Abstract
Accumulating evidence suggests a higher risk for cardiovascular diseases among individuals with mental disorders, but very little is known about the risk for overall and specific groups of cardiovascular diseases in people with attention‐deficit/hyperactivity disorder (ADHD). To fill this knowledge gap, we investigated the prospective associations between ADHD and a wide range of cardiovascular diseases in adults. In a nationwide population‐based cohort study, we identified 5,389,519 adults born between 1941 and 1983, without pre‐existing cardiovascular diseases, from Swedish registers. The study period was from January 1, 2001 to December 31, 2013. Incident cardiovascular disease events were identified according to ICD codes. Hazard ratios (HR) with 95% confidence intervals (CI) were calculated using Cox proportional hazards regression model, with ADHD as a time‐varying exposure. After an average 11.80 years of follow‐up, 38.05% of individuals with ADHD versus 23.57% of those without ADHD had at least one diagnosis of cardiovascular disease (p<0.0001). ADHD was significantly associated with increased risk of any cardiovascular disease (HR=2.05, 95% CI: 1.98‐2.13) after adjusting for sex and year of birth. Further adjustments for education level, birth country, type 2 diabetes mellitus, obesity, dyslipidemia, sleep problems and heavy smoking attenuated the association, which however remained significant (HR=1.84, 95% CI: 1.77‐1.91). Further adjustment for psychiatric comorbidities attenuated but could not fully explain the association (HR=1.65, 95% CI: 1.59‐1.71). The strongest associations were found for cardiac arrest (HR=2.28, 95% CI: 1.81‐2.87), hemorrhagic stroke (HR=2.16, 95% CI: 1.68‐2.77), and peripheral vascular disease/arteriosclerosis (HR=2.05, 95% CI: 1.76‐2.38). Stronger associations were observed in males and younger adults, while comparable associations were found among individuals with or without psychotropic medications and family history of cardiovascular diseases. These data suggest that ADHD is an independent risk factor for a wide range of cardiovascular diseases. They highlight the importance of carefully monitoring cardiovascular health and developing age‐appropriate and individualized strategies to reduce the cardiovascular risk in individuals with ADHD.
Keywords: Attention‐deficit/hyperactivity disorder, cardiovascular diseases, cardiac arrest, hemorrhagic stroke, peripheral vascular disease, arteriosclerosis, psychotropic medications, psychiatric comorbidities
Attention‐deficit/hyperactivity disorder (ADHD), characterized by pervasive and impairing inattention and/or hyperactivity‐impulsivity, is one of the most common neurodevelopmental disorders, with a global prevalence of 2‐7% in children and 2.5% in adults1, 2, 3. The disorder is often comorbid with a number of psychiatric (e.g., anxiety disorders and depression 4 ) and physical (e.g., obesity 5 and asthma 6 ) conditions.
Prospective studies have previously demonstrated that several psychiatric conditions (e.g., depression7, 8, schizophrenia 9 , bipolar disorder 10 , and anxiety disorders 11 ) as well as neurodevelopmental disorders (e.g., autism12, 13, intellectual disabilities 12 , and conduct disorder 12 ) are associated with a higher risk for cardiovascular diseases, the leading cause of mortality worldwide 14 . The mechanisms linking psychiatric illness to cardiovascular diseases are complex, but risky health behaviours (e.g., smoking, drinking alcohol, substance abuse, and sedentary lifestyles) 15 and prolonged use of psychotropic medications 16 have been proposed as potential contributors to the risk.
Little is known about the risk for overall and specific groups of cardiovascular diseases in individuals with ADHD. In these individuals, such diseases have mainly been studied as potential adverse effects of pharmacological treatment17, 18, as ADHD medications have been reported to be associated with elevated blood pressure and heart rate, which may increase the risk for severe cardiovascular events (e.g., stroke, myocardial infarction) 19 .
Only a few studies have explored the association between ADHD and cardiovascular diseases. A small Dutch study 20 with 231 older adults found no overall association, although elevated levels of ADHD symptoms were associated with increased risk for cardiovascular diseases. A recent Swedish register‐based cohort study 21 with 4,288,451 sibling pairs and 1,841,303 family clusters (age: 18‐81 years) showed that adults with ADHD were at increased risk for a wide range of physical health conditions, including cardiovascular diseases.
However, in these few previous studies, only broad measures of cardiovascular diseases were used20, 21. Thus, there are no data about the risk for specific groups of such diseases in ADHD. This is important to inform prevention and treatment strategies, which may vary substantially depending on which specific cardiovascular diseases are most strongly associated with this mental disorder. Furthermore, no previous study has explored the role of psychiatric comorbidities and the use of psychotropic medications in the development of cardiovascular diseases in ADHD. This is an important limitation, as adults with ADHD are frequently treated pharmacologically not only for that condition but also for other concomitant psychiatric disorders (e.g., mood disorders and substance use disorders), which in turn may influence the risk for cardiovascular diseases 16 .
In addition, the role of well‐established risk factors for cardiovascular diseases, such as low educational attainment 22 , smoking 23 , sleep problems 24 , metabolic conditions (e.g., obesity 25 , type 2 diabetes mellitus 26 , and dyslipidemia 27 ), as well as cardiovascular family history 24 , has never been considered in exploring the association between ADHD and cardiovascular diseases. These modifiable or non‐modifiable risk factors have the potential to be included in screening tools to identify people who are at increased risk for cardivascular diseases 28 .
Finally, although it is well‐established that the prevalence rates of ADHD and cardiovascular diseases are higher in males than in females29, 30, and that the core symptoms of ADHD often decline with increasing age 31 , while the incidence of cardiovascular diseases increases substantially with advancing age 32 , it is currently unknown if these patterns translate into sex and age differences in the associations of ADHD with cardiovascular diseases. A better understanding of such sex‐ and age‐specific associations is needed for risk stratification and individualized treatment recommendations in individuals with ADHD.
In this register‐based cohort study, we aimed to fill these knowledge gaps by investigating the prospective associations between ADHD and the risk of developing a broad range of cardiovascular diseases in adults. We also aimed to examine the extent to which any observed associations could be explained by common psychiatric comorbidities, well‐established risk factors for cardiovascular diseases (e.g., low education level, smoking, sleep disorders, and metabolic conditions), use of psychotropic medications, and cardiovascular family history. An additional exploratory aim was to assess the potential impact of sex and age.
METHODS
This study was approved by the regional ethical review board in Stockholm, Sweden (reference number: 2013/862‐31/5). The informed consent of the participants is not required for pseudo‐anonymized register‐based research according to Swedish law.
Study cohort and data sources
The study cohort included all individuals born in Sweden between 1941 and 1983, who were alive and residing in Sweden in 2001 (N=5,448,328), the year since which outpatient data became available. We excluded individuals who had a history of any cardiovascular disease before or at baseline, and those who died or emigrated before being diagnosed with ADHD, leaving 5,389,519 individuals aged 18‐60 years at baseline.
We followed these individuals from January 1, 2001 until their first diagnosis of any cardiovascular disease, death, emigration, or December 31, 2013 (whichever occurred first), with the oldest cohort member censored at 73 years of age.
Data were obtained by linking multiple Swedish registries with the unique personal identification number assigned to every individual registered in Sweden. The Total Population Register (TPR) 33 includes all individuals in Sweden born since 1932, who were alive in 1963 and later. TPR also contains information on all migrations in or out of Sweden since 1969. The Medical Birth Register (MBR) 34 covers more than 99% of births in Sweden since 1973. The National Patient Register (NPR) 35 contains data on inpatient care since 1973 and outpatient care since 2001. The Prescribed Drug Register (PDR) 36 includes detailed information on all dispensed drugs in Sweden, coded according to the Anatomical Therapeutic Chemical (ATC) classification, since July 1, 2005. The Longitudinal Integration Database for Health Insurance and Labor Studies (LISA) 37 covers the entire Swedish population aged 16 or older since 1990. The Multi‐Generation Register (MGR) 38 provides information on biological relationships for all residents in Sweden since 1932. The Cause of Death Register 39 contains information on all registered deaths since 1961.
Measures
ADHD
Individuals with ADHD were identified as those who had received their first ADHD diagnosis (ICD‐9 or ICD‐10: 314/F90) from the NPR at the age of 3 years or older, or their first prescription of an ADHD medication (ATC codes: N06BA01, N06BA02, N06BA04, N06BA12, N06BA09) from the PDR, or both, before or during the follow‐up period.
This approach to identify individuals with ADHD has been validated and is widely used in Swedish register‐based studies21, 40, 41. In a sensitivity analysis, we only used diagnoses of ADHD from NPR for case identification, to reflect clinically diagnosed cases.
Cardiovascular diseases
Consistent with previous studies42, 43, incident cardiovascular disease events were defined as the first diagnosis of cardiovascular disease from NPR (including any cardiovascular disease and specific diseases: ischemic heart disease, cerebrovascular disease, venous thrombo‐embolism, hypertensive diseases, heart failure, arrhythmias, cardiac arrest, and peripheral vascular disease/arteriosclerosis), or death from cardiovascular disease obtained from the Cause of Death Register (see also supplementary information).
Covariates
We collected information on year of birth, sex, and country of birth (Sweden, other Nordic country, other) from TPR, and on highest educational level – primary or lower secondary, upper secondary, post‐secondary, post‐graduate, unknown – from LISA as a proxy of socioeconomic status.
Type 2 diabetes mellitus, obesity, dyslipidemia, sleep disorders, heavy smoking (including tobacco abuse and nicotine dependence) and psychiatric comorbidities (including anxiety disorders, autism spectrum disorder, bipolar disorder, conduct disorder, depressive disorder, eating disorders, intellectual disability, personality disorders, schizophrenia, and substance use disorders) diagnosed before the diagnosis of cardiovascular diseases were identified from NPR (see also supplementary information).
Statistical analysis
Main analyses
Cox proportional hazard regression model was used to estimate hazard ratios (HR) with 95% confidence intervals (CI) expressing the rate/risk of cardiovascular diseases in individuals with ADHD, compared with individuals without ADHD, taking attained age as the underlying time scale. ADHD was modelled as a time‐varying exposure, that is, individuals were assigned to the unexposed group before the diagnosis of ADHD, and were assigned to the exposed group from the first diagnosis of ADHD or medication prescription for this disorder to the end of follow‐up.
The analysis was first conducted for “any cardiovascular disease” as an outcome, and then separately for six major categories (ischemic heart disease, cerebrovascular disease, venous thrombo‐embolism, hypertensive diseases, heart failure, and arrhythmias) and 17 individual cardiovascular diseases (acute coronary syndrome, ACS; chronic coronary syndrome without ACS; subarachnoidal bleeding, hemorrhagic stroke, ischemic stroke, other cerebrovascular disease, deep vein thrombosis, pulmonary emboli, essential hypertension, other hypertensive disease, heart failure, ischemic cardiomyopathy, other cardiomyopathy, bradyarrhythmias, takyarrhythmias, cardiac arrest, peripheral vascular disease/arteriosclerosis).
In addition to the underlying attained age, we adjusted for year of birth and sex in model 1. Model 2 further adjusted for education level, birth country, type 2 diabetes mellitus, obesity, dyslipidemia, sleep problems, and heavy smoking. We further adjusted for psychiatric comorbidities in model 3. Next, we conducted stratified analyses for “any cardiovascular disease” by sex and age bands (18‐30, 31‐40, 41‐50, 51‐60 and 61‐73 years) for models 1‐3.
The proportionality of hazards over underlying time scale was assessed using a Schoenfeld residuals‐based test. There was no evidence of violation of the assumption.
Cumulative incidence of any cardiovascular disease among individuals with or without ADHD was estimated using flexible parametric models that adjusted for attained age, year of birth and sex, and were visualized by standardized survival curves 44 . Cumulative incidence of any cardiovascular disease for each sex (adjusted for year of birth) and age band (adjusted for sex and year of birth) was also estimated.
To further explore the specific contribution of each psychiatric comorbidity to the association between ADHD and any cardiovascular disease, given that the magnitude of their associated cardiovascular disease risk is known to vary 12 , models 1 and 2 were repeated by comparing individuals without ADHD to individuals with ADHD only (without any psychiatric comorbidities), and those with ADHD plus each specific psychiatric comorbidity.
Sensitivity analyses
First, we only used the diagnosis from NPR, without information on ADHD medications, to identify individuals with ADHD. Second, because treatment with stimulants (ATC codes: N06BA01, N06BA02, N06BA04, N06BA12), antipsychotics (ATC code: N05A), anxiolytics, hypnotics and sedatives (ATC codes: N05B, N05C), and antidepressants (ATC code: N06A) are known to be associated with cardiovascular diseases16, 45, we excluded individuals with ADHD and ever treated with stimulants or other psychotropic medications during the follow‐up period, to rule out the potential impact of medication treatment on the studied associations.
Third, to control for the familial susceptibility to cardiovascular diseases, we excluded those with family history of these diseases, which was defined as any cardiovascular event among any first‐degree relative (biological parents and full siblings). Finally, we used ADHD as time‐invariant exposure (i.e., individuals with the diagnosis of ADHD were considered as exposed from the baseline to the end of the follow‐up, regardless of the timing of the ADHD diagnosis) to further test the robustness of the results.
Data management was performed using SAS, version 9.4 (SAS Institute, Cary, NC, USA). Data analyses were conducted with R, version 4.0.5 (R Foundation for Statistical Computing) and Stata (version 15.0; Stata Corp LP, College Station, TX).
RESULTS
Cohort description
We followed 5,389,519 individuals for a total of 64,084,464 person‐years. Among these, 2,750,621 (51.04%) were males and 2,638,898 (48.96%) were females, with a mean age at study entry of 38.44±12.32 years. A total of 37,027 (0.68%) individuals (55.30% male and 44.70% female) had a diagnosis of ADHD or an ADHD medication prescription (see Table 1).
Table 1.
Baseline characteristics of individuals with and without attention‐deficit/hyperactivity disorder (ADHD)
| Total | With ADHD | Without ADHD | |
|---|---|---|---|
| (N=5,389,519) | (N=37,027) | (N=5,352,492) | |
| Age (years, mean±SD) | 38.44±12.32 | 30.34±9.25 | 38.49±12.32 |
| Year of birth (%) | |||
| 1941‐1950 | 22.40 | 2.93 | 22.54 |
| 1951‐1960 | 21.63 | 12.24 | 21.70 |
| 1961‐1971 | 24.50 | 28.89 | 24.47 |
| 1977‐1983 | 31.46 | 55.94 | 31.29 |
| Sex (%) | |||
| Male | 51.04 | 55.30 | 51.01 |
| Female | 48.96 | 44.70 | 48.99 |
| Country of birth (%) | |||
| Sweden | 78.94 | 89.64 | 78.86 |
| Denmark, Finland, Norway or Iceland | 3.69 | 2.34 | 3.70 |
| Other | 17.37 | 8.01 | 17.44 |
| Educational attainment (%) | |||
| Primary or lower secondary | 14.87 | 25.87* | 14.79 |
| Upper secondary | 41.68 | 47.69 | 41.64 |
| Post‐secondary | 33.39 | 21.82 | 33.47 |
| Post‐graduate | 1.21 | 0.43 | 1.22 |
| Unknown | 8.85 | 4.19 | 8.88 |
| Well‐established risk factors for CVD (%) | |||
| Type 2 diabetes | 2.93 | 2.62 | 2.93 |
| Obesity | 2.19 | 6.15* | 2.16 |
| Dyslipidemia | 2.04 | 1.15* | 2.04 |
| Sleep problems | 2.69 | 11.20* | 2.64 |
| Heavy smoking | 0.94 | 2.82* | 0.93 |
| Psychiatric comorbidities (%) | |||
| Anxiety disorders | 3.81 | 41.58* | 3.54 |
| Autism spectrum disorder | 0.25 | 11.14* | 0.17 |
| Bipolar disorder | 0.84 | 14.05* | 0.75 |
| Conduct disorder | 0.04 | 1.22* | 0.03 |
| Depressive disorder | 4.96 | 43.15* | 4.69 |
| Eating disorders | 0.22 | 2.79* | 0.20 |
| Intellectual disability | 0.38 | 3.29* | 0.36 |
| Personality disorders | 1.23 | 21.56* | 1.09 |
| Schizophrenia | 0.55 | 2.39* | 0.54 |
| Substance use disorders | 4.09 | 37.80* | 3.85 |
Significantly higher in individuals with vs. without ADHD, p<0.0001
Individuals with ADHD were more likely to have primary or lower secondary as the highest educational attainment (25.87% vs. 14.79%, p<0.0001). Moreover, they were more likely to be diagnosed with obesity (6.15% vs. 2.16%, p<0.0001), sleep problems (11.20% vs. 2.64%, p<0.0001), heavy smoking (2.82% vs. 0.93%, p<0.0001), and all types of psychiatric comorbidities (p<0.0001), compared to people without ADHD.
The overall mean duration of follow‐up was 11.80±2.85 years. During this period, 746,572 individuals were newly diagnosed with cardiovascular diseases. The overall incidence rate of these diseases within the study period was 1.65 per 100 person‐years; it was 1.79% among individuals with ADHD and 1.16% among those without ADHD (p<0.0001).
Main analyses
At the end of the follow‐up, the cumulative incidence of any cardiovascular disease was 38.05% (95% CI: 34.87%‐41.52%) for individuals with ADHD and 23.57% (95% CI: 23.47%‐23.67%) for those without ADHD (p<0.0001) (see also supplementary information).
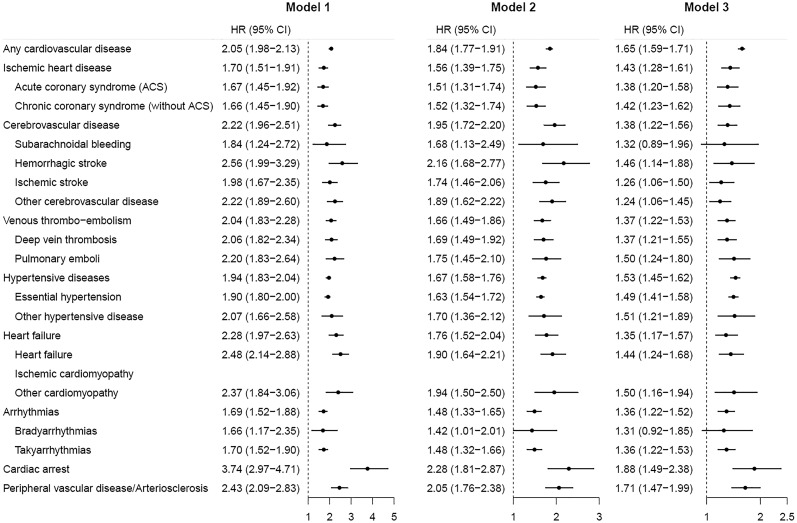
As shown in Figure 1, adults with ADHD had a more than two‐fold increased risk of any cardiovascular disease (HR=2.05, 95% CI: 1.98‐2.13), compared with those without ADHD, after adjusting for sex and year of birth (model 1). The association attenuated, but remained significant, when adjusted for education level, birth country, type 2 diabetes mellitus, obesity, dyslipidemia, sleep problems and heavy smoking in model 2 (HR=1.84, 95% CI: 1.77‐1.91). Further adjustments for psychiatric comorbidities (model 3) attenuated but did not fully explain the association (HR=1.65, 95% CI: 1.59‐1.71).
Figure 1.
Hazard ratio (HR) with 95% CI of developing different types of cardiovascular diseases among adults with attention‐deficit/hyperactivity disorder (ADHD), compared with those without ADHD. Model 1: adjusted for sex and year of birth; model 2: adjusted for sex, year of birth, education level, birth country, type 2 diabetes mellitus, obesity, dyslipidemia, sleep problems and heavy smoking; model 3: adjusted for sex, year of birth, education level, birth country, type 2 diabetes mellitus, obesity, dyslipidemia, sleep problems, heavy smoking and psychiatric comorbidities.
Significant associations were observed for all specific cardiovascular diseases across all adjusted models in individuals with ADHD compared with those without ADHD. The highest HRs were found for cardiac arrest (HR=2.28, 95% CI: 1.81‐2.87), hemorrhagic stroke (HR=2.16, 95% CI: 1.68‐2.77) and peripheral vascular disease/arteriosclerosis (HR=2.05, 95% CI: 1.76‐2.38) in model 2. When further adjusting for psychiatric comorbidities, most of the relative risks (20 out of 22 specific cardiovascular diseases) were slightly attenuated but remained statistically significant.
Subgroup analyses
The associations between ADHD and cardiovascular diseases were stronger across all levels of adjustments in males compared to females (HR=1.70, 95% CI: 1.62‐1.79 and HR=1.58, 95% CI=1.49‐1.68, respectively, in model 3, p<0.001) (see Table 2).
Table 2.
Association between attention‐deficit/hyperactivity disorder (ADHD) and cardiovascular diseases as hazard ratios (HRs) with 95% CI adjusted for covariates
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Overall | 2.05 (1.98‐2.13) | 1.84 (1.77‐1.91) | 1.65 (1.59‐1.71) |
| Sex | |||
| Male | 2.10 (2.00‐2.20) | 1.89 (1.80‐1.98) | 1.70 (1.62‐1.79) |
| Female | 2.00 (1.88‐2.13) | 1.76 (1.65‐1.87) | 1.58 (1.49‐1.68) |
| Age (years) | |||
| 18‐30 | 2.78 (2.42‐3.19) | 2.43 (2.12‐2.79) | 2.49 (2.17‐2.87) |
| 31‐40 | 2.74 (2.53‐2.96) | 2.36 (2.18‐2.55) | 2.14 (1.97‐2.32) |
| 41‐50 | 2.32 (2.18‐2.47) | 2.05 (1.93‐2.19) | 1.82 (1.71‐1.94) |
| 51‐60 | 1.67 (1.54‐1.80) | 1.54 (1.43‐1.66) | 1.43 (1.32‐1.54) |
| 61‐73 | 1.50 (1.33‐1.69) | 1.33 (1.18‐1.50) | 1.22 (1.08‐1.37) |
Model 1: adjusted for sex and year of birth; model 2: adjusted for sex, year of birth, education level, birth country, type 2 diabetes mellitus, obesity, dyslipidemia, sleep problems and heavy smoking; model 3: adjusted for sex, year of birth, education level, birth country, type 2 diabetes mellitus, obesity, dyslipidemia, sleep problems, heavy smoking and psychiatric comorbidities
When stratified by age bands, the highest adjusted HR was observed in the youngest adults (18‐30 years: HR=2.49, 95% CI: 2.17‐2.87, in model 3), while the lowest association was found in the oldest adults (61‐73 years: HR=1.22, 95% CI: 1.08‐1.37, in model 3, p<0.001) (see Table 2). At the end of the follow‐up, the prevalence of cardiovascular diseases was 5.89% among the youngest adults (18‐30 years) with ADHD, compared to 2.87% in the non‐ADHD group (p<0.0001), while it was 94.26% among the oldest adults (61‐73 years) with ADHD, compared to 73.55% for the non‐ADHD group (p<0.0001) (see also supplementary information).
Using individuals without ADHD as a reference group, we found that the relative risk of cardiovascular diseases was slightly higher among individuals with ADHD plus any psychiatric comorbidity (HR=1.87, 95% CI=1.79‐1.95), compared with ADHD only (HR=1.72, 95% CI=1.59‐1.86) (model 2). Specifically, an additional increase in the risk of cardiovascular diseases was found among those with comorbid depressive disorder, personality disorders, anxiety disorders, substance use disorders and eating disorders, compared with ADHD only. The strongest associations were found for eating disorders (HR=2.21, 95% CI: 1.72‐2.85) and substance use disorders (HR=2.20, 95% CI: 2.09‐2.33) (model 2) (see Table 3).
Table 3.
Psychiatric comorbidities and risk of cardiovascular diseases among individuals with attention‐deficit/hyperactivity disorder (ADHD) as hazard ratios (HRs) with 95% CI adjusted for covariates
| Model 1 | Model 2 | |
|---|---|---|
| No ADHD | 1.00 (reference) | 1.00 (reference) |
| ADHD only | 1.82 (1.68‐1.97) | 1.72 (1.59‐1.86) |
| ADHD plus any comorbidity | 2.13 (2.04‐2.22) | 1.87 (1.79‐1.95) |
| ADHD plus anxiety disorders | 2.22 (2.09‐2.36) | 1.92 (1.81‐2.04) |
| ADHD plus autism spectrum disorder | 1.55 (1.36‐1.77) | 1.34 (1.17‐1.53) |
| ADHD plus bipolar disorder | 1.87 (1.69‐2.07) | 1.64 (1.48‐1.81) |
| ADHD plus conduct disorder | 2.79 (2.00‐3.91) | 2.39 (1.71‐3.35) |
| ADHD plus depressive disorder | 2.05 (1.93‐2.17) | 1.77 (1.67‐1.88) |
| ADHD plus eating disorders | 2.75 (2.14‐3.54) | 2.21 (1.72‐2.85) |
| ADHD plus intellectual disability | 2.21 (1.79‐2.72) | 1.67 (1.36‐2.06) |
| ADHD plus personality disorders | 2.25 (2.08‐2.43) | 1.92 (1.77‐2.07) |
| ADHD plus schizophrenia | 1.87 (1.49‐2.35) | 1.59 (1.27‐2.00) |
| ADHD plus substance use disorders | 2.53 (2.40‐2.67) | 2.20 (2.09‐2.33) |
Model 1: adjusted for sex and year of birth; model 2: adjusted for sex, year of birth, education level, birth country, type 2 diabetes mellitus, obesity, dyslipidemia, sleep problems and heavy smoking
Sensitivity analyses
Results from sensitivity analyses are presented in Table 4. When ADHD was only defined by diagnosis from the NPR, the cardiovascular risk was similar to that of the main analysis, but with a stronger association (HR=1.76, 95% CI=1.68‐1.84 in model 3).
Table 4.
Results from sensitivity analyses on associations between attention‐deficit/hyperactivity disorder (ADHD) and cardiovascular diseases as hazard ratios (HRs) with 95% CI adjusted for covariates
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| ADHD diagnosis only | 2.22 (2.13‐2.32) | 1.99 (1.90‐2.07) | 1.76 (1.68‐1.84) |
| Excluding those treated with stimulants | 2.25 (2.16‐2.36) | 2.01 (1.92‐2.10) | 1.77 (1.69‐1.85) |
| Excluding those treated with other psychiatric medications | 2.29 (2.19‐2.40) | 2.06 (1.97‐2.16) | 1.83 (1.74‐1.91) |
| Excluding those with family history of cardiovascular diseases | 2.06 (1.98‐2.14) | 1.84 (1.77‐1.91) | 1.65 (1.59‐1.71) |
| ADHD as time‐invariant exposure | 2.05 (1.97‐2.13) | 1.83 (1.77‐1.90) | 1.64 (1.58‐1.70) |
Model 1: adjusted for sex and year of birth; model 2: adjusted for sex, year of birth, education level, birth country, type 2 diabetes mellitus, obesity, dyslipidemia, sleep problems and heavy smoking; model 3: adjusted for sex, year of birth, education level, birth country, type 2 diabetes mellitus, obesity, dyslipidemia, sleep problems, heavy smoking and psychiatric comorbidities
The estimates were similar when excluding individuals with ADHD diagnosis and treated with stimulants (HR=1.77, 95% CI: 1.69‐1.85 in model 3) or other psychiatric medications (including antipsychotics, anxiolytics, hypnotics and sedatives, and antidepressants) (HR=1.83, 95% CI:1.74‐1.91 in model 3).
When restricting our main analyses to individuals without family history of cardiovascular diseases, the risk estimates for cardiovascular diseases remained largely similar (HR=1.65, 95% CI: 1.59‐1.71 in model 3).
We generally found the same results across all levels of adjustment when using ADHD as time‐invariant exposure (HR=1.64, 95% CI: 1.58‐1.70 in model 3).
DISCUSSION
In this large‐scale, register‐based cohort study, we found that adults with ADHD were more than twice as likely to develop at least one cardiovascular disease, compared with those without ADHD, independently from treatment with psychotropic medications. The increased risk was present across all types of cardiovascular diseases, but the strength of the associations was greatest for cardiac arrest, hemorrhagic stroke and peripheral vascular disease/arteriosclerosis.
Well‐established risk factors for cardiovascular diseases (such as type 2 diabetes mellitus, obesity, dyslipidemia, sleep problems and heavy smoking) and psychiatric comorbidities could not fully explain the associations, indicating that ADHD is an independent risk factor for a wide range of cardiovascular diseases. This finding is consistent with a recent two‐sample Mendelian randomization study reporting a direct causal effect of ADHD on coronary artery disease 46 .
Although the underlying mechanisms remain unclear, plausible biological mechanisms could explain the observed association between ADHD and cardiovascular diseases, including immune system abnormalities47, 48, neuromodulator dysregulation49, 50, and dysregulation of the hypothalamic‐pituitary‐adrenal (HPA) axis51, 52. The observed associations could also be partly explained by shared etiological components, as suggested in a previous genetically‐informed study based on sibling pairs 21 .
The observed strength of associations between ADHD and cardiovascular diseases is largely comparable to estimates of associations between these diseases and schizophrenia 9 , depression 8 , and bipolar disorder 10 , and stronger than associations with anxiety disorders 11 , obsessive‐compulsive disorder 43 , and stress‐related disorders 42 . In contrast to the available evidence base for other psychiatric disorders 53 , the association between ADHD and cardiovascular diseases has been substantially understudied. Our findings call for enhanced clinical awareness of cardiovascular risk among adults with ADHD.
Sex differences in ADHD 29 and cardiovascular diseases 30 are well established, with higher prevalence estimates in males than in females for both conditions, but our study extends this knowledge base by showing that the association between ADHD and cardiovascular diseases is stronger in males than in females. The present study points to the potential value of screening for cardiovascular risk factors in ADHD, particularly targeting young adults and males.
In our study, comorbid eating disorders and substance use disorders significantly increased the risk of cardiovascular diseases among individuals with ADHD. As suggested in previous studies, around 80% of patients with an eating disorder are affected by a cardiac complication 54 , and prolonged heavy use of certain substances (e.g., amphetamines, alcohol, tobacco and heroin) substantially increases the risk for several serious cardiovascular problems, including hypertension, stroke and cardiac arrest 55 . Therefore, the appropriate identification and treatment of these psychiatric comorbidities is necessary to successfully impact cardiovascular health among adults with ADHD.
Our findings also suggest that the observed associations between ADHD and cardiovascular diseases are independent from the use of stimulants and other psychotropic medications. A slightly stronger association was even found among individuals with ADHD not treated with antipsychotics, anxiolytics, hypnotics and sedatives, and antidepressants. A seemingly protective effect of psychotropic medications for the risk of cardiometabolic conditions and mortality has been found in other mental disorders56, 57, 58, 59. However, confounding by indication needs to be carefully considered using other study designs (e.g., within‐individuals comparisons). Our results should not be interpreted as indicating that psychotropic medications are free from cardiovascular adverse effects, and they should continue to be used with caution in ADHD patients.
Our study has some limitations. First, the national registers mainly capture the most severe cases, which might have led to an underestimation of the number of patients with milder symptoms of ADHD or less severe cardiovascular diseases. On the other hand, a detection bias (i.e., individuals with ADHD and psychiatric comorbidities may be more likely to be diagnosed with cardiovascular diseases as they have more frequent contacts with the health care system than those without ADHD) cannot be ruled out. Second, the prevalence of ADHD tends to increase over time, reflecting changes in diagnostic practices, and the late inclusion of outpatient specialist care records in the NPR (since 2001) and information on medication in PDR (since 2005) might have led to a loss of early diagnoses of ADHD in our cohort, particularly in older adults. Delayed diagnosis may have resulted in misclassification from exposed to unexposed person‐time, which would be most likely to bias estimates towards the null.
Third, as the median age of the study population at the end of the follow‐up was 50.49 (range 31‐73) years, we might have mostly captured early onset cases of cardiovascular diseases. Therefore, future studies would be necessary to explore the association of ADHD with later onset cardiovascular diseases among older adults. Finally, we had no data on some lifestyle related factors (such as dietary intake and physical activities) that may contribute to the observed association as confounders or mediators. Our results suggest that heavy smoking and sleep problems could explain only a small portion of the associations, but tobacco use and sleep disorders identified from registers might only reflect the most severe cases. Thus, further studies are warranted to clarify the impact of lifestyle related factors on the association between ADHD and cardiovascular diseases.
In conclusion, in this large‐scale, register‐based cohort study, we found that ADHD is a risk factor for a wide range of cardiovascular diseases, independent from well‐established cardiovascular risk factors, psychiatric comorbidities, and psychotropic medication treatment. These findings underscore the importance of carefully monitoring cardiovascular health in adults with ADHD, and highlight a critical need for development of age‐appropriate and individualized strategies to reduce the risk of cardiovascular morbidity in ADHD people. Additional studies are needed to confirm our findings and to further explore the mechanisms underlying the association between ADHD and cardiovascular diseases.
ACKNOWLEDGEMENTS
This project has received funding from the European Union's Horizon 2020 research and innovation programme (grant nos. 667302 and 965381). H. Larsson acknowledges financial support from the Swedish Research Council (2018‐02599) and the Swedish Brain Foundation (FO2021‐0115); Z. Chang from the Swedish Council for Health, Working Life and Welfare (2019‐00176); M. Dobrosavljevic from the European Union's Horizon 2020 research and innovation programme (Marie Skłodowska‐Curie grant no. 754285); E. Du Rietz from the Swedish Research Council for Health, Working Life, and Welfare, and from the Swedish Society for Medical Research. Supplementary information on this study is available at https://osf.io/8f9xb/.
REFERENCES
- 1. Faraone SV, Asherson P, Banaschewski T et al. Attention‐deficit/hyperactivity disorder. Nat Rev Dis Primers 2015;1:15020. [DOI] [PubMed] [Google Scholar]
- 2. Davidson MA. Literature review: ADHD in adults: a review of the literature. J Atten Disord 2007;11:628‐41. [DOI] [PubMed] [Google Scholar]
- 3. Sayal K, Prasad V, Daley D et al. ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry 2018;5:175‐86. [DOI] [PubMed] [Google Scholar]
- 4. Kessler RC, Adler L, Barkley R et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006;163:716‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cortese S, Moreira‐Maia CR, St Fleur D et al. Association between ADHD and obesity: a systematic review and meta‐analysis. Am J Psychiatry 2016;173:34‐43. [DOI] [PubMed] [Google Scholar]
- 6. Cortese S, Sun S, Zhang J et al. Association between attention deficit hyperactivity disorder and asthma: a systematic review and meta‐analysis and a Swedish population‐based study. Lancet Psychiatry 2018;5:717‐26. [DOI] [PubMed] [Google Scholar]
- 7. Nicholson A, Kuper H, Hemingway H. Depression as an etiologic and prognostic factor in coronary heart disease: a meta‐analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J 2006;27:2763‐74. [DOI] [PubMed] [Google Scholar]
- 8. Hare DL, Toukhsati SR, Johansson P et al. Depression and cardiovascular disease: a clinical review. Eur Heart J 2014;35:1365‐72. [DOI] [PubMed] [Google Scholar]
- 9. Hennekens CH, Hennekens AR, Hollar D et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J 2005;150:1115‐21. [DOI] [PubMed] [Google Scholar]
- 10. Swartz HA, Fagiolini A. Cardiovascular disease and bipolar disorder: risk and clinical implications. J Clin Psychiatry 2012;73:1563‐5. [DOI] [PubMed] [Google Scholar]
- 11. Tully PJ, Harrison NJ, Cheung P et al. Anxiety and cardiovascular disease risk: a review. Curr Cardiol Rep 2016;18:120. [DOI] [PubMed] [Google Scholar]
- 12. Momen NC, Plana‐Ripoll O, Agerbo E et al. Association between mental disorders and subsequent medical conditions. N Engl J Med 2020;382:1721‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun X, Chen L, Wang Z et al. Association of autism spectrum disorder, neuroticism, and subjective well‐being with cardiovascular diseases: a two‐sample Mendelian randomization study. Front Cardiovasc Med 2021;8:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mc Namara K, Alzubaidi H, Jackson JK. Cardiovascular disease as a leading cause of death: how are pharmacists getting involved? Integr Pharm Res Pract 2019;8:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Escobar DF, Noll PR, Jesus TF et al. Assessing the mental health of Brazilian students involved in risky behaviors. Int J Environ Res Public Health 2020;17:3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vancampfort D, Stubbs B, Mitchell AJ et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta‐analysis. World Psychiatry 2015;14:339‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mick E, McManus DD, Goldberg RJ. Meta‐analysis of increased heart rate and blood pressure associated with CNS stimulant treatment of ADHD in adults. Eur Neuropsychopharmacol 2013;23:534‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cortese S. Pharmacologic treatment of attention deficit‐hyperactivity disorder. N Engl J Med 2020;383:1050‐6. [DOI] [PubMed] [Google Scholar]
- 19. Liu H, Feng W, Zhang D. Association of ADHD medications with the risk of cardiovascular diseases: a meta‐analysis. Eur Child Adolesc Psychiatry 2019;28:1283‐93. [DOI] [PubMed] [Google Scholar]
- 20. Semeijn EJ, Kooij JJ, Comijs HC et al. Attention‐deficit/hyperactivity disorder, physical health, and lifestyle in older adults. J Am Geriatr Soc 2013;61: 882‐7. [DOI] [PubMed] [Google Scholar]
- 21. Du Rietz E, Brikell I, Butwicka A et al. Mapping phenotypic and aetiological associations between ADHD and physical conditions in adulthood in Sweden: a genetically informed register study. Lancet Psychiatry 2021;8:774‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kubota Y, Heiss G, MacLehose RF et al. Association of educational attainment with lifetime risk of cardiovascular disease: the Atherosclerosis Risk in Communities Study. JAMA Intern Med 2017;177:1165‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 2004;43:1731‐7. [DOI] [PubMed] [Google Scholar]
- 24. Fan M, Sun D, Zhou T et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur Heart J 2019;41:1182‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Powell‐Wiley TM, Poirier P, Burke LE et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2021;143:e984‐1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Einarson TR, Acs A, Ludwig C et al. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol 2018;17:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hedayatnia M, Asadi Z, Zare‐Feyzabadi R et al. Dyslipidemia and cardiovascular disease risk among the MASHAD study population. Lipids Health Dis 2020;19:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Imes CC, Lewis FM. Family history of cardiovascular disease, perceived cardiovascular disease risk, and health‐related behavior: a review of the literature. J Cardiovasc Nurs 2014;29:108‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rucklidge JJ. Gender differences in attention‐deficit/hyperactivity disorder. Psychiatr Clin North Am 2010;33:357‐73. [DOI] [PubMed] [Google Scholar]
- 30. Bots SH, Peters SAE, Woodward M. Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ Glob Health 2017;2:e000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simon V, Czobor P, Balint S et al. Prevalence and correlates of adult attention‐deficit hyperactivity disorder: meta‐analysis. Br J Psychiatry 2009;194:204‐11. [DOI] [PubMed] [Google Scholar]
- 32. Yazdanyar A, Newman AB. The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clin Geriatr Med 2009;25:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ludvigsson JF, Almqvist C, Bonamy A‐KE et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016;31:125‐36. [DOI] [PubMed] [Google Scholar]
- 34. Axelson O. The Swedish medical birth register. Acta Obstet Gynecol Scand 2003;82:491‐2. [DOI] [PubMed] [Google Scholar]
- 35. Ludvigsson JF, Andersson E, Ekbom A et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wettermark B, Hammar N, Fored CM et al. The new Swedish Prescribed Drug Register – opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007;16:726‐35. [DOI] [PubMed] [Google Scholar]
- 37. Ludvigsson JF, Svedberg P, Olén O et al. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol 2019;34:423‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ekbom A. The Swedish Multi‐generation Register. Methods Mol Biol 2011;675:215‐20. [DOI] [PubMed] [Google Scholar]
- 39. Brooke HL, Talbäck M, Hörnblad J et al. The Swedish cause of death register. Eur J Epidemiol 2017;32:765‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Larsson H, Ryden E, Boman M et al. Risk of bipolar disorder and schizophrenia in relatives of people with attention‐deficit hyperactivity disorder. Br J Psychiatry 2013;203:103‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sun S, Kuja‐Halkola R, Faraone SV et al. Association of psychiatric comorbidity with the risk of premature death among children and adults with attention‐deficit/hyperactivity disorder. JAMA Psychiatry 2019;76:1141‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song H, Fang F, Arnberg FK et al. Stress related disorders and risk of cardiovascular disease: population based, sibling controlled cohort study. BMJ 2019;365:l1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Isomura K, Sidorchuk A, Brander G et al. Risk of specific cardiovascular diseases in obsessive‐compulsive disorder. J Psychiatr Res 2021;135:189‐96. [DOI] [PubMed] [Google Scholar]
- 44. Hinchliffe SR, Lambert PC. Flexible parametric modelling of cause‐specific hazards to estimate cumulative incidence functions. BMC Med Res Methodol 2013;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Correll CU, Detraux J, De Lepeleire J et al. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015;14:119‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leppert B, Riglin L, Wootton RE et al. The effect of attention deficit/hyperactivity disorder on physical health outcomes: a 2‐sample Mendelian randomization study. Am J Epidemiol 2020;190:1047‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fernández‐Ruiz I. Immune system and cardiovascular disease. Nat Rev Cardiol 2016;13:503. [DOI] [PubMed] [Google Scholar]
- 48. Hoekstra PJ. Attention‐deficit/hyperactivity disorder: is there a connection with the immune system? Eur Child Adolesc Psychiatry 2019;28:601‐2. [DOI] [PubMed] [Google Scholar]
- 49. Ahmad Banday A, Lokhandwala MF. Defective renal dopamine D1 receptor function contributes to hyperinsulinemia‐mediated hypertension. Clin Exp Hypertens 2006;28:695‐705. [DOI] [PubMed] [Google Scholar]
- 50. Misener V, Luca P, Azeke O et al. Linkage of the dopamine receptor D1 gene to attention‐deficit/hyperactivity disorder. Mol Psychiatry 2004;9:500‐9. [DOI] [PubMed] [Google Scholar]
- 51. Corominas M, Ramos‐Quiroga J, Ferrer M et al. Cortisol responses in children and adults with attention deficit hyperactivity disorder (ADHD): a possible marker of inhibition deficits. Atten Defic Hyperact Disord 2012;4:63‐75. [DOI] [PubMed] [Google Scholar]
- 52. Jokinen J, Nordström P. HPA axis hyperactivity and cardiovascular mortality in mood disorder inpatients. J Affect Disord 2009;116:88‐92. [DOI] [PubMed] [Google Scholar]
- 53. Nielsen RE, Banner J, Jensen SE. Cardiovascular disease in patients with severe mental illness. Nat Rev Cardiol 2021;18:136‐45. [DOI] [PubMed] [Google Scholar]
- 54. Casiero D, Frishman WH. Cardiovascular complications of eating disorders. Cardiol Rev 2006;14:227‐31. [DOI] [PubMed] [Google Scholar]
- 55. Schulte MT, Hser Y‐I. Substance use and associated health conditions throughout the lifespan. Public Health Rev 2013;35:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brander G, Isomura K, Chang Z et al. Association of Tourette syndrome and chronic tic disorder with metabolic and cardiovascular disorders. JAMA Neurol 2019;76:454‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Isomura K, Brander G, Chang Z et al. Metabolic and cardiovascular complications in obsessive‐compulsive disorder: a total population, sibling comparison study with long‐term follow‐up. Biol Psychiatry 2018;84:324‐31. [DOI] [PubMed] [Google Scholar]
- 58. Tiihonen J, Mittendorfer‐Rutz E, Torniainen M et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow‐up study. Am J Psychiatry 2016;173:600‐6. [DOI] [PubMed] [Google Scholar]
- 59. Taipale H, Tanskanen A, Mehtälä J et al. 20‐year follow‐up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry 2020;19:61‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]