Abstract
Background
The World Health Organization (WHO) recommends parasitological testing of all suspected malaria cases using malaria rapid diagnostic tests (mRDTs) or microscopy prior to treatment. Some governments have extended this responsibility to community health workers (CHWs) to reduce malaria morbidity and mortality through prompt and appropriate treatment. This is an update of a Cochrane Review first published in 2013.
Objectives
To evaluate community‐based management strategies for treating malaria or fever that incorporate both a definitive diagnosis with an mRDT and appropriate antimalarial treatment.
Search methods
We searched CENTRAL, MEDLINE, Embase, five other databases, and three trials registers up to 14 September 2021.
Selection criteria
We included individually randomized trials and cluster‐randomized controlled trials (cRCTs), controlled before‐after studies, and controlled interrupted time series studies in people living in malaria‐endemic areas, comparing programmes that train CHWs and drug shop vendors to perform mRDTs and provide appropriate treatment versus similar programmes that do not use mRDTs, and versus routine health facility care.
Data collection and analysis
We used standard Cochrane methods. For each dichotomous outcome, we extracted the number of participants with the event and the total number of participants in each group, unless studies presented results at a population level only. Primary outcomes were all‐cause mortality, hospitalizations, and number of people receiving an antimalarial within 24 hours. Secondary outcomes were malaria‐specific mortality, severe malaria, outcomes related to antimalarial treatments, antibiotic prescribing to people with a negative microscopy or polymerase chain reaction (PCR) result, parasitaemia, anaemia, and all adverse events.
Main results
We included eight studies from several African countries, Afghanistan, and Myanmar. Staff included CHWs and drug shop vendors.
Community use of malaria rapid diagnostic tests compared to clinical diagnosis
Compared to clinical diagnosis, mRDT diagnosis results in reduced prescribing of antimalarials to people who are found to be malaria parasite‐negative by microscopy or PCR testing (71 fewer per 100 people, 95% confidence interval (CI) 79 to 51 fewer; risk ratio (RR) 0.17, 95% CI 0.07 to 0.40; 3 cRCTs, 7877 participants; moderate‐certainty evidence). This reduction may be greater among CHWs compared to drug shop vendors. People diagnosed by mRDT are more likely to receive appropriate treatment; that is, an antimalarial if they are microscopy‐ or PCR‐positive and no antimalarial if they are microscopy‐ or PCR‐negative (RR 3.04, 95% CI 2.46 to 3.74, 3 cRCTs, 9332 participants; high‐certainty evidence). Three studies found that a small percentage of people with a negative mRDT result (as read by the CHW or drug shop vendors at the time of treatment) were nevertheless given an antimalarial: 38/1368 (2.8%), 44/724 (6.1%) and 124/950 (13.1%). Conversely, in two studies, a few mRDT‐positive people did not receive an antimalarial (0.5% and 0.3%), and one small cross‐over study found that 6/57 (10.5%) people classified as non‐malaria in the clinical diagnosis arm received an antimalarial. Use of mRDTs probably increases antibiotic use compared to clinical diagnosis (13 more per 100 people, 95% CI 3 to 29 more; RR 2.02, 95% CI 1.21 to 3.37; 2 cRCTs, 5179 participants; moderate‐certainty evidence). We were unable to demonstrate any effect on mortality.
Community use of malaria rapid diagnostic tests compared to health facility care
Results were insufficient to reach any conclusion.
Authors' conclusions
Use of mRDTs by CHWs and drug shop vendors compared to clinical diagnosis reduces prescribing of antimalarials to people without malaria. Deaths were uncommon in both groups. Antibiotic prescribing was higher in those with a negative mRDT than in those with a negative clinical diagnosis.
Plain language summary
Does adding rapid diagnostic tests to community‐based malaria programmes improve the treatment of people with malaria or fever?
Key messages
• In regions where malaria is a serious problem (malaria‐endemic areas), many people cannot access the treatment they need. • Rapid diagnostic tests for diagnosing malaria (mRDTs) are simple to use: they involve dropping a finger prick of blood onto a small cassette. • In the context of community‐based programmes in malaria‐endemic areas, when people without professional healthcare qualifications use mRDTs rather than providing a diagnosis based on physical signs and symptoms (clinical diagnosis), the treatment of malaria improves. • Further research is needed to understand the impact of mRDTs on how often antibiotics are prescribed.
How is malaria diagnosed and treated in community‐based programmes?
There are effective and safe treatments for malaria (antimalarial medicines, also known as antimalarials), but many people still cannot access the medicines they need, especially if they live far from health facilities. To improve this situation, local people without formal healthcare qualifications have been trained to diagnose and treat malaria either by recognising malaria signs and symptoms or using an mRDT. These people can be community health workers or vendors in non‐pharmacy medicine shops.
What did we want to find out?
We aimed to compare the effect of two different techniques for diagnosing malaria (mRDTs and clinical diagnosis) used by local people without formal healthcare qualifications, on the treatment given. We also wanted to compare the community use of mRDTs with the routine care provided in health facilities, such as hospitals, to find out which approach resulted in better treatment for people with suspected malaria.
What did we do?
This is an update of a published Cochrane Review. We searched online databases for studies that compared mRDT diagnosis to clinical diagnosis in the community, or mRDT diagnosis and treatment in the community to health facility care. We extracted information about the study designs, the people being treated, the type of non‐medically qualified health worker, their training, the mRDTs and treatments used, and the results (including deaths, number of people with or without malaria treated with an antimalarial, and use of antibiotics). Where possible, we combined results using statistical software.
What did we find?
We found six studies from Africa, one from Myanmar, and one from Afghanistan. Five studies compared community use of mRDT to community clinical diagnosis of malaria, and three compared community use of mRDT to health facility care. Five studies used laboratory tests to double‐check the community diagnosis of malaria (whether mRDT or clinical). All studies except one offered less than one week's training to the staff. The antimalarials used were mostly for taking by mouth, although two studies also trained staff to give medicine to very ill children by inserting it into their bottoms. Most studies also trained staff to send people who had a negative mRDT result, people who were very ill, young babies, and pregnant women to a health facility. The medicines were sometimes free to patients or customers. Customers who had to pay in medicine shops often paid a reduced price. The mRDTs were usually free.
When mRDTs were used in the community, far fewer people who did not actually have malaria received antimalarials (about 71 fewer per 100 people). Community health workers may be less likely than medicine shop vendors to give antimalarials to people without malaria.
Similarly, more people diagnosed by mRDT (about 45 more per 100) got the right treatment: an antimalarial if they definitely had malaria (proven by laboratory tests), no antimalarial if they did not. Some studies found that a few people with a negative mRDT result (as read by the community health worker or medicine shop vendor) received antimalarial anyway. One small study found that some people with a negative clinical diagnosis received an antimalarial. Conversely, other studies found that a few people with a positive mRDT result did not get an antimalarial.
We also found some increased antibiotic use in the mRDT group in people with a negative laboratory test result compared to the clinical diagnosis group (about 13 more uses of antibiotic per 100 people). We were unable to draw any conclusion about people's health or use of treatments when comparing use of mRDTs in the community with the usual health facility care.
There were very few deaths in the study population.
What are the limitations of the evidence?
We are moderately confident that fewer people without malaria receive antimalarials after an mRDT, and that more people diagnosed by mRDT get the right treatment, because the studies that provided these results included a large number of people, even if there were some differences in study methods.
How up to date is this evidence?
This evidence is up‐to‐date to 14 September 2021.
Summary of findings
Background
A previous version of this Cochrane Review examined home‐ and community‐based programmes for treating malaria (Okwundu 2013). Subsequent studies specifically examined adding a malaria rapid diagnostic test (mRDT) to the package of diagnosis and treatment. For this updated Cochrane Review, we narrowed the inclusion criteria to examine the effects of mRDT in community‐based programmes, and excluded home‐based management of malaria (HMM), which is unlikely to include use of an mRDT.
Description of the condition
In 2020, an estimated 241 million cases of malaria occurred worldwide, resulting in approximately 627,000 deaths (exacerbated by service disruptions due to COVID‐19). The greatest burden of this disease is in the African region, which accounts for about 95% of reported cases and 96% of malaria deaths (WHO 2021a). Management of malaria involves preventing transmission and promptly diagnosing and treating cases. Malaria prevention is centred on vector control strategies, such as the use of long‐lasting insecticide‐treated or insecticidal nets (Pryce 2018; WHO 2017), indoor residual spraying (Pluess 2010; Radeva‐Petrova 2014), and larval source management (Tusting 2014). Currently, the World Health Organization (WHO) also recommends intermittent preventive treatment (in infants in certain settings, in pregnant women, and in children under five years of age) as well as seasonal malaria chemoprevention (WHO 2010a; WHO 2012a; WHO 2012b; WHO 2021b; WHO 2022). Prompt diagnosis and appropriate treatment of malaria cases reduces malaria‐related morbidity and mortality, while also reducing the pool of individuals who contribute to transmission. Currently, the WHO recommends that all suspected malaria cases be confirmed by a parasitological test such as microscopy or mRDT before treatment (WHO 2015; WHO 2022).
Current evidence shows that artemisinin‐based combination therapies (ACTs) are safe and effective in the treatment of malaria (Sinclair 2009; Zani 2014). Consequently, they are recommended as the first‐line treatment for uncomplicated Plasmodium falciparum (P falciparum) malaria in children and adults, except in the first trimester of pregnancy (WHO 2021b; WHO 2022). For uncomplicated cases of Plasmodiumvivax (P vivax), Plasmodiumovale (P ovale), Plasmodiummalariae (P malariae), and Plasmodiumknowlesi (P knowlesi) malaria, the recommended first‐line treatment is ACTs or chloroquine in areas with chloroquine‐susceptible infections, and ACTs only in areas with chloroquine‐resistant infections (Gogtay 2013; WHO 2015; WHO 2022). The recommended treatment for severe malaria is intravenous or intramuscular artesunate for at least 24 hours, followed by three days of oral ACT as soon as the person can tolerate oral therapy. Pending transfer to an appropriate facility for further care, adults and children receive a single intramuscular dose of artesunate, artemether or quinine. Alternatively, a single rectal dose of artesunate (10 mg/kg bodyweight), can be administered to children under six years of age as prereferral treatment in remote areas where comprehensive treatment is unavailable (WHO 2015; WHO 2022).
Despite the availability of effective and safe interventions to treat malaria, the proportion of people who receive the recommended treatment is still low, especially in children under five years of age (WHO 2021a). The causes are multifactorial, including lack of physical access to prompt diagnosis and effective treatment services, especially for children in rural areas and hard‐to‐reach populations; lack of health personnel; and weak health systems (UNICEF 2014). Furthermore, not all people seek treatment at health facilities: treatment‐seeking behaviours vary and depend on socioeconomic, religious and cultural factors, amongst others (Kassam 2015).
Description of the intervention
Community‐based interventions, which originated in 1920s China and proliferated in the 1970s in many low‐income settings (Perry 2014), have been shown to improve maternal and paediatric health outcomes (Lassi 2010; Rosato 2008), and, increasingly, malaria outcomes (Christopher 2011; Salam 2014; Smith Paintain 2014). Community‐based programmes complement public health services to ensure prompt detection and treatment of malaria (ICF International 2012). It is imperative that health systems are able to accommodate the needs and preferences of the people they serve, to ensure the proper implementation of malaria treatment programmes. Community‐based management of malaria involves working with people from a particular community who are available to help manage malaria in their setting by performing a clinical or mRDT‐based diagnosis and administering prepackaged antimalarial medicines (Sunguya 2017; WHO 2004). mRDTs are simple, quick, and relatively inexpensive tests that detect parasite‐specific antigens or genus‐ or species‐specific enzymes in whole blood samples from finger pricks (WHO 2015). This recommendation is based on the evidence that private drug outlets and community health workers (CHWs) play a significant role in the treatment of malaria because of their proximity, affordability and convenience. Though these individuals do not generally receive professional or paraprofessional education, they do receive limited training to equip them for the tasks they are expected to perform in the community (Perry 2014). Owing to the success of community case management (CCM) of malaria, this intervention has been expanded to incorporate the management of other childhood illnesses, such as pneumonia and diarrhoea, under the integrated community case management (iCCM) approach (Young 2012).
How the intervention might work
The spectrum of activities involved in home‐ and community‐based management of suspected malaria is quite broad. Winch 2005 summarized interventions for the case management of children with malaria or pneumonia outside health facilities into seven models, six of which are relevant to the management of malaria. These models are constructed depending on who assesses the sick child (family versus CHW), method of diagnosis (microscopy, mRDT, or clinical assessment), provision of an antimalarial by CHW or family, and method of referral to the nearest health facility (Winch 2005). The duties of those involved often include prevention and health promotion specific to malaria, as well as case identification and management (Sunguya 2017). Other providers of antimalarials include registered or unregistered drug shops operating a private service with varying levels of diagnostic and case management capacity or training.
The WHO guidelines for the management of malaria have evolved considerably since 2006 (WHO 2006; WHO 2010b; WHO 2015; WHO 2021b; WHO 2022). Though ACTs have remained the recommended treatment for uncomplicated malaria, the guidelines increasingly emphasize the utility of parasitological testing of malaria using RDTs or microscopy prior to treatment. The aims of this approach are to improve care in parasite‐positive people, identify parasite‐negative people who may have other conditions so that they may also receive appropriate treatment, and thereby ensure rational use of antimalarials (WHO 2021b; WHO 2022). This approach has revolutionized the delivery of community‐based treatment of malaria, with increased incorporation of RDTs in these programmes to ensure accurate diagnosis of fever by CHWs and related workers. As summarized in Figure 1 and Figure 2, community‐based programmes for the management of malaria aim to reduce malaria‐related morbidity and mortality by increasing access to and availability of antimalarial treatment, and ensuring prompt and appropriate treatment of cases and adherence to treatment. The intervention also ensures timely identification and referral of cases of severe malaria and fevers from other causes (Amouzou 2014; WHO 2004). Despite the evidence presented in these reviews, uncertainty remains about the effectiveness of community‐based management strategies for treating malaria or fever specifically where mRDTs have been incorporated.
1.
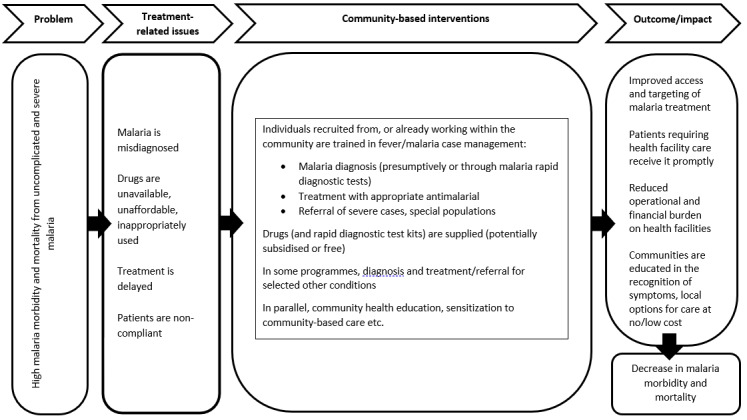
Conceptual framework for community‐based programmes for malaria.
2.
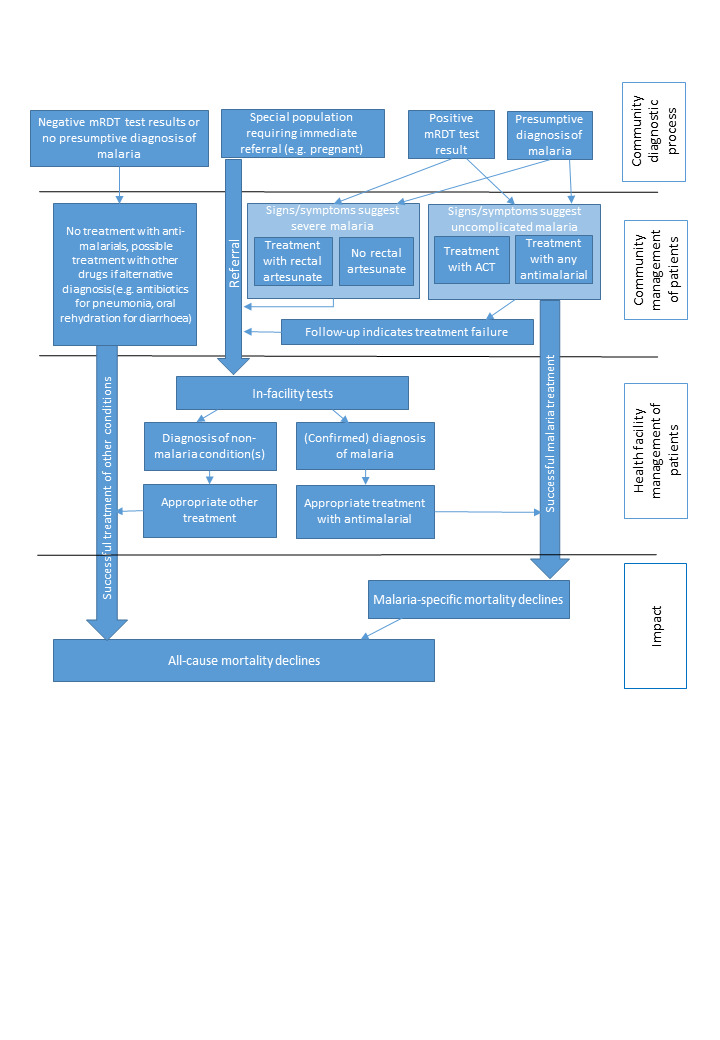
Logic model for community‐based programmes for malaria. mRDT: malaria rapid diagnostic test; ACT: artemisinin‐based combination therapy.
Why it is important to do this review
Several reviews have examined the evidence on home‐ and community‐based interventions for the management of malaria, as summarized in Table 3. The previous version of this Cochrane Review aimed to assess the effectiveness of home‐based and community‐based management strategies for treating malaria or fever (Okwundu 2013). It included 10 studies: eight involving presumptive treatment of fevers and two where CHWs were trained to use mRDTs to guide treatment decisions (Okwundu 2013). The single study involving retail outlet staff did not equip them with mRDTs (Kangwana 2011). There was moderate‐certainty evidence that home‐ and community‐based intervention for malaria reduced time to treatment with an effective antimalarial, and reduced all‐cause mortality. However, the intervention had little or no effect on the prevalence of anaemia, and there was uncertainty regarding the effect of the intervention on hospitalizations and parasitaemia prevalence. Gaps in the evidence included a paucity of studies examining:
1. Summary of systematic reviews on home‐ and community‐based interventions for malaria.
| Study ID | Objectives | Date last search / restriction | Types of studies | Population | Intervention | Comparison | Outcomes | No of studies | Summary of findings |
|
Okwundu 2013 Home‐ or community‐based programmes for treating malaria |
To evaluate home‐based and community‐based management strategies for treating malaria or fever | 2012 | cRCT, CBA, ITS | People living in malaria‐endemic areas | Any programme that trains relevant actors (mothers or caregivers, community‐based volunteers, community‐based health workers, or drug sellers) to recognize and treat fevers with AMs | Health facility‐based care, or alternative home‐ or community‐based programme for recognizing and treating malaria or fevers |
Primary: all‐cause mortality Secondary: malaria‐specific mortality, hospitalizations, severe malaria, treatment with recommended AM within 24 hours, treatment with any AM, parasitaemia, anaemia, AEs |
10 | Home‐ or community‐based interventions providing antimalarial drugs free of charge probably improve prompt access to AMs. Moderate‐quality evidence from rural Ethiopia shows they may reduce all‐cause mortality when implemented in appropriate settings. Programmes treating all fevers presumptively with AMs lead to overuse, and potentially undertreatment of other causes of fever. Incorporating mRDTs into home‐ or community‐based malaria programmes may help to reduce this overuse, and has been shown to be safe under trial conditions. |
|
Hopkins 2007 Impact of home‐based management of malaria (HMM) on health outcomes in Africa: a systematic review of the evidence |
To summarize the current evidence base for HMM, and to identify areas where further research could guide implementation of HMM in Africa | 2007 (Africa) |
cRCT, CBA, observational | Not specified | AM administered presumptively for febrile illness by local community members with no formal education in health care | — | Case presentations including malaria morbidity, mortality, or malariometric indices (parasite rates, haemoglobin or packed cell volume, spleen rates) | 6 | Presumptive treatment of febrile children with prepackaged antimalarials in HMM is likely to increase delivery of effective drugs, improve timing, adherence, and dosing of treatment. Evaluations of community acceptability and feasibility are encouraging, but further study of impact on morbidity and mortality will provide stronger evidence to support sustained implementation of community‐based interventions |
|
Christopher 2011 Thirty years after Alma‐Ata: a systematic review of the impact of community health workers delivering curative interventions against malaria, pneumonia and diarrhoea on child mortality and morbidity in sub‐Saharan Africa |
To systematically review randomized and non‐randomized studies of CHWs' impact on child mortality in sub‐Saharan Africa | 2007 (SSA) |
cRCT, CBA, ITS, observational | Children < 6 years | CHWs delivering curative care, with or without preventive services, to children for malaria, pneumonia or diarrhoea | — | Impact of CHW programme on mortality, morbidity or nutritional status in children < 6 years | 7 | CHW programmes can have large impacts on child mortality when they deliver ITNs or malarial chemoprophylaxis in an endemic malaria setting. However, there is still little evidence from Africa on the effectiveness of CHWs delivering curative interventions against pneumonia and diarrhoea or comprehensive packages of interventions against the major causes of mortality in children (pneumonia, diarrhoea, malaria, and, in some settings, HIV) |
|
UNICEF 2012 A systematic review of strategies to increase demand, uptake and quality of community‐based diagnosis and case management of malaria |
1. To assess and report the effectiveness of strategies to improve the quality of services provided by community health workers responsible for malaria case management. 2. To assess and report the effectiveness of strategies to strengthen referrals from CHWs to facility‐based providers, with a focus on the management of malaria. 3. To assess and report the effectiveness of strategies to strengthen the capacity of health systems to support case management, including universal diagnosis, at the community level. 4. To assess and report the effectiveness of strategies to integrate malaria diagnosis and case management with other health services at the community level. 5. To assess and report the effectiveness of strategies at the community level aiming to increase care seeking behaviour for fever |
2011 | RCT, CBA, ITS | Not specified | 1. Strategies to improve the quality of services provided by community health workers responsible for malaria case management. 2. Strategies to strengthen referrals from CHWs to facility‐based providers, with a focus on the management of malaria. 3. Strategies to strengthen the capacity of health systems to support case management, including universal diagnosis, at the community level. 4. Strategies to integrate malaria diagnosis and case management with other health services at the community level. 5. Strategies at the community level aiming to increase care seeking behaviour for fever. |
— | Clinical outcome; proportion of people seen by CHW diagnosed and treated correctly; correct use of mRDT by CHW and treatment according to result; correct referral to formal health facilities; referral completion; proportion of people with fever seeking care promptly (within 24 hours). | 42 | High adherence by CHWs to correct doses of AM in most studies, irrespective of diagnosis or AM policy, or strength of study design (due largely to prepackaged AMs and practical, interactive training techniques). Larger studies with less support had more modest results for prompt and effective treatment than rigorously controlled studies. CHWs demonstrated high ability for safe use of mRDTs and adherence to results, prescribing ACTs to most mRDT‐positive people (and to a minimum of mRDT‐negatives). Challenges remain with respect to action for mRDT‐negative people. Cost‐effectiveness of use of mRDTs depends on level of parasite prevalence. Evidence on CHW ability to diagnose and treat pneumonia is mixed. Few studies evaluated integration of malaria CCM with other interventions but no indication integration reduces quality of CHW malaria treatment. Few studies reported referrals between community and health facility. Very young children with severe disease and those given clear instructions by CHW more likely to comply with referral advice. Elements of health system capacity critical for effective CHW programmes include: ability to treat referred cases, regular supervision, reliable/consistent supply chain for essential medicines and equipment. Additional tasks do not seem to reduce quality of malaria CCM, provided sufficient training, supervision and support is maintained. Reporting on quality of delivery of other interventions is limited. Community mobilization activities to encourage prompt treatment seeking for fever more successful when conducted alongside an intervention to improve malaria treatment provision. Malaria CCM interventions with insufficient mobilization support resulted in low demand for CHW services. No conclusive evidence was found on the impact of user fees for consultations or treatment on CHW utilization or socioeconomic equity of access. Questions remain about relative effectiveness of interventions relating to particular aspects of CHW quality (e.g. relative importance of supervision or different models of training). |
|
Smith Paintain 2014 CHWs and stand‐alone or integrated case management of malaria: a systematic literature review |
1. To assess evidence for interventions to increase quality of services provided by CHWs responsible for malaria case management among children < 5 years 2. To integrate malaria diagnosis and case management with other health services at community level, with emphasis on case management of uncomplicated pneumonia 3. To increase capacity of health systems to support case management at community level 4. To strengthen referrals from community to facility‐based providers |
2013 |
cRCTs, pre‐post without control, ITS, some post‐only | Children < 5 years | Intervention to introduce or improve community‐based management (CHWs) of malaria where objective and standardized impact or outcome measures were reported | — | CHW performance (CCM, integration with other health interventions), strengthening of health system support for CCM and referrals from community to health facility, clinical outcomes (all‐cause and malaria mortality, severe malaria morbidity), hospitalizations, anaemia, haemoglobin, splenomegaly, treatment response | 43 | CHWs are able to provide good quality malaria care, including performing procedures such as mRDTs. Appropriate training, clear guidelines, and regular supportive supervision are important facilitating factors. Crucial to sustainable success of CHW programmes is strengthening health system capacity to support commodity supply, supervision, and appropriate treatment of referred cases. The little evidence available on referral from community to health facility level suggests that this is an area that needs urgent attention. The studies of integrated CCM suggest that additional tasks do not reduce the quality of malaria CCM provided sufficient training and supervision are maintained. Relatively little evidence that CHWs can impact mortality and morbidity. |
|
Ruizendaal 2014 Success or failure of critical steps in community case management of malaria with RDTs: a systematic review |
Provide a comprehensive overview of the success or failure of critical steps in malaria CCM with mRDTs | 2013 |
Various | Not specified | RDT‐based malaria CCM by CHWs. Excluded: studies on iCCM in which the individual effect of mRDT‐based malaria CCM on outcome cannot be identified |
Presumptive malaria CCM, no malaria CCM | Test performance, execution, interpretation, adherence to results, effect on morbidity and mortality, adherence to test results, referral completion, social acceptance, community uptake, stock‐outs, CHW incentives and motivation, cost‐effectiveness | 27 | Malaria CCM is generally well executed by CHWs, but there are several barriers for its success. Lower mRDT specificity could lead to missed diagnoses of non‐malarial fevers. Other threats for malaria CCM are non‐adherence to negative test results and low referral rates. Integrated CCM may overcome some of these barriers. Morbidity and mortality are not adequately investigated |
|
Amouzou 2014 Assessing the impact of integrated community case management (iCCM) programs on child mortality: review of early results and lessons learned in Sub‐Saharan Africa |
To review recent experience in documenting and attributing changes in under‐5 mortality to the specific interventions of a variety of iCCM programmes | 2013 (English) |
cRCTs, stepped‐wedge, quasi‐experimental | Children aged 2–59 months | iCCM (pneumonia with antibiotics, malaria with antimalarials, diarrhoea ORS/zinc) | — | Mortality | 8 | 6 of 8 studies showed a higher decline in mortality among children 2–59 months in program areas compared to comparison areas, although this was statistically significant in only 1 study with a decline of 76% larger in intervention than in comparison areas |
|
Awor 2014 Systematic literature review of integrated community case management and the private sector in Africa: relevant experiences and potential next steps |
To determine the extent to which the private sector has been utilized in providing integrated care for sick children < 5 years old with community‐acquired malaria, pneumonia or diarrhoea | 2014 | cRCT, quasi, pre/post with/without control | Children < 5 years | Any intervention with drugs or diagnostics for malaria (CCM), pneumonia or diarrhoea, or a combination of those illnesses (iCCM; private sector) | — | Descriptive only – number/titles of studies | 62 | While the private sector is an important source of care for children in low‐income countries, little has been done to harness the potential of this sector in improving access to care for non‐malaria fever in children within the community |
|
Boyce 2017 Use of malaria RDTs in various health contexts across sub‐Saharan Africa: a systematic review |
Assesses the diagnostic use of mRDTs in 4 different contexts: health facilities, the community, drug shops and schools | 2016 |
Various | Not specified | Diagnostic use of mRDTs in healthcare facilities, drug shops, schools, or by CHWs (N = 16) | — | Performance of mRDT, appropriateness of treatment | 16/52 | RDTs generally used well, though compliance with test results is variable, especially in formal healthcare sector. 7/16 studies showed CHWs displaying high levels of adherence to treatment guidelines. All studies showed CHWs providing appropriate treatment at least 80% of the time and CHWs rarely provided inappropriate treatment of mRDT‐negative people. |
|
Sunguya 2017 |
What is the role of CHWs and related cadres in malaria prevention, case management and health promotion in highly malaria‐endemic regions? What are the challenges encountered while implementing iCCM for malaria using CHWs and related cadres? | Not stated | cRCT, quasi, pre/post, observational, secondary | Not specified | iCCM, malaria CCM, SMC, and home‐based management of fever by CHWs and related cadres (e.g. village health volunteers and other lay health workers: home care providers and community medicine distributors) | — | Description of the roles and challenges faced by CHWs and related cadres | 66 | CHWs and related cadres have been taking roles similar to those of more qualified health workers. They are important actors in malaria control and elimination but suffer from the health system challenges including financing, logistics, human resource management, and stewardship. To meet targets in sustainable development in health and to save countless lives and morbidity, CHWs and related cadres must be well resourced and sustained |
|
Visser 2017 Introducing malaria rapid diagnostic tests in private medicine retail outlets: a systematic literature review |
1. Examine outcomes pertaining to mRDT uptake, provider adherence to test results, referral, cost and safety 2. Review characteristics of each intervention to introduce mRDT use to explore factors that are associated with mRDT uptake and provider adherence to test results |
2016 | Various | Any private medicine retail outlet providers and the people they serve | Any introduction of mRDTs with or without supporting interventions, where mRDTs were performed by private medicine retail outlet staff | Studies were included regardless of whether there was a comparison group, and whether the comparison group was randomly allocated | Proportion of people seeking treatment for fever or suspected malaria tested with mRDT; mRDT positivity; proportion of people seeking treatment for fever or suspected malaria sold ACTs, regardless of testing; adherence to negative or positive results; proportion of people sold ACTs in presence of positive result or not sold ACTs or other AM in presence of negative result; proportion of people sold antibiotics in presence of positive result; proportion of people sold antibiotics in presence of negative mRDT result; referrals; accuracy and safety; median retail price of mRDT | 12 | RDT uptake varied from 8%–100%. Provision of ACTs for people testing positive ranged from 30%–99% and was > 85% in 5 studies. Of those testing negative, provision of AMs varied from 2%–83% and was < 20% in 8 studies. Longer provider training, lower mRDT retail prices and frequent supervision appeared to have a positive effect on mRDT uptake and provider adherence to test results. Performance of mRDTs by PMR vendors was generally good, but disposal of medical waste and referral of patients to public facilities were common challenges. Expanding services of PMRs to include malaria diagnostic services may hold great promise to improve malaria case management and curb overtreatment with AMs. However, doing so will require careful planning, investment and additional research to develop and sustain effective training, supervision, waste management, referral and surveillance programmes beyond the public sector |
AE: adverse event; AM: antimalarial; CBA: controlled before‐after study; CCM: community case management; CHW: community health worker; cRCT: cluster‐randomized controlled trial; HMM: home‐based management of malaria; iCCM: integrated community case management; ITN: insecticide‐treated net; ITS: interrupted time series; mRDT: malaria rapid diagnostic test; PMR: private medicine retail outlet; RCT: randomized controlled trial; RDT: rapid diagnostic test; ORS: oral rehydration salt; SSA: sub‐Saharan Africa; SMC: seasonal malaria chemoprevention.
community‐based interventions including parasitological confirmation of malaria diagnosis with RDTs;
adverse events relating to the intervention;
severe malaria; and
malaria‐specific mortality.
Before and since the publication of Okwundu 2013, other reviews have examined the evidence on home‐ and community‐based interventions for the management of malaria, as summarized in Table 3. Hopkins 2007 synthesized the evidence on the impact of HMM on health outcomes in Africa, finding that the number of relevant studies was limited, that the impact on morbidity and mortality endpoints was mixed, and that the evidence base for HMM in Africa regarding the use of ACTs was narrow, requiring additional research. No included studies involved mRDTs.
Several other reviews have evaluated the evidence on related questions, including the effectiveness of CHWs in delivering iCCM (Amouzou 2014; Awor 2014; Christopher 2011). Again, there was limited evidence available for this assessment, highlighting the need for larger and more rigorous trials. Three published reviews narrowed the focus to examine the impact of mRDTs used by private medicine retail outlets and CHWs on CCM of malaria (Boyce 2017; Ruizendaal 2014; Visser 2017). Boyce 2017 found that CHWs rarely provided inappropriate treatment of mRDT‐negative participants. However, Ruizendaal 2014 found some non‐adherence to negative mRDT results and low referral rates. More recently, Visser 2017 found that mRDT uptake by consumers varied from 8% to 100%; and that among the people who tested negative, provision of antimalarials varied from 2% to 83% and was less than 20% in eight studies. Longer provider training, lower mRDT retail prices and frequent supervision appeared to have a positive effect on mRDT uptake and provider adherence to results. Performance of mRDTs by vendors was generally good, but disposal of medical waste and referral of customers to public facilities were common challenges. Visser 2017 concluded that expanding services to include malaria diagnostics may improve malaria case management and curb overtreatment with antimalarials.
However, doing this would require careful planning. Two reviews assessed the effectiveness of strategies to improve CCM of malaria (Smith Paintain 2014; UNICEF 2012). Though they included clinically important outcomes such as reduction in all‐cause mortality and severe malaria morbidity, these reviews were limited to studies carried out in sub‐Saharan Africa (Smith Paintain 2014), and from 2000 onwards (UNICEF 2012). They concluded that CHWs demonstrated high ability in the safe use of mRDTs and adherence to results, prescribing ACTs for most mRDT‐positive participants and few ACTs for mRDT‐negative participants. However, challenges remain with respect to management of mRDT‐negative people, and the long‐term success of CHW programmes requires strengthening of health system capacity to support commodity supply, supervision, and appropriate treatment of referred cases. Sunguya 2017 described the role of CHWs and related workers in malaria prevention and management in malaria‐endemic regions, and highlighted the challenges they encounter, which include lack of remuneration, stockouts of essential drug supplies, and poor supervision. Even after the publication of these reviews, uncertainty remains about the effectiveness of community‐based management strategies for treating malaria or fever.
Since Okwundu 2013, more studies have examined the impact of community‐based interventions on the treatment of malaria. Furthermore, due to a shift in research focus, there are newer areas of interest not included in the original review, such as the use of prereferral artesunate for severe cases. Other related reviews described in Table 3 fall short of answering our question despite overlaps in evidence. It is imperative that the evidence be kept up to date to inform policy. In the first edition of this review, the authors noted that adding mRDTs may reduce overuse of antimalarial drugs. Very little evidence was available on community programmes using mRDTs at that time, but a series of relevant studies have been published since. As mRDTs are increasingly adopted in line with recommendations, it is less likely that family members or caregivers can provide home‐based treatment for malaria, owing to supply, quality, and supervision issues. Therefore, this review update will not include home‐based management of fever or malaria.
The previous review also highlighted the importance of diagnosing and treating fever in people with a negative mRDT result. This concern has contributed to the development of iCCM, which includes the use of antibiotics in pneumonia, for example. This is covered in a separate Cochrane Review (Oliphant 2017).
Objectives
To evaluate community‐based management strategies for treating malaria or fever that incorporate both a definitive diagnosis with an mRDT and appropriate antimalarial treatment.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) for which the unit of randomization is the individual or cluster; controlled before‐after studies (CBAs) with a contemporaneous control group and at least two sites per arm; and controlled interrupted time series studies.
Types of participants
People living in malaria‐endemic areas.
Types of interventions
Intervention
Any programme that trains community‐based volunteers, CHWs, other non‐medically qualified providers, or sellers of drugs (drug shop vendors) to perform mRDT diagnosis and treat positive cases with an antimalarial.
Comparison
We considered studies that compared the intervention to:
community‐based clinical diagnosis and treatment of malaria; or
routine health facility‐based care (which is likely to use mRDTs).
Types of outcome measures
We considered the following outcomes in intervention and control arms.
Primary outcomes
All‐cause mortality
Hospitalizations
Number of people receiving an antimalarial within 24 hours
Secondary outcomes
Malaria‐specific mortality
Severe malaria
-
Other outcomes related to antimalarial treatment as reported by study authors:
Antimalarial prescribing to microscopy‐ or polymerase chain reaction (PCR)‐negative people
Appropriate treatment (a composite measure defined against the malaria microscopy or PCR result: positive malaria treated with an antimalarial and negative cases receiving no antimalarial)
Antimalarial treatment based on mRDT results
Antibiotic prescribing to microscopy‐ or PCR‐negative people
Parasitaemia
Anaemia
Any adverse event as reported in the included studies
Other outcomes considered relevant for the review
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Electronic searches
We searched the following databases using the search terms and strategy presented in Appendix 1.
Cochrane Infectious Diseases Group Specialized Register (14 September 2021).
Cochrane Central Register of Controlled Trials (CENTRAL, 2021, Issue 9, published in the Cochrane Library).
MEDLINE PubMed (1946 to 14 September 2021).
Embase Ovid (1947 to 14 September 2021).
Social Science Citation Index‐Expanded (Web of Science, 1900 to 14 September 2021).
CINAHL EBSCOHost (1982 to 14 September 2021).
PsycINFO EBSCOHost (1967 to 14 September 2021).
We also searched the WHO International Clinical Trials Registry Platform (ICTRP; who.int/ictrp), and ClinicalTrials.gov (clinicaltrials.gov), for trials in progress, on 14 September 2021, with the search terms 'malaria', 'home‐based', 'community‐based' and 'presumptive treatment'.
Searching other resources
We handsearched relevant conference proceedings including MIM Pan‐Africa Malaria and ASTMH Conferences from 2011 to 2021, and checked the reference lists of all identified studies and other relevant reviews.
Data collection and analysis
Selection of studies
Two review authors (EA, AB) independently assessed the titles and abstracts obtained from the searches to identify potentially eligible studies using a study selection form. We resolved any discrepancies through discussion, and when required, consulted a third person (Paul Garner (PG)). We obtained the full text articles of all selected abstracts to formally assess eligibility using the prespecified eligibility criteria. We used a reference manager to identify multiple publications from the same study. Reasons for excluding studies are summarized in the Characteristics of excluded studies table.
Data extraction and management
Two review authors (EA, AB) independently extracted data from the studies using a detailed, prepiloted data extraction form. Any discrepancies were resolved through discussion and consensus or, if necessary, by consulting a third person (PG). We extracted the following information.
-
Study data, comprising:
study duration;
study location;
study design;
source of funding;
study site; and
prevalence of malaria.
-
Participant data, comprising:
inclusion and exclusion criteria;
ages; and
sample size
-
Details of the interventions, including:
nature of the drug shop vendor, CHW, or other non‐medically qualified health worker;
content, type and duration of training (including details of the mRDT and treatments to be used, how mRDTs were provided and should be used);
supervision of the scheme (frequency of visits to the provider, if any; form of recording supervision);
incentives or payment for the provider;
use of charts, algorithms, posters and other materials to help with dosing; and
any other community activities organized to support the scheme.
-
Outcome‐related data, including:
all‐cause mortality;
malaria‐related mortality;
hospitalizations;
malaria parasitological prevalence;
adverse events;
anaemia;
parasitaemia;
severe malaria;
treatment with an antimalarial as reported by study authors; and
antibiotic prescribing to microscopy‐ or PCR‐negative people.
For each dichotomous outcome, we extracted the number of participants with the event and the total number of participants in each group.
Cluster‐randomized controlled trials
Where a trial adjusted for clustering, we extracted the adjusted measure of effect and its 95% confidence interval (CI). However, if the trial did not adjust for clustering, we attempted to contact study authors to request estimates for the intra‐cluster correlation coefficient (ICC) values, to make appropriate adjustments in our analysis using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). In case of no response, we sought estimates of ICC values from similar trials. Failing that, we conducted a sensitivity analysis imputing three different ICC values of 0.01, 0.05 and 0.1 to assess the robustness of the results as per the original review (Okwundu 2013).
Non‐randomized trials
For dichotomous outcomes in CBAs, we extracted event rates before and after the intervention for both treatment groups. Where studies presented measures of effect that compared intervention versus control, we extracted the result and noted whether the measure of effect was adjusted for any confounders. We found no interrupted time series studies, only CBAs.
Assessment of risk of bias in included studies
Two review authors (EA and MM) independently assessed risk of bias using different criteria depending on the study design, as indicated below. Any differences were resolved through discussion. We presented the results of this assessment in the risk of bias summary, risk of bias graph, and risk of bias tables.
Individually randomized trials
We assessed the risk of bias of individual RCTs, including cross‐over trials, using the Cochrane risk of bias tool (RoB 1; Higgins 2017). This approach assesses the risk of bias across the following five domains.
Bias arising from the randomization process.
Bias due to deviations from intended interventions.
Bias due to missing outcome data.
Bias in measurement of the outcome.
Bias in selection of the reported results.
Cluster‐randomized controlled trials
For cluster‐randomized controlled trials (cRCTs), we included additional aspects recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Non‐randomized trials
For non‐randomized trials, we used the suggested risk of bias criteria for Cochrane Effective Practice and Organisation of Care reviews (EPOC 2017).
Measures of treatment effect
We presented the measures of treatment effect as reported by the study authors, with 95% CIs and tests of statistical significance where available. We summarized dichotomous outcomes using risk ratios (RRs) with 95% CIs (or other measures of effect if RRs were not presented in the trial reports of non‐randomized or cRCTs).
Unit of analysis issues
Where cluster‐randomized controlled trials did not explicitly adjust for the cluster design, in order to make appropriate adjustments ourselves, we sought ICC values from the included trials or from similar trials on malaria. Where appropriately adjusted effect estimates were not available for a particular outcome, we adjusted our effect estimates (for binary outcomes) by dividing both the numerator and denominator by the design affect. The design effect is given by 1 + (m − 1) × ICC, where m is the average cluster size and ICC is the intra‐cluster correlation coefficient obtained from the study. Where no ICC was reported, we used a common ICC of 0.01. For one cross‐over cRCT, we were unable to adjust for the cross‐over effect (only for clustering), as there was insufficient information and no response from the corresponding authors (Mubi 2011).
Dealing with missing data
We contacted study authors where there were missing or unclear data. No imputation measures for missing data were applied. Participants with missing information (including losses to follow‐up) were excluded from the analysis.
Assessment of heterogeneity
We assessed statistical heterogeneity by visually inspecting the forest plots (to detect overlapping CIs), using the I² statistic (a value of 50% was considered to represent moderate heterogeneity; Higgins 2003; Deeks 2022), and applying the Chi² test (a P value of 0.10 was considered to indicate statistically significant heterogeneity).
Assessment of reporting biases
The likelihood of reporting bias was not examined using funnel plots, as we included fewer than 10 trials in this review update.
Data synthesis
We analysed the data using Review Manager Web (Review Manager Web 2022) and Stata 16 (Statacorp), and pooled trial results in meta‐analyses where appropriate. We used a random‐effects model throughout as we were estimating an average effect and expected heterogeneity across trials. A fixed‐effect model was only used if clinical heterogeneity was absent and statistical heterogeneity was not substantial (where substantial statistical heterogeneity was defined as unexplained I² statistic above 80% or Chi² below 0.001. For example, we used a fixed‐effect model where there was no clinical heterogeneity across study effect estimates (i.e. effect estimates were clinically equivalent) despite significant statistical heterogeneity due to small standard errors. Where possible and appropriate, we added the adjusted raw data (as dichotomous outcomes) in Review Manager Web (Review Manager Web 2022) for analysis or via the generic inverse variance method for reported cluster (or cross‐over) adjusted effect estimates and 95% CIs. When reporting total number of participants per arm, we reported the unadjusted totals. For meta‐analysis, we used ITT data where possible. We reported CBAs narratively and did not pool the results.
Statistical heterogeneity was explored via subgroup analysis per outcome, and reported without pooled effect estimates.
Subgroup analysis and investigation of heterogeneity
We conducted a subgroup analysis to explore reasons for significant heterogeneity between trials, dividing the results according to the role of the people who received training (drug shop vendors or CHWs). We were unable to create subgroups for transmission zone or different intervention methods, because we included a small number of trials, some of which did not present the relevant data separately. Additionally, we were unable to perform subgroup analysis by age as these strata were not mutually exclusive.
Sensitivity analysis
We conducted sensitivity analyses (post hoc) to determine the effect of collapsing different transmission zone trial estimates reported in Ndyomugyenyi 2016, and the effect of relaxing our exclusion criteria to include Mbonye 2015, which had a large proportion (50%) of healthcare professionals (nurses who owned drug shops). We performed the latter analysis in case other studies had also included healthcare professionals such as nurses.
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of the evidence across each outcome measure using the GRADE approach. The certainty rating across studies has four levels: high, moderate, low, or very low. RCTs are initially categorized as high certainty, but can be downgraded after assessment of five criteria: risk of bias, inconsistency, indirectness, imprecision, and publication bias. Observational studies are initially categorized as low certainty and can be downgraded by these same criteria. In exceptional circumstances they may be upgraded: where the effect size is large, where all plausible confounders are likely to reduce the effect size, and where there is evidence of a dose‐response effect (Guyatt 2011). We presented the results using informative statements combining size and certainty of effects, as recommended for systematic reviews (Santesso 2020).
Results
Description of studies
See Table 4 and Table 5 for a summary of each included study.
2. Summary of trials comparing rapid diagnostic tests to clinical diagnosis in community‐based interventions.
| Study ID | Design | Country (setting) | People trained | Length of training | Summary of intervention | Summary of control | Outcomes assessed | Drugs/mRDTs free to participants | Supervision |
| Ansah 2015 | cRCT | Ghana (rural) | Chemical sellers | 1 day over and above standard 3 days malaria case management training for both groups | Training to treat P falciparum malaria after positive mRDT with AL, AQAS, or DP, and to refer if negative mRDT | Current practice of chemical sellers dispensing medicines without test results | Mortality, appropriate treatment, treatment for malaria based on mRDT results, antibiotic after negative mRDT, referrals | Drugs: no, but subsidized mRDTs: yes | Fieldworkers and supervisors provided technical support. Accuracy of records of drugs dispensed validated by random checks of forms and 'mystery clients'. Direct observation of interactions between chemical sellers and customers by checklist on weekly basis for first month and a further week midway through trial |
| Cohen 2015 | cRCT | Uganda (unclear) | Drug shop vendors | 2 days | Training in use of mRDTs, and recommendation for purchase of first‐line ACT (no algorithms or details on referrals when negative mRDT) | Current practice of drug shops selling antimalarials generally without use of mRDTs | Number receiving an antimalarial | Drugs: no, but subsidized mRDTs: no | Monthly visits to track stock and usage of mRDTs and compliance with protocols for testing using a 17‐point checklist. Unused kits sent for lot testing every 3 months |
| Leslie 2017 | cRCT | Afghanistan (rural) | CHWs | Half day over and above standard 1 day refresher malaria case management training for both groups | Training to treat after positive P falciparum mRDT with SP/AS, and after pan‐specific (assumed P vivax) mRDT with CQ. If negative no drug, unclear about referral | Refresher workshop for CHWs on community‐based treatment of suspected malaria with CQ or SP | Number of people receiving an antimalarial, appropriate treatment, treatment for malaria based on mRDT results, antibiotic after negative mRDT, referrals | Not reported | CHWs normally supervised by a manager who supplies basic items including essential medicines |
| Mubi 2011 | Cross‐over cRCT | Tanzania (rural) | CHWs | 1 week | Training to treat P falciparum malaria after positive mRDT with AL. Unclear about referral if mRDT‐negative | Current practice of CHWs dispensing AL based on clinical diagnosis of malaria | Mortality, number of people receiving an antimalarial, treatment for malaria based on mRDT results, adverse events, referrals | Not reported | Regular, no details |
| Ndyomugyenyi 2016 | cRCT | Uganda (rural) | CHWs | 4 days | Trained to treat P falciparum malaria after positive mRDT with AL or rectal AS, and to refer if negative mRDT as appropriate | Current practice of CHWs dispensing AL or rectal AS based on presumptive diagnosis of malaria | Number of people receiving an antimalarial, appropriate treatment | Not reported | Close supervision by project staff for 6 months through meetings (promoting accurate and complete records, how to handle difficult situations), scaled back thereafter to when CHWs collected supplies |
ACT: artemisinin‐based combination therapy; AL: artemether‐lumefantrine; AQ: amodiaquine; AS: artesunate; CHW: community health worker; CQ: chloroquine; cRCT: cluster‐randomized controlled trial; DP: dihydroartemisinin‐piperaquine; mRDT: malaria rapid diagnostic test; P falciparum: Plasmodium falciparum; P vivax: Plasmodium vivax; SP: sulfadoxine‐pyrimethamine.
3. Summary of trials comparing community‐based interventions using rapid diagnostic tests with health facility‐based care.
| Study ID | Design | Country (setting) | People trained | Duration of training | Summary of intervention | Summary of control | Outcomes assessed | Drugs/mRDTs free to participants | Supervision |
| Ohnmar 2012 | cRCT | Myanmar (rural) | Unpaid volunteers | 2 days | Trained to treat P falciparum malaria after positive mRDT with AL or presumptive P vivax with CQ in those testing negative | Health facility care | Mortality, hospitalizations | Drugs: unclear mRDTs: no | Midwives in nearest health facility routinely monitored/supervised volunteers during their monthly immunization visits. Malaria supervisors also occasionally visited volunteers. |
| Swana 2016 | CBA | Democratic Republic of the Congo (rural) | CHWs | 3 days | Trained to treat P falciparum malaria after positive mRDT with AQAS or rectal AS, and to refer if negative mRDT as appropriate | Health facility care | Parasitaemia | Drugs: yes mRDTs: not reported | Initial observation of mRDTs by supervisors with corrective actions over 2–4 days, weekly visits thereafter, 3 monthly meetings with investigators |
| Thiam 2012 | CBA | Senegal (rural) | Home care providers | 3 days theory, 2 weeks practical | Trained to treat P falciparum malaria after positive mRDT with AQAS, and to refer if negative mRDT | Health facility care | Mortality, hospitalizations, referrals | Drugs: no (USD 0.6 adults, USD 0.3 children until May 2010) mRDTs: yes | Post‐training follow‐up, monthly supervision by health post head nurse, co‐ordinating meetings by district health teams and nurses in peripheral health facilities |
AL: artemether‐lumefantrine; AQ: amodiaquine; AS: artesunate; CBA: controlled before‐after study; CHW: community health worker; CQ: chloroquine; cRCT: cluster‐randomized controlled trial; mRDT: malaria rapid diagnostic test; P falciparum: Plasmodium falciparum; P vivax: Plasmodium vivax.
Results of the search
Figure 3 presents the study flow diagram. We retrieved 3060 records through database searching, and there were 2080 after removal of duplicates. After screening by title and abstract, we excluded 2011 records, and assessed 69 full‐text articles for eligibility. We excluded 61 articles, and one study was ongoing. In this review update, we included seven new studies and one study from the original Cochrane Review (Okwundu 2013).
3.
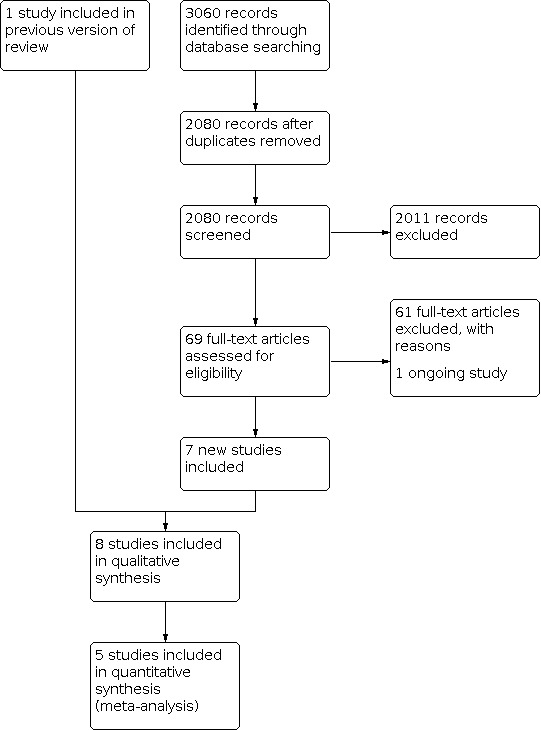
Study flow diagram.
Included studies
Settings
Of the eight included studies, six were conducted in Africa: one in Ghana (Ansah 2015), two in Uganda (Cohen 2015; Ndyomugyenyi 2016), one in Tanzania (Mubi 2011), one in the Democratic Republic of Congo (Swana 2016), and one in Senegal (Thiam 2012). The remaining two studies took place in Afghanistan (Leslie 2017), and Myanmar (Ohnmar 2012). All studies that described the setting indicated that it was rural; however, malaria endemicity and transmission intensities varied.
Study designs, populations, and personnel
There were five cRCTs (Ansah 2015; Cohen 2015; Leslie 2017; Ndyomugyenyi 2016; Ohnmar 2012), one cross‐over cRCT (Mubi 2011), and two CBAs (Swana 2016; Thiam 2012). All cRCTs adjusted for the cluster design, via random‐effects logistic regression, generalized linear mixed models, or adjustment of standard errors for clustering effects. Five studies compared community use of mRDT with community clinical diagnosis of malaria (Ansah 2015; Cohen 2015; Leslie 2017; Mubi 2011; Ndyomugyenyi 2016), while the other three compared community use of mRDT with health facility‐based care (Ohnmar 2012; Swana 2016; Thiam 2012). The people trained to use mRDTs to diagnose malaria were CHWs or equivalent workers with different job titles (paid or unpaid), hereinafter all considered CHWs (Leslie 2017; Mubi 2011; Ndyomugyenyi 2016; Ohnmar 2012; Swana 2016; Thiam 2012); or community‐based drug shop vendors (Ansah 2015; Cohen 2015). Seven studies included adults and children (typically with a minimum age of two to six months), while Ndyomugyenyi 2016 only included children under five years of age. Data collection involved various combinations of:
records kept by the community staff (Ansah 2015; Leslie 2017; Mubi 2011; Ndyomugyenyi 2016; Ohnmar 2012; Swana 2016; Thiam 2012);
household surveys (Cohen 2015; Ohnmar 2012); and
questionnaires for the drug shop vendors (Mubi 2011).
Swana 2016 used pre‐ and postintervention school malaria prevalence surveys for their primary endpoint, while Ohnmar 2012 used death registers and verbal autopsy surveys, and Thiam 2012 used pre‐ and postintervention routine data collected at health facility level. Five studies included microscopy or PCR tests to verify the diagnosis of malaria (Ansah 2015; Leslie 2017; Mubi 2011; Ndyomugyenyi 2016).
Intervention
There were variations in the detail of the intervention, but in all studies the intervention‐specific training (in combination with or over and above standard malaria case management training) offered to community staff lasted less than one week, except in Thiam 2012, where staff received two weeks of practical training after an initial three days of theory. The antimalarial drugs used to treat uncomplicated malaria were predominantly ACTs for P falciparum infection, or chloroquine for Pvivax in Afghanistan and Myanmar (Leslie 2017; Ohnmar 2012). Ndyomugyenyi 2016 and Swana 2016 trained staff to give rectal artesunate as prereferral treatment for severe malaria, and all studies trained staff to refer malaria‐negative people and at‐risk groups to a health facility. Antimalarial drugs were free of charge to participants in Swana 2016, but not in Ansah 2015, Cohen 2015 or Thiam 2012 (through the cost was subsidized in Ansah 2015 and Cohen 2015). The remaining trials did not provide this information. The mRDTs were free for patients or customers in Ansah 2015, Ohnmar 2012, and Thiam 2012, but not in Cohen 2015. All studies supervised community‐based staff through regular visits, with some observation of how they worked. Ansah 2015 also used mystery clients.
Outcomes measured
Four studies reported mortality (Ansah 2015; Mubi 2011; Ohnmar 2012; Thiam 2012), although only Thiam 2012 reported this at a population level. Most other outcomes concerned the use of antimalarials in relation to a test result: either the mRDT result as reported by the CHW or drug shop vendor (i.e. mRDT arm only), or the result of a subsequent laboratory test performed to determine true malaria positivity or negativity.
Three studies presented the composite outcome of appropriate treatment, which was the proportion of participants with a positive microscopy or molecular PCR result from a dried blood spot who received an antimalarial, combined with the proportion of participants with a negative microscopy or blood PCR result who did not receive an antimalarial (Ansah 2015; Leslie 2017; Ndyomugyenyi 2016). This outcome therefore relates to targeting of treatment; those with malaria receive the antimalarial they need, while those without malaria do not.
No study reported our predetermined primary outcome of the number of people receiving an antimalarial within 24 hours, although four reported the number of people receiving an antimalarial with no timeline (Leslie 2017; Mubi 2011; Ndyomugyenyi 2016; Ohnmar 2012), and Ndyomugyenyi 2016 reported appropriate treatment within 24 hours. Swana 2016 reported parasitaemia through school‐based prevalence surveys using a stratified, randomized and proportional sampling method, and Mubi 2011 reported adverse event outcomes, including severe malaria. Though not a predetermined review outcome, we also describe referrals and the operational validity of the mRDTs used, where relevant data were available.
Excluded studies
We assessed the full‐text of 70 articles, excluding 62. The reasons for excluding eight of these are described in the Characteristics of excluded studies table. In most cases, the intervention included case management of diseases other than malaria (Awor 2014; Biemba 2016; Kitutu 2017; Mukanga 2012; Yeboah‐Antwi 2010). In Maloney 2017, the difference between arms was recommended retail price, and Gaye 2020 had mRDTs in both trial arms. In Mbonye 2015, a large proportion of drug shop vendors had professional healthcare qualifications (nurses, midwives or clinical officers; 37.9% in the intervention arm and 56.7% in the control arm), while the remainder were auxiliary nurses or nursing aides. Therefore, we excluded this study from the review as per our eligibility criteria, but included it in a sensitivity analysis in case other studies had included some healthcare professionals without reporting this detail.
Risk of bias in included studies
See the Characteristics of included studies table for a description of risk of bias concerns, Figure 4 for the risk of bias summary, and Figure 5 for the risk of bias graph. As mentioned in Assessment of risk of bias in included studies, we applied different criteria depending on the study design, which is why the table, summary and graph have blank spaces (where a criterion is not relevant to the study design). Similarly, we performed no bias assessment where an outcome was not included for a particular study design.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Different criteria were applied depending on the study design, and we performed no bias assessment where an outcome was not included for a particular study design.
5.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Different criteria were applied depending on the study design, and we performed no bias assessment where an outcome was not included for a particular study design.
Allocation
Most cRCTs used adequate random sequence generation and so were considered at low risk of bias in this regard. An exception was Mubi 2011, where each CHW was assigned a unique number on a ticket to determine the order of cross‐over from use of mRDT to clinical diagnosis every other week. As the publication provided no details on how these unique numbers were assigned, we considered risk of bias to be unclear. Similarly, the limited information provided in Ohnmar 2012 made it impossible to assess risk of bias related to random sequence generation. The details of allocation concealment were insufficient to assess the corresponding risk of bias in all studies, except Mubi 2011, which we considered at low risk of bias, and Ohnmar 2012, which we judged at high risk of bias. We judged all CBAs at high risk of selection bias because of inherent limitations of this study design.
Blinding
While blinding of CHWs or drug shop vendors and participants themselves was not possible in any study, this is unlikely to have affected mortality rates. However, it is unclear whether lack of blinding may affect other outcomes, such as antimalarial prescribing to microscopy‐ or PCR‐negative people (or associated composite measure), or the number of people who receive an antimalarial. Again, the design used by Mubi 2011 may lend itself to a higher risk of bias, as it relied on CHWs themselves collecting data while alternating mRDTs and clinical diagnoses weekly. Outcome measures based on laboratory results (i.e. microscopy or PCR), where laboratory staff were blinded to the intervention, were considered at low risk of detection bias. This was the case for all cRCTs measuring prescribing of antimalarials to microscopy‐ or PCR‐negative participants, or associated composite results. We assessed CBAs at high risk of performance and detection bias because of inherent limitations of this study design.
Incomplete outcome data
We considered most studies at low risk of attrition bias for all outcomes. An exception was Ohnmar 2012, whose methods for capturing mortality data had to be modified mid‐study after the original plan led to under‐reporting. We also considered Cohen 2015 at high risk of attrition bias for number of people receiving an antimalarial, as 40% of shops did not buy the mRDTs and three shops accounted for 32% of mRDTs used. However, it is important to note that this attrition measure was the main outcome measure for the study. The two CBAs were difficult to assess for attrition bias due to limited information.
Selective reporting
We judged all studies at low risk of reporting bias as the publications appear to reflect registered details where available.
Other potential sources of bias
Other biases we considered for cRCTs were recruitment after randomization, and contamination. For recruitment after randomization, baseline imbalance was minimal, and we therefore assessed all studies as having some concerns. While few studies mentioned the possibility of contamination, the description of the study sites did not suggest a high risk of bias. The exception was Mubi 2011, where the cross‐over design naturally lends itself to more potential to bias. It was also unclear how the significant difference in baseline parasite prevalence between groups in Swana 2016 affected this outcome measurement. Not all studies reported on uptake of the mRDTs.
Effects of interventions
Summary of findings 1. Malaria rapid diagnostic test (mRDT) compared to clinical diagnosis in community‐based malaria programmes.
| Malaria rapid diagnostic test (mRDT) compared to clinical diagnosis in community‐based malaria programmes | ||||||
|
Patient or population: people with suspected malaria Setting: malaria‐endemic areas Intervention: mRDT Comparison: clinical diagnosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with clinical diagnosis | Risk with mRDT | |||||
| Mortality | There is very little evidence about the effect of community use of mRDTs on mortality compared to clinical diagnosis. 2 studies reported on mortality, but there were very few events (4 events in total, 3 in mRDT and 1 in clinical diagnosis) | — | 7678 (2 cRCTs) | ⊕⊝⊝⊝ Very lowa | — | |
| Antimalarial prescribing to microscopy‐ or PCR‐negative people | 85 per 100 | 15 per 100 (6 to 34) | RR 0.17 (0.07 to 0.40) | 7877 (3 cRCTs) | ⊕⊕⊕⊝ Moderateb,c | mRDT probably results in a large reduction in antimalarial prescribing to microscopy‐ or PCR‐negative people. |
| Composite appropriate treatment | 22 per 100 | 67 per 100 (54 to 83) | RR 3.04 (2.46 to 3.74) | 9332 (3 cRCTs) | ⊕⊕⊕⊕ Highc | mRDT results in a large increase in composite appropriate treatment. |
| Number of people receiving an antimalarial | 96 per 100 | 20 per 100 (13 to 33) | RR 0.21 (0.14 to 0.34) | 4729 (2 cRCTs) | ⊕⊕⊝⊝ Lowc,d,e | mRDT may result in a large reduction in the number of people receiving antimalarials. |
| Antibiotic prescribing to microscopy‐ or PCR‐negative people | 12 per 100 | 25 per 100 (15 to 41) | RR 2.02 (1.21 to 3.37) | 5179 (2 cRCTs) | ⊕⊕⊕⊝ Moderatef | mRDT probably results in increased antibiotic use. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; cRCT: cluster‐randomized controlled trial; mRDT: malaria rapid diagnostic test; PCR: polymerase chain reaction; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded three levels for extremely serious imprecision. bDowngraded one level for serious inconsistency despite heterogeneity partially explained in subgroup analysis. cNot downgraded for imprecision: optimal information size met, large sample size (more than 4000). dDowngraded one level for serious risk of bias due to lack of blinding for this outcome and overall unclear risk of bias (Leslie 2017; Ndyomugyenyi 2016) or high risk of bias (Mubi 2011) across trials. eDowngraded one level for serious inconsistency, although effects and CIs are marginally similar and in the same direction. fDowngraded one level for serious imprecision due to wide 95% CI (for the relative effect) and 95% CI limit including a trivial absolute benefit, despite optimal information size being met.
Summary of findings 2. Malaria rapid diagnostic test (mRDT) in community‐based care versus health facility care for people with suspected malaria.
| Malaria rapid diagnostic test (mRDT) in community‐based care versus health facility care for people with suspected malaria | |||
|
Patient or population: people with suspected malaria Setting: malaria‐endemic areas Intervention: mRDT in community‐based care Comparison: health facility care | |||
| Outcomes | Impact | Number of participants (studies) | Certainty of the evidence (GRADE) |
| All‐cause mortality and malaria mortality | No difference in all‐cause and malaria mortality across studies. We are uncertain whether mRDT in community‐based care has any effect on mortality compared to health facility care. | 9428 (1 cRCT, 1 CBA) |
⊕⊕⊝⊝ Lowa |
| Hospitalizations | No difference in hospitalizations across studies. We are uncertain whether mRDT in community‐based care has any effect on mortality compared to health facility care. | 9428 (1 cRCT, 1 CBA) |
⊕⊕⊝⊝ Lowa |
| CBA: controlled before‐after study; cRCT: cluster‐randomized controlled trial; mRDT: malaria rapid diagnostic test | |||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||
aDowngraded one level due to high risk of bias in randomization and allocation concealment (selection bias) in Thiam 2012 and Ohnmar 2012; and one level for imprecision due to wide CIs, including both protective and harmful effects.
Comparison 1: malaria rapid diagnostic test versus clinical diagnosis in community‐based care
See Table 1.
All the studies in this comparison were cRCTs; Mubi 2011 was the only study with a cross‐over cluster design.
All‐cause mortality
Two studies reported deaths (Ansah 2015; Mubi 2011), although the single death reported in Ansah 2015 actually occurred after the study period. Given the very low number of deaths, and the fact that these data were not adjusted for cluster or cross‐over designs, meta‐analysis was not possible. We do not know whether community use of mRDTs compared to clinical diagnosis affects mortality. In Mubi 2011, four participants died: three children under five years old who died within the first three days of follow‐up (two in the intervention arm, one in the control arm), and one adult in the intervention arm who died within seven days. Two of the children were mRDT‐ and microscopy‐positive (32,800 parasites/μL and 54,000 parasites/μL), and had been referred for further management of severe malaria after receiving ACT. The third child and the adult were both mRDT‐ and microscopy‐negative and had been referred without ACT treatment because of breathing problems (in the child) and stomach problems (in the adult).
Hospitalizations
No studies reported hospitalizations.
Number of people receiving an antimalarial within 24 hours
No studies reported the number of people receiving an antimalarial within 24 hours, although some studies reported the number of people receiving an antimalarial without a timescale (see Analysis 1.3).
1.3. Analysis.

Comparison 1: Malaria rapid diagnostic test (mRDT) compared to clinical diagnosis for community‐based programmes for treating malaria, Outcome 3: Number receiving antimalarials
Malaria‐specific mortality
There were two malaria‐related deaths in Mubi 2011.
Severe malaria
There were two cases of severe malaria in Mubi 2011.
Other outcomes related to antimalarial treatment as reported by study authors
Antimalarial prescribing to microscopy‐ or polymerase chain reaction‐negative people
Ansah 2015, Leslie 2017, and Ndyomugyenyi 2016 reported on the use of antimalarials comparing mRDTs with clinical diagnosis when the true malaria positivity status was evaluated and found to be negative by a blinded review of microscopy or PCR testing (result made available to researchers after mRDT/clinical diagnosis and prescribing decision, but not to CHWs or drug shop vendors). The evidence indicates that mRDT probably results in a large reduction in use of antimalarials in microscopy‐ or PCR‐negative people (RR 0.17, 95% CI 0.07 to 0.40; 3 cRCTs, 7877 participants; Analysis 1.1). There were 71 fewer unnecessary prescriptions per 100 in the mRDT arm compared to the clinical diagnosis arm (95% CI 79 to 51 fewer).
1.1. Analysis.

Comparison 1: Malaria rapid diagnostic test (mRDT) compared to clinical diagnosis for community‐based programmes for treating malaria, Outcome 1: Antimalarial prescribing to microscopy‐ or PCR‐negative people
In subgroup analyses, stratification by job title of the person providing the diagnosis and treatment (CHWs compared to drug shop vendors) provided some explanation for the high statistical heterogeneity observed (I² =97%): mRDT diagnosis, compared to clinical diagnosis, led to a larger reduction in the use of antimalarials in microscopy‐negative participants when the diagnosing and prescribing person was a CHW (RR 0.11, 95 % CI 0.08 to 0.14) than when they were a drug shop owner (RR 0.41, 95% CI 0.29 to 0.58). There were no mutually exclusive subgroups for age. However, both Ndyomugyenyi 2016, which only included children under five years old, and Ansah 2015, in a subgroup of children under 13 years old, found that the effect of mRDT versus clinical diagnosis was consistent with the overall result (Ndyomugyenyi 2016: RR 0.10, 95% CI 0.07 to 0.15; Ansah 2015: RR 0.52, 95% CI 0.36 to 0.74).
A sensitivity analysis revealed no significant differences in the pooled effects when collapsing effect estimates by transmission zone as reported in Ndyomugyenyi 2016. Additionally, sensitivity analysis imputing different ICC values did not significantly alter the pooled effects for this outcome.
Appropriate treatment
Several studies used a composite measure of appropriate treatment: use of antimalarials in people with malaria and no antimalarials in those without malaria. The study authors assessed this outcome through a blinded review of microscopy or molecular PCR testing, where the result was available to the study authors after the mRDT/clinical diagnosis and prescribing decision, not to CHWs or drug shop vendors. Three cRCTs contributed to the evidence (Ansah 2015; Leslie 2017; Ndyomugyenyi 2016); they showed that use of mRDT results in a large increase in appropriate treatment compared to clinical diagnosis (RR 3.04, 95% CI 2.46 to 3.74; 3 cRCTs, 9332 participants; Analysis 1.2), or 45 more appropriate prescriptions per 100 (95% CI 32 to 61 more). Subgroup analyses provided some explanation of the limited heterogeneity between studies (I² = 31%). When stratified by job title, the effect of mRDTs was larger for CHWs (RR 3.26, 95% CI 2.74 to 3.87) than for drug shop vendors (RR 2.39, 95% CI 1.69 to 3.38). There were no mutually exclusive subgroups for age, though Leslie 2017 found that 64/82 (91.4%) children under five years old in the intervention group and 6/46 (8.7%) children under five years old in the control group received appropriate treatment (P < 0.001). For children over five years old, the proportion of those receiving appropriate treatment was 753/1001 (80.8%) in the intervention group and 179/1007 (19.2%) in the control group (P < 0.001).
1.2. Analysis.

Comparison 1: Malaria rapid diagnostic test (mRDT) compared to clinical diagnosis for community‐based programmes for treating malaria, Outcome 2: Appropriate treatment
Ndyomugyenyi 2016 also presented data for appropriately targeted treatment within the first 24 hours, where the use of mRDTs had a much lower effect compared to clinical diagnosis in moderate‐ to high‐transmission areas (odds ratio (OR) 5.92, 95% CI 4.15 to 8.45) than in low‐transmission areas (OR 40.3, 95% CI 28.1 to 57.9). Sensitivity analysis revealed no significant differences in pooled effects when collapsing effect estimates by transmission zone. Additionally, sensitivity analysis imputing different ICC values did not significantly alter the pooled effects for this outcome.
Antimalarial treatment based on mRDT results
Several studies reported on concordance in treatment given by CHWs or drug shop vendors with the mRDT result they themselves obtained when diagnosing patients or customers. Ansah 2015 and Mubi 2011 found that a small number of people who tested negative by mRDT were nevertheless given an antimalarial (Ansah 2015: 38/1368 (2.8%); Mubi 2011: 44/724 (6.1%)). A larger proportion of mRDT‐negative participants received an antimalarial in Leslie 2017 (124/950, 13.1%). Conversely, a minority of mRDT‐positive participants in Ansah 2015 and Mubi 2011 were not treated with an antimalarial (Ansah 2015: 0.5%; Mubi 2011: 0.3%).
Mubi 2011 also reported that 6/57 (10.5%) participants classified as non‐malaria in the presumptive diagnosis arm were nevertheless given an ACT.
Number of people receiving an antimalarial
Meta‐analysis of two studies indicates that use of mRDTs may result in a large reduction in the number of people receiving an antimalarial (RR 0.21, 95% CI 0.14 to 0.34; 2 cRCTs, 4729 participants; Analysis 1.3), or 76 fewer per 100 (95% CI 82 to 63 fewer). Sensitivity analysis revealed no differences when relaxing inclusion criteria for type of staff (to include Mbonye 2015), or collapsing effect estimates by transmission zone as reported in Ndyomugyenyi 2016.
Mubi 2011 also found a large reduction in the number receiving an antimalarial in the weeks when mRDTs were used compared to the clinical diagnosis weeks (OR 0.39, 95% CI 0.29 to 0.53). There were no relevant raw data to extract from Cohen 2015. Additionally, sensitivity analysis imputing different ICC values did not significantly alter the pooled effects for this outcome.
Antibiotic prescribing to microscopy‐ or PCR‐negative people
Two cRCTs contributed to the evidence on antibiotic prescribing against negative microscopy or PCR (Ansah 2015; Leslie 2017). The evidence indicated that the use of mRDTs probably increased antibiotic use compared to clinical diagnosis (RR 2.02, 95% CI 1.21 to 3.37; 2 cRCTs, 5179 participants; Analysis 1.4), resulting in 13 more antibiotic prescriptions per 100 (95% CI 3 more to 29 more). Cohen 2015 reported reduced antibiotic use following mRDT diagnosis, though data could not be combined as it was obtained through a statistical model only. Additionally, sensitivity analysis imputing different ICC values did not significantly alter the pooled effects for this outcome.
1.4. Analysis.

Comparison 1: Malaria rapid diagnostic test (mRDT) compared to clinical diagnosis for community‐based programmes for treating malaria, Outcome 4: Antibiotic prescribing to microscopy‐ or PCR‐negative people
Parasitaemia
No studies reported parasitaemia.
Anaemia
No studies reported anaemia.
Adverse events
In Mubi 2011, 1.1% of all participants experienced drug‐related adverse events, most commonly nausea, weakness, headache, and diarrhoea. However, the study authors did not separate adverse event data by study arm.
Other outcomes considered relevant for the review
Referrals
Referral data could not be meta‐analysed, but are described here. There was no difference in the proportion referred in the mRDT and clinical diagnosis arms in Leslie 2017 (34.6% with mRDT versus 26.4% with clinical diagnosis; P = 0.116). Less than 1.5% of microscopy‐positive customers in Ansah 2015 said they were referred to a health facility, though more of these were in the mRDT arm (P = 0.024). Conversely, 80% of those who tested negative by mRDT were referred for further care. Referrals in Mubi 2011 were higher in the mRDT arm (OR 1.95, 95% CI 1.36 to 2.80; P < 0.001).
Accuracy of mRDTs
Several studies reported on the operational (i.e. field) sensitivity and specificity of the mRDTs against microscopy. There is considerable variation in the results, particularly for sensitivity, which was much lower in low transmission zones (Table 6).
4. Accuracy of malaria rapid diagnostic tests.
| Study | Job title | Method | Sensitivity (%) | 95% CIa | Specificity (%) | 95% CIa |
| Ansah 2015b | Drug seller | Microscopy | 96.0 | Range 98% to 100% | 70.0 | Range 73% to 98% |
| Leslie 2017 | CHW | PCR | ||||
| Pan (all species) overall | 54.2 | 47.5 to 60.9 | 91.5 | 89.5 to 93.2 | ||
| – Low transmission | 2.1 | 0.1 to 11.3 | 99.8 | 98.7 to 100 | ||
| – High transmission | 68.0 | 60.6 to 74.8 | 85.2 | 82.0 to 88.1 | ||
| Pfalciparum overall | 53.2 | 38.1 to 67.9 | 96.8 | 95.6 to 97.8 | ||
| – Low transmission | 0 | N/A | 0 | N/A | ||
| – High transmission | 62.5 | 45.8 to 77.3 | 94.7 | 92.8 to 96.3 | ||
| Mubi 2011 | CHW | Microscopy | 85.3 | Not known | 59.8 | Not known |
| Ndyomugyenyi 2016 | CHW | Microscopy | ||||
| Moderate to high transmission | 72.1 | Not known | 83.3 | Not known | ||
| Low transmission | 20.8 | Not known | 98.1 | Not known | ||
aUnless specified bAuthors reported variations by drug shop CHW: community health worker; CI: confidence interval; N/A: not applicable; PCR: polymerase chain reaction
Comparison 2: mRDTs in community‐based care versus health facility care
See Table 2.
Three studies compared use of mRDTs by community personnel versus usual health facility care: one cRCT (Ohnmar 2012), and two CBA studies (Swana 2016; Thiam 2012). However, none of the outcomes overlapped by study design (including measures of effect and the population level at which effects were measured), so were not meta‐analysed.
All‐cause and malaria‐specific mortality
Two of the studies investigated all‐cause and malaria‐specific mortality (Ohnmar 2012; Thiam 2012). In Ohnmar 2012, there were some problems with measurement of mortality as planned, with a meaningful level of missing or incomplete data. However, through other means, the team ascertained dates for deaths and found no difference between the arms in terms of all‐cause mortality (OR 1.18, 95% CI 0.82 to 1.70) or malaria‐specific mortality (OR 1.09, 95% CI 0.45 to 2.66). Thiam 2012 found that total deaths per 100,000 decreased by 15.4% (95% CI 5.4% to 25.4%), while malaria‐related mortality decreased by 62.5% (95% CI 43.8% to 81.2%) in the mRDT area. Overall, we are uncertain whether mRDT in community‐based care has any effect on mortality compared to health facility care (2 RCTs). The certainty of the evidence was downgraded by two levels for risk of bias and imprecision.
Hospitalizations
Ohnmar 2012 reported no difference between arms in relation to hospitalizations (OR 1.03, 95% CI 0.72 to 1.47). Similarly, Thiam 2012 found similar decreases in hospitalizations in the intervention and comparison areas: 23.6% (95% CI 21.6% to 25.6%) versus 24.7% (95% CI 21.4% to 28.0%) for total hospitalizations; and 43.1% (95% CI 39.6% to 46.6%) versus 40.9% (95% CI 34.6% to 47.3%) for malaria‐related hospitalizations. Overall, we are uncertain whether mRDT in community‐based care has any effect on hospitalizations compared to health facility care (two RCTs). We downgraded the certainty of the evidence by two levels for risk of bias and imprecision.
Number of people receiving an antimalarial within 24 hours
No studies reported the number of people receiving an antimalarial within 24 hours
Severe malaria
No studies reported severe malaria.
Other outcomes related to antimalarial treatment as reported by study authors
The included studies reported no other relevant outcomes related to antimalarial treatment.
Antibiotic prescribing to microscopy‐ or PCR‐negative people
No studies reported antibiotic prescribing to microscopy‐ or PCR‐negative people.
Parasitaemia
Swana 2016 used school‐based malaria prevalence surveys, finding no statistical difference between the mRDT intervention and comparison schools at endline. However, incidence was higher in the intervention area at baseline, with malaria prevalence decreasing significantly in postintervention compared to the pre‐intervention (Chi² = 17.00, P < 0.001).
Anaemia
No studies reported anaemia.
Adverse events
No studies reported adverse events.
Other outcomes considered relevant for the review
Referrals
Thiam 2012 reported referrals among mRDT‐negative participants, finding a proportion of 79.5% (3224/4054) in 2009 and 97.4% (3262/3348) in 2010.
Discussion
This review concerns the use of mRDTs by local people residing in malaria‐endemic communities who do not have professional healthcare qualifications but who have been trained to diagnose and treat malaria within specific limits of practice. Successful task‐shifting of such duties from the formal healthcare sector is expected to increase prompt access to antimalarials within under‐served communities, and help to better target antimalarials to those who have confirmed malaria, while ensuring those without malaria are referred for further investigation and management of their symptoms. Ultimately, this may reduce malaria‐related morbidity and mortality. It may also save costs at the primary healthcare level (Kyaw 2016).
We found very little evidence about the effect of community use of mRDTs on mortality compared to clinical diagnosis, as few included studies reported this as an outcome and, where data were available, proportions of deaths were small. Data were similarly sparse for mortality when comparing community use of mRDTs with routine health facility‐based care. One included study showed a reduction in all‐cause deaths (and a 62% reduction in deaths from malaria) in the mRDT arm over the study period, with no meaningful change in the health facility arm, although the population level design was different to other studies described (Thiam 2012).
We found that community use of mRDTs increases appropriate treatment of malaria compared to presumptive treatment: those with malaria are able to access a drug they need while those without malaria are not given a drug they do not need. The large reduction in the number of people receiving an antimalarial after mRDT compared to clinical diagnosis may seem counterintuitive at face value, as diagnostics are often used to detect more cases of an illness for treatment. For malaria, however, the default has been presumed malaria (and thus default use of an antimalarial drug) in everyone presenting with fever in malaria‐endemic regions. Therefore, the goal of using mRDTs is also to detect people who do not have the disease, so they do not receive a drug they do not need. This approach prevents unnecessary side effects and contribution to parasite resistance, and allows for referrals of non‐malaria fevers to healthcare professionals. The reduction in overtreatment with antimalarials is therefore to be expected and welcomed. In addition, while data about referrals were sparse and not amenable to meta‐analysis, a large proportion of people who tested mRDT‐negative were referred for further care in two included studies, suggesting triage at the community level worked well, and there were relatively low levels of antimalarial use in those who were found to be mRDT‐negative.
Diagnosis by mRDT was associated with a larger reduction in the use of antimalarials in microscopy‐negative people who were seen by a CHW compared to those who were seen by a shop vendor. Further research is needed to substantiate our findings and explore contributory factors. Regardless, both types of staff (and indeed healthcare professionals) may be concerned about mRDT false negatives, especially in a potentially fatal disease such as malaria, and this may be compounded by perceived pressure from patients or carers for staff to provide (or sell) a drug even when an mRDT indicates no malaria (Chandler 2008a; Chandler 2008b; Danquah 2016; Diggle 2014; Watson 2019). However, reviews have suggested that CHWs and drug shop vendors may actually adhere more closely to mRDT test results than healthcare professionals. The reasons are not clear, but it may be that healthcare professionals feel they can rely more on their clinical judgement (Kabaghe 2016).
It can take time for people to adapt to a new way of working, and the adaptation process in this case includes accepting and learning to use the new diagnostic tool. Ansah 2015 suggests behaviour change was quicker among the drug shop vendors included in their trial than among healthcare professionals from other studies who may have more ingrained behaviour; but the caveat is that free mRDTs were distributed to the shops in Ansah 2015 and then used under trial conditions. Outside these circumstances, recommended practices such as referrals of mRDT‐negative people may be less frequent (Kwarteng 2019). Good adherence to mRDT results will be undermined if uptake by providers is poor. In this sense, one study included in our review found that where mRDTs were merely introduced into the supply chain with vendors encouraged to buy them, uptake was variable: 40% did not buy any mRDTs, and many more bought only a few (Cohen 2015). Similarly, a review that examined the use of mRDTs in private retail outlets found varying results relating to mRDT uptake and, perhaps consequently, relating to clinical outcomes such as appropriateness of treatment (Visser 2017). Price may be influential for both shopkeepers and their customers, but one trial found no difference in the proportion of customers who purchased an mRDT with or without a subsidy (Maloney 2017). Cost‐effectiveness of subsidies for mRDTs is likely influenced by various factors, including local treatment practices and malaria transmission, and it has been proposed that the right price combined with intensive information, education, and counselling constitutes a more effective approach, while innovative methods such as text messaging to shopkeepers can help sustain the positive effects of introducing mRDTs (Aung 2015; Bath 2020; Bruxvoort 2014).
It is clear that the factors influencing community use of mRDTs are complex. CHWs operate in a context very different to that of drug shop vendors, with mRDTs and antimalarials supplied as part of a project or programme, usually limited financial reward, and often closer integration with the people they treat. While uptake of mRDTs among CHWs is less of a concern, their motivations for using mRDTs, and the contexts in which they use them, will likely differ from those of shop vendors or healthcare professionals in formal health facilities, and may vary considerably between regions or countries. Therefore, while our results are broadly encouraging, the introduction of mRDTs in any situation warrants careful consideration as regards training, support, and equipment, based on both the literature and, ideally, bespoke formative work. We were unable to explore specific components of the interventions in this review, yet even nuances in how they are applied, such as flexibility in treatment algorithms, may impact outcomes (Burchett 2017). Neither did we explore issues relating to the quality of care by staff, including correct and safe use of mRDTs, and how effects may change over time (Ruizendaal 2014; UNICEF 2012). Long‐term follow‐up is therefore important, as personal attributes, community dynamics, malaria transmission intensities, and health system or treatment policies may all impact the successful long term use of mRDTs in different community settings (Boyce 2018; Ruizendaal 2014). Cost‐effectiveness of CHW mRDT programmes may also be related to geographical remoteness (Kyaw 2016).
Aside from the effect on malaria management, there is moderate‐certainty evidence that community use of mRDTs results in an increase in antibiotic use in people who receive a negative diagnosis by mRDT compared to by clinical examination. This has been a growing concern, including in the formal healthcare setting, as non‐malarial fevers are challenging to diagnose: there is a large range of potential causes and people with malaria may be coinfected (Elven 2020). One review found nearly 70% of mRDT‐negative people were prescribed an antibiotic (in public, private retail, and community contexts) compared to 40% of mRDT‐positive people (Hopkins 2017). One Cochrane Review found mixed results among healthcare professionals (Odaga 2014). Regarding shop vendors, an anthropological exploration of mRDTs in retail outlets suggests that the status of drug shop vendors increases as this new technology is introduced, with shops becoming more attractive places to seek care; as a result, care provision moves from the formal healthcare sector into shops, where there is more unregulated use of medicines such as antibiotics (Hutchinson 2017). These influences may affect CHWs less, and may instead be harnessed with expansion of the community management of malaria to other illnesses, such as childhood pneumonias and diarrhoea within iCCM (Smith Paintain 2014). However, while CHWs appear to adhere well to algorithms, there still exists the potential to deviate from them and push up the use of antibiotics. For this reason, similar attention should be given to sustaining correct performance within each context. Otherwise, the much‐needed gains in appropriate use of antimalarials may inadvertently drive antimicrobial resistance to much‐needed antibiotics.
Summary of main results
Eight studies were included from several African countries, Afghanistan and Myanmar. Staff included CHWs or drug shop vendors. The evidence is very uncertain about the effect of community use of mRDTs on mortality compared to clinical diagnosis. However, there is moderate‐certainty evidence that their use leads to a large reduction in antimalarial prescribing to people who are malaria parasite‐negative by microscopy or PCR; and high‐certainty evidence of a large increase in appropriate treatment, whereby people who are microscopy‐ or PCR‐positive for malaria receive an antimalarial, while those who are negative do not. These positive effects may be greater in people seen by CHWs compared to those seen by drug shop vendors, though the difference was not statistically significant for appropriate treatment. In addition, there is moderate‐certainty evidence of an increase in antibiotic use with mRDT versus clinical diagnosis. See Table 1.
Overall completeness and applicability of evidence
We consider that the comprehensive search and screening using Cochrane methods should have uncovered all eligible studies. However, we were unable to meta‐analyse cRCTs with CBAs, and could only combine data for some outcomes. Six studies were conducted in Africa, one in South Asia (Afghanistan) and one in South East Asia (Myanmar). No studies were from South America. Studies predominantly concerned P falciparum (only the Asian studies identified Pvivax) and were conducted in rural areas (to be expected, as this is where access to health facilities is more difficult). These issues should be taken into account when interpreting the results for different epidemiological settings. Moreover, as discussed, there are multiple factors, including human behaviours, that may influence the effects of introducing mRDTs within the community but that could not be formally assessed.
Certainty of the evidence
We integrated risk of bias into a certainty of evidence assessment for each meta‐analysed outcome using the GRADE approach. This yielded very low‐certainty evidence for the primary mortality outcomes, due to extremely serious imprecision. However, there was moderate‐certainty evidence for the outcome of antimalarial use in microscopy‐ or PCR‐negative people, and high‐certainty evidence for the outcome of appropriateness of treatment. We downgraded the certainty of evidence on antimalarial use in microscopy‐ or PCR‐negative people for serious inconsistency, although heterogeneity was partially explained in the subgroup analysis. We downgraded the certainty of the evidence relating to the number of people treated with an antimalarial by two levels for risk of bias concerns due to lack of blinding and an overall unclear to high risk of bias, and for inconsistency. Finally, we downgraded the certainty of the evidence on use of antibiotics after a negative mRDT result by one level to moderate for serious imprecision, with a wide 95% CI including a trivial effect (estimate crosses the small effect threshold), despite the optimal information size being met.
Potential biases in the review process
Strengths of this review include the use of recognized Cochrane methods and tools, the absence of language or publication date filters in the search, and the overall completeness of data. Where appropriately adjusted effect estimates were not available, we adjusted our effect estimates (for binary outcomes) by dividing both the numerator and denominator by the design affect given by 1 + (m − 1) × ICC, where m is the average cluster size and ICC is the intra‐cluster correlation coefficient obtained from the study. Where no ICC was reported, we used a common ICC of 0.01. However, we were unable to take the cross‐over design into account for one cRCT, and we were unable to conduct a sensitivity analysis considering or imputing missing data.
Agreements and disagreements with other studies or reviews
This is an update of an existing Cochrane Review (Okwundu 2013). Due to shifts in research focus since the original publication date, including the rapid introduction of mRDTs into community management of malaria, we modified the protocol considerably for this update, excluding trials of HMM, which was an important aspect of other reviews (Okwundu 2011). We describe these and similar reviews in Table 3, as discussed in our Background section. UNICEF 2012 found high adherence to mRDT results, and Okwundu 2013 proposed that mRDTs may help reduce overuse of antimalarials. However, Boyce 2017 found variable adherence to mRDT results among CHWs, and Visser 2017 among drug shop staff, suggesting that training and sustained supervision are important factors for success. No review includes all the studies included in this update, but some focus on important aspects of introducing technology to people with limited healthcare training that we do not. We therefore consider that all reviews are complementary. One study was ineligible for this review update because a large percentage of the staff who provided diagnoses and treatments were qualified healthcare professionals working in drug shops (Mbonye 2015). Including this study in a sensitivity analysis revealed no significant differences in the relevant pooled effects.
Authors' conclusions
Implications for practice.
The results of this review update suggest that training community health workers (CHWs) and drug sellers to use malaria rapid diagnostic tests (mRDTs) and dispense or sell antimalarials for the diagnosis and treatment of malaria has important benefits. These strategies improve targeting of antimalarials, in that people who are malaria‐positive have access to a drug, while those who are malaria‐negative generally do not. These programmes seem to reduce the provision of antimalarials to uninfected people, which is to be expected, although a small proportion of customers testing negative by mRDT may receive an antimalarial, and some who test positive by mRDT may not receive an antimalarial. Indirect comparisons suggest programmes with mRDTs work better with CHWs than with drug sellers.
Implications for research.
These programmes appear to improve targeting of antimalaria treatment. Future research could examine how such programmes can be sustained, particularly in drug shops, or even among people testing themselves at home. One possible area of research is antibiotic use in mRDT‐negative and mRDT‐positive people, as the staff responsible for managing people with malaria symptoms need support in the challenging area of non‐malarial fevers or coinfections.
What's new
| Date | Event | Description |
|---|---|---|
| 30 August 2022 | New citation required and conclusions have changed | The authors revised the protocol to be more relevant for how community recognition and treatment of malaria is managed with randomized controlled trials. They amended the risk of bias methods. |
| 30 August 2022 | New search has been performed | This is an update of Okwundu 2013. A new author team performed this review update, and amended the review title from 'Home‐ or community‐based programmes for treating malaria' to 'Adding rapid diagnostic tests to community‐based programmes for treating malaria'. |
History
Protocol first published: Issue 12, 2011 Review first published: Issue 5, 2013
Acknowledgements
The following people were involved in the editorial process for this article.
Contact Editor: Dr Patricia Graves.
Sign‐off Editor (final editorial decision): Professor Paul Garner.
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Dr Deirdre Walshe.
Copy Editor (copy editing and production): Julia Turner, Cochrane Copy Edit Support.
Peer‐reviewers (provided comments and recommended an editorial decision): Dr Sarah Nevitt, University of Liverpool, UK (methods review); Dr Nicholas Nyaaba, Infectious Disease Center, 37 Military Hospital, Accra, Ghana (clinical review); Heidi Hopkins, MD, Associate Professor, London School of Hygiene & Tropical Medicine (clinical review). One additional peer reviewer provided clinical peer review but chose not to be publicly acknowledged.
The editorial base of the Cochrane Infectious Diseases Group is funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed in this review do not necessarily reflect the UK government's official policies.
We thank the authors of the original review, and the following people who commented on the revised protocol or review update: Paul Garner, Taryn Young, Tamara Kredo, Anke Rohwer, Samuel Gonahasa, Mary Kyohere, and Jerry Mulondo. We also thank Charles Okwundu for his contribution to the protocol development and screening of titles and abstracts in an earlier version; and Shahista Jaffer, who assisted with data extraction. Finally, grateful thanks to Vittoria Lutje (CIDG Information Specialist) for developing and applying the searches, and Deirdre Walshe (CIDG Managing Editor) for her support with review logistics.
Appendices
Appendix 1. Search strategy
| Search Name: Cochrane Central Register of Controlled Trials ID Search #1 malaria #2 MeSH descriptor: [Malaria] explode all trees #3 #1 or #2 #4 Plasmodium #5 #3 or #4 #6 treatment or management or therapy or prevention or ACT* or artemis* #7 #5 and #6 #8 MeSH descriptor: [Community Health Workers] explode all trees #9 MeSH descriptor: [Volunteers] explode all trees #10 MeSH descriptor: [Allied Health Personnel] explode all trees #11 "community worker*" OR "lay health worker*" OR "village health worker*" OR "health auxiliary" #12 "peer educator*" OR "peer counsellor*" OR "health extension worker*" OR "allied health worker*" OR "health promoter*" #13 "health assistant*" #14 "Community Health advisor*" or "Lay health workers*" #15 "voluntary worker*" #16 "community volunteer*" #17 "Community drug distributor*" or CDD or "drug seller*" #18 "drug dispensing outlet*" or ADDO or kiosk* #19 "Health extension worker*" or "Community directed distributor*" or "Community medicine distributor*" or "Community health animators" or "Community Implementer" or "Community led" or "Community directed" or "CDI" or "health center*" #20 MeSH descriptor: [Community Health Services] explode all trees #21 MeSH descriptor: [Delivery of Health Care] explode all trees #22 "capacity building" #23 MeSH descriptor: [Health Services Research] explode all trees #24 MeSH descriptor: [Education, Medical] explode all trees #25 MeSH descriptor: [Health Promotion] explode all trees #26 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 #27 #26 and #7 | |
| PubMed (MEDLINE) | |
| #1 | Search "Malaria"[Mesh] |
| #2 | Search malaria Field: Title/Abstract |
| #3 | Search plasmodium Field: Title/Abstract |
| #4 | Search (#3) OR #2 OR #1 |
| #5 | Search treatment or management or therapy or prevention or ACT* or artemis* Field: Title/Abstract |
| #6 | Search "drug therapy" [Subheading] |
| #7 | Search (#6) OR #5 |
| #8 | Search "Capacity Building"[Mesh] or "capacity building" [Title/abstract] or "Health Services Research"[Mesh] or "Education, Medical"[Mesh] or "Health Promotion"[Mesh] |
| #9 | Search (("Community Health Workers"[Mesh]) OR "Volunteers"[Mesh]) OR "Allied Health Personnel"[Mesh] |
| #10 | Search "community worker*" OR "lay health worker*" OR "village health worker*" OR "health auxiliary" Field: Title/Abstract |
| #11 | Search "peer educator*"□ OR "peer counsellor*" OR "health extension worker*" OR "allied health worker*" OR "health promoter*" Field: Title/Abstract |
| #12 | Search "health assistant*" or "Community Health advisor*" or "Lay health workers*" Field: Title/Abstract |
| #13 | Search "voluntary worker*" or "community volunteer*" Field: Title/Abstract |
| #14 | Search "drug dispensing outlet*" or ADDO or kiosk* Field: Title/Abstract |
| #15 | Search "Health extension worker*" or "Community directed distributor*" or "Community medicine distributor*" or "Community health animators" or "Community Implementer" or "Community led" or "Community directed" or "CDI" or "health center*" Field: Title/Abstract |
| #16 | Search ("Community Health Services"[Mesh]) OR "Delivery of Health Care"[Mesh] |
| #17 | Search "Community drug distributor*" or CDD or "drug seller*" Field: Title/Abstract |
| #18 | Search ((((((((((#17) OR #16) OR #15) OR #14) OR #13) OR #12) OR #11) OR #10) OR #9) OR #8) |
| #19 | Search (#18) AND #7 AND #4 |
| #20 | Search cohort OR longitudinal OR cross‐ sectional OR interrupted time series OR "before and after study" OR before‐after study OR cross‐sequential OR "control group*" OR "matched control*" OR " matched cohort*" Field: Title/Abstract |
| #21 | Search "Comparative Study" [Publication Type] |
| #22 | Search "Interrupted Time Series Analysis"[Mesh] OR "Controlled Before‐After Studies"[Mesh] OR "Historically Controlled Study"[Mesh] |
| #23 | Search randomized or placebo or randomly or trial Field: Title/Abstract |
| #24 | Search "Randomized Controlled Trial" [Publication Type] OR "Controlled Clinical Trial" [Publication Type] |
| #25 | Search ((((#24) OR #23) OR #22) OR #21) OR #20 |
| #26 | Search (#25) AND #19 |
| Embase 1947‐Present, updated daily 1 malaria/ or malaria.mp. 2 Plasmodium/ or plasmodium.mp. 3 1 or 2 4 (treatment or management or therapy or prevention or ACT* or artemis*).ab. or (treatment or management or therapy or prevention or ACT* or artemis*).ti. 5 3 and 4 6 community health workers.mp. or health auxiliary/ 7 volunteer/ 8 paramedical personnel/ 9 ("lay health worker*" or "village health worker*").ab. or ("lay health worker*" or "village health worker*").ti. 10 ("peer educator*" or "peer counsellor*" or "health extension worker*" or "health promoter*").ab. or ("peer educator*" or "peer counsellor*" or "health extension worker*" or "health promoter*").ti. 11 ("Community Health advisor*" or "Lay health workers*").ab. or ("Community Health advisor*" or "Lay health workers*").ti. 12 ("Community drug distributor* or CDD or drug seller* ).ab. OR (Community drug distributor* or CDD" or "drug seller*").ti. 13 ("drug dispensing outlet*" or ADDO or kiosk*).ab. or ("drug dispensing outlet*" or ADDO or kiosk*).ti. 14 ("community health" or "community program" or "community programme" or "community management" or "door to door" or "door‐to‐door").mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 15 health care delivery/ 16 capacity building/ or capacity building.mp. 17 health promotion/ 18 ("peer educator*" or "peer counsellor*" or "health extension worker*" or "health promoter*").ab. 19 ("peer educator*" or "peer counsellor*" or "health extension worker*" or "health promoter*").ti. 20 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21 5 and 20 22 randomized controlled trial.mp. or Randomized Controlled Trial/ 23 controlled clinical trial.mp. or Controlled Clinical Trial/ 24 comparative study/ 25 Interrupted Time Series Analysis.mp. 26 (Controlled Before and After Study).mp. 27 "time series".mp. or time series analysis/ 28 cohort analysis/ or cohort.mp. 29 longitudinal study/ 30 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 31 21 and 30 | |
| Social Science Citation Index (Web of Science) | |
| #10 | #9 AND #8 Indexes=SSCI, CPCI‐SSH Timespan=All years |
| #9 |
TOPIC: (randomized or controlled or trial or double‐blind or single‐blind) ORTOPIC: (controlled or crossover) Indexes=SSCI, CPCI‐SSH Timespan=All years |
| #8 | #7 AND #3 Indexes=SSCI, CPCI‐SSH Timespan=All years |
| #7 | #6 OR #5 OR #4 Indexes=SSCI, CPCI‐SSH Timespan=All years |
| #6 |
TOPIC: ("Health extension worker*" or "Community directed distributor*" or "Community medicine distributor*" or "Community health animators" or "Community Implementer" or "Community led" or "Community directed" or "CDI" or "health center*") Indexes=SSCI, CPCI‐SSH Timespan=All years |
| #5 |
TOPIC: ("community health worker*") Indexes=SSCI, CPCI‐SSH Timespan=All years |
| #4 |
TOPIC: ("community worker*" OR "lay health worker*" OR "village health worker*" OR "health auxiliary") ORTOPIC: ("peer educator*" OR "peer counsellor*" OR "health extension worker*" OR "allied health worker*" OR "health promoter*") ORTOPIC: ("Community drug distributor*" or CDD or "drug seller*") Indexes=SSCI, CPCI‐SSH Timespan=All years |
| #3 | #2 AND #1 Indexes=SSCI, CPCI‐SSH Timespan=All years |
| #2 |
TOPIC: (treatment or management or therapy or prevention or ACT* or artemis*) Indexes=SSCI, CPCI‐SSH Timespan=All years |
| #1 |
TOPIC: (malaria or plasmodium) Indexes=SSCI, CPCI‐SSH Timespan=All years |
| CINAHL and PsycINFO (EBSCO Host) | |
| # | Query |
| S5 | S1 AND S4 |
| S4 | S2 OR S3 |
| S3 | TX "Health extension worker*" or "Community directed distributor*" or "Community medicine distributor*" or "Community health animators" or "Community Implementer" or "Community led" or "Community directed" or "CDI" or "health center*" |
| S2 | TX ( "community worker*" OR "lay health worker*" OR "village health worker*" OR "health auxiliary" ) OR TX ( "peer educator*" OR "peer counsellor*" OR "health extension worker*" OR "allied health worker*" OR "health promoter*" ) OR TX ( "Community drug distributor*" or CDD or "drug seller*" ) |
| S1 | TX malaria AND ( treatment or management or therapy or prevention or ACT* or artemis* ) |
Data and analyses
Comparison 1. Malaria rapid diagnostic test (mRDT) compared to clinical diagnosis for community‐based programmes for treating malaria.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Antimalarial prescribing to microscopy‐ or PCR‐negative people | 3 | 7877 | Risk Ratio (IV, Random, 95% CI) | 0.17 [0.07, 0.40] |
| 1.1.1 Community health workers (CHWs) | 2 | 4453 | Risk Ratio (IV, Random, 95% CI) | 0.11 [0.08, 0.14] |
| 1.1.2 Drug shop vendors | 1 | 3424 | Risk Ratio (IV, Random, 95% CI) | 0.41 [0.29, 0.58] |
| 1.2 Appropriate treatment | 3 | 9332 | Risk Ratio (IV, Random, 95% CI) | 3.04 [2.46, 3.74] |
| 1.2.1 Community health workers (CHWs) | 2 | 4729 | Risk Ratio (IV, Random, 95% CI) | 3.26 [2.74, 3.87] |
| 1.2.2 Drug shop vendors | 1 | 4603 | Risk Ratio (IV, Random, 95% CI) | 2.39 [1.69, 3.38] |
| 1.3 Number receiving antimalarials | 2 | 4729 | Risk Ratio (IV, Random, 95% CI) | 0.21 [0.14, 0.34] |
| 1.4 Antibiotic prescribing to microscopy‐ or PCR‐negative people | 2 | 5179 | Risk Ratio (IV, Fixed, 95% CI) | 2.02 [1.21, 3.37] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ansah 2015.
| Study characteristics | ||
| Methods | Trial design: cRCT Unit of randomization: community with ≥ 1 chemical shop Number of clusters: 24 (12 per arm) to obtain 80 adults and 114 children per cluster Data collection: seller kept records on test results, medications dispensed, and whether customer was referred. Slide for parasitology reading at laboratory. Length of follow‐up: 17 months Adjustment for clustering: yes |
|
| Participants | Target treatment group: adults and children > 6 months Sample size: 4208 (2719 intervention, 2029 comparison) Exclusion criteria: pregnancy, age < 6 months, signs of severe disease, prescription from health facility |
|
| Interventions | Staff who received training: chemical sellers Duration of training: 1 day over and above standard 3‐day malaria case management training for both groups Content of training: treating Pfalciparum malaria after positive mRDT with AL, AQAS, or DP, and referring after negative mRDT Supervision: fieldworkers and supervisors provided technical support. Accuracy of records of drugs dispensed validated by random checks of forms and 'mystery clients'. Direct observation of interactions between chemical sellers and customers by checklist on weekly basis for first month and a further week midway through trial Antimalarials free to participants: no, but subsidized through the Affordable Medicines Facility malaria mRDTs free to participants: yes Additional details: chemical sellers can also sell analgesics, antibiotics (co‐trimoxazole), multivitamins/minerals, haematinics, and antacids. |
|
| Outcomes | All‐cause mortality and malaria mortality (risk of bias combined), use of antimalarial when microscopy‐negative, appropriate treatment (defined as antimalarial provision to microscopy‐positive participants and no antimalarial provision to microscopy‐negative participants), number receiving an antimalarial | |
| Notes | Control: chemical sellers dispensing medicines without test results (community‐based treatment of suspected malaria by clinical diagnosis) Country: Ghana Setting: rural Malaria endemicity: not stated Study dates: August 2011–January 2013 Study sponsor: the Malaria Capacity Development Consortium of the London School of Hygiene & Tropical Medicine, with funding from the Welcome Trust and the Bill and Melinda Gates Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a programme written in R by a statistician not otherwise involved. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) Mortality | Low risk | Not blinded, but mortality unlikely to be affected by knowledge of intervention. |
| Blinding of participants and personnel (performance bias) Antimalarial prescribing to microscopy‐ or PCR‐negative people, appropriate treatment | Unclear risk | Not blinded, but unclear how this could affect outcome. |
| Blinding of participants and personnel (performance bias) Number receiving an antimalarial | Unclear risk | Not blinded, but unclear how this could affect outcome. |
| Blinding of outcome assessment (detection bias) Mortality | Unclear risk | Not blinded, but unclear who assessed mortality. |
| Blinding of outcome assessment (detection bias) Antimalarial prescribing to microscopy‐ or PCR‐negative people, appropriate treatment | Low risk | Those preparing and reading slides were blind to allocation and mRDT result. |
| Blinding of outcome assessment (detection bias) Number receiving an antimalarial | Unclear risk | Not blinded, but unclear how this could affect outcome. |
| Incomplete outcome data (attrition bias) Mortality | Low risk | 1 cluster closed in control arm (8%), and some people refused consent in control, through unclear why. 91.3% positives were successfully followed up. Percentage of participant samples analysed was not similar in both arms. |
| Incomplete outcome data (attrition bias) Antimalarial prescribing to microscopy‐ or PCR‐negative people, appropriate treatment | Low risk | 1 cluster closed in control arm (8%), and some people refused consent in control, through unclear why. 91.3% positives were successfully followed up. Percentage of participant samples analysed was not similar in both arms. |
| Incomplete outcome data (attrition bias) Number receiving an antimalarial | Low risk | 1 cluster closed in control arm (8%), and some people refused consent in control, through unclear why. 91.3% positives were successfully followed up. Percentage of participant samples analysed was not similar in both arms. |
| Selective reporting (reporting bias) | Low risk | Reporting appears as per the statistical analysis plan. Additional outcomes were registered on Clincaltrials.gov, but are not relevant to this review. |
| Other bias | Unclear risk | Recruitment after randomization, which may have influenced selection, though little baseline imbalance. Arms similar, though control sellers more likely to have formal training in dispensing, range differences for number of customers per cluster, refresher training. Contamination and uptake of mRDTs were not described. |
Cohen 2015.
| Study characteristics | ||
| Methods | Trial design: cRCT Unit of randomization: village Number of clusters: 79 Data collection: baseline, every month for 9 months and endline surveys with 25 randomly‐selected households in each target village. At endline, test for malaria by RDT if consenting. Length of follow‐up: 13 months Adjustment for clustering: yes |
|
| Participants | Target treatment group: not described; all ages Sample size: 79 villages Exclusion criteria: any person who tested positive who had taken an antimalarial in previous 4 weeks was referred for microscopy |
|
| Interventions | Staff who received training: drug shop vendors Duration of training: 2 days Content of training: using mRDTs, and recommending purchase of first‐line ACT (no algorithms) Supervision: monthly visits to track stock and usage of mRDTs and compliance with protocols for testing using a 17‐point checklist. Unused kits sent for lot testing every 3 months Antimalarials free to participants: no, but subsidized mRDTs free to participants: no |
|
| Outcomes | Number receiving an antimalarial | |
| Notes | Control: chemical sellers dispensing medicines without test results (community‐based treatment of suspected malaria by clinical diagnosis) Country: Uganda Setting: unclear Malaria endemicity: annual transmission rates > 100 bites/person Study dates: March 2011–April 2012 Study sponsor: the Clinton Health Access Initiative and the Bill & Melinda Gates Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple random draw generated by Stata/SE version 11 for selection of study villages/households and assignment of villages to arm. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) Number receiving an antimalarial | Unclear risk | No mention of blinding, unclear how this could affect outcome. |
| Blinding of outcome assessment (detection bias) Number receiving an antimalarial | Unclear risk | No mention of blinding, unclear how this could affect outcome. |
| Incomplete outcome data (attrition bias) Number receiving an antimalarial | High risk | 6 villages that were included in the baseline survey dropped out postrandomization from the intervention arm, and 92/108 shops completed training (15% attrition). Then 40% of shops did not actually buy any mRDTs and 3 shops accounted for 32% of mRDTs used. Generally each survey covered the same number of households. |
| Selective reporting (reporting bias) | Low risk | No major differences with clinicaltrials.gov relating to this review. |
| Other bias | Low risk | Households in target villages listed before launch. Subsequently, 25 in each village randomly selected, baseline survey to record basic demographic characteristics, health behaviours, revisited monthly. No significant differences aside from significantly less malaria testing/antimalarial use among cases of fever in intervention versus control at baseline; likely due to relatively larger fraction of participants from control villages seeking treatment at a public health facility. Contamination not described. |
Leslie 2017.
| Study characteristics | ||
| Methods | Trial design: cRCT Unit of randomization: clinics (Basic Health Centres or Comprehensive Health Centres) Number of clusters: 22 (11 per arm) Data collection: pretested semi‐pictorial forms for data at participant consultation, blood filter spot for PCR at national laboratory Length of follow‐up: 7 months Adjustment for clustering: yes |
|
| Participants | Target treatment group: not described; all ages Sample size: 79 villages, 2542 participants Exclusion criteria: sought care for the episode of illness from any other source, or referred directly to clinic for any reason prior to diagnosis |
|
| Interventions | Staff who received training: CHWs Duration of training: half day over and above standard 1‐day refresher malaria case management training for both groups Content of training: treating people who have a positive Pfalciparum mRDT result with SP/AS, and treating those with a pan‐specific (assumed Pvivax) mRDT result with CQ Supervision: as normally supervised by a manager who supplies basic items including essential medicines Antimalarials free to participants: not reported mRDTs free to participants: not reported Additional details: training by national trainers who were not part of the study team, some sessions observed by study staff to ensure they were done according to curriculum. Cotrimoxazole for treatment of pneumonia in children included in standard package. |
|
| Outcomes | Use of antimalarial when microscopy‐negative, appropriate treatment (defined as antimalarial provision to PCR‐positive participants and no antimalarial provision to PCR‐negative participants), number receiving an antimalarial | |
| Notes | Control: CHWs dispensing medicines without test results (community‐based treatment of suspected malaria by clinical diagnosis) Country: Afghanistan Setting: rural Malaria endemicity: moderate‐ and low‐transmission areas Study dates: October 2011–May 2012 Study sponsor: the ACT Consortium through a grant from the Bill & Melinda Gates Foundation to the London School of Hygiene & Tropical Medicine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by trial statistician not otherwise involved, using program in R. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) Antimalarial prescribing to microscopy‐ or PCR‐negative people, appropriate treatment | Unclear risk | No mention of blinding, unclear how this could affect outcome. |
| Blinding of participants and personnel (performance bias) Number receiving an antimalarial | Unclear risk | No mention of blinding, unclear how this could affect outcome. |
| Blinding of outcome assessment (detection bias) Antimalarial prescribing to microscopy‐ or PCR‐negative people, appropriate treatment | Low risk | PCR analysis was blinded to allocation. |
| Blinding of outcome assessment (detection bias) Number receiving an antimalarial | Unclear risk | No mention of blinding, unclear how this could affect outcome. |
| Incomplete outcome data (attrition bias) Antimalarial prescribing to microscopy‐ or PCR‐negative people, appropriate treatment | Low risk | 86% CHWs received training, consented and enrolled participants. 10% missing data judged acceptable. |
| Incomplete outcome data (attrition bias) Number receiving an antimalarial | Low risk | 86% CHWs received training, consented and enrolled participants. 10% missing data judged acceptable. |
| Selective reporting (reporting bias) | Low risk | Primary outcome is per protocol, some additional secondary outcomes and no report on cost‐effectiveness. |
| Other bias | Unclear risk | Recruitment after randomization, which may have influenced selection, though little baseline imbalance. Control arm had more CHWs with tertiary education, more missing treatment or diagnosis data, and more participants seen at home, although differences in participants were minimal. Contamination and mRDT uptake not described. |
Mubi 2011.
| Study characteristics | ||
| Methods | Trial design: cross‐over cRCT Unit of randomization: CHW alternated intervention and control weekly Number of clusters: 22 active CHWs in 5 villages Data collection: questionnaires completed by CHWs, blood smears, assessment of compliance through returns Length of follow‐up: 20 weeks |
|
| Participants | Target treatment group: adults and children > 3 months Sample size: 5 villages, 360 participants per group (3005 potential participants, 75 excluded, giving 2930 included participants) Exclusion criteria: pregnancy, severe disease, study inclusion within previous 28 days |
|
| Interventions | Staff who received training: CHWs Duration of training: 1 week Content of training: treating Pfalciparum malaria after positive mRDT with AL Supervision: regular supervision, but no details Antimalarials free to participants: not reported mRDTs free to participants: not reported Additional details: CHWs given a monthly allowance, community sensitization meetings about the study |
|
| Outcomes | All‐cause mortality and malaria mortality (risk of bias combined), and number receiving an antimalarial | |
| Notes | Control: CHWs dispensing medicines without test results (community‐based treatment of suspected malaria by clinical diagnosis) Country: Tanzania Setting: rural Malaria endemicity: holoendemic, high transmission Study dates: March–August 2006 Study sponsor: Swedish International Development Cooperation Agency and SAREC |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Systematic random sampling: each CHW was assigned a unique number on a ticket which determined the order of arm, though no details on how the unique numbers were assigned. |
| Allocation concealment (selection bias) | Low risk | 11 tickets picked blindly from a box after mixing, with these selected CHWs allocated to start using mRDT the first week and alternating thereafter. |
| Blinding of participants and personnel (performance bias) Mortality | Low risk | Not blinded, but mortality unlikely to be affected by knowledge of intervention. |
| Blinding of participants and personnel (performance bias) Number receiving an antimalarial | High risk | Not possible to blind, potential for impact on outcome due to cross‐over design. |
| Blinding of outcome assessment (detection bias) Mortality | Unclear risk | Not blinded, participants who did not return on day 3 or day 7 were actively followed up, and told to return if deterioration/fever. |
| Blinding of outcome assessment (detection bias) Number receiving an antimalarial | Unclear risk | Not blinded, but unclear how this could affect outcome. |
| Incomplete outcome data (attrition bias) Mortality | Low risk | All enrolled participants completed day 28. |
| Incomplete outcome data (attrition bias) Number receiving an antimalarial | Low risk | All enrolled participants completed day 28. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | High risk | CHWs were to provide ACT based on symptoms during clinical diagnosis weeks and with an mRDT during mRDT weeks. While characteristics in each arm appear similar, this design is open to bias in how the intervention is applied. Uptake of mRDT was unclear. |
Ndyomugyenyi 2016.
| Study characteristics | ||
| Methods | Trial design: cRCT Unit of randomization: village Number of clusters: 127 Data collection: registers kept by CHWs prospectively on all children seeking treatment for fever and blood slide results Length of follow‐up: 12 months Adjustment for clustering: yes |
|
| Participants | Target treatment group: children < 5 years old Sample size: 127 (63 Bwambara, 64 Nyakishenyi) Exclusion criteria: referral of children with danger signs or other signs for referral |
|
| Interventions | Staff who received training: CHWs Duration of training: 4 days Content of training: treating Pfalciparum malaria after positive mRDT with AL or rectal AS, and referring if negative mRDT as appropriate Supervision: close supervision by project staff for 6 months through meetings (promoting accurate and complete records, how to handle difficult situations), scaled back thereafter to when CHWs collected supplies Antimalarials free to participants: not reported mRDTs free to participants: not reported Additional details: community sensitization on diagnostic testing for malaria |
|
| Outcomes | Use of antimalarial when microscopy‐negative, appropriate treatment (defined as microscopy‐positive, received antimalarial and microscopy‐negative did not receive an antimalarial), number receiving an antimalarial | |
| Notes | Control: CHWs dispensing medicines without test results (community‐based treatment of suspected malaria by clinical diagnosis) Country: Uganda Setting: rural Malaria endemicity: in Bwambara, lower altitudinal area meso‐endemic with moderate‐to‐high transmission; in Nyakishenyi, epidemic‐prone highland with low transmission, hypo‐endemic Study dates: July 2011–December 2011 Study sponsor: the ACT Consortium, through a grant from the Bill and Melinda Gates Foundation to the London School of Hygiene and Tropical Medicine. Sian Clarke supported by the Wellcome Trust through a Research Career Development Fellowship (084933) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Within each transmission area, villages (clusters) were randomly allocated using a random number table in Epi Info to intervention or control (though no mention of independent statistician). |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) Antimalarial prescribing to microscopy‐ or PCR‐negative people, appropriate treatment | Unclear risk | No mention of blinding, unclear how this could affect outcome. |
| Blinding of participants and personnel (performance bias) Number receiving an antimalarial | Unclear risk | No mention of blinding, unclear how this could affect outcome. |
| Blinding of outcome assessment (detection bias) Antimalarial prescribing to microscopy‐ or PCR‐negative people, appropriate treatment | Low risk | Reference microscopy blinded to results. |
| Blinding of outcome assessment (detection bias) Number receiving an antimalarial | Unclear risk | No mention of blinding, unclear how this could affect outcome. |
| Incomplete outcome data (attrition bias) Antimalarial prescribing to microscopy‐ or PCR‐negative people, appropriate treatment | Unclear risk | After training, CHWs in villages located close to the border of the study area started to receive febrile children from outside the study district; these villages were subsequently withdrawn from the trial: 1/31 clusters in the intervention arm, 2/32 in control for moderate/high transmission – modified ITT analysis. |
| Incomplete outcome data (attrition bias) Number receiving an antimalarial | Unclear risk | After training, CHWs in villages located close to the border of the study area started to receive febrile children from outside the study district; these villages were subsequently withdrawn from the trial: 1/31 clusters in the intervention arm, 2/32 in the control for moderate/high transmission – modified ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Appears to correlate with NCT01048801, however that record refers to a different paper which could be incorrect. |
| Other bias | Unclear risk | Recruitment after randomization, which could have influenced selection, though little baseline imbalance. Possible differences in low transmission, unclear how this would affect outcome (e.g. sex, CHW previous experience). Contamination and mRDT uptake were not described (no explanation of why the number of consultations in the control arm usually exceeded those in the mRDT arm). |
Ohnmar 2012.
| Study characteristics | ||
| Methods | Trial design: cRCT Unit of randomization: village Number of clusters: 59 Data collection: pre‐ and postintervention surveys (either self‐completed or interviews) of participants reporting fever when screened at household and individual level, death registers and verbal autopsy surveys (at endline assessment), volunteer and midwives' logbooks (at endline) Length of follow‐up: 11 months Adjustment for clustering: yes |
|
| Participants | Target treatment group: adults and children Sample size: 59 villages (21 intervention, 17 comparison 1, 21 comparison 2) Exclusion criteria: infants (no age given), pregnancy, symptoms such as sore throat, difficult urination, ear discharge, cough, loose stools, skin ulcers |
|
| Interventions | Staff who received training: unpaid volunteers Duration of training: 2 days Content of training: treating P falciparum malaria after positive mRDT with AL, and treating presumptive P Vivax with CQ Supervision: midwives in nearest health facility routinely monitored/supervised volunteers during their monthly immunization visits. Malaria supervisors also occasionally visited volunteers. Antimalarials free to participants: unclear mRDTs free to participants: yes Additional details: volunteers displayed posters announcing availability of free mRDTs; there were educational flip charts in local languages for volunteers to educate participants; supervisors demonstrated impregnation of nets, though not as part of training; 1 co‐intervention village with paid mRDT services |
|
| Outcomes | All‐cause and malaria mortality, hospitalization | |
| Notes | Control: unpaid volunteers dispensing medicines without test results (community‐based treatment of suspected malaria by clinical diagnosis) Country: Myanmar Setting: rural Malaria endemicity: moderate endemicity all year round, transmission peak June/July Study dates: March 2009–February 2010 Study sponsor: WHO/SEARO‐TDR Small Grants Scheme, New Delhi, India |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | High risk | No allocation concealment. |
| Blinding of participants and personnel (performance bias) Mortality | Low risk | Not blinded, but mortality unlikely to be affected by knowledge of intervention. |
| Blinding of participants and personnel (performance bias) Antimalarial prescribing to microscopy‐ or PCR‐negative people, appropriate treatment | Low risk | Not blinded, hospitalizations unlikely to be affected by knowledge of intervention. |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Not blinded, unlikely to be affected by knowledge of intervention. |
| Blinding of outcome assessment (detection bias) Antimalarial prescribing to microscopy‐ or PCR‐negative people, appropriate treatment | Low risk | Not blinded, unlikely to be affected by knowledge of intervention. |
| Incomplete outcome data (attrition bias) Mortality | High risk | 4/21 intervention villages dropped out (2 before, 2 after training). Problems capturing mortality data as planned. |
| Incomplete outcome data (attrition bias) Antimalarial prescribing to microscopy‐ or PCR‐negative people, appropriate treatment | Unclear risk | 4/21 intervention villages dropped out (2 before, 2 after training), unclear how this would affect outcome. |
| Other bias | Unclear risk | Recruitment after randomization, which could have influenced selection, though little baseline imbalance. Intervention villages smaller, closer to health facility than controls, otherwise similar. Participants similar except intervention group less likely to be Bamar/Buddhist. Villages had to be ≥ 2 hours away from nearest selected villages, however there was evidence of contamination. 9 volunteers had ≥ 1‐month period without any mRDT activity. It is not known whether this inactive period was due to his/her absence, mRDT shortage, or no cases of fever. |
Swana 2016.
| Study characteristics | ||
| Methods | Trial design: CBA (called quasi‐experimental by study authors) Data collection: weekly CHW records, pre‐ and postintervention school malaria prevalence surveys (and qualitative studies) Length of follow‐up: 13 months |
|
| Participants | Target treatment group: all ages > 2 months Sample size: not known Exclusion criteria: pregnancy |
|
| Interventions | Staff who received training: CHWs Duration of training: 3 days Content of training: treating P falciparum malaria after positive mRDT with AQAS or rectal AS, and referring if negative mRDT as appropriate Supervision: initial observation of mRDTs by supervisors with corrective actions over 2–4 days, weekly visits thereafter, 3 monthly meetings with investigators Antimalarials free to participants: yes mRDTs free to participants: not reported Additional details: CHWs given bicycles (which they could keep) and food baskets at end of study. Community sensitization campaign at start and ongoing information sessions by CHWs. Periodic IRS and larval source management |
|
| Outcomes | Parasitaemia | |
| Notes | Control: health facility care Country: Democratic Republic of the Congo Setting: rural Malaria endemicity: high prevalence, perennial Study dates: November 2011–August 2013 Study sponsor: Freeport McMoRan and Tenke Fungurume Mining, US President's Malaria Initiative |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomized, quasi‐experimental with 1 intervention and 1 comparison area. |
| Allocation concealment (selection bias) | High risk | Not randomized, quasi‐experimental with 1 intervention and 1 comparison area. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Unclear risk | Few details, however net ownership/use was slightly higher in intervention regions, and malaria prevalence at the beginning of the study was significantly higher in the intervention arm compared to the comparison arm. Uptake of mRDTs was unclear. |
Thiam 2012.
| Study characteristics | ||
| Methods | Trial design: CBA (not explicit) Data collection: pre‐ and postintervention data routinely collected at health facility level on a standard form validated at district level before submission to national malaria control programme. Home care providers used standard registers for participant demographics, test results, treatment and follow‐up, drug consumption. Length of follow‐up: not clear |
|
| Participants | Target treatment group: all ages > 2 months Sample size: 861 villages Exclusion criteria: pregnancy, severe disease |
|
| Interventions | Staff who received training: home care providers Duration of training: 3 days theory, 2 weeks practical Content of training: treating Pfalciparum malaria after positive mRDT with AQAS, and referring if negative mRDT Spervision: post‐training follow‐up, monthly supervision by health post head nurse, co‐ordinating meetings by district health team and nurses in peripheral health facilities Antimalarials free to participants: no (USD 0.6 adults, USD 0.3 children until May 2010) mRDTs free to participants: yes Additional details: incentives for home care providers, sensitization in villages, community mobilization events/radio broadcasts, link between head of village and home care provider, simultaneous scale‐up of malaria control interventions (nets) |
|
| Outcomes | All‐cause and malaria mortality, hospitalization | |
| Notes | Control: health facility care Country: Senegal Setting: rural Malaria endemicity: endemic malaria with high transmission during rainy season of July–November Study dates: unclear 2008–May 2010 Study sponsor: the Global Fund for AIDS, TB and Malaria and the President's Malaria Initiative |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | CBA (not explicit). |
| Allocation concealment (selection bias) | High risk | CBA (not explicit). |
| Blinding of participants and personnel (performance bias) Mortality | Low risk | Not blinded, but mortality unlikely to be affected by knowledge of intervention. |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | Not blinded, but mortality unlikely to be affected by knowledge of intervention. |
| Incomplete outcome data (attrition bias) Mortality | Low risk | Routine data with data quality assurance system at 3 stages. |
| Other bias | Low risk | The same malaria control strategies in both areas, slightly higher bed net use in comparison area. mRDTs were used in 92% of people with suspected malaria in the intervention area. |
ACT: artemisinin‐based combination therapy; AL: artemether‐lumefantrine; AQAS: amodiaquine‐artesunate; CBA: controlled before‐after study; CHW: community health worker; CQ: chloroquine; cRCT: cluster‐randomized controlled trial; DP: dihydroartemisinin‐piperaquine; IRS: indoor residual spraying; ITT: intention‐to‐treat; mRDT: malaria rapid diagnostic test; PCR: polymerase chain reaction; Pfalciparum: Plasmodiumfalciparum; Pvivax: Plasmodium; SP/AS: sulfadoxine‐pyrimethamine and artesunate.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Awor 2014 | Intervention not limited to malaria (iCCM of malaria, pneumonia and diarrhoea). |
| Biemba 2016 | Intervention not limited to malaria (iCCM of malaria, pneumonia and diarrhoea). |
| Gaye 2020 | mRDTs in both arm, active versus passive. |
| Kitutu 2017 | Intervention not limited to malaria (iCCM of malaria, pneumonia). |
| Maloney 2017 | Difference between arms only related to recommended retail price. |
| Mbonye 2015 | A large proportion of vendors had professional healthcare qualifications (nurses, midwives or clinical officers; 37.9% in the intervention arm and 56.7% in the control arm), while the remainder were auxiliary nurses or nursing aides. |
| Mukanga 2012 | Intervention not limited to malaria (included pneumonia). |
| Yeboah‐Antwi 2010 | Intervention not limited to malaria (included pneumonia). |
iCCM: integrated community case management; mRDT: malaria rapid diagnostic test.
Characteristics of ongoing studies [ordered by study ID]
Soniran 2020.
| Study name | Evaluating interventions to improve test, treat, and track (T3) malaria strategy among over‐the‐counter medicine sellers (OTCMS) in some rural communities of Fanteakwa North district, Ghana: study protocol for a cluster randomized controlled trial |
| Methods | cRCT |
| Participants | Adults and children |
| Interventions | Subsidized mRDT kits; training on malaria diagnosis, treatment, and tracking of cases; supportive visits; community sensitization; and malaria surveillance tool |
| Outcomes | Primary outcome: proportion of children < 10 with fever/suspected malaria visiting CHW and tested before treatment. Secondary outcome: proportion of children < 10 receiving antimalarial drugs without testing; adherence to treatment guidelines; adherence to mRDT retail price; sensitivity, specificity, and predictive values of mRDTs |
| Starting date | September 2019 |
| Contact information | cahorlu@noguchi.ug.edu.gh |
| Notes |
CHW: community health worker; cRCT: cluster‐randomized controlled trial; mRDT: malaria rapid diagnostic test.
Differences between protocol and review
Differences between review and review update
This is an update of Okwundu 2013.
A new author team performed this review update, and we amended the review title from 'Home‐ or community‐based programmes for treating malaria' to 'Adding rapid diagnostic tests to community‐based programmes for treating malaria'.
We revised the protocol to be more relevant for how community recognition and treatment of malaria is managed with randomized controlled trials. We amended the risk of bias methods.
Contributions of authors
EA and AW revised the protocol, based on an earlier published review (Okwundu 2013), and led screening, selection, and data extraction. MM joined the team to lead the statistical analysis, with input from EA. EA and MM drafted the review. All authors reviewed the text.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK
External sources
-
Foreign, Commonwealth and Development Office (FCDO), UK
Project number 300342‐104
Declarations of interest
EA: none AW: none MM: none
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Ansah 2015 {published data only}
- Ansah EK, Narh-Bana S, Affran-Bonful H, Bart-Plange C, Cundill B, Gyapong M, et al. The impact of providing rapid diagnostic malaria tests on fever management in the private retail sector in Ghana: a cluster randomized trial. BMJ 2015;350:h1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2015 {published data only}
- Cohen J, Fink G, Maloney K, Berg K, Jordan M, Svoronos T, et al. Introducing rapid diagnostic tests for malaria to drug shops in Uganda: a cluster-randomized controlled trial. Bulletin of the World Health Organization 2015;93:142-51. [Google Scholar]
Leslie 2017 {published data only}
- Leslie T, Rowland M, Mikhail A, Cundill B, Willey B, Alokozai A, et al. Use of malaria rapid diagnostic tests by community health workers in Afghanistan: cluster randomised trial. BMC Medicine 2017;15:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mubi 2011 {published data only}
- Mubi M, Janson A, Warsame M, Mårtensson A, Källander K, Petzold MG, et al. Malaria rapid testing by community health workers is effective and safe for targeting malaria treatment: randomised cross-over trial in Tanzania. PLOS One 2011;6(7):e19753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ndyomugyenyi 2016 {published data only}
- Ndyomugyenyi R, Magnussen P, Lal S, Hansen K, Clarke SE. Appropriate targeting of artemisinin-based combination therapy by community health workers using malaria rapid diagnostic tests: findings from randomized trials in two contrasting areas of high and low malaria transmission in south-western Uganda. Tropical Medicine and International Health 2016;21(9):1157-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ohnmar 2012 {published data only}
- Ohnmar, Tun-Min, San-Shwe, Than-Win, Chongsuvivatwong V. Effects of malaria volunteer training on coverage and timeliness of diagnosis: a cluster randomized controlled trial in Myanmar. Malaria Journal 2012;11:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Swana 2016 {published data only}
- Swana EK, Makan GY, Mukeng CK, Mupumba HI, Kalaba GM, Luboya ON, et al. Feasibility and implementation of community-based malaria case management with integrated vector control in the Democratic Republic of Congo. Malaria Journal 2016;15:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thiam 2012 {published data only}
- Thiam S, Thwing J, Diallo I, Fall FB, Diouf MB, Perry R, et al. Scale-up of home-based management of malaria based on rapid diagnostic tests and artemisinin-based combination therapy in a resource-poor country: results in Senegal. Malaria Journal 2012;11:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Awor 2014 {published data only}
- Awor P, Wamani H, Tylleskar T, Jagoe G, Peterson S. Increased access to care and appropriateness of treatment at private sector drug shops with integrated management of malaria, pneumonia and diarrhoea: a quasi-experimental study in Uganda. PLOS One 2014;9(12):e115440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Biemba 2016 {published data only}
- Biemba G, Yeboah-Antwi K, Vosburg KB, Prust ML, Keller B, Worku Y, et al. Effect of deploying community health assistants on appropriate treatment for diarrhoea, malaria and pneumonia: quasi-experimental study in two districts of Zambia. Tropical Medicine and International Health 2016;21(8):985-94. [DOI] [PubMed] [Google Scholar]
Gaye 2020 {published data only}
- Gaye S, Kibler J, Ndiaye JD, Diouf MB, Linn A, Gueye AB, et al. Proactive community case management in Senegal 2014–2016: a case study in maximizing the impact of community case management of malaria. Malaria Journal 2020;19:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kitutu 2017 {published data only}
- Kitutu FE, Kalyango JN, Mayora C, Selling KE, Peterson S, Wamani H. Integrated community case management by drug sellers influences appropriate treatment of paediatric febrile illness in South Western Uganda: a quasi-experimental study. Malaria Journal 2017;16(1):425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maloney 2017 {published data only}
- Maloney K, Ward A, Krenz B, Petty N, Bryson L, Dolkart C, et al. Expanding access to parasite-based malaria diagnosis through retail drug shops in Tanzania: evidence from a randomized trial and implications for treatment. Malaria Journal 2017;16(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mbonye 2015 {published data only}
- Mbonye AK, Magnussen P, Lal S, Hansen KS, Cundill B, Chandler C, et al. A cluster randomised trial introducing rapid diagnostic tests into registered drug shops in Uganda: impact on appropriate treatment of malaria. PLOS One 2015;10(7):e0129545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mukanga 2012 {published data only}
- Mukanga D, Tiono AB, Anyorigiya T, Källander K, Konaté AT, Oduro AR, et al. Integrated community case management of fever in children under five using rapid diagnostic tests and respiratory rate counting: a multi-country cluster randomized trial. American Journal of Tropical Medicine and Hygiene 2012;87(5):21-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeboah‐Antwi 2010 {published data only}
- Yeboah-Antwi K, Pilingana P, Macleod WB, Semrau K, Siazeele K, Kalesha P, et al. Community case management of fever due to malaria and pneumonia in children under five in Zambia: a cluster randomized controlled trial. PLOS Medicine 2010;7(9):e1000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Soniran 2020 {published data only}
- Soniran OT, Abuaku B, Ahorlu CS. Evaluating interventions to improve test, treat, and track (T3) malaria strategy among over-the-counter medicine sellers (OTCMS) in some rural communities of Fanteakwa North district, Ghana: study protocol for a cluster randomized controlled trial. Trials 2020;21(1):623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Amouzou 2014
- Amouzou A, Morris S, Moulton LH, Mukanga D. Assessing the impact of integrated community case management (iCCM) programs on child mortality: review of early results and lessons learned in sub-Saharan Africa. Journal of Global Health 2014;4(2):020411. [DOI: 10.7189/jogh.04.020411] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aung 2015
- Aung T, White C, Montagu D, McFarland W, Hlaing T, Khin HS, et al. Improving uptake and use of malaria rapid diagnostic tests in the context of artemisinin drug resistance containment in eastern Myanmar: an evaluation of incentive schemes among informal private healthcare providers. Malaria Journal 2015;14:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Awor 2014
- Awor P, Miller J, Peterson S. Systematic literature review of integrated community case management and the private sector in Africa: relevant experiences and potential next steps. Journal of Global Health 2014;4(2):020414. [DOI: 10.7189/jogh.04.020414] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bath 2020
- Bath D, Goodman C, Yeung S. Modelling the cost-effectiveness of introducing subsidised malaria rapid diagnostic tests in the private retail sector in sub-Saharan Africa. BMJ Global Health 2020;5(5):e002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyce 2017
- Boyce MR, O'Meara WP. Use of malaria RDTs in various health contexts across sub-Saharan Africa: a systematic review. BMC Public Health 2017;17(1):470. [DOI: 10.1186/s12889-017-4398-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyce 2018
- Boyce MR, Menya D, Turner EL, Laktabai J, Prudhomme-O'Meara W. Evaluation of malaria rapid diagnostic test (RDT) use by community health workers: a longitudinal study in western Kenya. Malaria Journal 2018;17(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruxvoort 2014
- Bruxvoort K, Festo C, Kalolella A, Cairns M, Lyaruu P, Kenani M, et al. Cluster randomized trial of text message reminders to retail staff in Tanzanian drug shops dispensing artemether-lumefantrine: effect on dispenser knowledge and patient adherence. American Journal of Tropical Medicine and Hygiene 2014;91(4):844-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burchett 2017
- Burchett HE, Leurent B, Baiden F, Baltzell K, Björkman A, Bruxvoort K, et al. Improving prescribing practices with rapid diagnostic tests (RDTs): synthesis of 10 studies to explore reasons for variation in malaria RDT uptake and adherence. BMJ Open 2017;7(3):e012973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chandler 2008a
- Chandler CI, Jones C, Boniface G, Juma K, Reyburn H, Whitty CJ. Guidelines and mindlines: why do clinical staff over-diagnose malaria in Tanzania? A qualitative study. Malaria Journal 2008;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chandler 2008b
- Chandler CI, Mwangi R, Mbakilwa H, Olomi R, Whitty CJ, Reyburn H. Malaria overdiagnosis: is patient pressure the problem? Health Policy and Planning 2008;23(3):170-8. [DOI] [PubMed] [Google Scholar]
Christopher 2011
- Christopher JB, Le May A, Lewin S, Ross DA. Thirty years after Alma-Ata: a systematic review of the impact of community health workers delivering curative interventions against malaria, pneumonia and diarrhoea on child mortality and morbidity in sub-Saharan Africa. Human Resources for Health 2011;9:27. [DOI: 10.1186/1478-4491-9-27] [DOI] [PMC free article] [PubMed] [Google Scholar]
Danquah 2016
- Danquah DA, Buabeng KO, Asante KP, Mahama E, Bart-Plange C, Owusu-Dabo E. Malaria case detection using rapid diagnostic test at the community level in Ghana: consumer perception and practitioners' experiences. Malaria Journal 2016;15:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Diggle 2014
- Diggle E, Asgary R, Gore-Langton G, Nahashon E, Mungai J, Harrison R, et al. Perceptions of malaria and acceptance of rapid diagnostic tests and related treatment practises among community members and health care providers in Greater Garissa, North Eastern Province, Kenya. Malaria Journal 2014;13:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elven 2020
- Elven J, Dahal P, Ashley EA, Thomas NV, Shrestha P, Stepniewska K, et al. Non-malarial febrile illness: a systematic review of published aetiological studies and case reports from Africa, 1980–2015. BMC Medicine 2020;18(1):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2017
- Cochrane Effective Practice and Organisation of Care. Suggested risk of bias criteria for EPOC reviews. EPOC resources for review authors, 2017. Available at epoc.cochrane.org/resources/epoc-resources-review-authors (accessed 19 February 2019).
Gogtay 2013
- Gogtay N, Kannan S, Thatte UM, Olliaro PL, Sinclair D. Artemisinin-based combination therapy for treating uncomplicated Plasmodium vivax malaria. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD008492. [DOI: 10.1002/14651858.CD008492.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI: 10.1016/j.jclinepi.2010.04.026] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2019
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized controlled trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Hopkins 2007
- Hopkins H, Talisuna A, Whitty CJ, Staedke SG. Impact of home-based management of malaria on health outcomes in Africa: a systematic review of the evidence. Malaria Journal 2007;6:134. [DOI: 10.1186/1475-2875-6-134] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hopkins 2017
- Hopkins H, Bruxvoort KJ, Cairns ME, Chandler CI, Leurent B, Ansah EK, et al. Impact of introduction of rapid diagnostic tests for malaria on antibiotic prescribing: analysis of observational and randomised studies in public and private healthcare settings. BMJ 2017;356:j1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutchinson 2017
- Hutchinson E, Hutchison C, Lal S, Hansen K, Kayendeke M, Nabirye C, et al. Introducing rapid tests for malaria into the retail sector: what are the unintended consequences? BMJ Global Health 2017;2(1):e00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICF International 2012
- ICF International LSHTM. Independent Evaluation of the Affordable Medicines Facility – malaria (AMFm) Phase 1. Multi-country independent evaluation report: final report. 28 September 2012. unitaid.org/assets/Mid-term-evaluation-Affordable-medicines-for-malaria-facility-AMFm-Phase-1.pdf (accessed 30 November 2019).
Kabaghe 2016
- Kabaghe AN, Visser BJ, Spijker R, Phiri KS, Grobusch MP, Vugt M. Health workers' compliance to rapid diagnostic tests (RDTs) to guide malaria treatment: a systematic review and meta-analysis. Malaria Journal 2016;15:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kangwana 2011
- Kangwana BP, Kedenge SV, Noor AM, Alegana VA, Nyandigisi AJ, Pandit J, et al. The impact of retail-sector delivery of artemether-lumefantrine on malaria treatment of children under five in Kenya: a cluster randomized controlled trial. PLOS Medicine 2011;8(5):e1000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kassam 2015
- Kassam R, Collins JB, Liow E, Rasool N. Narrative review of current context of malaria and management strategies in Uganda (Part I). Acta Tropica 2015;152:252-68. [DOI: 10.1016/j.actatropica.2015.07.028] [DOI] [PubMed] [Google Scholar]
Kwarteng 2019
- Kwarteng A, Malm KL, Febir LG, Tawiah T, Adjei G, Nyame S, et al. The accuracy and perception of test-based management of malaria at private licensed chemical shops in the middle belt of Ghana. American Journal of Tropical Medicine and Hygiene 2019;100(2):264-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kyaw 2016
- Kyaw SS, Drake T, Thi A, Kyaw MP, Hlaing T, Smithuis FM, et al. Malaria community health workers in Myanmar: a cost analysis. Malaria Journal 2016;15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lassi 2010
- Lassi ZS, Haider BA, Bhutta ZA. Community-based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes. Cochrane Database of Systematic Reviews 2010, Issue 11. Art. No: CD007754. [DOI: 10.1002/14651858.CD007754.pub2] [DOI] [PubMed] [Google Scholar]
Maloney 2017
- Maloney K, Ward A, Krenz B, Petty N, Bryson L, Dolkart C, et al. Expanding access to parasite-based malaria diagnosis through retail drug shops in Tanzania: evidence from a randomized trial and implications for treatment. Malaria Journal 2017;16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Odaga 2014
- Odaga J, Sinclair D, Lokong JA, Donegan S, Hopkins H, Garner P. Rapid diagnostic tests versus clinical diagnosis for managing people with fever in malaria endemic settings. Cochrane Database of Systematic Reviews 2014, Issue 4. Art. No: CD008998. [DOI: 10.1002/14651858.CD008998.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliphant 2017
- Oliphant NP, Daniels K, Odendaal WA, Besada D, Manda S, Kinney M, et al. Integrated community case management of childhood illness in low- and middle-income countries. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD012882. [DOI: 10.1002/14651858.CD012882] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perry 2014
- Perry HB, Zulliger R, Rogers MM. Community health workers in low-, middle-, and high-income countries: an overview of their history, recent evolution, and current effectiveness. Annual Review of Public Health 2014;35:399-421. [DOI: 10.1146/annurev-publhealth-032013-182354] [DOI] [PubMed] [Google Scholar]
Pluess 2010
- Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database of Systematic Reviews 2010, Issue 4. Art. No: CD006657. [DOI: 10.1002/14651858.CD006657.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pryce 2018
- Pryce J, Richardson M, Lengeler C. Insecticide-treated nets for preventing malaria. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD000363. [DOI: 10.1002/14651858.CD000363.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Radeva‐Petrova 2014
- Radeva-Petrova D, Kayentao K, ter Kuile FO, Sinclair D, Garner P. Drugs for preventing malaria in pregnant women in endemic areas: any drug regimen versus placebo or no treatment. Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No: CD000169. [DOI: 10.1002/14651858.CD000169.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager Web 2022 [Computer program]
- The Cochrane Collaboration Review Manager Web (RevMan Web). Version 4.10.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rosato 2008
- Rosato M, Laverack G, Grabman LH, Tripathy P, Nair N, Mwansambo C, et al. Community participation: lessons for maternal, newborn, and child health. Lancet 2008;372(9642):962-71. [DOI: 10.1016/S0140-6736(08)61406-3] [DOI] [PubMed] [Google Scholar]
Ruizendaal 2014
- Ruizendaal E, Dierickx S, Peeters Grietens K, Schallig HD, Pagnoni F, Mens PF. Success or failure of critical steps in community case management of malaria with rapid diagnostic tests: a systematic review. Malaria Journal 2014;13:229. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salam 2014
- Salam RA, Das JK, Lassi ZS, Bhutta ZA. Impact of community-based interventions for the prevention and control of malaria on intervention coverage and health outcomes for the prevention and control of malaria. Infectious Diseases of Poverty 2014;3:25. [DOI: 10.1186/2049-9957-3-25] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI] [PubMed] [Google Scholar]
Sinclair 2009
- Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD007483. [DOI: 10.1002/14651858.CD007483.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith Paintain 2014
- Smith Paintain L, Willey B, Kedenge S, Sharkey A, Kim J2, Buj V, et al. Community health workers and stand-alone or integrated case management of malaria: a systematic literature review. American Journal of Hygiene and Tropical Medicine 2014;91(3):461-70. [DOI: 10.4269/ajtmh.14-0094] [DOI] [PMC free article] [PubMed] [Google Scholar]
Statacorp [Computer program]
- Stata. Version 16. College Station, TX, USA: StataCorp, 2019. Available at www.stata.com.
Sunguya 2017
- Sunguya BF, Mlunde LB, Ayer R, Jimba M. Towards eliminating malaria in high endemic countries: the roles of community health workers and related cadres and their challenges in integrated community case management for malaria: a systematic review. Malaria Journal 2017;16:10. [DOI: 10.1186/s12936-016-1667-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tusting 2014
- Tusting LS. Larval source management – a supplementary measure for malaria vector control. An operational manual. Outlooks on Pest Management 2014;25:41-3. [DOI: 10.1564/v25_feb_13] [DOI]
UNICEF 2012
- UNICEF. A systematic review of strategies to increase demand, uptake and quality of community-based diagnosis and case management of malaria. December 2012. chwcentral.org/wp-content/uploads/2013/07/A-systematic-review-of-strategies-to-increase-demand-uptake-and-quality-of-community-based-diagnosis-and-case-management-of-malaria_0.pdf (accessed 30 November 2018).
UNICEF 2014
- UNICEF. Committing to Child Survival: A Promise Renewed. Progress Report 2015. www.unicef.org/media/50721/file/APR_2015_9_Sep_15.pdf (accessed 30 November 2018).
Visser 2017
- Visser T, Bruxvoort K, Maloney K, Leslie T, Barat LM, Allan R, et al. Introducing malaria rapid diagnostic tests in private medicine retail outlets: a systematic literature review. PLOS One 2017;12:e0173093. [DOI: 10.1371/journal.pone.0173093] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 2019
- Watson OJ, Sumner KM, Janko M, Goel V, Winskill P, Slater HC, et al. False-negative malaria rapid diagnostic test results and their impact on community-based malaria surveys in sub-Saharan Africa. BMJ Global Health 2019;4(4):e001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2004
- World Health Organization. Scaling up home-based management of malaria. From research to implementation. Available at apps.who.int/iris/bitstream/handle/10665/42918/WHO_HTM_MAL_2004.1096.pdf?sequence=1&isAllowed=y (accessed 30 November 2018).
WHO 2006
- World Health Organization. Guidelines for the treatment of malaria. First edition. 2006. Available at infonet-biovision.org/sites/default/files/1571.treatmentguidelines20061.pdf (accessed 30 November 2018).
WHO 2010a
- World Health Organization. WHO policy recommendation on intermittent preventive treatment during infancy with sulfadoxine-pyrimethamine (SP-IPTi) for Plasmodium falciparum malaria control in Africa. 2010. Available at apps.who.int/iris/bitstream/handle/10665/337977/WHO-HTM-GMP-2010.01-eng.pdf (accessed 10 February 2019).
WHO 2010b
- World Health Organization. Guidelines for the Treatment of Malaria. Second edition. 2010. Available at www.paho.org/en/node/50095 (accessed 30 November 2018).
WHO 2012a
- World Health Organization. Intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP): updated WHO policy recommendation. Available at apps.who.int/iris/bitstream/handle/10665/337990/WHO-HTM-GMP-2012.05-eng.pdf (accessed 10 February 2019).
WHO 2012b
- World Health Organization. WHO policy recommendation: seasonal malaria chemoprevention (SMC) for Plasmodium falciparum malaria control in highly seasonal transmission areas of the Sahel sub-region in Africa. Available at apps.who.int/iris/bitstream/handle/10665/337978/WHO-HTM-GMP-2012.02-eng.pdf (accessed 10 February 2019).
WHO 2015
- World Health Organization. Guidelines for the treatment of malaria. Third edition. 2015. Available at apps.who.int/iris/bitstream/handle/10665/162441/9789241549127_eng.pdf (accessed 30 November 2018).
WHO 2017
- World Health Organization. Achieving and maintaining universal coverage with long-lasting insecticidal nets for malaria control. 2017. Available at apps.who.int/iris/bitstream/handle/10665/259478/WHO-HTM-GMP-2017.20-eng.pdf (accessed 30 November 2018).
WHO 2021a
- World Health Organization. World Malaria Report 2021. Available at apps.who.int/iris/handle/10665/350147 (accessed 17 February 2021).
WHO 2021b
- World Health Organization. WHO guidelines for malaria, 13 July 2021. Available at apps.who.int/iris/bitstream/handle/10665/343751/WHO-UCN-GMP-2021.01-Rev.1-eng.pdf (accessed 17 February 2022).
WHO 2022
- World Health Organization. WHO guidelines for malaria. 3 June 2022. Available at who.int/publications/i/item/guidelines-for-malaria (accessed 07 September 2022).
Winch 2005
- Winch PJ, Gilroy KE, Wolfheim C, Starbuck ES, Young MW, Walker LD, et al. Intervention models for the management of children with signs of pneumonia or malaria by community health workers. Health Policy Plan 2005;20(4):199-212. [DOI: 10.1093/heapol/czi027] [DOI] [PubMed] [Google Scholar]
Young 2012
- Young M, Wolfheim C, Marsh DR, Hammamy D. World Health Organization/United Nations Children's Fund joint statement on integrated community case management: an equity-focused strategy to improve access to essential treatment services for children. American Journal of Tropical Medicine and Hygiene 2012;87:6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zani 2014
- Zani B, Gathu M, Donegan S, Olliaro PL, Sinclair D. Dihydroartemisinin-piperaquine for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No: CD010927. [DOI: 10.1002/14651858.CD010927] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Okwundu 2011
- Okwundu CI, Nagpal S, Musekiwa A. Home or community programmes for treating malaria. Cochrane Database of Systematic Reviews 2011, Issue 12. Art. No: CD009527. [DOI: 10.1002/14651858.CD009527] [DOI] [PMC free article] [PubMed] [Google Scholar]
Okwundu 2013
- Okwundu CI, Nagpal S, Musekiwa A, Sinclair D. Home- or community-based programmes for treating malaria. Cochrane Database of Systematic Reviews 2011, Issue 5. Art. No: CD009527. [DOI: 10.1002/14651858.CD009527.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


