Abstract

Artesunate is a widely used drug in clinical treatment of malaria. The aim of this study was to investigate the therapeutic mechanism of artesunate on malaria using an integrated strategy of network pharmacology and serum metabolomics. The mice models of malaria were established using 2 × 107 red blood cells infected with Plasmodium berghei ANKA injection. Giemsa and hematoxylin-eosin (HE) staining were used to evaluate the efficacy of artesunate on malaria. Next, network pharmacology analysis was applied to identify target genes. Then, a metabolomics strategy has been developed to find the possible significant serum metabolites and metabolic pathways induced by artesunate. Additionally, two parts of the results were integrated to confirm each other. Giemsa and HE staining results showed that artesunate significantly inhibited the proliferation of Plasmodium and reduced liver and spleen inflammation. Based on metabolomics, 18 differential endogenous metabolites were identified as potential biomarkers related to the artesunate for treating malaria. These metabolites were mainly involved in the relevant pathways of biosynthesis of unsaturated fatty acids; aminoacyl-tRNA biosynthesis; valine, leucine, and isoleucine biosynthesis; and phenylalanine, tyrosine, and tryptophan biosynthesis. The results of the network pharmacology analysis showed 125 potential target genes related to the treatment of malaria with artesunate. The functional enrichment was mainly associated with lipid and atherosclerosis; pathways of prostate cancer and proteoglycans in cancer; and PI3K-Akt, apoptosis, NF-κB, Th17 cell, and AGE-RAGE signaling pathways. These findings were partly consistent with the findings of the metabolism. Our results further suggested that artesunate could correct the inflammatory response caused by malaria through Th17 cell and NF-κB pathways. Meanwhile, our work revealed that cholesterol needed by Plasmodium berghei came directly from serum. Cholesterol and palmitic acid may be essential in the growth and reproduction of Plasmodium berghei. In summary, artesunate may have an effect on anti-malarial properties through multiple targets.
1. Introduction
The World Malaria Report 2021 issued by the World Health Organization (WHO) pointed out that the number of malaria cases increased to 2.41 million in 2020. Since 2000, the number of deaths caused by malaria has decreased year by year, although the number of deaths has increased in 2020.1 Clearing malaria is still a great challenge. Malaria is an insect-borne infectious disease caused by Anopheles carrying Plasmodium (P.) biting or inputting the blood carrying P. There are two hosts during P. life: Anopheles and vertebrates. The stage of asexual reproduction in spinal animals is divided into two stages: intrahepatic phase and erythrocyte phase.2,3 The metabolism of P. in the red phase is accelerated by the continuous consumption of hemoglobin to provide nutrients for their own division and proliferation. When P. develops into mature schizonts, a large number of schizonts are released from hepatocytes.4 The proliferation of P. and the destruction of red blood cells caused a series of clinical symptoms, such as fever, anemia, and so forth.5,6
There are five main kinds of P. that infect humans: Plasmodium falciparum, Plasmodium vivax, Plasmodium ovum, P. vivax, and Plasmodium knowlesi.7 There are two main species which infect rodents: Plasmodium berghei and Plasmodium yoelii.4 The structure, physiology, and life cycle of Plasmodium. berghei ANKA (Pb) are similar to those of P. in primates.8 Pb is often used to establish the model of mice malaria in the laboratory.
Artemisinin and its derivatives are the most important class of anti-malarial drugs.9 Artesunate is a semi-synthetic derivative of artemisinin (Figure 1a).10 The mechanism of artemisinin and its derivatives, the most potent of the anti-malarial drugs, is not completely understood.11 Most scholars believed that artesunate killed P. via the cleavage of their endoperoxide bridge by heme or iron.12 There were some studies on specific targets of malaria. Wang et al., showed that heme was predominantly responsible for artemisinin activation. When artemisinin drugs were activated by heme, many proteins of P. were misfolded, resulting in irreversible protein damage.13 In addition, artemisinin drugs could destroy multiple organelles such as mitochondria, endoplasmic reticulum, and digestive vacuoles of P.(14−16) However, the mechanism of artesunate in anti-malarial activity is not completely clear. Thus, artemisinin drugs may act on multiple organs and multiple targets to produce anti-malarial effects. However, the mechanism underlying the parasite’s infection is largely unknown.
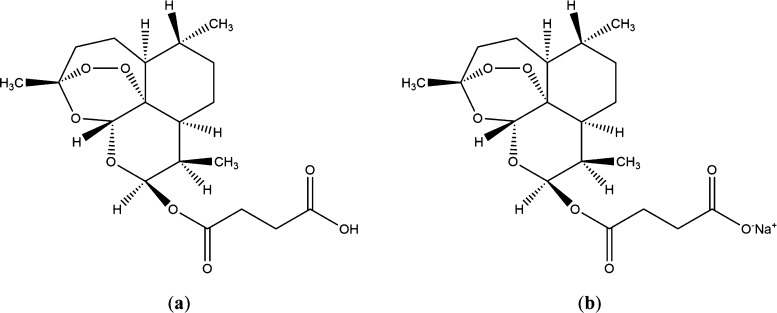
Figure 1.
Artesunate structure (a); SA structure (b).
Our study used sodium artesunate (SA), which is the sodium salt form of artesunate (Figure 1b), and SA has better solubility than artesunate. Giemsa and hematoxylin and eosin (HE) staining were used to evaluate the efficacy of SA on malaria. Then, the network pharmacology was utilized to elucidate its molecular mechanisms in malaria and to construct the key “component–targets–pathways” network. In addition, the metabolomic was applied to study changes in endogenous substances induced by treatment with artesunate so that experimental evidence related to the metabolic mechanism of artesunate in treating Pb could be provided. This paper revealed the potential complex relationship between multiple metabolites and multiple targets by studying the effects of artesunate on endogenous metabolites and metabolic pathways in infected mice.
2. Results
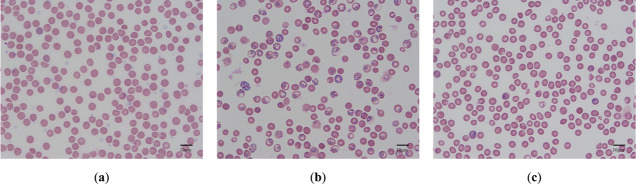
2.1. Giemsa Staining and Infection Rate
According to the results of Giemsa staining (Figure 2), it was observed that the model group contained late trophozoites and mass ring forms. In the model group, it was observed that the red blood cell structure was fragile, and the infection rate was 78.44 ± 12.64%. The percentage parasitemia of the SA group was significantly lower than that of the model group, and the infection rate was 7.82 ± 4.12%. The SA group contained a small amount of ring forms.
Figure 2.
Red blood cell Giemsa staining in the blank group (a), model group (b), and SA group (c).
2.2. Histopathological
Three groups’ mice showed a significant difference in color and shape of liver and spleen (Figure 3). The hepatomegaly and spleen of the model group for 5 days were enlarged, and the color of liver and spleen deepened. The organs were structurally fragile and easy to break during the experimental operation. However, the above symptoms were alleviated in the SA group.
Figure 3.

Liver and spleen morphology of Pb in the blank group (a), model group (b), and SA group (c).
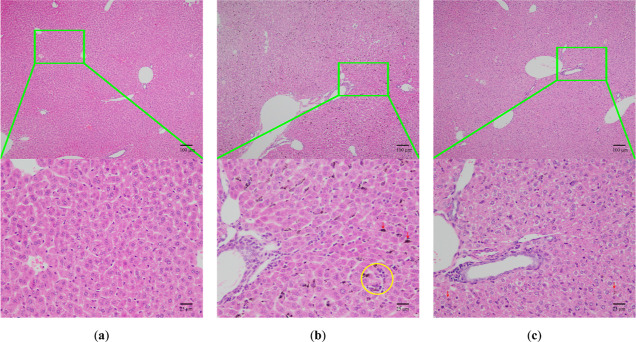
Inflammation and necrotic lesions (yellow circle) (Figure 4) were observed in the livers of model group mice. Furthermore, the liver cells with uneven sizes, extensive swelling, and loose tissues of hepatocytes were seen in the livers of the model group, and malarial pigment deposition was found in the livers of malarial mice (red arrow). However, all symptoms were relieved in the SA group.
Figure 4.
Morphology of the liver tissue of the blank group (a), model group (b), and SA group (c) infected by HE (100×, 400×). Red arrow: Pb remnant; yellow circle: inflammatory infiltration.
The HE staining (Figure 5) of the spleen tissue showed a clear distinction between the red pulp and white pulp. Severe hyperemia and enlargement of the red pulp were observed in the model group. The structure of the white pulp was destroyed, and the clear marginal zones surrounding follicles became inapparent in spleens of infected mice. Furthermore, extensive vacuolation in the red pulp was acquired in spleens of model group mice (yellow circle). The malarial pigment deposition was observed in the livers of model mice on day 5 after infection (red arrow). However, all symptoms were relieved in the SA group.
Figure 5.
Morphology of the spleen tissue of the blank group (a), model group (b), and SA group (c) infected by HE (100×, 400×). Red arrow: Pb remnant; yellow circles: vacuolated lesions.
2.3. Metabolomics
2.3.1. Multivariable Data Analysis
Figure 6 shows the typical ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) total ion current chromatogram of serum samples. Meanwhile, quality control (QC) samples could cluster together, indicating that the instrument was stable (Figure 7). PCA and PLS-DA (ESI+: R2 = 0.0, 0.995, Q2 = 0.0, −0.0985; ESI–: R2 = 0.0, 0.677, Q2 = −0.503) showed that the blank group, model group, and SA group were separated from each other and reflected the regulatory effect of SA on metabolites.
Figure 6.
Typical UHPLC-MS/MS total ion currents of serum samples. Black and blue indicate the total ion flow diagrams of the blank group; red and yellow indicate the total ion flow diagrams of the model group; green and purple indicate the total ion flow diagrams of the SA group.
Figure 7.
Results of multivariate statistical analysis. The positive ion mode (a); the negative ion mode (b).
2.3.2. Identification of Differential Biomarkers
VIP was used to identify characteristic metabolites in the three groups, namely, ions with VIPs > 1.0, P < 0.05, and FC > 1.5 or FC < 0.67, which were considered potential biomarkers. In the positive ion mode, eight differential endogenous metabolites were putatively identified among the blank group, model group, and SA group. Similarly, 11 metabolites were identified in the negative ion mode as differential metabolites among three groups. Detailed information regarding each differential metabolite is provided in Table 1.
Table 1. Differential Metabolites Characterized in the Serum Profile and Their Change Trend after SA Treatment.
| no. | RT | metabolite identification | HMDB | formula | m/z | ion from | P (SAb/Mc) | SA/M | P (M/Bd) | M/B |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 11.060 | 11,12-epoxyeicosatrienoic acid | HMDB0004673 | C20H32O3 | 319.2279 | [M – H]− | 1.40 × 10–1 | 2.04 × 10–2 | ↓ | |
| 2 | 0.849 | l-histidine | HMDB0000177 | C6H9N3O2 | 154.2000 | [M – H]− | 1.84 × 10–10 | ↓ | 3.25 × 10–12 | ↑ |
| 3 | 10.299 | palmitic acid | HMDB0000220 | C16H32O2 | 256.2350 | [M – H]− | 3.61 × 10–2 | ↑a | 4.88 × 10–2 | ↓ |
| 4 | 12.430 | stearic acid | HMDB0000827 | C18H36O2 | 283.2647 | [M – H]− | 3.98 × 10–2 | ↑ | 3.60 × 10–2 | ↓ |
| 5 | 17.930 | linoleic acid | HMDB0000673 | C18H32O2 | 279.4000 | [M – H]− | 4.39 × 10–2 | ↑ | 4.66 × 10–2 | ↓ |
| 6 | 8.660 | tetradecanedioic acid | HMDB0000872 | C14H26O4 | 257.1758 | [M – H]− | 5.90 × 10–3 | ↑ | 3.26 × 10–2 | ↓ |
| 7 | 1.110 | d-leucine | HMDB0013773 | C6H13NO2 | 130.0874 | [M – H]− | 3.22 × 10–2 | ↓ | 1.59 × 10–2 | ↑ |
| 8 | 1.110 | l-leucine | HMDB0000687 | C6H13NO2 | 130.0874 | [M – H]− | 3.22 × 10–2 | ↓ | 1.59 × 10–2 | ↑ |
| 9 | 10.780 | oleic acid | HMDB0000207 | C18H34O2 | 282.2509 | [M – H]− | 8.24 × 10–4 | ↑ | 2.70 × 10–3 | |
| 10 | 1.040 | l-phenylalanine | HMDB0000159 | C9H11NO2 | 163.9000 | [M – H]− | 1.43 × 10–2 | ↓ | 1.28 × 10–3 | ↑ |
| 11 | 0.890 | l-isoleucine | HMDB0000172 | C6H13NO2 | 131.9010 | [M + H]+ | 2.99 × 10–2 | ↓ | 1.24 × 10–2 | ↑ |
| 12 | 0.903 | l-ornithine | HMDB0003374 | C5H12N2O2 | 132.9620 | [M + H]+ | 1.06 × 10–4 | ↓ | 6.79 × 10–4 | ↑ |
| 13 | 1.000 | d-proline | HMDB0003411 | C5H9NO2 | 114.0560 | [M + H]+ | 1.41 × 10–5 | ↓ | 5.87 × 10–4 | ↑ |
| 14 | 0.910 | proline betaine | HMDB0004827 | C7H13NO2 | 144.1019 | [M + H]+ | 2.60 × 10–5 | ↓ | 9.47 × 10–3 | ↑ |
| 15 | 9.770 | eicosapentaenoic acid | HMDB0001999 | C20H30O2 | 303.2319 | [M + H]+ | 1.96 × 10–2 | ↑ | 8.57 × 10–3 | ↓ |
| 16 | 7.770 | cytidine | HMDB0000089 | C9H13N3O5 | 243.9400 | [M + H]+ | 1.23 × 10–2 | ↓ | 2.32 × 10–5 | ↑ |
| 17 | 9.110 | choline | HMDB0000097 | C5H13NO | 105.5000 | [M + H]+ | 2.81 × 10–2 | ↑ | 6.68 × 10–2 | ↓ |
| 18 | 7.780 | 17-HDoHE | HMDB0010213 | C23H32O3 | 357.2424 | [M + H]+ | 1.13 × 10–1 | 2.43 × 10–2 | ↓ |
Arrow ↑ means a relative increase in signal; arrow ↓ means a relative decrease in signal.
SA means sodium artesunate.
M means the model group.
B means the blank group.
In negative ion and positive ion modes, contrary to the blank group, the integral levels of l-histidine, d-leucine, l-leucine, and l-phenylalanine were increased in the model group. Notably, the levels of these metabolites were decreased in the SA group. In contrast to the blank group, expressions of 11,12-epoxyeicosatrienoic acid, palmitic acid, stearic acid, linoleic acid, and tetradecanedioic acid were downregulated in the model group. After treatment with SA, the levels of these metabolites increased. Similarly, in the positive ion mode, the levels of l-isoleucine, d-ornithine, d-proline, proline betaine, and cytidine were increased in the model group compared with the blank group. In contrast, the integral levels of eicosapentaenoic acid and 17-HDoHE were decreased in the model group compared with the blank group. Interestingly, intervention with SA reversed these alterations induced by the model group.
The heat maps average normalized amount of differentially expressed metabolites in the three groups and showed that metabolite expression varied significantly between different groups (Figure 8).
Figure 8.
Results of multivariate statistical analysis. The positive ion mode (a); the negative ion mode (b).
2.3.3. Metabolic Pathways Analysis
A total of 18 differential endogenous metabolites were found among the three groups. They were associated mainly with fatty acids, amino acid, and phosphatide. Metabolic pathway analysis was carried out by importing these differential endogenous metabolites into MetaboAnalyst 5.0 to further explore the possible mechanisms through which there is SA treatment of malaria. We obtained 25 metabolic pathways through this analytical method (Figure 9) by setting the screening threshold as raw P < 0.05 and pathway impact value > 0.1.17 We obtained the biosynthesis of unsaturated fatty acids; aminoacyl-tRNA biosynthesis; valine, leucine, and isoleucine biosynthesis; and phenylalanine, tyrosine, and tryptophan biosynthesis as key metabolic pathways.
Figure 9.

Pathways analyses of differential metabolites in the serum sample.
2.4. Targets Prediction of Artesunate on Malaria
We retrieved the targets of artesunate from databases and related publications. Moreover, 317 predicted targets of artesunate were identified based on the obtained compounds. Matching them with the malaria-related targets acquired through OMIM and GeneCard, we finally identified 122 potential targets of artesunate related to malaria treatment. We acquired 125 pathways including lipid and atherosclerosis; pathways in cancer, prostate cancer, and proteoglycans in cancer; PI3K-Akt signaling pathway; apoptosis; Hepatitis B, IL-17 signaling pathway, AGE-RAGE signaling pathway in diabetic complications and so on (Figure 10a).
Figure 10.
Drug–targets-pathways; the red, orange, and blue nodes represent the active drug, target genes, and pathways, respectively (a). Protein–protein interaction network of artesunate acting on malaria, the ranking of artesunate target genes’ importance for treating malaria. The size and color of nodes were proportional to their degree value (b).
After extracting the relationship information for the 122 potential target genes of artesunate, a protein–protein interaction network was constructed, as shown in Figure 10b. There were some central nodes in the network, such as heat shock protein HSP 90-alpha, protooncogene tyrosine-protein kinase Src, caspase-3, epidermal growth factor receptor, and signal transducer and activator of transcription 3. These results suggested that amino acid metabolism and inflammatory genes may be the key target genes for artesunate to treatment malaria (Figure 10b).
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were undertaken to explore the potential functions of the 122 potential target genes of artesunate acting on malaria. The top 10 enriched GO terms ranked by P-value in biological processes, cell components, and molecular functions are presented in Figure 11a. The top three terms in GO biological processes were regulation of programmed cell death (GO:0043067), regulation of the apoptotic process (GO:0042981), and the apoptotic process (GO:0006915). In terms of GO cell components, they were mainly enriched in ficolin-1-rich granules (GO:0101002), ficolin-1-rich granule lumen (GO:1904813), and the cytoplasmic part (GO:0044444). Regarding GO molecular functions, the terms were catalytic activity (GO:0003824), enzyme binding (GO:0019899), and peptidase activity, acting on l-amino acid peptides (GO:0070011). GO and KEGG pathway enrichment analyses were accomplished for further investigation of the potential functions of the detected targets (P < 0.05). According to the KEGG enrichment analysis, lipid and atherosclerosis (hsa05417) and pathways in cancer (hsa05200) were significantly enriched, as shown in Table 2 and Figure 11b.
Figure 11.
GO functional analysis (a). Bubble diagram of the KEGG pathway: the abscissa corresponds to the ratio of the pathway, the ordinate represents the KEGG term, the color of the sports corresponds to the −lg P, and the size of the dots corresponds to the number of genes annotated (b).
Table 2. Main KEGG Pathways Significantly Relating to Major Hubs.
| term | pathway | count | pop hits | P-value | term | pathway | count | pop hits | P-value |
|---|---|---|---|---|---|---|---|---|---|
| hsa05417 | lipid and atherosclerosis | 24 | 215 | 4.88 × 10–15 | hsa04020 | calcium signaling pathway | 12 | 240 | 3.49 × 10–4 |
| hsa05200 | pathways in cancer | 31 | 531 | 5.72 × 10–12 | hsa04520 | adherens junction | 7 | 71 | 3.54 × 10–4 |
| hsa05215 | prostate cancer | 15 | 97 | 2.82 × 10–11 | hsa05169 | Epstein–Barr virus infection | 11 | 202 | 3.56 × 10–4 |
| hsa05205 | proteoglycans in cancer | 18 | 205 | 1.44 × 10–9 | hsa05218 | melanoma | 7 | 72 | 3.82 × 10–4 |
| hsa04151 | PI3K-Akt signaling pathway | 22 | 354 | 6.42 × 10–9 | hsa04115 | p53 signaling pathway | 7 | 73 | 4.12 × 10–4 |
| hsa04210 | apoptosis | 14 | 136 | 2.64 × 10–8 | hsa05165 | human papillomavirus infection | 14 | 331 | 4.50 × 10–4 |
| hsa05161 | hepatitis B | 15 | 162 | 2.71 × 10–8 | hsa04660 | T-cell receptor signaling pathway | 8 | 104 | 4.64 × 10–4 |
| hsa04657 | IL-17 signaling pathway | 12 | 94 | 4.06 × 10–8 | hsa04910 | insulin signaling pathway | 9 | 137 | 4.77 × 10–4 |
| hsa04933 | AGE-RAGE signaling pathway in diabetic complications | 12 | 100 | 7.78 × 10–8 | hsa04659 | Th17 cell differentiation | 8 | 108 | 5.83 × 10–4 |
| hsa01521 | EGFR tyrosine kinase inhibitor resistance | 11 | 79 | 8.12 × 10–8 | hsa05152 | tuberculosis | 10 | 180 | 6.59 × 10–4 |
| hsa04926 | relaxin signaling pathway | 13 | 129 | 1.27 × 10–7 | hsa04621 | NOD-like receptor signaling pathway | 10 | 184 | 7.72 × 10–4 |
| hsa05010 | Alzheimer disease | 21 | 384 | 1.36 × 10–7 | hsa04670 | leukocyte transendothelial migration | 8 | 114 | 8.06 × 10–4 |
| hsa05163 | human cytomegalovirus infection | 16 | 225 | 2.66 × 10–7 | hsa04215 | apoptosis—multiple species | 5 | 32 | 8.19 × 10–4 |
| hsa05418 | fluid shear stress and atherosclerosis | 13 | 139 | 2.90 × 10–7 | hsa05134 | legionellosis | 6 | 57 | 9.44 × 10–4 |
| hsa05223 | non-small cell lung cancer | 10 | 72 | 4.17 × 10–7 | hsa05213 | endometrial cancer | 6 | 58 | 1.02 × 10–3 |
| hsa01522 | endocrine resistance | 11 | 98 | 6.39 × 10–7 | hsa04071 | sphingolipid signaling pathway | 8 | 119 | 1.04 × 10–3 |
| hsa04914 | progesterone-mediated oocyte maturation | 11 | 102 | 9.29 × 10–7 | hsa04935 | growth hormone synthesis, secretion, and action | 8 | 119 | 1.04 × 10–3 |
| hsa04625 | C-type lectin receptor signaling pathway | 11 | 104 | 1.11 × 10–6 | hsa04722 | neurotrophin signaling pathway | 8 | 119 | 1.04 × 10–3 |
| hsa04931 | insulin resistance | 11 | 108 | 1.58 × 10–6 | hsa04014 | Ras signaling pathway | 11 | 232 | 1.05 × 10–3 |
| hsa04066 | HIF-1 signaling pathway | 11 | 109 | 1.72 × 10–6 | hsa04932 | non-alcoholic fatty liver disease | 9 | 155 | 1.08 × 10–3 |
| hsa04915 | estrogen signaling pathway | 12 | 138 | 2.07 × 10–6 | hsa04217 | necroptosis | 9 | 159 | 1.27 × 10–3 |
| hsa04668 | TNF signaling pathway | 11 | 112 | 2.21 × 10–6 | hsa04611 | platelet activation | 8 | 124 | 1.32 × 10–3 |
| hsa05145 | toxoplasmosis | 11 | 112 | 2.21 × 10–6 | hsa05203 | viral carcinogenesis | 10 | 204 | 1.60 × 10–3 |
| hsa05162 | measles | 12 | 139 | 2.22 × 10–6 | hsa05132 | Salmonella infection | 11 | 249 | 1.79 × 10–3 |
| hsa05235 | PD-L1 expression and PD-1 checkpoint pathway in cancer | 10 | 89 | 2.58 × 10–6 | hsa05164 | Influenza A | 9 | 171 | 2.02 × 10–3 |
| hsa05415 | diabetic cardiomyopathy | 14 | 203 | 2.79 × 10–6 | hsa04664 | Fc epsilon RI signaling pathway | 6 | 68 | 2.09 × 10–3 |
| hsa05120 | epithelial cell signaling in Helicobacter pylori infection | 9 | 70 | 3.78 × 10–6 | hsa04920 | adipocytokine signaling pathway | 6 | 69 | 2.23 × 10–3 |
| hsa05022 | pathways of neurodegeneration—multiple diseases | 21 | 476 | 4.04 × 10–6 | hsa05230 | central carbon metabolism in cancer | 6 | 70 | 2.38 × 10–3 |
| hsa05207 | chemical carcinogenesis—receptor activation | 14 | 212 | 4.52 × 10–6 | hsa05206 | MicroRNAs in cancer | 12 | 310 | 2.81 × 10–3 |
| hsa05133 | pertussis | 9 | 76 | 7.06 × 10–6 | hsa05140 | leishmaniasis | 6 | 77 | 3.61 × 10–3 |
| hsa05167 | Kaposi sarcoma-associated herpesvirus infection | 13 | 194 | 9.89 × 10–6 | hsa04072 | phospholipase D signaling pathway | 8 | 148 | 3.62 × 10–3 |
| hsa04370 | VEGF signaling pathway | 8 | 59 | 1.22 × 10–5 | hsa05226 | gastric cancer | 8 | 149 | 3.75 × 10–3 |
| hsa04510 | focal adhesion | 13 | 201 | 1.42 × 10–5 | hsa04726 | serotonergic synapse | 7 | 115 | 4.33 × 10–3 |
| hsa05219 | bladder cancer | 7 | 41 | 1.54 × 10–5 | hsa04150 | mTOR signaling pathway | 8 | 156 | 4.83 × 10–3 |
| hsa04936 | alcoholic liver disease | 11 | 142 | 1.86 × 10–5 | hsa04010 | MAPK signaling pathway | 11 | 294 | 5.87 × 10–3 |
| hsa05222 | small cell lung cancer | 9 | 92 | 2.92 × 10–5 | hsa04540 | gap junction | 6 | 88 | 6.37 × 10–3 |
| hsa04152 | AMPK signaling pathway | 10 | 120 | 3.01 × 10–5 | hsa00591 | linoleic acid metabolism | 4 | 29 | 6.62 × 10–3 |
| hsa04912 | GnRH signaling pathway | 9 | 93 | 3.16 × 10–5 | hsa04650 | natural killer cell-mediated cytotoxicity | 7 | 126 | 6.74 × 10–3 |
| hsa04919 | thyroid hormone signaling pathway | 10 | 121 | 3.21 × 10–5 | hsa00590 | arachidonic acid metabolism | 5 | 61 | 8.83 × 10–3 |
| hsa05208 | chemical carcinogenesis—reactive oxygen species | 13 | 223 | 3.99 × 10–5 | hsa04024 | cAMP signaling pathway | 9 | 221 | 9.42 × 10–3 |
| hsa04613 | neutrophil extracellular trap formation | 12 | 190 | 4.38 × 10–5 | hsa04750 | inflammatory mediator regulation of TRP channels | 6 | 98 | 9.95 × 10–3 |
| hsa05160 | hepatitis C | 11 | 157 | 4.42 × 10–5 | hsa04371 | apelin signaling pathway | 7 | 139 | 1.07 × 10–2 |
| hsa01524 | platinum drug resistance | 8 | 73 | 5.00 × 10–5 | hsa05146 | amebiasis | 6 | 102 | 1.17 × 10–2 |
| hsa05171 | coronavirus disease—COVID-19 | 13 | 232 | 5.87 × 10–5 | hsa05017 | spinocerebellar ataxia | 7 | 143 | 1.22 × 10–2 |
| hsa05214 | glioma | 8 | 75 | 5.96 × 10–5 | hsa05221 | acute myeloid leukemia | 5 | 67 | 1.22 × 10–2 |
| hsa04068 | FoxO signaling pathway | 10 | 131 | 6.02 × 10–5 | hsa04064 | NF-kappa B signaling pathway | 6 | 104 | 1.26 × 10–2 |
| hsa05130 | pathogenic Escherichia coli infection | 12 | 197 | 6.10 × 10–5 | hsa04620 | toll-like receptor signaling pathway | 6 | 104 | 1.26 × 10–2 |
| hsa05142 | chagas disease | 9 | 102 | 6.16 × 10–5 | hsa04960 | aldosterone-regulated sodium reabsorption | 4 | 37 | 1.30 × 10–2 |
| hsa05212 | pancreatic cancer | 8 | 76 | 6.49 × 10–5 | hsa05204 | chemical carcinogenesis—DNA adducts | 5 | 69 | 1.35 × 10–2 |
| hsa05020 | prion disease | 14 | 273 | 6.68 × 10–5 | hsa05224 | breast cancer | 7 | 147 | 1.38 × 10–2 |
| hsa05225 | hepatocellular carcinoma | 11 | 168 | 7.85 × 10–5 | hsa05202 | transcriptional misregulation in cancer | 8 | 193 | 1.47 × 10–2 |
| hsa05135 | Yersinia infection | 10 | 137 | 8.53 × 10–5 | hsa04921 | oxytocin signaling pathway | 7 | 154 | 1.71 × 10–2 |
| hsa04140 | autophagy—animal | 10 | 141 | 1.07 × 10–4 | hsa04630 | JAK-STAT signaling pathway | 7 | 162 | 2.13 × 10–2 |
| hsa05131 | Shigellosis | 13 | 247 | 1.07 × 10–4 | hsa04810 | regulation of actin cytoskeleton | 8 | 218 | 2.67 × 10–2 |
| hsa04015 | Rap1 signaling pathway | 12 | 210 | 1.08 × 10–4 | hsa04380 | osteoclast differentiation | 6 | 128 | 2.83 × 10–2 |
| hsa05170 | human immunodeficiency virus 1 infection | 12 | 212 | 1.18 × 10–4 | hsa05032 | morphine addiction | 5 | 91 | 3.34 × 10–2 |
| hsa04012 | ErbB signaling pathway | 8 | 85 | 1.33 × 10–4 | hsa04614 | renin–angiotensin system | 3 | 23 | 3.74 × 10–2 |
| hsa05210 | colorectal cancer | 8 | 86 | 1.43 × 10–4 | hsa04923 | regulation of lipolysis in adipocytes | 4 | 56 | 3.88 × 10–2 |
| hsa04360 | axon guidance | 11 | 182 | 1.53 × 10–4 | hsa04550 | signaling pathways regulating pluripotency of stem cells | 6 | 143 | 4.26 × 10–2 |
| hsa04218 | cellular senescence | 10 | 156 | 2.30 × 10–4 | hsa04062 | chemokine signaling pathway | 7 | 192 | 4.37 × 10–2 |
| hsa05231 | choline metabolism in cancer | 8 | 98 | 3.23 × 10–4 | hsa04723 | retrograde endocannabinoid signaling | 6 | 148 | 4.82 × 10–2 |
| hsa04917 | prolactin signaling pathway | 7 | 70 | 3.28 × 10–4 | hsa04213 | longevity regulating pathway—multiple species | 4 | 62 | 5.00 × 10–2 |
| hsa04930 | type II diabetes mellitus | 6 | 46 | 3.47 × 10–4 |
We focused on the correlation of metabolites and possible targets to integrate the results of metabolomics and network pharmacology and comprehensively explored the underlying mechanisms. We specifically referred to the relationship between metabolites of crucial pathways and their related proteins. Ultimately, a network containing relationships between metabolites, metabolic pathways, and enzymes was established (Figure 12).
Figure 12.
Proposed metabolic pathways for explanation relationship between SA and malaria treatment in serum. Red represents the upregulation of metabolites. Green represents the downregulation of metabolites.
3. Discussion
Malaria is a disease caused by P. parasites transmitted through the bites by the infected Anopheles. After 1 to 3 h, the sporozoites leave the skin and are brought to the liver cells.18 The Trap like protein was necessary to P. to enter liver cells.19 The first replication of P. needed some specific proteins, such as sporozoite microneme protein essential for cell traversal, perforin-like protein 1, phosphlipase A2, and so on.19−21 In summary, erythrocyte invasion by P. is a tight process involving a series of receptor–ligand interactions.
Studies suggested that the possible endogenous metabolite biomarkers in the development and progression of malaria include histidine, linoleic acid, tetradecanedioic acid, leucine, oleic acid, phenylalanine, isoleucine, ornithine, proline, proline betaine, eicosapentaenoic acid, cytidine, choline, and so on. From Table 1, we could know that the differential metabolites in serum were mainly concentrated in the process of amino acid and lipid metabolism. Moreover, four metabolic pathways were predicted to be associated with SA treatment by malaria. The results of the network pharmacology analysis showed 122 potential target genes and 125 pathways related to the treatment of malaria with artesunate. The results of network pharmacology were partly consistent with the experimental results. The disease mainly affects amino acid and fatty acid metabolism in mice.
Proliferation of P. in host cells requires a large amount of protein synthesis, which might be the reason for the disorder of amino acid metabolism caused by P. Wang et al., who identified 124 target proteins of artemisinin by chemical proteomics.13 Through the experimental results (Figure 12), we found that the synthetic pathways of l-histidine, l-phenylalanine, l-isoleucine, and l-ornithine were blocked under disease conditions. They could synthesize anserine, 5-formimidoyltetrahydrofolic acid, 4-fumarylacetoacetate, 5,6,7,8-tetrahydrobiopterin, and S-adenosylmethioninaminium. These compounds have the functions of antioxidant, anti-anemia, anti-nervous degeneration, anti-metabolism disorder, and maintenance of DNA methylation.22−26 These synthetic products might play a vital role in the anti-malarial properties of SA.
HE staining showed that SA could inhibit liver and spleen inflammation caused by Pb. SA could be rapidly hydrolyzed to dihydroartemisinin in vivo.27 SA regulated NF-κB, ERK, AMPK/SIRT1, and ROS-JNK1/2 inflammatory pathways.28−31 Besides, the network pharmacology result showed that the therapeutic effect of artesunate on malaria was related to Th17 cell differentiation. Th17 cells played an important role in the autoimmune diseases and tissue inflammation. Th17 cells could increase the secretion of proinflammatory factors such as TNF, IL-1β, IL-6, and IL-8 by secreting inflammatory mediators IL-17. These inflammatory factors mediated the local infiltration of inflammatory cells and induce tissues.32 Serum metabolomics results showed that l-phenylalanine was significantly increased in the model group, which may be a result of Pb inhibiting the synthesis of 3-(indol-3-yl) pyruvic acid. Studies demonstrated that 3-(indol-3-yl) pyruvic acid was an aromatic receptor activator.33 Previously, scholars found that aromatic hydrocarbon receptor-mediated inflammation control was an important target of immunoregulation. Ligand activated aromatic hydrocarbon receptors could inhibit the differentiation, maturation, and function of dendritic cells. At the same time, the activated aromatic hydrocarbon receptor could reduce the expression of cytokines closely related to Th17 cell differentiation, such as IL-1β, IL-6, IL-23, and TNF-α. In this way, Th17 cell differentiation was inhibited.34 When Pb infected mice, these acquired an increased accumulation of IL-17+ cells in the spleen tissue.35 Artesunate attenuated inflammation by downregulating Th17 cell responses in mice.10 This pathway may be a key for the artesunate to reduce inflammation and produce the anti-malarial effect.
Merozoites were released into the bloodstream from erythrocytes infected by Pb, and they enabled attachment to nearby erythrocytes.5,36 Pb could not only change the structure membrane but also change the function of red blood cells, and these changes made red blood cells more suitable for the growth and reproduction of Pb.37−39 Pb increased phosphatidylcholine content in the host cell membrane.40 Phosphatidylcholine was the main membrane phospholipids of P. parasites and could be generated by the Kennedy CDP-choline pathway; the Kennedy pathways initiate from choline.41 However, the exact source of choline was not clear, and most scholars believed that it came directly from the serum.42 Network pharmacology results showed that artesunate had anti-malarial effects by affecting choline metabolism. Our experimental results reinforced the hypothesis that choline required for Pb came from serum. The content of choline in the host serum of the model group decreased significantly, so we speculated that the phenomenon may be related to the synthesis of phosphatidylcholine by Pb using the choline of the host.40 The content of phosphatidylcholine in infected red blood cells will increase.43 At the same time, the excessive synthesis of phosphatidylcholine reduced the content of choline in serum, while a lack of choline affected the synthesis of acetylcholine.44 Acetylcholine was released at nerve terminals, and the neurotransmitters could activate immune cells located in close proximity to the nerve terminals such as macrophages.45 Expression of receptors for neurotransmitters was found in the α7 subunit of the nicotinic acetylcholine receptor on macrophages. Activation of the receptor inhibited the release of proinflammatory factors such as tumor necrosis factor-α.46 In summary, the cholinergic anti-inflammatory pathway produced anti-inflammatory effects by regulating the NF-κB pathway.47 Pb could activate the host NF-κB pathway.48 Inflammatory mice treated with SA showed downregulating NF-κB phosphorylation.49 These results are consistent with our experimental results. Our experimental results showed that SA reduced the release of inflammatory factors through downregulating the NF-κB pathway to reduce the release of inflammatory factors and then to improve the inflammation of the host liver and spleen.
In contrast to the blank group, palmitic acid was downregulated in the model group. After treatment with SA, the levels of palmitic acid increased. Previous studies have shown an increased content of palmitic acid in infected erythrocyte plasma infected with Pb.50 We speculate that palmitic acid plays an important role in the growth and reproduction of Pb. However, this speculation still needs further verification.
Combining previous research and our studies, we hypothesized that artesunate may act on malaria through different biological pathways. Three limitations should be considered in the interpretation of our findings. First, further pharmacological experiments are necessary to validate the accurate mechanisms of artesunate predicted by the network pharmacology analysis. Second, comprehensive metabolic changes in other structures (e.g., spinal fluid, liver) should be determined to more accurately reflect the mechanism through which artesunate acts on malaria. Third, enhanced metabolomics technologies, such as targeted metabolomics, are required to detect metabolite biomarkers purposefully.
4. Materials and Methods
4.1. Materials and Reagents
Plasmodium berghei ANKA strain artesunate (100 g, batch number: X03511Y123220) was purchased from source leaf organisms (Shanghai, China). UHPLC grade methanol was provided by Merck (Canada). Formic acid and acetonitrile were supplied by Thermo Fisher Scientific (Fair Lawn, NJ, USA). Water was obtained from Watsons (Guangdong, China).
Vanquish UHPLC (Thermo Scientific, Germany) equipped with a vanquish autosampler, a vanquish pump, and a vanquish column compartment; a Q-Exactive Plus Orbitrap MS (Thermo Scientific, Germany), and an X-30R all-purpose high-speed freeze centrifuge (Berle X-mark, USA) were used.
4.2. Experimental Design
The study protocol was approved by the Animal Experimental Ethics Committee of Shandong Academy of Traditional Chinese Medicine (protocol code SDZYY20200415002 and 15 Apr 2020 of approval).
SPF female KM mice (18 ± 2 g) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd., license number: SCXK (Beijing) 2016-0011. All mice were raised in the Shandong Institute of Traditional Chinese Medicine animal room and were housed under standard conditions (55 ± 5% relative humidity, 12 h light–dark cycle, 22 ± 2 °C) and had free access to standard mice chow and water. A total of 18 mice were divided equally and randomly into a blank group, model group, and SA group. All animals that died because of Pb were disposed by incineration.
After 7 days of adaptive feeding, each model group and SA group mice were infected with 2 × 107 Pb infected erythrocytes, whereas the blank group received an equal amount of normal saline. We injected the mice with drugs 2 h after the Pb infection. Mice in the SA group were received i.p. of 1 mg artesunate/20 g mice bodyweight, while the blank group and model group received an equal amount of normal saline. All mice were continuously i.p. administrated for 4 days.
4.3. Giemsa Staining
The 5 days post-infection levels of parasitemia were determined by microscopic examination of Giemsa-stained thin blood smears of peripheral tail blood of each mouse.51,52 All the smears were air dried with high purity nitrogen and fixed in methanol. Finally, samples were stained for 20 min. The formula % parasitemia = [(total number of infected RBC counted/total number of RBC counted) × 100] was used.
4.4. HE Staining
Mice livers and spleens were fixed in 4% paraformaldehyde at room temperature for ≥24 h. After paraffin embedding and slicing, HE staining was performed to determine changes of hepatic and splenic architectures.53
4.5. UHPLC-MS/MS-Based Serum Metabolomics
4.5.1. Collection and Preparation of Samples
After Giemsa staining, mice were followed by a cardiac puncture to collect blood in a centrifuge tube. Samples were stored at room temperature for more than half an hour. Subsequently, the serum was separated by centrifugation (3000 rpm, 10 min, 4 °C) and stored at −200 °C until use. The process of sample preparation for UHPLC-MS/MS was very specific. First, methanol (300 μL) was added to a serum sample (100 μL), after which it was vortex-mixed for 1 min; then the sample was separated by centrifugation (12,000 rpm, 10 min, 4 °C). Subsequently, the supernatant was subjected to nitrogen drying and redissolved in methanol (100 μL). The experimenter took 20 μL of each sample and mixed it as the QC sample of UHPLC-MS/MS.
4.5.2. Acquisition of UHPLC-MS/MS Data
The column temperature was 25 °C, the flow rate was 0.3 mL/min, the injection volume was 2 μL, and the post-equilibrium time was 3 min. The composition of the mobile phase, the flow rate, and the column temperature were optimized for better resolution. Finally, (A) deionized water containing 0.1% formic acid and (B) acetonitrile were chosen as the mobile phase. The gradient of the mobile phase was 0–4.5 min, 95% B; 4.5–6.0 min, 40.0–95% B; 6–17 min, 25–40% B; 17–19 min, 5–25% B.
The ionization modes detected by positive and negative ions of the electrospray ion source were as follows: a positive ion mode collision energy voltage of 30 V and a negative ion mode collision energy voltage of 35 V. The atomization gas was high purity nitrogen (N2), and the collision gas was high-purity helium (He). The scanning range was 80–1200 m/z, the sheath pressure was 40 arb, the auxiliary pressure was 5 arb, the spray voltage was 3.0 kV, the ion transport tube temperature was 300 °C, the auxiliary gas heating temperature was 320 °C, the lens voltage was 50 V, and the resolution was 70,000 (primary mass spectrometry) 17,500 (secondary mass spectrometry) high energy collision dissociation.
4.6. Statistical Analysis
Raw data were processed with the Compound Discoverer 3.1 software (Thermo Scientific) for baseline filtering, peak identification, the peak extraction, integration, correction of retention time, peak alignment, combined ion merging, missing value filling, and peak area correction. Data processing for ESI+ and ESI– was performed separately. Thresholds for peak detection included a minimum of six data points per peak. Metabolites were scored against the Human Metabolome Database (https://hmdb.ca/) spectra from the mzCloud database (www.mzcloud.org). For stability study data analysis, QC samples were analyzed to determine the extent of retention time variability and verify consistent mass accuracy throughout the analysis. The quality control samples with a relative standard deviation for peak area was 30%, and the signal-to-noise ratio (S/N) threshold was 3.
Data were exported from the UHPLC-MS/MS into SIMCA-P 13.0. PCA and PLS-DA were used to differentiate the samples. PCA scores were unsupervised as a basis for PLS-DA which was supervised. R2 and Q2 provided a measure of model reliability of PCA and PLS-DA.54 The PLS-DA model was assessed by the intercepts of R2 and Q2 in the permutation test to avoid overfitting. The criteria for model validity included two conditions: (1) all Q2 values on the permuted data set to the left were lower than the Q2 values on the actual data set to the right and (2) the regression line (line joining the point of observed Q2 to the centroid of a cluster of permuted Q2 values) has a negative value of intercept on the y-axis.55 Through the variable importance for the projection (VIP) of the PLS-DA model, the potential biomarker was found with VIP > 1. A larger VIP indicated a more important biomarker.
Data processing of each software package yielded a multidimensional peak table including accurate m/z, molecular weights, retention times, compound formulas, peak areas, and other statistical information. The differential metabolites with statistical significance were screened according to the difference multiple (fold change, FC) FC > 1.5 or FC < 0.67 and t-test (test level α = 0.05). In this study, compounds with VIP > 1, P < 0.05, and FC > 1.5 or FC < 0.67 were considered as differential metabolites.
4.7. Network Pharmacology Analysis
The PubChem (https://pubchem.ncbi.nlm.nih.gov/) database was used to retrieve artesunate 3D/2D structure information, SMILES, and CID numbers. The putative targets of artesunate were obtained from the online prediction platforms of SwissTargetPrediction (http://swisstargetprediction.ch/), Charit.ed - About SuperPred (https://prediction.charite.de/), PharmMapper (http://lilab-ecust.cn/pharmmapper/index.html), and published literature studies. All the retrieved targets were converted to their official symbols using the Uniprot (https://www.uniprot.org/).
The malaria-related target proteins were collected from GeneCard (https://www.genecards.org/). This was a comprehensive database of human genes and covered the analytical data of genes in multiple databases.56 By intersecting the putative target genes of artesunate with malaria-related target proteins, genes that correspond simultaneously to disease and treatment were considered potential targets of artesunate.
GO enrichment and KEGG pathway enrichment analyses were undertaken to probe the biological function and potential mechanisms of detected targets (https://david.ncifcrf.gov/). Meanwhile, the acquired target genes were submitted to the Search Tool for the Retrieval of Interacting Genes (https://www.stringdb.org/) to obtain a protein–protein interaction network.57 Furthermore, a compound–potential targets–potential pathways network of artesunate for treating malaria was constructed by utilizing the network-visualization software Cytoscape 3.7.1 (http://cytoscape.org/).
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (82004037), the Key Technology Research and Development Program of Shandong Province (2018GSF119018), the Inheritance Project of Traditional Chinese Medicine Academic Schools of Qilu Medical School “Establishment of Shandong Research Model of Artemisinin and its Derivatives” and the Traditional Chinese Medicine Science and Technology Development Project of Shandong Province (M-2022173).
Glossary
Abbreviations
- Hal
histidine ammonia-lyase
- Amdhd1
probable imidazolonepropionase
- Fah
fumarylacetoacetate hydrolase
- Odc1
ornithine decarboxylase
- Sms
spermine synthase
- Sat1
diamine acetyltransferase 1
- UCK2 tetramer
uridine-cytidine kinase 2
- CK
choline kinase
- Chdh
choline dehydrogenase, mitochondrial
- CCT, CTP
phosphocholine cytidylyltransferase
- Aldh7a1
alpha-aminoadipic semialdehyde dehydrogenase A
- CEPT
choline/ethanolaminephosphotransferase
- ART
aryl hydrocarbon receptor
- ChAT
choline acetyl transferase
Author Contributions
∥ F.W. and J.S. contributed equally.
The authors declare no competing financial interest.
References
- World Health O.World Malaria Report 2021; World Health Organization: Geneva, 2021; pp 9–21.
- Winzeler E. A. Malaria research in the post-genomic era. Nature 2008, 455, 751–756. 10.1038/nature07361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman M. J.; Carruthers V. B. Recent insights into apicomplexan parasite egress provide new views to a kill. Curr. Opin. Microbiol. 2013, 16, 459–464. 10.1016/j.mib.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicole K.; Jae-Yeon C.; Dennis R. V.; Choukri B. M. Role of phospholipid synthesis in the development and differentiation of malaria parasites in the blood. J. Biol. Chem. 2018, 293, 17308–17316. 10.1074/jbc.R118.003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman A. F.; Healer J.; Marapana D.; Marsh K. Malaria: Biology and Disease. Cell 2016, 167, 610–624. 10.1016/j.cell.2016.07.055. [DOI] [PubMed] [Google Scholar]
- Kasturi H.; Narla M. Malaria, erythrocytic infection, and anemia. Hematology. Am. Soc. Hematol. Educ Program. 2009, 2009, 87–93. 10.1182/asheducation-2009.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgorski R. M.; Goff K. A.; Penney T. P.; Maness N. J.; Keating J.; Yukich J. O.; Marx P. A. DNA analysis reveals non-falciparum malaria in the Democratic Republic of the Congo. Acta Trop. 2020, 212, 105557. 10.1016/j.actatropica.2020.105557. [DOI] [PubMed] [Google Scholar]
- Wykes M. N.; Good M. F. What have we learnt from mouse models for the study of malaria?. Eur. J. Immunol. 2009, 39, 2004–2007. 10.1002/eji.200939552. [DOI] [PubMed] [Google Scholar]
- Wang J. G.; Xu C. C.; Wong Y. K.; Li Y. J.; Liao F. L.; Jiang T. L.; Tu Y. Y. Artemisinin, the Magic Drug Discovered from Traditional Chinese Medicine. Engineering 2019, 5, 32–39. 10.1016/j.eng.2018.11.011. [DOI] [Google Scholar]
- Bai X. Y.; Liu P.; Chai Y. W.; Wang Y.; Ren S. H.; Li Y. Y.; Zhou H. Artesunate attenuates 2, 4-dinitrochlorobenzene-induced atopic dermatitis by down-regulating Th17 cell responses in BALB/c mice. Eur. J. Pharmacol. 2020, 874, 173020. 10.1016/j.ejphar.2020.173020. [DOI] [PubMed] [Google Scholar]
- Dai Y. F.; Zhou W. W.; Meng J.; Du X. L.; Sui Y. P.; Dai L.; Wang P. Q.; Huo H. R.; Sui F. The pharmacological activities and mechanisms of artemisinin and its derivatives: a systematic review. Med. Chem. Res. 2017, 26, 867–880. 10.1007/s00044-016-1778-5. [DOI] [Google Scholar]
- Li S.; Li C. H.; Jiang T. L. [Reasearch on lipid metabolism of Plasmodium and antimalarial mechanism of artemisinin]. Zhongguo Zhongyao Zazhi 2021, 46, 4849–4864. 10.19540/j.cnki.cjcmm.20210610.703. [DOI] [PubMed] [Google Scholar]
- Wang J. G.; Zhang C. J.; Chia W. N.; Loh C. C. Y.; Li Z. J.; Lee Y. M.; He Y.; Yuan L. X.; Lim T. K.; et al. Haem-activated promiscuous targeting of artemisinin in Plasmodium falciparum. Nat. Commun. 2015, 6, 10111. 10.1038/ncomms10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig C. L.; Rosenthal A. S.; D’Angelo J.; Griffin C. E.; Posner G. H.; Cooper R. A. Accumulation of artemisinin trioxane derivatives within neutral lipids of Plasmodium falciparum malaria parasites is endoperoxide-dependent. Biochem. Pharmacol. 2009, 77, 322–336. 10.1016/j.bcp.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Huang L.; Li J.; Fan Q. W.; Long Y. C.; Li Y.; Zhou B. Artemisinin directly targets malarial mitochondria through its specific mitochondrial activation. PLoS One 2010, 5, e9582 10.1371/journal.pone.0009582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna S.; Uhlemann A. C.; Haynes R. K. Artemisinins: mechanisms of action and potential for resistance. Drug Resistance Updates 2004, 7, 233–244. 10.1016/j.drup.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Wang W. B.; Zhao L. L.; He Z. Y.; Wu N.; Li Q. X.; Qiu X. J.; Zhou L.; Wang D. S. Metabolomics-based evidence of the hypoglycemic effect of Ge-Gen-Jiao-Tai-Wan in type 2 diabetic rats via UHPLC-QTOF/MS analysis. J. Ethnopharmacol. 2018, 219, 299–318. 10.1016/j.jep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- Chora Â. F.; Mota M. M.; Miguel P. The reciprocal influence of the liver and blood stages of the malaria parasite’s life cycle. Int. J. Parasitol. 2022, 30, 00054–00056. 10.1016/j.ijpara.2022.02.002. [DOI] [PubMed] [Google Scholar]
- Ishino T.; Yano K.; Chinzei Y.; Yuda M. Cell-Passage Activity Is Required for the Malarial Parasite to Cross the Liver Sinusoidal Cell Layer. PLoS Biol. 2004, 2, e4 10.1371/journal.pbio.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot P.; Schauer K.; Coppens I.; Nussenzweig V. A Surface Phospholipase Is Involved in the Migration of Plasmodium Sporozoites through Cells. J. Biol. Chem. 2005, 280, 6752–6760. 10.1074/jbc.m411465200. [DOI] [PubMed] [Google Scholar]
- Risco-Castillo V.; Topçu S.; Marinach C.; Manzoni G.; Bigorgne A. E.; Briquet S.; Baudin X.; Lebrun M.; Dubremetz J. F.; Silvie O. Malaria Sporozoites Traverse Host Cells within Transient Vacuoles. Cell Host Microbe 2015, 18, 593–603. 10.1016/j.chom.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Al-Dhalimy M.; Overturf K.; Finegold M.; Grompe M. Long-Term Therapy with NTBC and Tyrosine-Restricted Diet in a Murine Model of Hereditary Tyrosinemia Type I. Mol. Genet. Metab. 2002, 75, 38–45. 10.1006/mgme.2001.3266. [DOI] [PubMed] [Google Scholar]
- Cyr A.; Lauryn K.; Lauran C.; Sladjana S.; Brian Z.; Cristina A. Circulating Metabolomic Analysis following Cecal Ligation and Puncture in Young and Aged Mice Reveals Age-Associated Temporal Shifts in Nicotinamide and Histidine/Histamine Metabolic Pathways. Oxid. Med. Cell. Longevity 2021, 2021, 5534241. 10.1155/2021/5534241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan M.; Hill-Yardin E. L.; Bornstein J. C.; Foong J. P. P. Endogenous Glutamate Excites Myenteric Calbindin Neurons by Activating Group I Metabotropic Glutamate Receptors in the Mouse Colon. Front. Neurosci. 2019, 13, 00426. 10.3389/fnins.2019.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misselbeck K.; Marchetti L.; Priami C.; Stover P. J.; Field M. S. The 5-formyltetrahydrofolate futile cycle reduces pathway stochasticity in an extended hybrid-stochastic model of folate-mediated one-carbon metabolism. Sci. Rep. 2019, 9, 4322. 10.1038/s41598-019-40230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y. L.; Yan Q. J.; Qian L.; Jiang Y. P.; Chen X.; Zeng S. S.; Xu Z. J.; Gong Z. C. S-adenosylmethionine administration inhibits levodopa-induced vascular endothelial growth factor-A expression. Aging 2020, 12, 21290–21307. 10.18632/aging.103863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geditz M. C. K.; Heinkele G.; Ahmed A.; Kremsner P. G.; Kerb M. S.; Schwab M.; Hofmann U. LC-MS/MS method for the simultaneous quantification of artesunate and its metabolites dihydroartemisinin and dihydroartemisinin glucuronide in human plasma. Anal. Bioanal. Chem. 2014, 406, 4299–4308. 10.1007/s00216-014-7820-x. [DOI] [PubMed] [Google Scholar]
- Li N.; Sun W. J.; Zhou X.; Gong H.; Chen Y. Q.; Chen D. F.; Xiang F. Dihydroartemisinin Protects against Dextran Sulfate Sodium-Induced Colitis in Mice through Inhibiting the PI3K/AKT and NF- κ B Signaling Pathways. BioMed Res. Int. 2019, 2019, 1415809. 10.1155/2019/1415809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou L.; Qin K.; Yang Z. X.; Bie M. J. The Effects and Mechanisms of Dihydroartemisinin on Influenza A Virus H1N1 Induces TNF-α and IL-6 Expression in Bronchial Epithelial Cells. Sichuan Daxue Xuebao, Yixueban 2020, 51, 171–177. 10.12182/20200360604. [DOI] [PubMed] [Google Scholar]
- Cetrullo S.; D’Adamo S.; Tantini B.; Borzi R. M.; Flamigni F. mTOR, AMPK, and Sirt1: Key Players in Metabolic Stress Management. Crit. Rev. Eukaryotic Gene Expression 2015, 25, 59–75. 10.1615/critreveukaryotgeneexpr.2015012975. [DOI] [PubMed] [Google Scholar]
- Zhang Z. L.; Guo M.; Zhao S. F.; Shao J. J.; Zheng S. Z. ROS-JNK1/2-dependent activation of autophagy is required for the induction of anti-inflammatory effect of dihydroartemisinin in liver fibrosis. Free Radical Biol. Med. 2016, 101, 272–283. 10.1016/j.freeradbiomed.2016.10.498. [DOI] [PubMed] [Google Scholar]
- Gu D. D.; Nan Q.; Miao Y. L.; Yang H. L.; Li M. J.; Ye Y.; Miao J. R. KT2 alleviates ulcerative colitis by reducing Th17 cell differentiation through the miR-302c-5p/STAT3 axis. Eur. J. Cell Biol. 2022, 101, 151223. 10.1016/j.ejcb.2022.151223. [DOI] [PubMed] [Google Scholar]
- Aoki R.; Ayako A. Y.; Chise S.; Yoshiharu T. Indole-3-Pyruvic Acid, an Aryl Hydrocarbon Receptor Activator, Suppresses Experimental Colitis in Mice. J. Immunol. 2018, 201, 3683–3693. 10.4049/jimmunol.1701734. [DOI] [PubMed] [Google Scholar]
- Xie J. M.; Wang Z. T.; Wang W. Semaphorin 4D Induces an Imbalance of Th17/Treg Cells by Activating the Aryl Hydrocarbon Receptor in Ankylosing Spondylitis. Front. Immunol. 2020, 11, 2151. 10.3389/fimmu.2020.02151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani T.; Bhattacharyya A. Differential role of T regulatory and Th17 in Swiss mice infected with Plasmodium berghei ANKA and Plasmodium yoelii. Exp. Parasitol. 2014, 141, 82–92. 10.1016/j.exppara.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Bannister L. H.; Hopkins J. M.; Fowler R. E.; Krishna S.; Mitchell G. H. A Brief Illustrated Guide to the Ultrastructure of Plasmodium falciparum Asexual Blood Stages. Parasitol. Today 2000, 16, 427–433. 10.1016/s0169-4758(00)01755-5. [DOI] [PubMed] [Google Scholar]
- Flammersfeld A.; Lang C.; Flieger A.; Pradel G. Phospholipases during membrane dynamics in malaria parasites. Int. J. Med. Microbiol. 2018, 308, 129–141. 10.1016/j.ijmm.2017.09.015. [DOI] [PubMed] [Google Scholar]
- Pessi G.; Guillermo K.; Choukri B. M. A pathway for phosphatidylcholine biosynthesis in Plasmodium falciparum involving phosphoethanolamine methylation. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 6206–6211. 10.1073/pnas.0307742101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. H.; Ackerman H. C.; Su X. Z.; Wellems T. E. Malaria biology and disease pathogenesis: insights for new treatments. Nat. Med. 2013, 19, 156–167. 10.1038/nm.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran P. N.; Brown S. H. J.; Rug M.; Ridgway M. C.; Mitchell T. W.; Maier A. G. Changes in lipid composition during sexual development of the malaria parasite Plasmodium falciparum. Malar. J. 2016, 15, 73. 10.1186/s12936-016-1130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déchamps S.; Wengelnik K.; Berry-Sterkers L.; Cerdan R.; Vial H. J.; Gannoun-Zaki L. The Kennedy phospholipid biosynthesis pathways are refractory to genetic disruption in Plasmodium berghei and therefore appear essential in blood stages. Mol. Biochem. Parasitol. 2010, 173, 69–80. 10.1016/j.molbiopara.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Ancelin M. L.; Parant M.; Thuet M. J.; Philippot J. R.; Vial H. J. Increased permeability to choline in simian erythrocytes after Plasmodium knowlesi infection. Biochem. J. 1991, 273, 701–709. 10.1042/bj2730701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibmooh N.; Pipitaporn B.; Wilairatana P.; Dangdoungjai J.; Udomsangpetch R.; Looareesuwan S.; Chantharaksri U. Effect of Artemisinin on Lipid Peroxidation and Fluidity of the Erythrocyte Membrane in Malaria. Biol. Pharm. Bull. 2000, 23, 1275–1280. 10.1248/bpb.23.1275. [DOI] [PubMed] [Google Scholar]
- Maruyama T.; Asuka M.; Toshiyuki I.; Yoshihiko K.; Makoto K. P2X2 receptors supply extracellular choline as a substrate for acetylcholine synthesis. FEBS Open Bio 2021, 12, 250–257. 10.1002/2211-5463.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarczyk J.; Benito A. Y.; Simone H. The Cholinergic Anti-Inflammatory Pathway as a Conceptual Framework to Treat Inflammation-Mediated Renal Injury. Kidney Blood Pressure Res. 2019, 44, 435–448. 10.1159/000500920. [DOI] [PubMed] [Google Scholar]
- Inoue T.; Tanaka S.; Okusa M. D. Neuroimmune Interactions in Inflammation and Acute Kidney Injury. Front. Immunol. 2017, 8, 945. 10.3389/fimmu.2017.00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nullens S.; Staessens M.; Peleman C.; Schrijvers D. M.; Malhotra-Kumar S.; Francque S.; Matteoli G.; Boeckxstaens G. E.; De Man J. G. D.; De Winter B. Y. D. Effect of Gts-21, An Alpha7 Nicotinic Acetylcholine Receptor Agonist, on Clp-Induced Inflammatory, Gastrointestinal Motility, and Colonic Permeability Changes in Mice. Shock 2016, 45, 450–459. 10.1097/shk.0000000000000519. [DOI] [PubMed] [Google Scholar]
- Bando H.; Pradipta A.; Iwanaga S.; Okamoto T.; Okuzaki D.; Tanaka S.; Vega-Rodríguez J.; Lee Y.; Ma J. S.; Sakaguchi N.; et al. CXCR4 regulates Plasmodium development in mouse and human hepatocytes. J. Exp. Med. 2019, 216, 1733–1748. 10.1084/jem.20182227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.; Du H. J.; Liu X.; Fu X.; Li X. D.; Cao Q. Artemisinin alleviates atherosclerotic lesion by reducing macrophage inflammation via regulation of AMPK/NF-κB/NLRP3 inflammasomes pathway. J. Drug Targeting 2020, 28, 70–79. 10.1080/1061186x.2019.1616296. [DOI] [PubMed] [Google Scholar]
- Hsiao L. L.; Howard R. J.; Aikawa M.; Taraschi T. F. Modification of host cell membrane lipid composition by the intra-erythrocytic human malaria parasite Plasmodium falciparum. Biochem. J. 1991, 274, 121–132. 10.1042/bj2740121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsel S. M.; Gustafson S. A.; Friedlander E.; Shnyra A. A.; Adegbulu A. J.; Liu Y.; Parrish N. M.; Jamal S. A.; Lofthus E.; Ayuk L.; et al. Malaria over-diagnosis in Cameroon: diagnostic accuracy of Fluorescence and Staining Technologies (FAST) Malaria Stain and LED microscopy versus Giemsa and bright field microscopy validated by polymerase chain reaction. Infect. Dis. Poverty 2017, 6, 32–40. 10.1186/s40249-017-0251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitali L.; Miller J. M.; Mwenda M. C.; Bridges D. J.; Hawela M. B.; Hamainza B.; Chizema-Kawesha E.; Eisele T. P.; Chipeta J.; Lindtjørn B. Distribution of Plasmodium species and assessment of performance of diagnostic tools used during a malaria survey in Southern and Western Provinces of Zambia. Malar. J. 2019, 18, 130–139. 10.1186/s12936-019-2766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H.; Jin C. X.; Peng A. P.; Xie H. Y.; Xie S. H.; Feng Y. F.; Xie A. Q.; Li J. J.; Fang C.; Yang Q.; et al. Characterization of γδT cells in lung of Plasmodium yoelii-infected C57BL/6 mice. Malar. J. 2021, 20, 89–104. 10.1186/s12936-021-03619-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. M.; Guang P. W.; Li L.; Song H.; Huang J.; Li Y.; Wang L.; Hu J. Early rapid diagnosis of Alzheimer’s disease based on fusion of near- and mid-infrared spectral features combined with PLS-DA. Optik 2021, 241, 166485. 10.1016/j.ijleo.2021.166485. [DOI] [Google Scholar]
- Mahadevan S.; Shah S. L.; Marrie J. M.; Slupsky C. M. Analysis of Metabolomic Data Using Support Vector Machines. Anal. Chem. 2008, 80, 7562–7570. 10.1021/ac800954c. [DOI] [PubMed] [Google Scholar]
- Stelzer G.; Rosen N.; Plaschkes I.; Zimmerman S.; Twik M.; Fishilevich S.; Stein T. I.; Nudel R.; Lieder I.; Mazor Y..; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinf. 2016, 54, 1.30.1. 10.1002/cpbi.5. [DOI] [PubMed] [Google Scholar]
- Szklarczyk D.; Andrea F.; Stefan W.; Kristoffer F.; Davide H.; Jaime H. C.; Milan S.; Alexander R.; Alberto S.; Tsafou K. P.; et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]