886. doi: 10.1172/JCI154886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grist JT, Collier GJ, Walters H, Kim M, Chen M, Abu Eid G, et al. Lung abnormalities depicted with hyperpolarized xenon MRI in patients with long COVID. Radiology. 2022 doi: 10.1148/radiol.220069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pairo-Castineira E, Clohisey S, Klaric L, Bretherick AD, Rawlik K, Pasko D, et al. Genetic mechanisms of critical illness in COVID-19. Nature. 2021;591(7848):92–98. doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- 10.Fadista J, Kraven LM, Karjalainen J, Andrews SJ, Geller F, Baillie JK, et al. Shared genetic etiology between idiopathic pulmonary fibrosis and COVID-19 severity. EBioMedicine. 2021;65:103277. doi: 10.1016/j.ebiom.2021.103277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peljto AL, Zhang Y, Fingerlin TE, Ma SF, Garcia JG, Richards TJ, et al. Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA. 2013;309(21):2232–2239. doi: 10.1001/jama.2013.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Moorsel CHM, van der Vis JJ, Duckworth A, Scotton CJ, Benschop C, Ellinghaus D, et al. The MUC5B promoter polymorphism associates with severe COVID-19 in the European population. Front Med (Lausanne) 2021;8:668024. doi: 10.3389/fmed.2021.668024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wendisch D, Dietrich O, Mari T, von Stillfried S, Ibarra IL, Mittermaier M, et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell. 2021;184(26):6243–61.e27. doi: 10.1016/j.cell.2021.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinha S, Castillo V, Espinoza CR, Tindle C, Fonseca AG, Dan JM, et al. COVID-19 lung disease shares driver AT2 cytopathic features with Idiopathic pulmonary fibrosis. EBioMedicine. 2022;82:104185. doi: 10.1016/j.ebiom.2022.104185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta P, Fajgenbaum DC. Is severe COVID-19 a cytokine storm syndrome: a hyperinflammatory debate. Curr Opin Rheumatol. 2021;33(5):419–430. doi: 10.1097/BOR.0000000000000822. [DOI] [PMC free article] [PubMed] [Google Scholar]