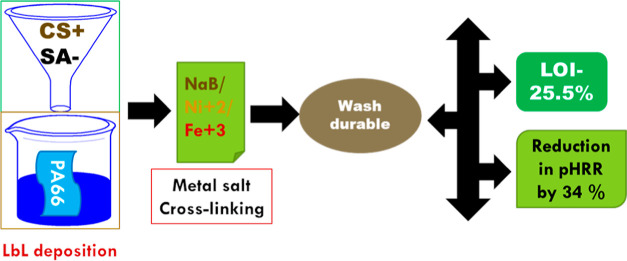
Abstract
Bio-derived polysaccharides, namely, chitosan (CS) and sodium alginate (SA) were considered in a layer-by-layer (LbL) deposition to construct flame retardant coatings onto the polyamide 66 (PA66) fabric surfaces. The as-prepared coatings were further modified in the impregnation process with a number of inorganic salts containing boron, nickel, and iron elements. Obtained results revealed that the simultaneously LbL-assembled and metal salt-treated fabric samples exhibited superior flame retardant performance compared to the only LbL-deposited fabric samples. The limiting oxygen index (LOI) value reached up to 25.5% of the CS–SA–iron salt treated fabric sample and the dripping tendency was completely diminished only for the LbL-metal salt modified fabric samples. Among the treated fabric samples, the CS–SA—iron-salt-modified fabric sample exhibited a maximum reduction in the peak heat release rate by 34% and handed improved and higher quality char residues, indicating a possible condensed phase flame retardant mechanism of this applied finishing. Moreover, metal salt-induced cross-linking could enhance the coating stability and durable finishes against regular home laundering where an iron-salt-treated fabric sample could retain anti-dripping properties even up to 10 laundering cycles. Thus, this pairing of bio-macromolecules (i.e., charring agent) with the metal salts in a hybrid system showed efficacy in improving the fire performance of polyamide textiles via the synergistic involvement between them.
Introduction
The greener application technique has increased recently considering harsh impacts of synthetic compounds onto the environment. Thus, the researchers are highly encouraged to discover the potential of naturally derived compounds in imparting numerous functionalities to the polymeric substrates. In the flame retardant field, this trend is also realized and already reported. Among them, some novel bio-based polysaccharides, namely, chitosan (CS) and alginate have been frequently paired or solely applied or compounded with other additives (i.e., phosphorus compounds) in imparting flame retardant properties to varied polymeric substrates such as polylactic acid (PLA),1,2 polyester,3 cotton,4,5 and polyamide 66 (PA66)6 textiles. In the meantime, the presence of some inorganic metal or metalloid compounds with these bio-compounds and their subsequent cross-linking can further intensify the fire performance of polymeric materials, including textiles.3,5,7−10 Here, the CS and sodium alginate (SA) act as a carbon source in a typical flame retardant mechanism due to its long-chain aromatic or poly-alcoholic structure, while the metal ions or metal elements available from some selected inorganic salts catalyze the dehydrogenation reaction to escalate the amount of charring4 and finally, their combined interactions boost up the total flame retardancy. In several applications, a wide range of metal ions, namely, Ni2+, Co2+, Mg2+, Ba2+, and Cu2+ have been used to prepare alginate based cross-linked films and fibers,9,11−14 which exhibit inherent flame retardant properties.
In line, the co-application of bio-polymers along with these metal compounds eases the application method. The direct use of metal ions as an additive as well as a synergist does not require us to synthesize them with the organic bio-polymers or flame retardants. Moreover, the synergistic effects of metal ions can limit the requirement of weight gain % by the textile substrates. For example, in a typical flame retardant treatment carried out in a layer-by-layer (LbL) deposition technique, it is usually required to deposit a large number of bilayers (i.e., 20/30) to achieve a satisfactory level of flame retardancy. Such deposition is simultaneously tedious and time-consuming while posing a higher weight gain % (i.e., >10%), which ultimately deteriorates physical properties (i.e., hand-feel, strength, drape, and so forth) of treated textiles. In addition, the metal-ion-induced cross-linking can equally enhance the wash durability of LbL-assembled coatings5 as the coatings developed in the LbL deposition through ionic interaction exhibit poor resistance in laundering. This is because the applied polyelectrolytes are basically water soluble compounds and their bonding property is weaker. This may cause a considerable loss of flame retardant performance during laundering cycles, which is a great concern and need to be addressed accordingly.
Thus, to overcome the abovementioned shortcomings in applying flame retardant functionality to the textile substrates, the hybridization of metal based inorganic salts with the organic bio-macromolecules can be considered a potential application method. Being inspirited from these, we do come up to experiment some novel polysaccharides with a good number of varied metal salts in improving the fire performance of polyamide 66 (PA66) textiles along with the durability enhancement of applied coatings as this kind of formulation is not yet reported and considered for imparting flame retardant properties to PA66 textiles.
Experimental Section
Materials
CS (viscosity 50–800 m Pa s, degree of deacetylation 80–95%) and SA (Mw: 50,075 kDa) were purchased from Sinopharm Reagent Co., Ltd. (Shanghai, China). Sodium tetra-borate decahydrate (Na2B4O7·10H2O), nickel acetate tetrahydrate (C4H6O4Ni·4H2O), and ferric chloride hexahydrate (FeCl3·6H2O) were also acquired from Sinopharm Chemical Reagent Co., Ltd. Sodium hydroxide and concentrated hydrochloric acid (HCl, 36.5–38%) were received from Changzheng Chemical Reagent Corporation. 100% PA66 woven fabrics with a yarn count 135 denier, Ends per inch (EPI) 85, Picks per inch (PPI) 81, and density of 100 g/m2 were obtained from Yangzhou City Jiaxing Weaving Industry Co. Ltd. (China). The scoured and air-dried fabric samples were used in the experimental process.
LbL Deposition
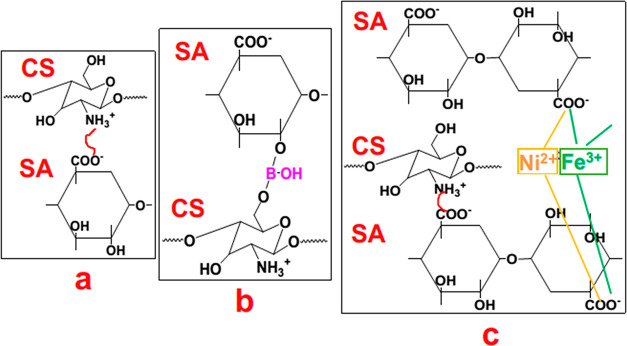
The polyelectrolyte solutions were prepared prior to the LbL deposition process. First, CS solution with a concentration of 10 g/L was made in the deionized water using acetic acid. Later, the SA solution with a concentration of 5 g/L was obtained in the deionized water. Both of the as prepared solutions were kept under magnetic stirring overnight under an ambient condition. In the next section, the fabric samples were immersed successively into the solutions containing positive CS and negative SA species. Initial immersion time was 5 min each and subsequent immersion was for 1 min. Each immersion step was followed by the rinsing of the substrates with deionized water in order to remove weakly adsorbed polyelectrolytes. The adsorption and rinsing step times were set at 1 min. It is worth to mention that the samples were not blown dry between successive deposition steps. After the deposition of desired number of bilayers, the samples were immersed in a 50 g/L Na2B4O7·10H2O,C4H6O4Ni·4H2O, or FeCl3·6H2O aqueous solution for 2 h, respectively. Here, the borate salt will be cross-linked with the amino and hydroxyl groups of CS and SA through covalent interactions, whereas the Ni and Fe salts will form positively charged divalent and trivalent ions, respectively, and these ions will go through the ionic cross-linking interaction with the negatively charged carboxylate ions (COO–) of SA. Then, the fabric samples were washed in deionized water to remove the unreacted salt compounds, and subsequently, fabric samples were dried in a convection oven at 70 °C overnight. Subsequently, weight measurement was carried out to determine add on % using the following formula
where W and W1 were the weight of the pure and treated PA66 fabric samples, respectively. The corresponding formulations and add on % are summarized in Table 1. Meanwhile, the LbL deposition and the subsequent cross-linking process are presented in Scheme 1.
Table 1. Formulations and dry Weight Pick-Up of PA66 Fabric Samples.
| sample | Salt concentration (g/L) | Dry weight pickup (added-on) (%) |
|---|---|---|
| PA66-Control | 0.0 | |
| PA66-10BL | 6.1 | |
| PA66-10BL-NaB | 50 | 7.0 |
| PA66-10BL-Ni2+ | 50 | 6.3 |
| PA66-10BL-Fe3+ | 50 | 8.3 |
Scheme 1. Schematic Representation of Ionic Interaction in a LbL Deposition (a), Covalent Cross-Linking in the Presence of Borate Salt (b), and Ionic Cross-Linking in the Presence of Ni2+ and Fe3+ Salts (c).
Characterization Techniques
The scanning electron microscopy (SEM) images of PA66 fabric samples were taken in a JSM-6700F, JEOL instrument and the elemental analysis was done in an energy-dispersive X-ray (EDX) analyzer. The fabric samples were sputter-coated with a conductive gold layer before taking images. Limiting oxygen index (LOI) test was carried out according to ASTM D2863 in a HC-2 oxygen index meter (Jiangning Analysis Instrument Company, China). The test specimens were with a dimension of 150 × 58 × 0.2 mm. Vertical burning test was done according to ASTM D6413-08 in a vertical burning tester (CZF-3, Nanjing Jiangning Analytical Instrument Factory, China). The fabric samples with a dimension of 300 × 76 × 0.2 mm, held 19 mm over the Bunsen burner, were first exposed to the flame for a period of 12 s and then rapidly detached from the fire source. Combustion properties were measured according to ISO 5660-1 standard in a cone calorimeter (Fire Testing Technology, UK). The fabric samples with a dimension of 100 mm × 100 mm × 0.6 mm were irradiated under the heat flux of 35 kW m–2 and in the presence of air flow of 24 l/s. The thermogravimetric analysis (TGA) was done in the TGA Q5000 IR thermogravimetric analyzer (TA Instruments) with a heating rate of 20 °C/min both in the air and nitrogen atmospheres. Raman spectra of collected char residues were recorded in a SPEX-1403 laser Raman spectrometer (SPEX Co., USA) in a wavenumber range from 500 to 2000 cm–1. Wash durability was investigated according to AATCC test method 61 (2A)-1996 in the presence of non-ionic detergent in an ambient condition at 38 ± 3 °C. The flame retardant performance of washed fabric samples was measured via the vertical burning test after 5 and 10 laundering cycles.
Results and Discussion
Surface Characterization
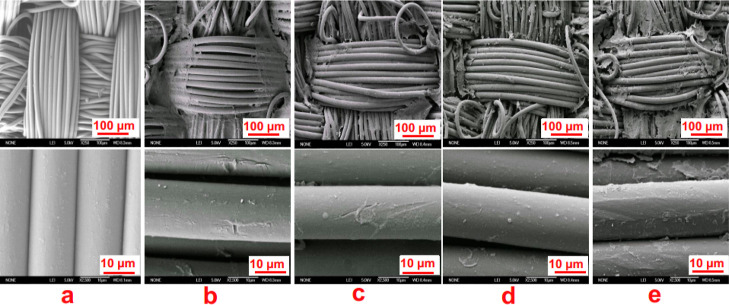
The surface morphological images of PA66 fabric samples are shown in Figure 1. From the images, it is revealed that the pure PA 66 exhibits a smooth and clean surface (see Figure 1a). Meanwhile, with the deposition of applied polyelectrolytes in a LbL fashion, the surface roughness gradually been visible for all the coated fabric samples. It is also observed that a kind of thin film is grown onto the only LbL deposited fabric sample (see Figure 1b), which is extended over the surface and cover a large portion of it. However, with the introduction of metal salts and their subsequent cross-linking converts the regular appearance of the surfaces into a broken and rough one (see Figure 1c–e). In addition, some sort clogging between adjacent fibers is also noticed, which in turn confirms the deposition of metal and metalloid compounds and their interactions with the previously deposited polyelectrolytes.
Figure 1.
SEM images of PA66-Control (a), PA66-10BL (b), PA66-10BL-NaB (c), PA66-10BL-Ni2+, (d) and PA66-10BL-Fe3+ (e) under different magnifications.
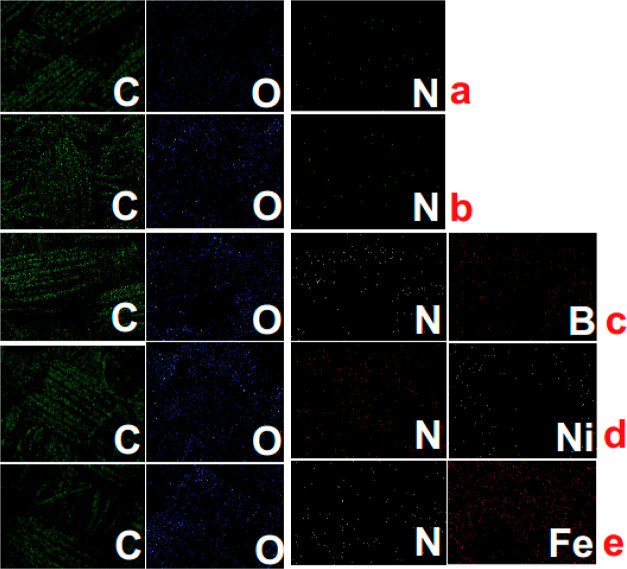
To further characterize the surface properties, the energy-dispersive X-ray analysis (EDX) is done and the corresponding images and atomic % available from the elemental mapping of some selected elements, namely, C, O, N, B, Ni, and Fe, are presented in Figure 2 and Table 2, respectively. First, from the images, it is obviously seen that the selected elements are evenly distributed both onto the only LbL assembled and LbL assembled-metal ion cross-linked fabric samples. It is also interesting to observe that the Fe element is densely packed onto the fabric surface (see Figure 2e) compared to the fabric samples treated with a B or Ni salt, which is also evident from the weight gain %, SEM image and the corresponding element % of this fabric sample. Meanwhile, the dissimilar atomic % of PA66 fabric samples as noted in Table 2 also indicates the successful deposition of desired compounds. In addition, from Table 2, it is also noticed that among three different metal salts, the Fe-salt is deposited and absorbed onto the fabric surface in a higher proportion. This is due to the higher valence of the Fe ion, which helps the Fe ion to interact with the applied polyelectrolytes in a great way and thus, maximize the rate of deposition of the same.
Figure 2.
EDX mapping of PA66-Control (a), PA66-10BL (b), PA66-10BL-NaB (c), PA66-10BL-Ni2+ (d), and PA66-10BL-Fe3+ (e).
Table 2. Atomic Percentage From EDS Test.
| sample | C | O | N | B | Ni | Fe |
|---|---|---|---|---|---|---|
| PA66-Control | 76.04 | 16.24 | 7.18 | |||
| PA66-10BL | 68.11 | 23.10 | 8.54 | |||
| PA66-10BL-NaB | 62.75 | 24.48 | 11.12 | 1.02 | ||
| PA66-10BL-Ni2+ | 68.61 | 22.32 | 8.13 | 0.53 | ||
| PA66-10BL-Fe3+ | 64.04 | 21.60 | 10.93 | 2.09 |
Flame Retardant Properties
LOI values of the PA66 fabric samples are presented in Table 3. It is seen that the LBL-deposited fabric sample as well as the B and Ni salt-treated fabric samples exhibit no significant changes in LOI value than that of pure PA66. Among the treated fabric samples, only the Fe-salt treated fabric sample (i.e., PA66-10BL-Fe3+) reveals a substantial escalation in LOI value by 25.5%.
Table 3. LOI and Vertical Burning Test Data of PA66 Fabric Samples.
| sample | LOI (%) | after flame time (s) | after glow time (s) | char length (cm) | dripping | cotton ignition |
|---|---|---|---|---|---|---|
| PA66-Control | 19.5 | 11 ± 9 | 0 | 10 | yes | yes |
| PA66-10BL | 20.0 | 47 ± 5 | 0 | 30 | yes | yes |
| PA66-10BL-NaB | 20.5 | 41 ± 7 | 0 | 30 | no | no |
| PA66-10BL-Ni2+ | 20.0 | 50 ± 3 | 0 | 30 | no | no |
| PA66-10BL-Fe3+ | 25.5 | 18 ± 6 | 0 | 30 | no | no |
The vertical burning test data are also listed in Table 3. It can be seen that the pure PA66 drips vigorously and even the LbL deposition of CS-SA cannot stop this dripping (i.e., PA66-10BL fabric sample). However, the introduction of cross-linking in the deposition process using metalloid (i.e., boron) and metal ions catalyze the burning process and thus no melting tendency is observed. The presence of metal and metalloid salts accelerates the degradation process, where the coated materials decompose ahead of PA66 fabric, resulting in the formation of char residues to support the molten. During the burning process, the char layer inhibits the transmission of heat, energy, and O2 between flame and PA66 fabric and retards the flowing of the melting PA66 and decomposed components. Thus, the treated fabric samples attain a longer burning time than that of the pure fabric; therefore, both the flame retardant and anti-dripping properties have been improved for the coated fabrics.
The combustion properties of pure and treated PA66 fabric samples are measured using the cone calorimetry test. The corresponding test data are summarized in Table 4 and the obtained heat release rate (HRR) curves are presented in Figure 3. From Table 4, it is observed that all the treated fabric samples show a lower time to ignition (TTI) value compared to that of the control PA66. This is primarily due to the earlier decomposition of applied coating ingredients, which also complies with the lower initial decomposition temperature as appeared in TGA curves. Meanwhile, from Table 4 and Figure 3, it is seen that all the treated fabric samples have a lower peak heat release rate (pHRR) value compared to that of pure PA66. Here, the only LbL assembled fabric sample (i.e., PA66-10BL) exhibits a marginal reduction in pHRR by ca. 22%. This is because the applied polyelectrolytes, being a polysaccharide-based carbon source, tend to decompose ahead of PA66 and thus, in turn, forms a protective carbon layer onto the polymer surface to hinder the permeation of combustible volatiles into the underneath of textile substrate and, subsequently, hands a reduced rate of heat release. Meanwhile, the inclusion of metal salts into the above formulation further escalates the reduction % in pHRR. All three different types of metal salts reveal a higher reduction % compared to the only LbL-treated fabric, and among them, the Fe-salt treated fabric sample (i.e., PA66-10BL-Fe3+) exhibits a significant reduction in pHRR by 34%. This is because the co-presence of carbon sources and metal ions in a typical formulation provides a synergistic effect to offer better flame retardant performance via enhancing char formation ability, which is also evident from the obtained char yield % in TG analysis. Here, metal ions usually generate metal oxides or metal carbonates during the combustion process and lay around the fiber surfaces to form a protective layer to safeguard them from the combustion source. Meanwhile, among the metal salt treated fabric sample, the Fe-salt treated fabric sample (i.e., PA66-10BL-Fe3+) exhibits better performance as it can effectively catalyze the carbon sources (i.e., CS and SA) to produce more noncombustible gases (i.e., NH3, CO2, and so forth) and less gaseous compounds (i.e., hydrocarbons) to release a lower amount of heat,12 which is also evident from the TG-IR analysis. In addition, the total heat release (THR) value of all the treated fabric samples is lower than that of the pristine PA66 where a maximum reduction in THR by ca. 22% is realized. Thus, the applied finishing seems to be effective to impart better flame retardant properties to PA66 textiles.
Table 4. Cone Calorimetry Test Data of PA66 Fabric Samples.
| sample | TTI (s) | pHRR (kW/m2) | THR (MJ/m2) | % reduction of pHRR |
|---|---|---|---|---|
| PA66-Control | 32 ± 5 | 249 ± 11 | 11.8 ± 0.9 | |
| PA66-10BL | 16 ± 3 | 193 ± 13 | 9.2 ± 0.7 | 22 |
| PA66-10BL-NaB | 20 ± 5 | 185 ± 10 | 10.4 ± 0.3 | 26 |
| PA66-10BL-Ni2+ | 11 ± 2 | 188 ± 7 | 9.3 ± 0.5 | 24 |
| PA66-10BL-Fe3+ | 16 ± 3 | 164 ± 9 | 11.3 ± 0.5 | 34 |
Figure 3.
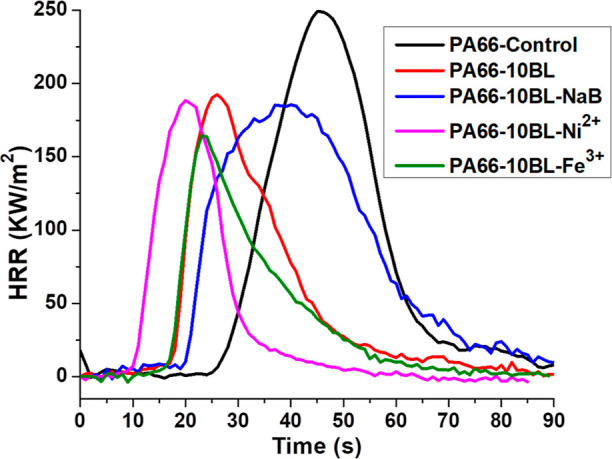
HRR curves of PA66 fabric samples.
Thermal Properties
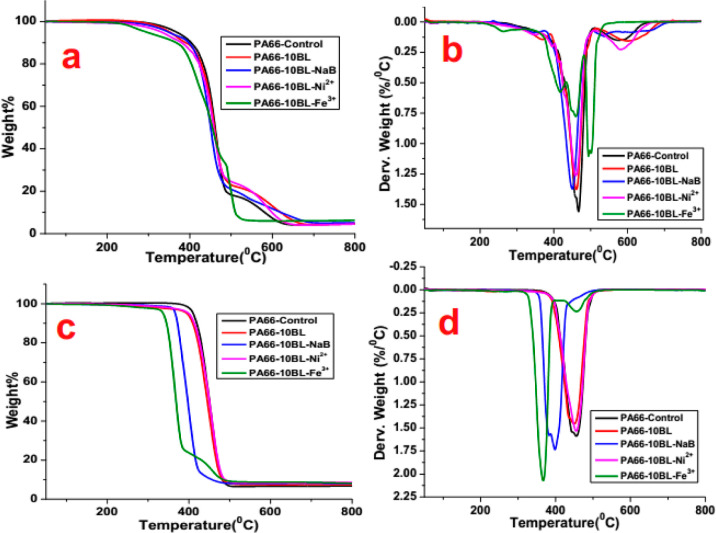
TGA shows the thermal degradation behaviors of the virgin and coated PA66 fabrics in different atmospheres. The experimental TGA and DTG curves of pure and treated PA66 fabrics under air and nitrogen atmosphere are shown in Figure 4, and the corresponding data are summarized in Table 5. The control PA66 exhibits two apparent degradation steps: the first step of degradation is assigned to the main chain breakdown, releasing water, NH3, CO2, hydrocarbon fragments, and CO, while the second weight loss was attributed to the thermo-oxidative degradation of the residues.15 However, all the coated PA66 fabric samples show a lower initial degradation temperature compared to the control PA66 fabric both in the air and N2 atmosphere, which is mostly due to the earlier decomposition of the coating materials. For, CS-SA treated fabrics, lower initial decomposition temperature is due to the earlier decomposition of CS (i.e., 250 °C) and SA (i.e., 190 °C) than the pristine PA66 (around 340 °C).16−18 Next, the carbon sources (CS and SA) are converted into char by a dehydration reaction at a higher temperature range and this char is then expanded by gases produced by the decomposition of CS; it also serves as a blowing agent.
Figure 4.
TGA and DTG [(a,b) under air and (c,d) under nitrogen] curves of PA66 fabric samples.
Table 5. TGA Data of PA66 Fabric Samples.
| temperature at specific weight loss (°C) |
residue at 800 °C (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
|
T–5% |
Tmax1 |
Tmax2 |
Tmax3 |
|||||
| sample | air | N2 | air | N2 | air | N2 | air | N2 |
| PA66-Control | 368 | 411 | 467 | 456 | 576 | 4.6 | 6.7 | |
| PA66-10BL | 352 | 395 | 461 | 450 | 601 | 4.9 | 7.5 | |
| PA66-10BL-NaB | 349 | 367 | 450 | 398 | 536 | 5.2 | 8.0 | |
| PA66-10BL-Ni2+ | 332 | 400 | 460 | 455 | 581 | 4.5 | 8.6 | |
| PA66-10BL-Fe3+ | 296 | 337 | 417 | 367 | 459 | 494 | 6.3 | 8.2 |
From the TGA curves, it can also be seen that the T–5% values of the borate cross-linked CS-SA deposited fabric sample (i.e., PA66-10BL-NaB) is further decreased with the inclusion of boron compounds. Furthermore, a rapid boost up in char yield % is observed, which is due to the generation of thermally stable inorganic boron-containing products. It is assumed that the boron-bearing moieties in the char layers exhibit excellent thermo-oxidative stability at an elevated temperature range to stabilize the residues and thus, an increased char yield % is realized. Among the metal ion cross-linked PA66 fabric samples, Fe ion cross-linked fabric sample (PA66-10BL-Fe3+) obtains the lowest initial decomposition temperature (i.e., 296 °C in the air and 337 °C in N2), while the nickel ion cross-linked fabric sample shows an initial decomposition of 332 °C in air and 400 °C in a N2 atmosphere. It is believed that the addition of metal ions accelerates the thermal degradation of alginate and the PA66 fabrics and changes the thermal degradation mechanism. This lower decomposition temperature is favorable for char formation to initiate the early decomposition, thus preventing further pyrolysis of the PA66, which in turn retards the flame spread. In an air atmosphere, the final char residue of nickel alginate treated fabric (avg. 4.5% in air for PA66-10BL-Ni2+) is inferior to the iron alginate fabric (6.3% in air for PA66-10BL-Fe3+), which may be due to the poor thermal stability of nickel alginate at a higher temperature. However, the nickel alginate coating shows much better thermal stability in a N2 atmosphere, which is evident from its higher char yield % (avg. 8.6% in N2 for PA66-10BL-Ni2+).
From the DTG curves, it can be seen that the temperatures at the main decomposition peak for all the coated fabric samples are lower than that of the control PA66. Moreover, the corresponding maximum mass loss rate of the coated fabric samples is also reduced than that of the control sample. This behavior is in agreement with the condensed-phase flame retardant mechanism: the CS-SA coatings form an insulating protective layer that inhibits the heat permeation and slows down the mass loss rate and thereby improved the resistance against thermal decomposition.19 However, the main loss happens in three different stages for the iron alginate cross-linked fabric sample (i.e., PA66-10BL-Fe3+) in an air atmosphere as it begins to degrade slowly with a quite lower thermal degradation rate compared to other coated fabrics. The appearance of Tmax3 both in TGA and DTG curves for the PA66-10BL-Fe3+ fabric sample is probably due to the strong oxidative nature of Fe ion, and thus, the generated residues of this fabric sample follow several oxidative degradation steps in an air atmosphere with the increase of temperature. Here, the weight loss is ascribed to the intensive evolution of small gas molecules and the thermal degradation of alginate with the fracture of glycosidic bonds, releasing incombustible gases such as CO2 and H2O. Then, the formed intermediate compounds further decompose to produce a more stable intermediate product at a slower rate of decomposition with the increase of temperature, and thus, the amount of char residue has increased in the air atmosphere. These results indicate that the addition of boron, nickel, and iron salts accelerates the thermal degradation of PA66 fabrics to form stable char residues and decrease the degradation rate of PA66.
Analysis of Char Residues
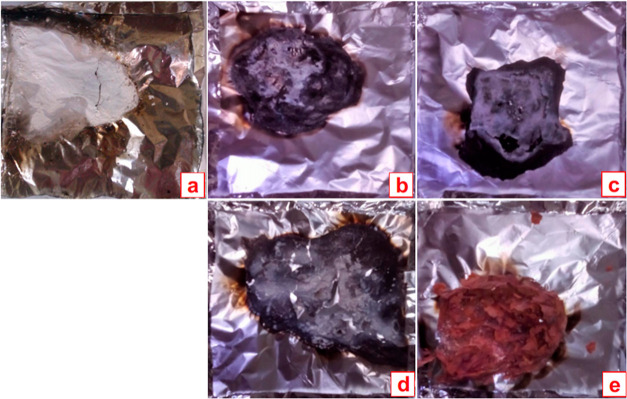
The digital images of char residues collected after the cone test are presented in Figure 5. From these images, it is observed that the pure PA66 produces a kind of white residue, indicating a fewer extent of carbon species. Meanwhile, for the treated fabric samples, carbon-rich residues are realized. In line, some sort of holes and cracks are found in the char residues of PA66-10BL, PA66-10BL-NaB, and PA66-10BL-Ni2+ fabric samples (see Figure 5b–d), whereas char residues of the Fe-salt-treated fabric sample becomes reddish in color and a kind of coherent and intact residues are formed (see Figure 5e). In turn, this quality residues form a protective layer onto the textile surfaces to offer superior flame retardant performance via providing a strong barrier effect,20 which is also realized from the improved LOI value and lowered pHRR, THR found in the earlier section.
Figure 5.
Digital images of char residues of PA66-Control (a), PA66-10BL (b), PA66-10BL-NaB (c), PA66-10BL-Ni2+ (d), and PA66-10BL-Fe3+ (e) after the cone test.
To support/validate the possible flame retardant mechanism as discussed earlier, the Raman spectroscopy test is carried out and the obtained char residues are further analyzed. Here, the corresponding graphitization degree as presented in Figure 6 indicates the quality of char residues. From Figure 6, it is seen that among the treated fabric samples, the char residues of the Fe-salt modified fabric sample (i.e., PA66-10BL-Fe3+) exhibits the lowest ID/IG value; signifying a good char structure with few defects. This can be explained that the metal ion acts as a catalyst in this system to induce the cross-linking mechanism to form compact and better quality char residues,4 which is also evident from the digital image of this char residue and this lowest value of ID/IG compared to other metal-salt-treated fabric samples is probably connected to its higher valence and its better cross-linking efficacy. Thus, the improved charring of these treated fabric samples evident from the TG analysis as well as their corresponding images and relevant ID/IG value obtained from Raman spectroscopy reveal the possible condensed phase activity of this applied finishing.
Figure 6.
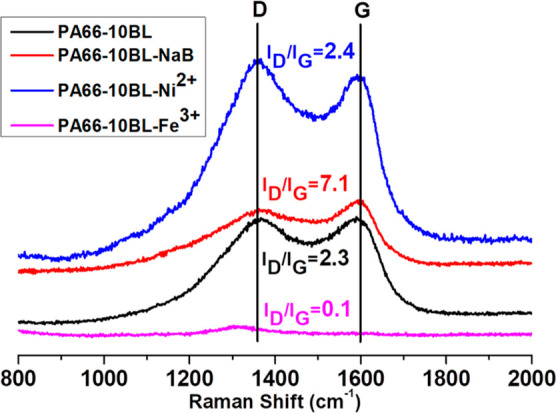
Digital images of char residues of PA66 fabric samples.
In this system, metal ions act as a catalyst to initiate the dehydration reaction of applied polysaccharides and carbon sources, namely, CS and alginate and catalyze the char formation of the same. Meanwhile, metal ions also offer cross-linked char residues with a high-quality char layer, which ultimately imparts a more effective barrier effect to protect the underneath intact polymer. Thus, it is further perceived that this applied finishing dominantly works in the condensed phase mechanism via char formation.
Wash Durability and Flame Retardant Performance
The wash durability of as prepared coatings against home laundering as well as their flame retardant performance after the wash process are measured in a vertical burning test. The related test data are noted in Table 6 and it is revealed that the fabric sample treated solely with CS and SA (i.e., PA66-10BL) in the LbL assembly shows poor resistance in laundering and cannot retain its anti-dripping properties just after five wash cycles; indicating lesser stability of ionic assembled LbL coatings in the alkaline detergent. At this point, boron salt-treated fabric sample also follows the similar trend. Meanwhile, after the treatment of LbL-assembled coatings with Ni and Fe metal salts, this scenario changes a lot. The Ni-salt-treated fabric sample (i.e., PA66-10BL-Ni2+) withstands up to five wash cycles, while the Fe-salt-treated fabric sample (i.e., PA66-10 BL-Fe3+) can retain its anti-dripping performance even after 10 wash cycles. This phenomenon indicates that the metal-ion-induced cross-linking among the polyelectrolytes imparts a substantial coating stability in the wash process. Nonetheless, it is also perceived that the Fe-ion-induced cross-linking exhibits improved resistance in the laundering, which may be connected to its higher valence (i.e., trivalent) offering superior cross-linking density. Thus, it is realized that this kind of metal-ion based cross-linking can surely imply better stability of LbL-assembled coatings and also can offer durable flame retardancy to the textile substrates.
Table 6. Vertical Burning Test Data of PA66 Fabric Samples before and after Washing.
| sample | extent of dripping before washing | extent of dripping after 5 times washing | extent of dripping after 10 times washing |
|---|---|---|---|
| PA66-Control | yes | Yes | yes |
| PA66-10BL | yes | Yes | yes |
| PA66-10BL-NaB | no | Yes | yes |
| PA66-10BL-Ni2+ | no | No | yes |
| PA66-10BL-Fe3+ | no | No | no |
Conclusions
In this work, a kind of hybrid coating composed of organic bio-derived polysaccharides and inorganic metal salts was constructed in imparting flame retardant properties to the polyamide 66 (PA66) textiles. Here, the CS and SA were deposited onto the PA66 fabric surfaces in a LbL assembly to construct the primary coating. Later, this coating was impregnated in a good number of metal salt solutions to further modify the coating properties, especially to intensify the flame retardant performance and to strengthen the coating structure. Obtained results indicated that the metal-salt-treated fabric samples exhibited superior flame retardant performance compared to the only LbL deposited fabric samples in a varied proportion. For example, the fabric sample simultaneously LbL treated and Fe-salt modified attained a maximum escalation in the LOI value by 25.5%. Meanwhile, the metal-salt modification could improve the anti-dripping performance irrespective of their types, structure, and valence. Among the treated fabric samples, the CS–SA–Fe-salt modified fabric sample exhibited a maximum reduction in pHRR by 34% and produced improved and higher quality char residues. Moreover, the metal salt-induced cross-linking enhanced the coating stability as the Fe-salt treated fabric sample could retain anti-dripping properties even up to 10 laundering cycles. Thus, it is perceived that this kind of hybridization between bio-derived organic polysaccharides and inorganic metal salts is quite effective in improving the fire performance of PA66 textiles and thus could be extended to other synthetic textiles as well..
Acknowledgments
The authors declare no funding for this work.
The authors declare no competing financial interest.
References
- Zhang Y.; Xiong Z.; Ge H.; Ni L.; Zhang T.; Huo S.; Song P.; Fang Z. Core-Shell Bioderived Flame Retardants Based on Chitosan/Alginate Coated Ammonia Polyphosphate for Enhancing Flame Retardancy of Polylactic Acid. ACS Sustainable Chem. Eng. 2020, 8, 6402–6412. 10.1021/acssuschemeng.0c00634. [DOI] [Google Scholar]
- Xiong Z.; Zhang Y.; Du X.; Song P.; Fang Z. Green and Scalable Fabrication of Core–Shell Biobased Flame Retardants for Reducing Flammability of Polylactic Acid. ACS Sustainable Chem. Eng. 2019, 7, 8954–8963. 10.1021/acssuschemeng.9b01016. [DOI] [Google Scholar]
- Liu J.; Xiao C. Fire-retardant multilayer assembled on polyester fabric from water soluble chitosan, sodium alginate and divalent metal ion. Int. J. Biol. Macromol. 2018, 119, 1083–1089. 10.1016/j.ijbiomac.2018.08.043. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Ma Z.; Leng Q.; Wang Y. Eco-Friendly Flame Retardant Coating Deposited on Cotton Fabrics from Bio-Based Chitosan, Phytic Acid and Divalent Metal Ions. Int. J. Biol. Macromol. 2019, 140, 303–310. 10.1016/j.ijbiomac.2019.08.049. [DOI] [PubMed] [Google Scholar]
- Pan Y.; Wang W.; Liu L.; Ge H.; Song L.; Hu Y. Influences of Metal Ions Crosslinked Alginate Based Coatings on Thermal Stability and Fire Resistance of Cotton Fabrics. Carbohydr. Polym. 2017, 170, 133–139. 10.1016/j.carbpol.2017.04.065. [DOI] [PubMed] [Google Scholar]
- Kundu C. K.; Wang W.; Zhou S.; Wang X.; Sheng H.; Pan Y.; Song L.; Hu Y. A Green Approach to Constructing Multilayered Nanocoating for Flame Retardant Treatment of Polyamide 66 Fabric from Chitosan and Sodium Alginate. Carbohydr. Polym. 2017, 166, 131–138. 10.1016/j.carbpol.2017.02.084. [DOI] [PubMed] [Google Scholar]
- Pan Y.; Liu L.; Zhao H. Recyclable Flame Retardant Paper Made from Layer-by-Layer Assembly of Zinc Coordinated Multi-Layered Coatings. Cellulose 2018, 25, 5309–5321. 10.1007/s10570-018-1922-0. [DOI] [Google Scholar]
- Zhang Q.; Zhang W.; Geng C.; Xue Z.; Xia Y.; Qin Y.; Zhang G. Study on the Preparation and Flame Retardant Properties of an Eco-Friendly Potassium-Calcium Carrageenan Fiber. Carbohydr. Polym. 2019, 206, 420–427. 10.1016/j.carbpol.2018.10.058. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Zhao J.-C.; Zhang C.-J.; Guo Y.; Zhu P.; Wang D.-Y. Effect of Manganese and Cobalt Ions on Flame Retardancy and Thermal Degradation of Bio-Based Alginate Films. J. Mater. Sci. 2016, 51, 1052–1065. 10.1007/s10853-015-9435-9. [DOI] [Google Scholar]
- Kundu C. K.; Wang X.; Song L.; Hu Y. Borate Cross-Linked Layer-by-Layer Assembly of Green Polyelectrolytes on Polyamide 66 Fabrics for Flame-Retardant Treatment. Prog. Org. Coat. 2018, 121, 173–181. 10.1016/j.porgcoat.2018.04.031. [DOI] [Google Scholar]
- Liu Y.; Zhang C.-J.; Zhao J.-C.; Guo Y.; Zhu P.; Wang D.-Y. Bio-Based Barium Alginate Film: Preparation, Flame Retardancy and Thermal Degradation Behavior. Carbohydr. Polym. 2016, 139, 106–114. 10.1016/j.carbpol.2015.12.044. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Zhao J.-C.; Zhang C.-J.; Guo Y.; Cui L.; Zhu P.; Wang D.-Y. Bio-Based Nickel Alginate and Copper Alginate Films with Excellent Flame Retardancy: Preparation, Flammability and Thermal Degradation Behavior. RSC Adv. 2015, 5, 64125–64137. 10.1039/c5ra11048c. [DOI] [Google Scholar]
- Zhang J.; Ji Q.; Wang F.; Tan L.; Xia Y. Effects of Divalent Metal Ions on the Flame Retardancy and Pyrolysis Products of Alginate Fibres. Polym. Degrad. Stab. 2012, 97, 1034–1040. 10.1016/j.polymdegradstab.2012.03.004. [DOI] [Google Scholar]
- Liu Y.; Wang J.-S.; Zhu P.; Zhao J.-C.; Zhang C.-J.; Guo Y.; Cui L. Thermal Degradation Properties of Biobased Iron Alginate Film. J. Anal. Appl. Pyrolysis 2016, 119, 87–96. 10.1016/j.jaap.2016.03.014. [DOI] [Google Scholar]
- Siat C.; Bourbigot S.; Le Bras M. Thermal Behaviour of Polyamide-6-Based Intumescent Formulations—a Kinetic Study. Polym. Degrad. Stab. 1997, 58, 303–313. 10.1016/s0141-3910(97)00066-9. [DOI] [Google Scholar]
- Beyler C. L.; Hirschler M. M.. Thermal Decomposition of Polymers. SFPE Handbook of Fire Protection Engineering; National Fire Protection Association Quincy: MA, 2002; Chapter 7, Vol. 2, pp 111–131. [Google Scholar]
- Reda S. Y. Evaluation of Antioxidants Stability by Thermal Analysis and Its Protective Effect in Heated Edible Vegetable Oil. Food Sci. Technol. 2011, 31, 475–480. 10.1590/s0101-20612011000200030. [DOI] [Google Scholar]
- Liu Y.; Zhao J.-C.; Zhang C.-J.; Cui L.; Guo Y.; Zhu P.; Zhang H.; Zheng Z.-W.; wang D.-Y. Flame Retardancy and Thermal Degradation Properties of Cotton/Alginate Fabric. J. Therm. Anal. Calorim. 2017, 127, 1543–1551. 10.1007/s10973-016-5418-6. [DOI] [Google Scholar]
- Wang X.; Song L.; Yang H.; Xing W.; Kandola B.; Hu Y. Simultaneous Reduction and Surface Functionalization of Graphene Oxide with POSS for Reducing Fire Hazards in Epoxy Composites. J. Mater. Chem. 2012, 22, 22037–22043. 10.1039/c2jm35479a. [DOI] [Google Scholar]
- Chu F.; Ma C.; Zhang T.; Xu Z.; Mu X.; Cai W.; Zhou X.; Ma S.; Zhou Y.; Hu W.; et al. Renewable Vanillin-Based Flame Retardant Toughening Agent with Ultra-Low Phosphorus Loading for the Fabrication of High-Performance Epoxy Thermoset. Composites, Part B 2020, 190, 107925. 10.1016/j.compositesb.2020.107925. [DOI] [Google Scholar]