Abstract
Purpose of Review
Histoplasmosis remains a challenging infection in solid organ transplantation. This review provides a topic update with emphasis on the changing Histoplasma epidemiology, along with new diagnostic and treatment innovations.
Recent Findings
Recent years have observed expanding Histoplasma geographic distribution due to climate change, environmental disruption, and host factors. Early clinical experience also suggests a relationship between COVID-19 infection and histoplasmosis, particularly among immunocompromised individuals. Advances in diagnostic methods, such as newer enzyme immunoassays and molecular techniques, have broadened the capability for expedient and highly specific pathogen identification. Novel drug innovations, including the development of new formulations of existing antifungal agents, extended-spectrum azoles and new antifungal drug classes have expanded therapeutic options.
Summary
Advances in organ transplantation have largely outpaced those for histoplasmosis. However, these emerging insights enhance our understanding of this pathogen and management of clinical infection, particularly for transplant recipients with a higher incidence and severity of disease.
Keywords: Histoplasmosis, Solid organ transplantation, Infection, Endemic mycoses, Antifungal therapy, Immunocompromised, COVID-19
Introduction
Histoplasmosis is a common endemic mycosis caused by the thermally dimorphic fungus Histoplasma capsulatum which exists as a mold in the environment with conversion to a yeast at body temperature within the human host. Most infections result from inhalation of aerosolized microconidia from the environment, most commonly affecting the lung. Solid organ transplant (SOT) recipients and other immunocompromised hosts have a propensity for severe infection inclusive of extrapulmonary and disseminated disease given their inability to contain the infection, with significant associated morbidity and mortality. This review provides a general topic update, with emphasis on (1) the expanding geographic distribution of histoplasmosis; (2) the relationship between coronavirus disease 2019 (COVID-19) infection and histoplasmosis; (3) advances in fungal diagnostics; and (4) novel antifungal therapies, approved or in the pipeline.
Epidemiology and Risk Factors
Histoplasmosis is the most common endemic mycosis reported in the SOT population and is among the most common endemic mycoses in the United States (US) and throughout the world [1, 2•]. Three varieties of Histoplasma have been identified, though H. capsulatum var. capsulatum and H. capsulatum var. duboisii predominate among humans. The former variety is most common with the widest geographic distribution, whereas the latter is localized to Africa [3••, 4]. Importantly, genomic sequencing and phylogenetic analyses demonstrate marked diversity within the Histoplasma genus. The identification of multiple cryptic species with varying geography and virulence has led to modifications of Histoplasma taxonomy [5, 6].
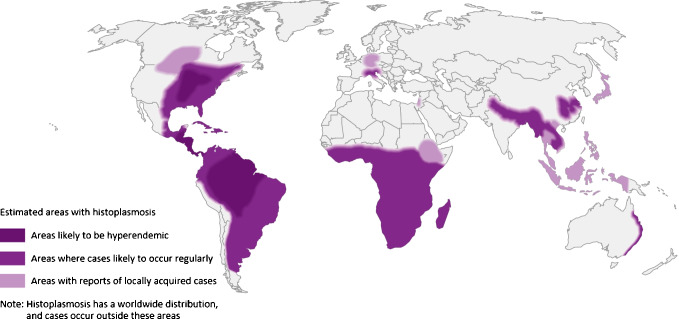
The geographic distribution and overall burden of disease worldwide is grossly underestimated due to lack of access to available diagnostics and infection recognition, as well as insufficient surveillance and reporting [2•]. For example, histoplasmosis is not a nationally notifiable disease in the US. Prior to 2016, it was reportable in only 12 states and lacked a standardized case definition [7]. In addition, maps detailing the geographic distribution of histoplasmosis date as far back as the 1940s based on skin testing surveillance studies [2•]. Figure 1 reflects ongoing efforts to detail the expansion of histoplasmosis beyond previously defined high endemicity regions such as the Ohio and Mississippi River Valleys in the US, Central and South America, areas of Africa and Asia [3••]. The revised maps draw from many sources including autochthonous case reports and series, skin testing surveillance, and environmental sampling. Explanations proposed for the geographic expansion of histoplasmosis include improved diagnostics and clinical recognition, climate change, environmental disruption, and expanding at-risk hosts [2•, 3••].
Fig. 1.
Estimated areas of histoplasmosis worldwide. Legend: from reference [3••] under a Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/)
Histoplasmosis is uncommon among SOT recipients despite their increased susceptibility to infection. Prospective epidemiologic data from 23 transplant centers in the Transplant-Associated Infection Surveillance Network (TRANSNET) reported a 1-year cumulative incidence of histoplasmosis of 0.1% [1]. Transplant-associated histoplasmosis may occur de novo after environmental exposure, from reactivation of latent infection, or rarely via transmission from the donor. Although lung transplant recipients are at greater risk for acquiring other opportunistic fungi from the environment via the respiratory tract, such as Aspergillus spp., case series describe histoplasmosis most commonly after renal transplantation, likely as it reflects the largest transplant group worldwide [1, 8, 9].
Risks for post-transplant histoplasmosis include applied immunosuppression and environmental exposure. Human-to-human transmission has not been described. Histoplasma is commonly found in soil with high nitrogen content due to bird and bat droppings from nearby trees, coops, or caves [10]. Outdoor recreational activities, particularly spelunking, are a known risk. A review of 105 outbreaks of histoplasmosis in the US spanning 1938 to 2013 demonstrated that nearly one-third were work-related involving occupations in construction, demolition, or maintenance. Exposure to birds, bats, or “droppings” were reported in 86% [11]. Enhanced histoplasmosis surveillance in nine US states also identified handling of plants and trees and digging soil to be common potential exposures [12], though no obvious geographic or other exposure was identified in 22% of the cases. This point highlights the pitfalls of relying on a lack of relevant exposures to exclude histoplasmosis, as doing so could risk delayed or even missed diagnoses resulting in poor outcomes [13].
Clinical Manifestations
Clinical manifestations of histoplasmosis are highly variable and often non-specific making diagnosis challenging. Infection is often reported within the first 2 years following transplant, presumably due to higher levels of immunosuppression in this earlier transplant period. However, histoplasmosis can occur at any interval after transplant, having been reported as late as 20 to 30 years post-transplant [1, 8, 14••, 15, 16]. Infections occurring in the immediate post-transplant period, particularly the first month, should signal an investigation for donor-derived infection [8, 17••].
Signs and symptoms are dependent on the organ(s) involved and disease severity. Most infections present subacutely, with progressive disseminated infection representing the most common manifestation among SOT recipients, ranging from 64 to 81% in large multicenter evaluations [1, 8, 15]. Fevers, night sweats, malaise, cough, dyspnea, weight loss, hepatosplenomegaly, and other gastrointestinal symptoms are often present [10, 18, 19]. The most common sites of dissemination include bone marrow, lymph nodes, spleen, liver, gastrointestinal tract, skin, and central nervous system (CNS). Fungemia is common [8, 15, 20]. In the largest series of CNS histoplasmosis (n = 77), the most common findings included headaches (60%), altered mental status (42%), and focal neurologic deficits (30%) [21].
Common laboratory findings include pancytopenia, transaminitis, hyperbilirubinemia, elevated inflammatory markers (e.g., erythrocyte sedimentation rate, C-reactive protein, and ferritin), and hypercalcemia [10, 20, 22]. Histoplasmosis-associated hemophagocytic lymphohistiocytosis has also been rarely described in the SOT population [23]. Findings on chest imaging are varied, including isolated nodules, miliary infiltrates, multifocal consolidation, and cavitary lesions [10, 24]. Additional common radiologic abnormalities include intra-thoracic and abdominal lymphadenopathy, and hepatosplenomegaly [10, 18]. Computed tomography or magnetic resonance imaging in CNS disease often reveal mass lesions, meningeal enhancement, and/or infarcts though may be normal [21].
Diagnostic Strategies
Establishing the diagnosis of histoplasmosis in transplant recipients typically requires a multifaceted approach. The first step is suspecting the diagnosis by recognizing the various manifestations of progressive disseminated histoplasmosis, the most common presentation in SOT recipients. Table 1 summarizes available diagnostics for histoplasmosis. The diagnostic gold standard is growth of Histoplasma from clinical specimens. While definitive, cultures from blood and tissue may take up to 4 to 6 weeks to demonstrate growth, which may delay actionable clinical intervention [25]. Direct visualization of yeast forms with morphologic features consistent with Histoplasma from suspected sites of involvement is more timely and, unlike culture, can impact clinical decision making in real time. Though morphologic features may overlap with other fungi, careful attention to differences in yeast size, shape, budding, and capsular characteristics highly support the diagnosis of histoplasmosis in the right clinical context. Positive biopsy specimens are most commonly obtained from liver, lung, lymph nodes, bone marrow, and skin. Associated granuloma formation may also be seen, though less consistently in immunosuppressed hosts owing to the less robust host response to infection. With a high burden of infection, fungal forms within neutrophils may be identified on peripheral blood smear [26]. Cytopathologic examination of fine needle aspirate specimens from involved tissues may also be positive and provide a non-invasive, economical, and expedient diagnostic approach [27•]. The sensitivity of culture and direct visualization from histopathologic and cytopathic specimens is variable and dependent on the organism burden, site of involvement, and host factors [28•].
Table 1.
Laboratory diagnosis for histoplasmosis [19, 21, 25, 26, 27•, 28•, 29–34, 36, 37, 44•, 45, 47, 48••]
| Diagnostic assay | Methodology | Specimen sources | Advantages | Limitations |
|---|---|---|---|---|
| Fungal culture |
Selective fungal media: brain heart infusion agar, Sabouraud dextrose agar Lysis centrifugation for blood cultures |
• Blood • Other body fluids • Tissue |
Gold standard |
• Prolonged incubation required for growth (4–6 weeks) • Variable sensitivity, limited by infection burden |
| Histopathology |
PAS staining GMS staining |
Tissue biopsy |
• Timely results • Supports tissue invasive infection |
• Variable sensitivity, limited by infection burden • Fungal morphology not confirmatory • Reviewer dependent |
| Cytopathology |
• Tissue aspirate • BAL fluid • Peripheral blood smear |
• Timely results • Minimally invasive • Economical |
||
| Serology |
Complement fixation Immunodiffusion |
• Serum • CSF |
• Minimally invasive • Positive CSF result sufficient to diagnose meningitis • Economical |
• Less useful for acute infection due to 4–8-week lag for seroconversion • Poor sensitivity in immunocompromised hosts • Background seropositivity in endemic areas • Hard to delineate past versus active infection • Cross-reactivity with other fungal infections, TB, and sarcoidosis |
| Antigen testing | Enzyme immunoassay |
• Urine • Serum • Other body fluids |
• High sensitivity for disseminated infection • Minimally invasive • Faster turnaround time than culture • Quantitative • Serial monitoring capability for treatment response |
• Cross-reactivity with other fungal infections • Less sensitivity with localized infection |
| Lateral flow assay | Urine |
• High sensitivity for disseminated infection • Minimally invasive • Rapid result at POC • Economical |
• Cross-reactivity with other fungal infections • Less sensitivity with localized infection • Qualitative only |
|
| Molecular testing | Chemiluminescence-labeled DNA probe directed at H. capsulatum ribosomal RNA | Culture isolates |
• High specificity • Streamlined isolate identification |
• Requires culture growth, delaying results • Limited availability |
| MALDI-ToF MS | ||||
| Fungal RNA sequencing | • Streamlined isolate identification to the species level |
• Requires culture growth, delaying results • Available only through a reference laboratory |
||
|
Tissue-based PCR testing Broad-range PCR of fungal 28S ribosome |
• Fresh tissue • Formalin-fixed, paraffin-embedded tissue blocks • Blood • Other body fluids |
• Provides an alternative diagnostic option when other testing is inconclusive |
• Heterogeneous assay methodology limits test interpretation • Variable sensitivity, generally low for tissue specimens • Available only through a reference laboratory • Expensive |
BAL, bronchoalveolar lavage; CSF, cerebrospinal fluid; DNA, deoxyribonucleic acid; GMS, Gomori methenamine silver; MALDI-ToF MS, matrix-assisted laser desorption ionization time-of-flight mass spectrometry; PAS, periodic acid-Schiff; PCR, polymerase chain reaction; POC, point-of-care; RNA, ribonucleic acid; TB, tuberculosis
Prior to the development of more rapid diagnostic assays for histoplasmosis, acute and convalescent antibody detection was used to establish the diagnosis. The two available methods, testing by immunodiffusion and complement fixation, have differing sensitivity and specificity characteristics and are most useful in subacute and chronic forms of histoplasmosis [29]. However, in addition to the 4 to 8 weeks lag in antibody detection after acute infection, background seropositivity in individuals with prior exposure to endemic areas or past infection confound test interpretation. Serologic testing in immunosuppressed individuals is even more challenging as the effects of immunosuppressive therapy or underlying disease may blunt the antibody response to infection and decrease test sensitivity. Data demonstrate that only 25–30% of SOT recipients develop a serum antibody response [19]. However, CSF anti-Histoplasma antibody detection was found to be a useful adjunct to CSF antigen detection in diagnosing Histoplasma meningitis, even in immunosuppressed individuals [21, 30].
Owing to the challenges of culture identification and serologic testing, which are particularly problematic in transplant recipients and other immunocompromised hosts, antigen testing has become the primary modality for diagnosing histoplasmosis. The first antigen assay was introduced in 1986, with the development of newer iterations of enzyme immunoassays (EIAs) over time with quantitative capabilities and improved performance. Currently there are two commercially available EIAs. Most published data and clinical experience stems from use of the MiraVista® (MiraVista Diagnostics, Indianapolis, IN, USA) assay, which was the first assay developed, now in its third generation. The IMMY® (Immuno-Mycologics [IMMY], Norman, OK, USA) ALPHA EIA assay, now replaced with the subsequently developed IMMY® clarus Histoplasma galactomannan monoclonal EIA assay, has the advantage of the ability to be processed in local facilities rather than requiring shipment to a reference laboratory. These EIA assays demonstrate reasonable concordance, with overall sensitivity dependent on the type of specimen sent, the underlying burden of infection, and clinical manifestation [31–34].
Owing to the concentration of antigen in urine, urine testing is marginally more sensitive than serum antigen testing, with some data suggesting enhanced sensitivity with combined testing [35]. Antigen testing may also be performed on other body fluids, including bronchoalveolar lavage (BAL), CSF, and pleural fluid, though result interpretation is less established. The development of the quantitative component allows for serial monitoring to assess antigen clearance as a response to therapy, best supported in people living with human immunodeficiency virus (PLWH) [36].
Cross-reactivity with other fungal antigens is well demonstrated and limits EIA test interpretation, emphasizing the importance of interpreting the assay in the appropriate clinical context [32, 37]. False-positive BAL Histoplasma antigen results were found in 8% (5/60 patients) of pulmonary aspergillosis cases with high concentrations of Aspergillus galactomannan, though notably at low concentrations [38]. Conversely, the Aspergillus galactomannan assay was positive in 50% of serum and BAL samples from individuals with histoplasmosis, typically in those specimens with very high Histoplasma antigen concentrations. In this setting, the results could incorrectly lead to the conclusion there is co-infection and potentially impact treatment [39, 40]. The (1–3)-β-D-glucan assay as a screening tool for invasive fungal infection is widely used in clinical settings, but notably the assay does not have a US Food and Drug Administration (FDA)-approved indication for the diagnosis of histoplasmosis. Limited data demonstrate a sensitivity of serum (1–3)-β-D-glucan assay of 87 to 89% and specificity of 68% in disseminated histoplasmosis cases, with quantitative values correlating with urine antigen EIA concentrations [41, 42]. CSF (1–3)-β-D-glucan assay test performance was poor in Histoplasma meningitis cases [43].
While the development and broad availability of the Histoplasma antigen EIA assays have advanced the timely identification of histoplasmosis infections, logistical barriers related to the requirement of specialized laboratories, equipment, and personnel present challenges, particularly in resource limited areas. This recognition has led to the recent development of a qualitative lateral flow assay (LFA) for Histoplasma antigen detection. This assay has the advantage of using urine specimens at the point of care and providing results within 40 min of specimen collection without the need for specialized equipment. Direct cross comparison of results from urine specimens between the LFA and EIA assay has demonstrated high concordance between the two assays, with high test sensitivity in immunosuppressed individuals with a higher burden of infection [44•, 45]. However, both assays are limited by positive results due to cross-reactions with other fungal pathogens.
Currently, molecular detection of Histoplasma in the clinical setting is largely limited to fungal identification from culture isolates [46]. Matrix-assisted laser desorption ionization time-of-flight is increasingly applied in clinical microbiology laboratories for pathogen identification from culture isolates including both fungal morphologic stages of H. capsulatum [47]. While these approaches offer the advantage of rapid identification from primary cultures without needing a more prolonged and laborious method, they still require culture growth, which may require weeks to occur. Wide availability and applicability of molecular detection methods for H. capsulatum directly from clinical specimens remains elusive. Currently, there are no FDA-approved assays for detecting Histoplasma species directly from human specimens. Tissue-based polymerase chain reaction (PCR) testing and sequencing, along with broad-range PCR of fungal 28S ribosome, are available at reference laboratories, but are largely cost prohibitive for routine use. Available data are largely limited to case reports and small case series. Cross comparisons of studies are challenging due to the use of heterogeneous molecular assays using different molecular targets, multiple specimen sources, and various comparison assays [48••]. As with other diagnostic assays, the overall sensitivity of molecular techniques is superior with higher organism burden [49]. Performance evaluation and clinical validation of these assays require further refinement, but emerging data suggest molecular methods will play an increasing future role in our diagnostic armamentarium for histoplasmosis.
Management of Histoplasmosis
Most Histoplasma infections in immunocompetent individuals do not necessitate directed therapy [50••]. In contrast, SOT recipients can develop severe and disseminated infection warranting timely initiation of antifungal therapy and reduction of immunosuppression [14••]. While infections in SOT recipients are best categorized as mild, moderate, or severe, well-defined characteristics of severity in this population are lacking as are randomized controlled trials comparing the efficacy of antifungal therapies.
Available antifungal therapies used to treat histoplasmosis are detailed in Table 2; however, the mainstays of therapy have historically been amphotericin B (AmB) and/or itraconazole (ITZ). Intravenous AmB remains the initial drug of choice for severe infection. Liposomal AmB is preferred over conventional AmB deoxycholate (AmB-d) due to its more favorable safety and tolerability profile and improved efficacy as demonstrated in other immunocompromised populations such as PLWH [51]. The American Society of Transplantation (AST) guidelines recommend step down to oral ITZ therapy following at least 1 to 2 weeks of AmB alongside evidence of clinical stabilization [14••]. For patients with CNS disease, longer courses of AmB therapy (i.e., 4 to 6 weeks) are recommended [50••, 52•]. Mild to moderate disease can be treated with ITZ monotherapy. In general, antifungal therapy should be continued for a minimum of 12 months irrespective of initial disease severity. Indefinite therapy may be considered in patients necessitating continued high-level immunosuppression or after relapsed disease, particularly with CNS involvement [53].
Table 2.
| Antifungal agentsa | Usual dosage | Common adverse reactionsb | Therapeutic drug monitoring | Major drug interactionsb,c | Additional comments |
|---|---|---|---|---|---|
| Polyenes | |||||
| AmB-d (Fungizone) (IV only) | 0.7–1 mg/kg/day |
• Acute infusion reactions (e.g. fever, chills, hypotension, nausea, vomiting, headache) • Anemia • Electrolyte imbalance (low potassium, magnesium, calcium, and sodium) • GI effects • Hepatotoxicity • Nephrotoxicity |
Not recommended |
• Digitalis glycosides, skeletal muscle relaxants, and antiarrhythmic agents (possible increased toxicity due to AmB-mediated hypokalemia) • Nephrotoxic medications (possible increased drug-induced nephrotoxicity) • Leukocyte transfusions (acute pulmonary toxicity reported when given concomitant to AmB; should not be given concurrently) |
• Infusion-related reactions can be reduced with pre-infusion acetaminophen and diphenhydramine; meperidine may be used for rigors • Nephrotoxicity can be minimized with pre- and post-infusion hydration and lipid-based AmB formulations • Close monitoring of electrolytes and renal function required |
| Liposomal AmB (AmBisome)* (IV only) | 3–5 mg/kg/day | ||||
|
AmB Lipid Complex (ABLC, Abelcet)* (IV only) *Lipid-based AmB formulations |
5 mg/kg/day | ||||
| Azoles | |||||
| ITZ (PO only) |
• Adrenal insufficiency (long-term use, rare) • CHF (avoid use if ventricular dysfunction/CHF) • GI effects • Headache • Hearing loss • Hepatotoxicity • Neuropathy • Peripheral edema • QT prolongation • Rash |
• Random concentration after ≥ 2 weeks of therapy; goal ≥ 1.0 µg/mL (via HPLC) • For HPLC, the goal concentration is sum of ITZ and active metabolite hydroxy-ITZ |
• ITZ is an inhibitor of CYP3A4 and p-glycoprotein • Potentiation of QT prolongation when used with other QT prolonging drugs |
For C-ITZ • Capsules: take with food and acidic beverage (e.g., cola); avoid proton pump inhibitors and H-2 blockers which reduce absorption • Solution: take on empty stomach For SUBA-ITZ • Take with food • Has not been adequately studied in Histoplasma CNS infections |
|
|
C-ITZ (Sporanox) Capsule/tablets and oral solution |
Loading dose: 200 mg TID × 3 days Maintenance dose: 200 mg BID |
||||
|
SUBA-ITZ (Tolsura) Capsule |
Initial dose: 130 mg daily (max dose 130 mg BID) | ||||
|
FCZ (Diflucan) IV/PO |
Recommendations vary based on site of infection |
• Alopecia (with prolonged therapy) • Exfoliative skin disorders • GI effects • Hepatotoxicity • Headaches • QT prolongation |
Not recommended |
• FCZ is an inhibitor of CYP2C9 and CYP3A4 • Potentiation of QT prolongation when used with other QT prolonging drugs |
Concerns with use for histoplasmosis include reduced in vitro activity, delayed fungemia clearance, and resistance emergence (see text) |
|
VCZ (Vfend) IV/PO |
IV: 6 mg/kg BID × 2 doses then 4 mg/kg BID PO: 400 mg BID × 2 doses then 200 mg BID |
• Fluorosis and periostitis • GI effects • Headache • Hepatotoxicity • QT prolongation • Skin rash, photosensitivity • Visual disturbances (e.g., photopsia, color vision change, photophobia, other visual hallucinations), rare optic neuritis, and papilledema • Long-term use associated with skin cancer |
• Trough concentration ≥ day 5 of therapy; goal trough ≥ 1.0 to 5.5 µg/mL • Recommendations extrapolated from IFIs such as aspergillosis; target trough values for histoplasmosis have not been defined |
• VCZ is metabolized by, and an inhibitor of CYP2C19, CYP2C9, and CYP3A4 • Potentiation of QT prolongation when used with other QT-prolonging drugs |
• Take 1 h before or after a meal • IV formulation contains SBECD and typically avoided when CrCl < 50 mL/min unless risk:benefit assessed • Caution is advised when utilizing VCZ for histoplasmosis given increased early mortality demonstrated in comparison to ITZ (see text) |
|
PCZ (Noxafil) IV/PO |
Loading dose: 300 mg BID × 2 doses Maintenance dose: 300 mg daily |
• GI effects • Headache • Hepatotoxicity • QT prolongation |
• Trough concentration > 1.0 µg/mL; measured after 7 days of therapy • Recommendations extrapolated from IFIs such as aspergillosis; target trough values for histoplasmosis have not been defined |
• PCZ is a substrate of p-glycoprotein and an inhibitor of CYP3A4 • Potentiation of QT prolongation when used with other QT-prolonging drugs |
• PCZ suspension—take with high-fat meal, acidic beverage (e.g., cola, ginger ale), and avoid proton pump inhibitors • PCZ delayed-release tablet—take with food • IV formulation contains SBECD and typically avoided when CrCl < 50 mL/min unless risk:benefit assessed |
|
ISZ (Cresemba) IV/PO |
Loading dose: 372 mg Q8h for 6 doses Maintenance dose: 372 mg daily |
• GI effects • Headache • Hypokalemia • Peripheral edema • Hepatotoxicity • Cardiac effects: shortening of the QT interval; contraindicated in familial short QT syndrome |
Not routinely recommended | • ISZ is a substrate of CYP3A4 and inhibitor of CYP3A4, p-glycoprotein, and organic cation transporter 2 | • IV formulation does not contain SBECD |
Table 2 adapted with permissions from [100]
ABLC, amphotericin B lipid complex; AmB, amphotericin B; AmB-d, amphotericin B deoxycholate; BID, twice daily; C-ITZ, conventional itraconazole; CHF, congestive heart failure; CNS, central nervous system; CrCl, creatinine clearance; CYP, cytochrome P450 enzyme system; FCZ, fluconazole; GI, gastrointestinal; H2, histamine-2 receptor; HPLC, high-performance liquid chromatography; ISZ, isavuconazole; IFI, invasive fungal infections; ITZ, itraconazole; IV, intravenous; MIC, minimal inhibitory concentration; LAmB, liposomal amphotericin B; PO, by mouth; PCZ, posaconazole; spp, species; SBECD, sulfobutyl ether beta-cyclodextrin sodium; SUBA-ITZ, super bioavailable itraconazole; TID, three times daily; VCZ, voriconazole
aAmB and C-ITZ are recommended first-line therapies (see the “Management of Histoplasmosis” section). Data with other extended-spectrum azoles is limited to case series and reports
bThis is not an all-inclusive list
cCritical to assess for drug interactions with concomitant medications that either share or modify the activity of involved metabolic pathways. See package insert and drug interactions screening databases for important drug interactions
Conventional ITZ (C-ITZ) is available as both an oral capsule and solution [54, 55]. C-ITZ capsules have variable bioavailability, which is particularly poor when administered with concomitant acid-suppressive therapies. Absorption can be optimized when C-ITZ is taken with a fatty meal and/or acidic drink. Conversely C-ITZ solution is minimally impacted by acid-suppressive therapies but requires administration on an empty stomach. C-ITZ solution has generally been preferred owing to its superior bioavailability though palatability can be problematic. Therapeutic drug monitoring is essential with both formulations to ensure adequate exposure [56]. Super bioavailable itraconazole (SUBA-ITZ) is a novel formulation of ITZ developed to address issues of tolerability and bioavailability observed with C-ITZ formulations [57]. It is available as an oral capsule formulation and applies a solid dispersion of ITZ in a polymetric matrix. Studies of SUBA-ITZ in healthy adults demonstrated no significant reductions in bioavailability in the presence of acid-suppressive therapies or in fed versus fasted states [58]. A compilation of seven crossover studies in healthy adults receiving SUBA-ITZ and C-ITZ capsules demonstrated improved bioavailability of SUBA-ITZ (173%) compared to C-ITZ capsules with less variability in ITZ exposure [59]. In addition, steady-state findings from an open-label crossover pharmacokinetic analysis of SUBA-ITZ and C-ITZ capsules showed greater attainment of therapeutic ITZ trough values (i.e., > 1000 ng/mL) in the SUBA-ITZ than C-ITZ group (81 versus 44%, respectively) [60]. SUBA-ITZ was approved by the FDA in 2018 for the treatment of specific fungal infections including histoplasmosis. Published results from a Mycosis Study Group-sponsored phase 3 multicenter, randomized, open-label study of invasive endemic fungal infections evaluating the pharmacokinetics, safety, efficacy, tolerability, and health economics of oral SUBA-ITZ compared to C-ITZ capsules are highly anticipated (NCT03572049).
Echinocandins are not currently recommended for histoplasmosis treatment due to a lack of supportive clinical data. However, fluconazole (FCZ) has been used as an alternative to ITZ in settings such as intolerance. Concerns with application of FCZ for Histoplasma include reduced in vitro activity, delayed fungemia clearance, and resistance emergence [50••, 61, 62]. Newer-generation extended-spectrum azoles including voriconazole (VCZ), posaconazole (PCZ), and isavuconazole (ISZ) may be suitable alternatives to ITZ though data are limited to case reports and series [63–66]. A particular advantage may exist in CNS infection, as VCZ has improved CNS penetration compared to ITZ but necessitates further study. Hendrix et al. published the only data to date comparing outcomes with VCZ and ITZ for histoplasmosis in a single-center, retrospective evaluation in 194 cases that included 21 (10.8%) transplant recipients [67••]. Survival analysis in patients receiving ITZ (90.2%) and VCZ (9.8%), either as primary or step-down therapy following AmB, demonstrated a statistically significant association of VCZ therapy with early mortality during the first 6 weeks (hazard ratio, 4.3; 95% confidence interval, 1.3–13.9; P = 0.015). Results of therapeutic drug monitoring were not reported and only one case with CNS disease was treated with VCZ. Potential explanations for poorer survival with VCZ include suboptimal drug exposure and resistance emergence. As previously mentioned, resistance has been demonstrated with FCZ and is due to a point mutation in cytochrome P450 enzyme 14α-demethylase (CYP51p) [68]. This mutation results in increased minimal inhibitor concentrations (MIC) for both FCZ and VCZ but does not appear to impact the MIC for the other extended-spectrum azoles [68, 69]. Despite the limitations of this retrospective evaluation, caution is advised when utilizing VCZ for histoplasmosis, particularly in the setting of preceding FCZ exposure. In situations of ITZ intolerance, the authors prefer PCZ based on resistance concerns and improved tolerability despite the limited clinical data. Prospective controlled trials are necessary to determine whether VCZ and other extended-spectrum azoles are effective therapeutic options for histoplasmosis, including CNS disease, and to further understand the potential for resistance emergence.
There are additional important considerations when managing histoplasmosis in the SOT recipient. Drug toxicities and drug interactions, especially those related to the cytochrome P450 isoenzyme system, remain a significant issue with azole therapies (see Table 2). Polypharmacy is common in the transplanted host and often includes other narrow therapeutic index agents such as calcineurin inhibitors (CNIs) impacting the CYP450 isoenzyme system. Thus, azole use necessitates careful dose adjustment of relevant medications and close monitoring. Reducing immunosuppression during acute infection is also paramount. However, an optimal strategy is lacking, with decisions typically individualized to the transplant recipient based on the transplant type and timeline, status of allograft rejection, and net state of immunosuppression. Mycophenolate has been identified as a risk factor for severe disease and failure to reduce CNI exposure has been associated with relapsed disease; hence, reductions in these agents should be prioritized [8]. While immune reconstitution syndrome has been described during treatment of disseminated histoplasmosis, it is uncommon in SOT and other immunocompromised populations such as PLWH [70, 71]. Finally, monitoring of urine and serum Histoplasma antigen levels during and after therapy is recommended to assess response to infection and monitor for relapse. The Infectious Diseases Society of America (IDSA) and AST guidelines recommend monitoring antigen levels at regular intervals, including at therapy initiation, 2 weeks, 1 month, and approximately every 3 months thereafter during therapy and for at least 6 to 12 months post-cessation [14••, 50••]. Additional testing should be performed as clinically indicated, such as scenarios when there is concern for relapse or with marked augmentation of immunosuppressive therapy (e.g., re-transplantation and severe allograft rejection). Not all SOT recipients will completely clear antigenemia and/or antigenuria at the end of appropriate therapy; however, most can still safely stop antifungal therapy without relapse, assuming all other indicators support clinical response [15, 72]. Of note, quantitative antigen values may vary depending on the specific EIA assay applied; hence, a consistent laboratory should be utilized for serial monitoring when feasible.
Novel Fungal Therapies for Histoplasmosis
There are additional drugs either in the antifungal pipeline or FDA-approved for other indications that have Histoplasma activity. These agents may ultimately supplement the limited fungal armamentarium for histoplasmosis and other endemic mycoses. For example, new polyene formulations in development include encochleated amphotericin B deoxycholate (MAT2203; Matinas BioPharma Nanotechnologies, Bedminster, NJ) which utilizes a novel lipid nanocrystal to allow for oral administration. This therapy lacks many of the adverse effects associated with intravenous AmB including infusion reactions, nephrotoxicity, and electrolyte disarray [73, 74]. Tetrazoles are next-generation azoles in development to abrogate management issues such as drug interactions given their greater selectivity for fungal Cyp51 compared with the mammalian cytochrome P450 enzyme system [75, 76]. Among these, VT-1598 (Viamet Pharmaceuticals, Durham, NC) has a broad spectrum of activity including Histoplasma and was granted orphan drug designation for the treatment of coccidioidomycosis. Ibrexafungerp is an oral triterpenoid antifungal with a mechanism of action similar to echinocandins, inhibiting 1,3-β-D-glucan synthase [75, 77, 78]. It received FDA approval in 2021 for treatment of vulvovaginal candidiasis. An ongoing phase 3 multicenter, open-label study evaluating the efficacy and safety of ibrexafungerp in patients with fungal diseases refractory to or intolerant of standard treatment was expanded to include histoplasmosis (FURI, NCT03059992) [77]. Other novel agents with Histoplasma activity include olorofim, fosmanogepix, and nikkomycin Z [76, 78, 79•, 80–83]. Both olorofim and fosmanogepix have activity against Histoplasma, are available in oral and intravenous formulations, and are actively being pursued for multiple fungal applications. Olorofim, an antifungal in the orotomide class, inhibits dihydroorotate dehydrogenase in the pyrimidine biosynthesis pathway [81]. Fosmanogepix, the oral prodrug of manogepix, inhibits the fungal enzyme Gwt1 important for mannoprotein adherence to the cell wall during establishment of infection [79•]. Finally, nikkomycin Z is a chitin synthase inhibitor and impacts chitin production, an important structural component of fungal cell walls. It demonstrates activity against histoplasmosis both as monotherapy and in combination with FCZ [83]. Renewed interest in nikkomycin Z for coccidioidomycosis treatment alongside the development of an extended-release formulation to offset its short half-life may hold promise in future application for endemic mycoses including histoplasmosis [84].
COVID-19 and Histoplasmosis
Emerging reports have described a temporal relationship between COVID-19 infection and invasive fungal infections, including histoplasmosis [85•]. Reports of histoplasmosis developing during and after COVID-19 infection are limited to small series and case reports, occurring among both immunocompromised and immunocompetent individuals [86–90]. Only one such case has been reported in a kidney transplant recipient, though additional anecdotal cases in SOT recipients have occurred (author personal communication) [90].
There are two main proposed mechanisms to explain an association between histoplasmosis and COVID-19 infection. First, parenchymal lung injury related to COVID-19 infection may predispose individuals to developing acute pulmonary histoplasmosis after an inhalation of environmental Histoplasma conidia. Second, SARS-CoV-2-associated immune modulation or that related to corticosteroid therapy used for COVID-19 infection management may lead to reactivation of latent Histoplasma infection in those individuals previously infected. Further, these factors may act synergistically in an infected individual. In many of the reported cases, interpretation of the diagnostic testing in the clinical context is challenging such that firm conclusions regarding a true link between Histoplasma and COVID-19 infections are lacking. More data are needed, but these early cases suggest clinicians should maintain heightened clinical suspicion for histoplasmosis, especially in endemic areas, among patients with concurrent or recent COVID-19 infection.
Peri-transplant Donor and Recipient Considerations
Recipient Evaluation
General consensus based on data compiled over 2 decades support that pre-transplant screening for histoplasmosis in transplant candidates is not indicated, even for those in endemic areas. Early support for this approach came from a retrospective review of 449 SOT recipients from a hyperendemic region, where approximately one quarter of SOT candidates had either a positive Histoplasma serology or chest radiograph evidence of prior infection [91]. None developed post-transplant histoplasmosis despite no antifungal prophylaxis, suggesting the risk for reactivation infection is negligible in this setting. Transplant candidates with active histoplasmosis should be deferred at least until there is significant clinical improvement, or if feasible, until therapy is completed. Active histoplasmosis within the prior 2 years may be a basis for antifungal prophylaxis after transplantation, though the duration is not established [50••]. Subsequent case series have further supported this approach, with targeted post-transplant antifungal prophylaxis for other selected recipients [8, 15, 19, 72].
Donor Evaluation
Given the low incidence of histoplasmosis in healthy donors and rarity of reported donor transmission events, even in endemic areas, routine donor screening is not indicated. However, if the donor demonstrates clinical findings such as non-hemorrhagic neurological disease, unexplained fever, and/or pneumonia, histoplasmosis should be considered and testing performed. Though procurement may proceed before tests result, organs should be carefully inspected for findings suspicious for histoplasmosis, such as hepatosplenomegaly, granuloma formation, and lymphadenopathy. In addition to obtaining tissue specimens for histopathology and culture, serology and antigen EIA testing should be performed. The collective results will inform recipient management following allograft implantation. Potential living donors with active histoplasmosis should be deferred and treated with antifungal therapy at least 3–6 months prior to consideration for organ donation [17••].
Management Recommendations
The optimal management approach for clinical scenarios where is there is concern for possible donor Histoplasma transmission and pre-existing recipient infection remain undefined. Accordingly, there exists significant practice variability among individual transplant centers, geographic regions, and organ transplanted. Recognizing the paucity of data, in 2012, the AST published guidance [17••]. Per these recommendations, azole prophylaxis should be offered if the donor tissue fungal stains are positive but cultures and antigen assays are negative and/or the donor complement fixation serology is ≥ 1:32. The recommended prophylaxis duration is 3 to 6 months. For lower risk scenarios, observation without azole prophylaxis with antigen EIA monitoring every 3 months is a reasonable approach. If active histoplasmosis is confirmed in the donor after allograft implantation, the recipient should be treated with azole therapy for at least 1-year post-transplant.
While appreciating the attempt to provide a construct for uniform practice across the transplant community, these recommendations were based largely on expert opinion and now date back a decade, leaving leeway for alternative present day approaches. Without updated data, testing and management of donors and recipients remain variable and individualized based on the infection burden and underlying recipient risk. Lung transplant recipients are considered higher risk for developing active histoplasmosis based on requirements for relatively higher baseline immunosuppression compared to other transplant groups as well as the pulmonary reservoir of latent or active Histoplasma organisms. Newer, extended-spectrum azoles with improved tolerability also introduce expanded options for prophylaxis and treatment. It is important to recognize that histoplasmosis remains a rare infection (< 1%) in the post-transplant setting, even in endemic areas [1].
Conclusions
Though an uncommon infection in the SOT population, histoplasmosis has significant clinical implications. Given the myriad of clinical manifestations associated with infection in SOT recipients and the expanding worldwide distribution, histoplasmosis should increasingly be suspected in the appropriate clinical context. Laboratory testing continues to evolve to optimize timely diagnosis and monitoring and the antifungal armamentarium is growing. With diligent monitoring for active infection and targeted post-transplant antifungal prophylaxis, serious infection can largely be averted in the highest risk transplant recipients.
Declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Jennifer L. Saullo declares that she has no conflicts of interest. Rachel A. Miller receives research support from Scynexis (institutional principal investigator in the FURI clinical trial, NCT03059992) and has no other conflicts of interest to disclose.
Footnotes
This article is part of the Topical Collection on Fungal Infections in Transplantation.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jennifer L. Saullo, Email: jennifer.horan@duke.edu
Rachel A. Miller, Email: rachel.a.miller@duke.edu
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Kauffman CA, Freifeld AG, Andes DR, Baddley JW, Herwaldt L, Walker RC, et al. Endemic fungal infections in solid organ and hematopoietic cell transplant recipients enrolled in the Transplant-Associated Infection Surveillance Network (TRANSNET) Transpl Infect Dis. 2014;16(2):213–224. doi: 10.1111/tid.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.•.Benedict K, Toda M, Jackson BR. Revising conventional wisdom about histoplasmosis in the United States. Open Forum Infect Dis. 2021;8(7):ofab306. 10.1093/ofid/ofab306. Excellent review of the evolving epidemiology of histoplasmosis including expanding geographic distribution and recent insights into changes in clinical spectrum and underlying conditions associated with the severe forms of histoplasmosis. [DOI] [PMC free article] [PubMed]
- 3.••.Ashraf N, Kubat RC, Poplin V, Adenis AA, Denning DW, Wright L, et al. Re-drawing the maps for endemic mycoses. Mycopathologia. 2020;185(5):843–865. doi: 10.1007/s11046-020-00431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Develoux M, Amona FM, Hennequin C. Histoplasmosis caused by Histoplasma capsulatum var. duboisii: a comprehensive review of cases from 1993 to 2019. Clin Infect Dis. 2021;73(3):e543–e9. doi: 10.1093/cid/ciaa1304. [DOI] [PubMed] [Google Scholar]
- 5.Sepúlveda VE, Márquez R, Turissini DA, Goldman WE, Matute DR. Genome sequences reveal cryptic speciation in the human pathogen Histoplasma capsulatum. mBio. 2017;5(8):e01339–17. doi: 10.1128/mBio.01339-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor ML, Reyes-Montes MDR, Estrada-Bárcenas DA, Zancopé-Oliveira RM, Rodríguez-Arellanes G, Ramírez JA. Considerations about the geographic distribution of histoplasma species. Appl Environ Microbiol. 2022;88(7):e0201021. doi: 10.1128/aem.02010-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong PA, Jackson BR, Haselow D, Fields V, Ireland M, Austin C, et al. Multistate epidemiology of histoplasmosis, United States, 2011–2014. Emerg Infect Dis. 2018;24(3):425–431. doi: 10.3201/eid2403.171258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assi M, Martin S, Wheat LJ, Hage C, Freifeld A, Avery R, et al. Histoplasmosis after solid organ transplant. Clin Infect Dis. 2013;57(11):1542–1549. doi: 10.1093/cid/cit593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gajurel K, Dhakal R, Deresinski S. Histoplasmosis in transplant recipients. Clin Transplant. 2017;31(10). 10.1111/ctr.13087. [DOI] [PubMed]
- 10.Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007;20(1):115–132. doi: 10.1128/cmr.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benedict K, Mody RK. Epidemiology of histoplasmosis outbreaks, United States, 1938–2013. Emerg Infect Dis. 2016;22(3):370–378. doi: 10.3201/eid2203.151117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedict K, McCracken S, Signs K, Ireland M, Amburgey V, Serrano JA, et al. Enhanced surveillance for histoplasmosis-9 states, 2018–2019. Open Forum Infect Dis. 2020;7(9):ofaa343. doi: 10.1093/ofid/ofaa343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buitrago MJ, Martín-Gómez MT. Timely diagnosis of histoplasmosis in non-endemic countries: a laboratory challenge. Front Microbiol. 2020;11:467. doi: 10.3389/fmicb.2020.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.••.Miller R, Assi M; AST Infectious Diseases Community of Practice. Endemic fungal infections in solid organ transplant recipients-guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13553. 10.1111/ctr.13553. AST endorsed guidelines based on expert opinion detailing the endemic mycoses including histoplasmosis and issues specific to the solid organ transplant population. [DOI] [PubMed]
- 15.Grim SA, Proia L, Miller R, Alhyraba M, Costas-Chavarri A, Oberholzer J, et al. A multicenter study of histoplasmosis and blastomycosis after solid organ transplantation. Transpl Infect Dis. 2012;14(1):17–23. doi: 10.1111/j.1399-3062.2011.00658.x. [DOI] [PubMed] [Google Scholar]
- 16.Majeed A, Kapoor V, Latif A, Zangeneh T. A 30-year delayed presentation of disseminated histoplasmosis in a heart transplant recipient: diagnostic challenges in a non-endemic area. BMJ Case Rep. 2017: bcr2017222012. 10.1136/bcr-2017-222012. [DOI] [PMC free article] [PubMed]
- 17.••.Singh N, Huprikar S, Burdette SD, Morris MI, Blair JE, Wheat LJ. Donor-derived fungal infections in organ transplant recipients: guidelines of the American Society of Transplantation, infectious diseases community of practice. Am J Transplant. 2012;12(9):2414–28. 10.1111/j.1600-6143.2012.04100.x. AST endorsed guidelines based on expert opinion which provide detailed recommendations for donor evaluation and recipient peri-transplant screening, monitoring, and prophylaxis for histoplasmosis. [DOI] [PubMed]
- 18.Franklin AD, Larson L, Rauseo AM, Rutjanawech S, Hendrix MJ, Powderly WG, et al. A comparison of presentations and outcomes of histoplasmosis across patients with varying immune status. Med Mycol. 2021: myaa112. 10.1093/mmy/myaa112. [DOI] [PubMed]
- 19.Cuellar-Rodriguez J, Avery RK, Lard M, Budev M, Gordon SM, Shrestha NK, et al. Histoplasmosis in solid organ transplant recipients: 10 years of experience at a large transplant center in an endemic area. Clin Infect Dis. 2009;49(5):710–716. doi: 10.1086/604712. [DOI] [PubMed] [Google Scholar]
- 20.Kauffman CA, Miceli MH. Histoplasmosis and blastomycosis in solid organ transplant recipients. J Fungi (Basel) 2015;1(2):94–106. doi: 10.3390/jof1020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wheat J, Myint T, Guo Y, Kemmer P, Hage C, Terry C, et al. Central nervous system histoplasmosis: multicenter retrospective study on clinical features, diagnostic approach and outcome of treatment. Medicine (Baltimore) 2018;97(13):e0245. doi: 10.1097/md.0000000000010245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gani I, Barrett A, Mulloy L, Kapoor R. Disseminated histoplasmosis presenting as weight loss and hypercalcemia in a renal transplant patient with prior history of subtotal parathyroidectomy. Am J Med Sci. 2021;361(3):383–387. doi: 10.1016/j.amjms.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Jabr R, El Atrouni W, Male HJ, Hammoud KA. Histoplasmosis-associated hemophagocytic lymphohistiocytosis: a review of the literature. Can J Infect Dis Med Microbiol. 2019;2019:7107326. doi: 10.1155/2019/7107326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verdecia J, Kunz Coyne AJ, Patel S, Oye M, Ravi M, Sands M. Miliary histoplasmosis in a renal transplant patient. Cureus. 2021;13(11):e19338. doi: 10.7759/cureus.19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kauffman CA. Histoplasmosis. Clin Chest Med. 2009;30(2):217–25. doi: 10.1016/j.ccm.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, McCracken J, Wang E. Yeast-like organisms phagocytosed by circulating neutrophils: evidence of disseminated histoplasmosis. Int J Lab Hematol. 2022;44(1):51–52. doi: 10.1111/ijlh.13693. [DOI] [PubMed] [Google Scholar]
- 27.•.Kapatia G, Saha A, Rohilla M, Gupta P, Gupta N, Srinivasan R, et al. Clinical and morphological spectrum of histoplasmosis on cytology along with the review of literature. Acta Cytol. 2020;64(6):532–8. 10.1159/000509151. An evaluation of 17 histoplasmosis cases describing the cytopathological characteristics of fine needle aspirate specimens from commonly involved sites of infection, with a review of existing literature on the topic. Cytology is proposed as an economical, rapid, and accurate histoplasmosis diagnostic method. [DOI] [PubMed]
- 28.•.Azar MM, Hage CA. Laboratory diagnostics for histoplasmosis. J Clin Microbiol. 2017;55(6):1612–1620. doi: 10.1128/jcm.02430-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hage CA, Ribes JA, Wengenack NL, Baddour LM, Assi M, McKinsey DS, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53(5):448–454. doi: 10.1093/cid/cir435. [DOI] [PubMed] [Google Scholar]
- 30.Bloch KC, Myint T, Raymond-Guillen L, Hage CA, Davis TE, Wright PW, et al. Improvement in diagnosis of histoplasma meningitis by combined testing for histoplasma antigen and immunoglobulin G and immunoglobulin M anti-histoplasma antibody in cerebrospinal fluid. Clin Infect Dis. 2018;66(1):89–94. doi: 10.1093/cid/cix706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Persaud SP, Lawton T, Burnham CD, Anderson NW. Comparison of urine antigen assays for the diagnosis of Histoplasma capsulatum infection. J Appl Lab Med. 2019;4(3):370–382. doi: 10.1373/jalm.2018.028910. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Lei GS, Lee CH, Hage CA. Evaluation of two new enzyme immunoassay reagents for diagnosis of histoplasmosis in a cohort of clinically characterized patients. Med Mycol. 2015;53(8):868–873. doi: 10.1093/mmy/myv062. [DOI] [PubMed] [Google Scholar]
- 33.Theel ES, Jespersen DJ, Harring J, Mandrekar J, Binnicker MJ. Evaluation of an enzyme immunoassay for detection of Histoplasma capsulatum antigen from urine specimens. J Clin Microbiol. 2013;51(11):3555–3559. doi: 10.1128/JCM.01868-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez-Gamboa A, Niembro-Ortega MD, Torres-Gonzalez P, Santiago-Cruz J, Velazquez-Zavala NG, Rangel-Cordero A, et al. Diagnostic accuracy of antigen detection in urine and molecular assays testing in different clinical samples for the diagnosis of progressive disseminated histoplasmosis in patients living with HIV/AIDS: a prospective multicenter study in Mexico. PLoS Negl Trop Dis. 2021;15(3):e0009215. doi: 10.1371/journal.pntd.0009215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swartzentruber S, Rhodes L, Kurkjian K, Zahn M, Brandt ME, Connolly P, et al. Diagnosis of acute pulmonary histoplasmosis by antigen detection. Clin Infect Dis. 2009;49(12):1878–1882. doi: 10.1086/648421. [DOI] [PubMed] [Google Scholar]
- 36.Myint T, Anderson AM, Sanchez A, Farabi A, Hage C, Baddley JW, et al. Histoplasmosis in patients with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS): multicenter study of outcomes and factors associated with relapse. Medicine (Baltimore) 2014;93(1):11–18. doi: 10.1097/MD.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maphanga TG, Naicker SD, Gomez BL, Mhlanga M, Mpembe RS, Schwartz IS, et al. Cross-reactivity of a Histoplasma capsulatum antigen enzyme immunoassay in urine specimens from persons with emergomycosis in South Africa. Med Mycol. 2021;59(7):672–682. doi: 10.1093/mmy/myaa100. [DOI] [PubMed] [Google Scholar]
- 38.Hage CA, Davis TE, Fuller D, Egan L, Witt JR, 3rd, Wheat LJ, et al. Diagnosis of histoplasmosis by antigen detection in BAL fluid. Chest. 2010;137(3):623–628. doi: 10.1378/chest.09-1702. [DOI] [PubMed] [Google Scholar]
- 39.Vergidis P, Walker RC, Kaul DR, Kauffman CA, Freifeld AG, Slagle DC, et al. False-positive Aspergillus galactomannan assay in solid organ transplant recipients with histoplasmosis. Transpl Infect Dis. 2012;14(2):213–217. doi: 10.1111/j.1399-3062.2011.00675.x. [DOI] [PubMed] [Google Scholar]
- 40.Wheat LJ, Hackett E, Durkin M, Connolly P, Petraitiene R, Walsh TJ, et al. Histoplasmosis-associated cross-reactivity in the BioRad Platelia Aspergillus enzyme immunoassay. Clin Vaccine Immunol. 2007;14(5):638–640. doi: 10.1128/cvi.00479-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egan L, Connolly P, Wheat LJ, Fuller D, Dais TE, Knox KS, et al. Histoplasmosis as a cause for a positive Fungitell (1 –> 3)-beta-D-glucan test. Med Mycol. 2008;46(1):93–95. doi: 10.1080/13693780701642235. [DOI] [PubMed] [Google Scholar]
- 42.Girouard G, Lachance C, Pelletier R. Observations on (1–3)-beta-D-glucan detection as a diagnostic tool in endemic mycosis caused by Histoplasma or Blastomyces. J Med Microbiol. 2007;56(Pt 7):1001–1002. doi: 10.1099/jmm.0.47162-0. [DOI] [PubMed] [Google Scholar]
- 43.Myint T, Chow FC, Bloch KC, Raymond-Guillen L, Davis TE, Wright PW, et al. Detection of (1,3)-β-d-glucan in cerebrospinal fluid in Histoplasma meningitis. J Clin Microbiol. 2018;56(10). doi: 10.1128/jcm.00663-18. [DOI] [PMC free article] [PubMed]
- 44.•.Abdallah W, Myint T, LaRue R, Minderman M, Gunn S, Wheat LJ, et al. Diagnosis of histoplasmosis using the MVista Histoplasma galactomannan antigen qualitative lateral flow-based immunoassay: a multicenter study. Open Forum Infect Dis. 2021;8(9):ofab454. 10.1093/ofid/ofab454. A multicenter study of 352 patients with and without histoplasmosis, comparing the performance of the MiraVista enzyme immunoassay with the lateral flow immunoassay. The assays demonstrated high concordance with similar cross-reactivity with other endemic mycoses, supporting a role for the lateral flow immunoassay as a rapid, accurate, and economical diagnostic option. [DOI] [PMC free article] [PubMed]
- 45.Cáceres DH, Gómez BL, Tobón ÁM, Minderman M, Bridges N, Chiller T, et al. Validation and concordance analysis of a new lateral flow assay for detection of histoplasma antigen in urine. Journal of Fungi. 2021;7(10):799. doi: 10.3390/jof7100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hologic. 2022. AccuProbe Culture Identification Tests. [online] Available at: http://www.hologic.ca/products/clinical-diagnostics-and-blood-screening/assays-and-tests/accuprobe-culture-identification. [Accessed 20 July 2022].
- 47.Valero C, Buitrago MJ, Gago S, Quiles-Melero I, García-Rodríguez J. A matrix-assisted laser desorption/ionization time of flight mass spectrometry reference database for the identification of Histoplasma capsulatum. Med Mycol. 2017;56(3):307–314. doi: 10.1093/mmy/myx047. [DOI] [PubMed] [Google Scholar]
- 48.••.Vasconcellos I, Dalla Lana DF, Pasqualotto AC. The role of molecular tests in the diagnosis of disseminated histoplasmosis. J Fungi (Basel). 2019;6(1):1. 10.3390/jof6010001. A systematic literature review of studies comparing immunologic diagnostic methods with molecular tests for the diagnosis of histoplasmosis. The authors also summarize published studies evaluating the performance of varied Histoplasma PCR assays both from clinical samples and fungal culture. [DOI] [PMC free article] [PubMed]
- 49.Alanio A, Gits-Muselli M, Lanternier F, Sturny-Leclere A, Benazra M, Hamane S, et al. Evaluation of a new Histoplasma spp. quantitative RT-PCR assay. J Mol Diagn. 2021;23(6):698–709. doi: 10.1016/j.jmoldx.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 50.••.Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807–825. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]
- 51.Johnson PC, Wheat LJ, Cloud GA, Goldman M, Lancaster D, Bamberger DM, et al. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann Intern Med. 2002;137(2):105–109. doi: 10.7326/0003-4819-137-2-200207160-00008. [DOI] [PubMed] [Google Scholar]
- 52.•.Riddell JT, Wheat LJ. Central nervous system infection with Histoplasma capsulatum. J Fungi (Basel). 2019;5(3):70. 10.3390/jof5030070. A comprehensive review of CNS infection with histoplasmosis including challenging diagnostic issues and management strategies. [DOI] [PMC free article] [PubMed]
- 53.Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am. 2016;30(1):207–227. doi: 10.1016/j.idc.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Sporanox Oral Capsule [package insert]. Titusville: Janssen Pharmaceuticals, Inc; 2019.
- 55.Sporanox Oral Solution [package insert]. Titusville: Janssen Pharmaceuticals, Inc; 2019.
- 56.Wiederhold NP, Pennick GJ, Dorsey SA, Furmaga W, Lewis JS, 2nd, Patterson TF, et al. A reference laboratory experience of clinically achievable voriconazole, posaconazole, and itraconazole concentrations within the bloodstream and cerebral spinal fluid. Antimicrob Agents Chemother. 2014;58(1):424–431. doi: 10.1128/aac.01558-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tolsura [package insert]. Greenville: Mayne Pharma; 2018.
- 58.Lindsay J, Mudge S, Thompson GR., 3rd Effects of food and omeprazole on a novel formulation of super bioavailability itraconazole in healthy subjects. Antimicrob Agents Chemother. 2018;62(12):e01723–e1818. doi: 10.1128/aac.01723-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abuhelwa AY, Foster DJ, Mudge S, Hayes D, Upton RN. Population pharmacokinetic modeling of itraconazole and hydroxyitraconazole for oral SUBA-itraconazole and sporanox capsule formulations in healthy subjects in fed and fasted states. Antimicrob Agents Chemother. 2015;59(9):5681–5696. doi: 10.1128/aac.00973-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson GR, 3rd, Lewis P, Mudge S, Patterson TF, Burnett BP. Open-Label crossover oral bioequivalence pharmacokinetics comparison for a 3-day loading dose regimen and 15-day steady-state administration of SUBA-itraconazole and conventional itraconazole capsules in healthy adults. Antimicrob Agents Chemother. 2020;64(8). 10.1128/aac.00400-20. [DOI] [PMC free article] [PubMed]
- 61.La Hoz RM, Loyd JE, Wheat LJ, Baddley JW. How i treat histoplasmosis. Current Fungal Infect Reports. 2013;7(1):36–43. doi: 10.1007/s12281-012-0117-7. [DOI] [Google Scholar]
- 62.Wheat J, MaWhinney S, Hafner R, McKinsey D, Chen D, Korzun A, et al. Treatment of histoplasmosis with fluconazole in patients with acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Acquired Immunodeficiency Syndrome Clinical Trials Group and Mycoses Study Group. Am J Med. 1997;103(3):223–32. doi: 10.1016/s0002-9343(97)00151-4. [DOI] [PubMed] [Google Scholar]
- 63.Freifeld A, Proia L, Andes D, Baddour LM, Blair J, Spellberg B, et al. Voriconazole use for endemic fungal infections. Antimicrob Agents Chemother. 2009;53(4):1648–1651. doi: 10.1128/aac.01148-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodriguez JA, Ivancic S, Eckardt PA, Lemos-Ramirez JC, Niu J. A case of pulmonary histoplasmosis presenting with hypercalcemia and altered mental status in a patient following allogeneic hematopoietic stem cell transplantation. Am J Case Rep. 2020;21:e919724. doi: 10.12659/ajcr.919724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakamura A, Tawara I, Ino K, Matsumoto T, Hayashi A, Imai H, et al. Achievement of long-term remission of disseminated histoplasmosis in an AIDS patient. Med Mycol Case Rep. 2020;27:25–28. doi: 10.1016/j.mmcr.2019.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mazzella A, Stone NRH, Pool ERM, García Mingo A, Bolache S, Wood C. HIV-associated disseminated histoplasmosis successfully treated with isavuconazole consolidation therapy. Med Mycol Case Rep. 2020;27:42–43. doi: 10.1016/j.mmcr.2019.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.••.Hendrix MJ, Larson L, Rauseo AM, Rutjanawech S, Franklin AD, Powderly WG, et al. Voriconazole versus itraconazole for the initial and step-down treatment of histoplasmosis: a retrospective cohort. Clin Infect Dis. 2021;73(11):e3727-e32. 10.1093/cid/ciaa1555. This single-center, retrospective evaluation of 194 cases of histoplasmosis is the first comparative study to evaluate outcomes with ITZ versus VCZ and demonstrated increased early mortality with VCZ therapy. [DOI] [PMC free article] [PubMed]
- 68.Wheat LJ, Connolly P, Smedema M, Durkin M, Brizendine E, Mann P, et al. Activity of newer triazoles against Histoplasma capsulatum from patients with AIDS who failed fluconazole. J Antimicrob Chemother. 2006;57(6):1235–1239. doi: 10.1093/jac/dkl133. [DOI] [PubMed] [Google Scholar]
- 69.Spec A, Connolly P, Montejano R, Wheat LJ. In vitro activity of isavuconazole against fluconazole-resistant isolates of Histoplasma capsulatum. Med Mycol. 2018;56(7):834–837. doi: 10.1093/mmy/myx130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jazwinski A, Naggie S, Perfect J. Immune reconstitution syndrome in a patient with disseminated histoplasmosis and steroid taper: maintaining the perfect balance. Mycoses. 2011;54(3):270–272. doi: 10.1111/j.1439-0507.2009.01796.x. [DOI] [PubMed] [Google Scholar]
- 71.Melzani A, de Reynal de Saint Michel R, Ntab B, Djossou F, Epelboin L, Nacher M, et al. Incidence and trends in immune reconstitution inflammatory syndrome associated with Histoplasma capsulatum among people living with human immunodeficiency virus: a 20-year case series and literature review. Clinical Infect Dis. 2019;70(4):643–52. doi: 10.1093/cid/ciz247. [DOI] [PubMed] [Google Scholar]
- 72.Freifeld AG, Iwen PC, Lesiak BL, Gilroy RK, Stevens RB, Kalil AC. Histoplasmosis in solid organ transplant recipients at a large Midwestern university transplant center. Transpl Infect Dis. 2005;7(3–4):109–115. doi: 10.1111/j.1467-8365.2005.00105.x. [DOI] [PubMed] [Google Scholar]
- 73.Aigner M, Lass-Flörl C. Encochleated amphotericin B: is the oral availability of amphotericin B Finally reached? J Fungi (Basel) 2020;6(2):66. doi: 10.3390/jof6020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Skipper CP, Atukunda M, Stadelman A, Engen NW, Bangdiwala AS, Hullsiek KH, et al. Phase I EnACT trial of the safety and tolerability of a novel oral formulation of amphotericin B. Antimicrob Agents Chemother. 2020;64(10):e00838–e920. doi: 10.1128/aac.00838-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wiederhold NP, Patterson HP, Tran BH, Yates CM, Schotzinger RJ, Garvey EP. Fungal-specific Cyp51 inhibitor VT-1598 demonstrates in vitro activity against Candida and Cryptococcus species, endemic fungi, including Coccidioides species, Aspergillus species and Rhizopus arrhizus. J Antimicrob Chemother. 2017;73(2):404–408. doi: 10.1093/jac/dkx410. [DOI] [PubMed] [Google Scholar]
- 76.Rauseo AM, Coler-Reilly A, Larson L, Spec A. Hope on the horizon: novel fungal treatments in development. Open Forum Infect Dis. 2020;7(2):ofaa016. doi: 10.1093/ofid/ofaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brexafemme [package insert]. Jersey CIty: Scynexis, Inc; 2022.
- 78.McCarthy MW. Pharmacokinetics and Pharmacodynamics of Ibrexafungerp. Drugs R D. 2022;22(1):9–13. doi: 10.1007/s40268-021-00376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.•.Hoenigl M, Sprute R, Egger M, Arastehfar A, Cornely OA, Krause R, et al. The antifungal pipeline: fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs. 2021;81(15):1703–1729. doi: 10.1007/s40265-021-01611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Logan A, Wolfe A, Williamson JC. Antifungal resistance and the role of new therapeutic agents. Curr Infect Dis Rep. 2022:1–12. 10.1007/s11908-022-00782-5. [DOI] [PMC free article] [PubMed]
- 81.Oliver JD, Sibley GEM, Beckmann N, Dobb KS, Slater MJ, McEntee L, et al. F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc Natl Acad Sci U S A. 2016;113(45):12809–12814. doi: 10.1073/pnas.1608304113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnson MD. Antifungals in clinical use and the pipeline. Infect Dis Clin N Am. 2021;35(2):341–371. doi: 10.1016/j.idc.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 83.Larwood DJ. Nikkomycin Z—ready to meet the promise? J Fungi. 2020;6(4):261. doi: 10.3390/jof6040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sass G, Larwood DJ, Martinez M, Shrestha P, Stevens DA. Efficacy of nikkomycin Z in murine CNS coccidioidomycosis: modelling sustained-release dosing. J Antimicrob Chemother. 2021;76(10):2629–2635. doi: 10.1093/jac/dkab223. [DOI] [PubMed] [Google Scholar]
- 85.•.Casalini G, Giacomelli A, Ridolfo A, Gervasoni C, Antinori S. Invasive fungal infections complicating COVID-19: a narrative review. J Fungi (Basel). 2021;7(11):921. 10.3390/jof7110921. This narrative review of 4099 cases of invasive fungal infections in COVID-19 patients, which includes four histoplasmosis cases, and highlights the heightened risk in this setting. [DOI] [PMC free article] [PubMed]
- 86.de Macedo PM, Freitas AD, Bartholo TP, Bernardes-Engemann AR, Almeida MA, Almeida-Silva F, et al. Acute pulmonary histoplasmosis following COVID-19: novel laboratorial methods aiding diagnosis. J Fungi (Basel) 2021;7(5):346. doi: 10.3390/jof7050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Messina FA, Marin E, Caceres DH, Romero M, Depardo R, Priarone MM, et al. Coronavirus disease 2019 (COVID-19) in a patient with disseminated histoplasmosis and HIV-a case report from Argentina and literature review. J Fungi (Basel) 2020;6(4):275. doi: 10.3390/jof6040275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taylor M, Ghodasara A, Ismail A, Gauhar U, El-Kersh K. Disseminated histoplasmosis in an immunocompetent patient after COVID-19 pneumonia. Cureus. 2021;13(8):e17269. doi: 10.7759/cureus.17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Toscanini MA, Barberis F, Benedetti MF, Garrido AV, Posse GB, Capece P, et al. Detection of anti-Histoplasma capsulatum antibodies and seroconversion patterns in critically ill patients with COVID-19: an underdiagnosed fungal entity complicating COVID-19? Med Mycol. 2022;60(3):myac012. doi: 10.1093/mmy/myac012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maldonado I, Elisiri M, Fernández Canigia L, Sánchez A, López L, Toranzo A, et al. COVID-19 asociado a histoplasmosis diseminada en un paciente trasplantado renal. Rev Argent Microbiol. 2021;S0325–7541(21):00121–128. doi: 10.1016/j.ram.2021.10.006. [DOI] [Google Scholar]
- 91.Vail GM, Young RS, Wheat LJ, Filo RS, Cornetta K, Goldman M. Incidence of histoplasmosis following allogeneic bone marrow transplant or solid organ transplant in a hyperendemic area. Transpl Infect Dis. 2002;4(3):148–151. doi: 10.1034/j.1399-3062.2002.01016.x. [DOI] [PubMed] [Google Scholar]
- 92.Fungizone [package insert]. Montreal: Bristol Myers Squibb; 2018.
- 93.Ambisome [package insert]. Foster City: Gilead Sciences; 2020.
- 94.Abelcet [package insert]. Gaithersberg: Sigma-Tau Pharmaceuticals; 2013.
- 95.Diflucan [package insert]. New York: Pfizer Inc; 2019.
- 96.VFEND [package insert]. New York: Pfizer Inc; 2022.
- 97.Noxafil [package insert]. Whitehouse Station: Merck & Co, Inc; 2022.
- 98.Cresemba [package insert]. Northbrook: Astellas Pharma US, Inc; 2022.
- 99.Kably B, Launay M, Derobertmasure A, Lefeuvre S, Dannaoui E, Billaud EM. Antifungal drugs TDM: trends and update. Ther Drug Monit. 2022;44(1):166–197. doi: 10.1097/ftd.0000000000000952. [DOI] [PubMed] [Google Scholar]
- 100.Saullo JS, Alexander BD. Fungal infections: opportunistic. In: Broaddus VC, Ernst JD, King TE et al, editors. Murray and Nadel’s textbook of respiratory medicine, 6th edn. Philadephia: Elsevier, Inc; 2022. eTable 57.1, Comparison of antifungal agents.