Abstract

The paper reports on the performances of cross-linked amidoxime hosted into mesoporous silica (AMOX) in the removal of As(III) and As(V). The optimum pH for sorption of As(III) and As(V) was pH 8 and pH 5, respectively. The PFO kinetic model and the Sips isotherm fitted the best the experimental data. The thermodynamic parameters were evaluated using the equilibrium constant values given by the Sips isotherm at different temperatures and found that the adsorption process of As(III) and As(V) was spontaneous and endothermic on all AMOX sorbents. The spent AMOX sorbents could be easily regenerated with 0.2 mol/L HCl solution and reused up to five sorption/desorption cycles with an average decrease of the adsorption capacity of 18%. The adverse effect of the co-existing inorganic anions on the adsorption of As(III) and As(V) onto the sorbent with the highest sorption capacity (AMOX3) was arranged in the following order: H2PO4– > HCO3– > NO3– > SO42–.
1. Introduction
Arsenic is one of the most harmful pollutants present in the surface water and groundwater as a side effect of the ore exploitation, activity of volcanoes, and some anthropogenic sources such as mining.1−4 Industrial wastewaters containing arsenic are generated by the metallurgical industry, glassware and ceramic production, tannery operation, dyestuff and pigments manufacture, wood preservatives, and pesticide manufacturing, petroleum refining, and rare-earth industries.5,6 Ingestion of contaminated drinking water can cause gastrointestinal damage, cardiovascular and endocrine disorders, skin cancer, and kidney cancer.6,7 To protect the human health, the World Health Organization decreased the maximum concentration of arsenic in drinking water to 10 μg/L.8 Arsenic exists mostly in two inorganic forms as H3AsO3 and H3AsO4, with the dominant arsenic species in groundwater being As(III), which is 60 times more toxic than As(V).9 Therefore, there is a stringent need to develop highly efficient technologies to remove arsenic species from groundwater and wastewaters. Removal of arsenic ions is usually performed by nanofiltration and reverse osmosis,10 electrocoagulation,11 chemical precipitation,12 ion exchange,8,13−17 and adsorption.2,7,9,18−23 Among the most accessed technologies for removal of arsenic, adsorption is obviously the most attractive by its accessible costs, easy operation, and large potential for the development of new generations of adsorbents.9,18−23
Various silica-based composites with good performances in the removal of heavy metal ions, or oxyanions, have been lately reported.24−28 Among them, selective and efficient adsorption of arsenic ions by the amino-functionalized silica,24,25 surface-ion imprinted silica,26 or quaternary amine-functionalized silica28 have been reported in the last decade. Porous silica has been used as a suitable host for numerous functional polymers, such as anionic hydrogels,29 anion exchangers,30 and amidoxime ligands.31,32 Amidoxime-based sorbents demonstrated high potential in the removal of heavy metal ions,32,33 or radionuclides.31,34 However, the possibility of using sorbents containing amidoxime functional groups in the reversible adsorption of arsenic from water has not been sufficiently explored.35 This lack of information prompted us to prepare and characterize some composites consisting of amidoxime resin entrapped into the pores of a commercial mesoporous silica and to demonstrate their performances in the removal of As(III) and As(V) as a function of the amidoxime content and textural characteristics of the composites. The effect of various adsorption parameters, such as pH, contact duration, the initial concentration of arsenic, temperature, and the presence of competitive anions on the sorption capacity, was deeply investigated by changing one parameter at a time, keeping the others constant. At the time of writing the paper, no information about the sorption performances of silica/amidoxime composites toward As(III) and As(V) has been reported.
2. Materials and Methods
2.1. Chemical Reagents
Mesoporous silica having textural characteristics of specific surface area, Ssp = 95 m2/g; pore volume, Vp = 1.02 cm3/g; and average pore radius, rp = 26.7 nm was purchased from Daiso Co. (Osaka, Japan). Acrylonitrile (AN), ethyleneglycol dimethacrylate (EGDMA), azoisobutyronitrile (AIBN) (recrystallized three times from methanol), and N,N-dimethylformamide (DMF) were purchased from Sigma-Aldrich Chemie (GmbH, Germany). Toluene, methanol, NaOH, and hydroxylamine hydrochloride (HA) were purchased from Chemical Company (Romania) and used as received. NaAsO2 (≥90%) and Na2HAsO4·7H2O (≥98%) were purchased from Sigma-Aldrich and used as received. NaHCO3, NaNO3, and Na2SO4 were purchased from Chemical Company, and KH2PO4 was purchased from Fluka and used as received.
2.2. Preparation of Silica/Amidoxime Composites
The synthesis of silica/amidoxime composites was performed as previously described31 with some changes. Cross-linked poly(acrylonitrile) (PAN) embedded into the silica pores was prepared using AIBN as an initiator (1 wt % vs monomers) and EGDMA as a cross-linker (10 wt % vs monomers), with toluene as a porogen at a ratio of 1:1 to the volume of monomers. After the adsorption of the monomer mixture into the silica pores, the polymerization was conducted 2 h at 60 °C, 3 h at 70 °C, and 7 h at 85 °C. The PAN homopolymer and toluene were removed by extraction with DMF and finally with methanol. Three PAN/silica samples were prepared in this work: SiO2/PAN22, containing 22 wt % PAN, SiO2/PAN15, containing 15 wt % PAN, and SiO2/IPN, which represents the composite having two networks of PAN (homo-IPN) constructed in a sequential manner, with PAN15 as the first network.36 The synthesis of silica/amidoxime (AMOX) from silica/PAN composites was performed by the reaction of the nitrile groups in PAN with HA as follows: 40 mL of methanolic solution of HA solution with a concentration of about 15% was added to 5 g of silica/PAN composite, the reaction being conducted at 70 °C, 5 h. The codes of the composites are as follows: AMOX1 resulted from SiO2/PAN22; AMOX2 from SiO2/PAN15; and AMOX3 from SiO2/IPN.
2.3. Characterization of Silica/Amidoxime Composites
The thermogravimetric curves were recorded on a STA 449F1 Jupiter device (Netzsch, Selb, Germany) in a nitrogen atmosphere (50 mL min–1). The sample (20 mg) was heated in alumina crucibles at a heating rate of 10 °C min–1. FTIR spectra were recorded with a Bruker Vertex FTIR spectrometer (Bruker, Ettlingen, Germany), resolution 2 cm–1, in the range of 4000–400 cm–1 by the KBr pellet technique. The specific surface area (Ssp) and the pore size distribution were estimated from N2 adsorption–desorption experiments conducted at 77 K using an Autosorb-1-MP surface area analyzer (Quantachrome Company, Boynton Beach, FL, USA). Ssp was determined by the Brunauer–Emmett–Teller (BET) method, whereas the Barrett–Joyner–Halenda (BJH) theory was used to evaluate the pore size distribution. The external surface and the internal morphology of the composite microspheres having amidoxime resin into the silica pores were observed by using an environmental scanning electron microscope (ESEM) type (FEI Company, Hillsboro, Oregon, USA) Quanta 200, operating at 20 kV with secondary electrons, in the low vacuum mode. Potentiometric titration was performed using a PCD-03 particle charge detector (PCD 03; Mütek GmbH, Germany) to determine the pHPZC values of the composites, defined as the pH where the potential is 0 mV. It was carried out between pH ≈ 3 and ≈11 by adjusting the pH of an aqueous suspension of microparticles using 0.1 mol·L–1 HCl and NaOH, respectively. The determination of As was carried out on a contrAA 800, High-Resolution Continuum Source Atomic Absorption Spectrometer (Analytic Jena AG, Germany), working in a flame mode, and equipped with a Xenon short arc lamp and an echelle grating monochromator (High-Resolution Optics). The characteristic wavelength of As (193 nm) was stabilized by using an integrated neon radiator.
2.4. Batch Sorption Experiments
Batch sorption of As(III) and As(V) onto the AMOX composites was carried out using 10 mg of sorbent and 10 mL of metalloid solution. The aqueous solutions of various concentrations were prepared from a stock solution with a concentration of 1000 mg/L. The pH of the solutions was adjusted with 0.1 M HCl or 0.1 M NaOH. After shaking for a certain time, the sorbent was separated by filtering through a 0.45 μm membrane filter. The samples collected at different contact times and at equilibrium were analyzed, after the adequate dilution, in duplicate, and the mean of three readings for each sample was used for the calculation of the sorption results.
The adsorption capacity at equilibrium, qe (mg/g), was calculated using eq 1
| 1 |
where Co—the initial concentration of As(III) or As(V) (mg/L), Ce—the concentration of arsenic in the aqueous solution at equilibrium (mg/L), V—the volume of the aqueous solution (L), and m—the mass of the sorbent (g).
The removal efficiency (RE) was calculated using eq 2
| 2 |
where Co and Ce have the same meaning as in eq 1.
The evaluation of kinetics and isotherm parameters was performed by a nonlinear regression method using two error functions to assess the level of fit: the correlation coefficient of determination (R2) and the nonlinear Chi-square (χ2) test, calculated by eq 3
| 3 |
where qe,exp and qe,cal represent the experimental data (mg/g) and the data calculated by models (mg/g), respectively.
All experiments were repeated three times, and the data were reported as the average of three independent measurements.
2.5. Reusability
To assess the sorbent recyclability, 10 mg of sorbent and 10 mL of metal ion solution with a concentration of 100 mg/L were stirred 8 h at 22 °C at pH 5.0 for As(V) and pH 8.0 for As(III). After that, the arsenic species loaded onto the composite sorbents were eluted with 0.2 M HCl aqueous solution (20 mL) for 8 h, washed several times with distilled water, and then regenerated with 0.1 M NaOH aqueous solution (20 mL) for 6 h. After washing with distilled water, the sorbents were reused in another cycle of sorption.
2.6. Effect of Competitive Ions
For the investigation of the effect of co-existing anions on the sorption capacity of the sorbents, the following anions were considered: NO3–, HCO3–, H2PO4–, and SO42–. The concentration of arsenic ions was 100 mg/L and that of the competing anions was 10–3, 10–2, and 10–1 M, and the ratio between the concentration of competing anions and arsenic species increased from 1 mM co-ion: 1.33 mM As up to 100 mM co-ion: 1.33 mM As.
3. Results and Discussion
3.1. Characterization of AMOX Sorbents
The effective amount of organic part immobilized into silica pores after the amidoximation of the nitrile groups was determined by TGA. As can be seen in Figure S1, all AMOX composite sorbents displayed a thermal degradation pattern in four stages. The first stage corresponds to loss of physically absorbed water and other residual traces, summing up 2.51, 1.54, and 1.52% for AMOX1, AMOX2, and AMOX3, respectively. In the second stage of thermal degradation, the mass loss values were 7.17, 3.9, and 4.76% for the same order of the composites. The main thermal degradation stages were the third and fourth, occurring above 253.24, 242.24, and 241.87 °C for AMOX1, AMOX2, and AMOX3, respectively. The total percentage of weight loss in the third and fourth stages, up to 700 °C, was 20.35% for AMOX1, 14.15% for AMOX2, and 17.34% for AMOX3. These thermal degradation stages are associated with the disruption of cross-links between the main chains and main chain degradation.
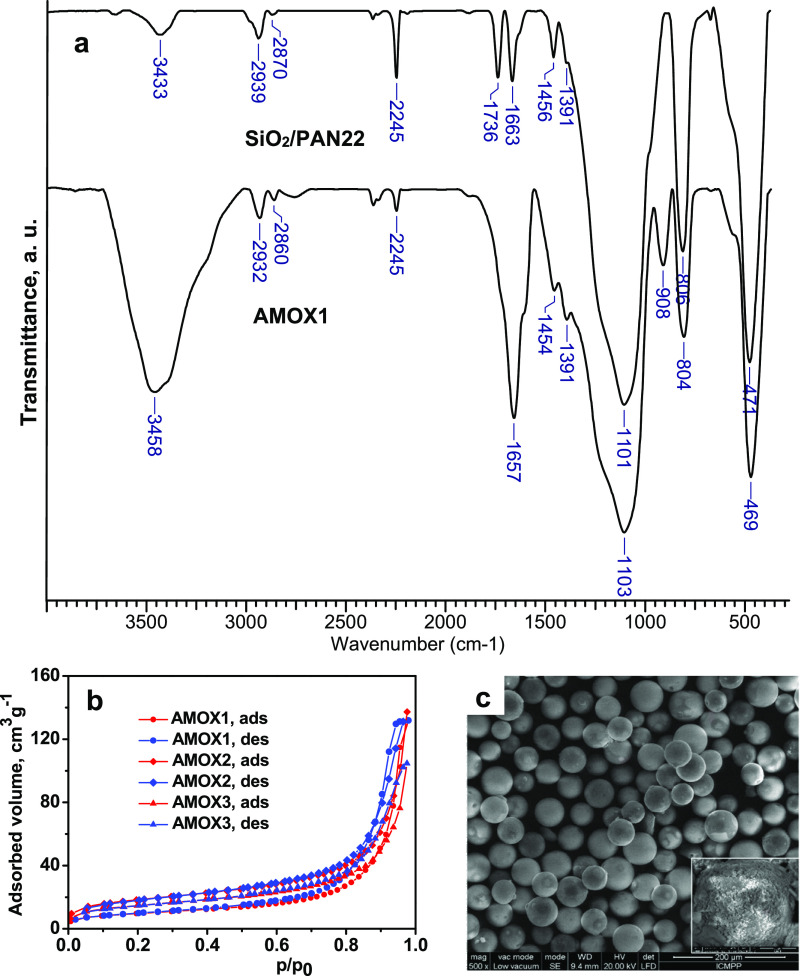
Figure 1a presents the FTIR spectra of the composite SiO2/PAN22 and of the composite AMOX1 resulted by the transformation of the nitrile groups in amidoxime. This pair of composites was taken as an example, the main bands being present in the other samples of SiO2/PAN and AMOX.
Figure 1.
Characterization of AMOX composite sorbents: (a) FTIR spectra of SiO2/PAN22 and of the AMOX1 sorbent; (b) BET isotherm of AMOX composites; and (c) SEM image of the AMOX1 sorbent; mag 500× (the inset image shows the interior morphology of AMOX1; mag. 5000×).
The characteristic bands of PAN are visible at 2928 and 2860 cm–1 attributed to the stretching vibrations of −CH3 and methylene groups in PAN cross-linked with EGDMA; the band located at 2245 cm–1 is characteristic to the stretching of the −C≡N groups; the band at 1736 cm–1 was assigned to the C=O groups in the ester groups of EGDMA used as a cross-linker; the band at 1456 cm–1 was attributed to the stretching vibrations of −CH2- groups, while the band of medium intensity situated at 1391 cm–1 was assigned to the symmetrical deformation mode in CH2 and CH3 groups.37 The large band at 1101 cm–1 and the sharp bands located at 806 and 471 cm–1 were assigned to the stretching vibration of Si–O–Si bonds, to the bending vibrations of Si–O bonds, and to the out-of-plane vibrations of Si–O bonds, respectively. After amidoximation, the main bands visible in the spectrum of AMOX1 are as follows: 2932 and 2860 cm–1 attributed to the stretching vibrations of −CH3 and methylene groups; a small peak at 2245 cm–1, which shows that some residual −C≡N groups are still present in the composite; the large band situated at 1657 cm–1, which screen the stretching vibration band of the C=O groups, was assigned to the amidoxime groups; the small peak at 1454 cm–1 was attributed to the stretching vibrations of −CH2– groups, while the band of medium intensity situated at 1391 cm–1 was assigned to the symmetrical deformation mode in CH2 and CH3 groups. The characteristic bands of silica are located at 1103, 804, and 469 cm–1.
It was demonstrated that the adsorption of various ions in an aqueous solution was influenced by the textural characteristics of the sorbent.38 The representative plots of BET isotherms for AMOX composites are presented in Figure 1b. As can be seen, all isotherms correspond to type IV, as categorized by the IUPAC classification, indicating the uniformity and regularity of the textural properties of all composites. The textural parameters, evaluated based on the isotherms in Figure 1b, are summarized in Table 1.
Table 1. Specific Surface Area and Pore Volume of the AMOX Composites Evaluated by the BET Method.
| sample code | Ssp, m2/g | Vp, cm3/g, | R2 |
|---|---|---|---|
| AMOX1 | 34.00 | 0.178, pores with d < 44.8 nm | 0.9998 |
| AMOX2 | 64.06 | 0.158, for pores with d < 46.8 nm | 0.9998 |
| AMOX3 | 51.31 | 0.118, for pores with d < 44.7 nm | 0.9996 |
As can be seen in Table 1, the BET surface area of the AMOX composites, calculated by applying the BET equation to the linear part (0.05 < P/P0 < 0.305) of the adsorption isotherm, is 34, 64.06, and 51.31 m2/g for AMOX1, AMOX2, and AMOX3, respectively. It is obvious that the specific surface area of the AMOX composites is much lower than that of the pristine mesoporous silica (Ssp = 95 m2/g), all the more so as a higher amount of amidoxime resin was embedded into the silica pores (AMOX1 compared with AMOX2). Also, the construction of the second network of PAN, which after the amidoximation conducted to the second network of amidoxime, is associated with the decrease of the specific surface area (AMOX3 compared with AMOX2). The majority of pores are located in the range of 5–20 nm, indicating that all composites have a mesoporous structure as can be seen from the pore size distribution profiles (Figure S2). The external surface of the composite microspheres is visible in Figure 1d. The internal morphology of the composite AMOX1 can be seen in the inset of Figure 1d.
3.2. Arsenic Removal in the Batch Mode
3.2.1. Effect of pH
The adsorption of arsenic is expected to be strongly influenced by the solution pH due to the different anionic species, which could be generated when the pH changes. The effect of the initial solution pH on the adsorption of As(III) and As(V) onto AMOX composites is presented in Figure 2a.
Figure 2.
Effect of initial solution pH on the sorption of As(III) and As(V) oxyanions onto AMOX1 (a); streaming potential as a function of pH for the composite sorbents (b); sorption kinetics of As(III) (c) and As(V) (d) oxyanions onto AMOX sorbents fitted by PFO, PSO, and Elovich models; and IPD model fitted on the sorption of As(III) (e) and As(V) (f) oxyanions onto AMOX sorbents. Sorption conditions: sorbent dose 0.010 g; Ci = 100 mg/L; Vsol = 10 mL; pH = 8, for As(III), and 5 for As(V); temp. 22 °C.
As can be seen, the optimum initial pH value for the removal of As(V) from aqueous solution was 5.0 while for As(III) was 8.0. At pH lower than 2.24, only nondissociated molecules of H3AsO4 are present in solution.9,15,39−41 Increasing the pH from 2 to 5, the adsorption of As(V) oxoanions increased and then monotonously decreased; this would indicate that at pH near to 5, there is an optimum concentration of H+ for the adsorption of As(V) as H2AsO4–.7,8,40,42 It was reported that the concentration of H2AsO4– as dominant species increases up to pH 6.96 (pKa2 for H3AsO4).15,39 Yu et al.42 have also reported the optimal pH for the removal of arsenate by cellulose-g-glycidyl methacrylate-b-tetraethylenepentamine at pH 5.0; the diminish of the As(V) adsorption at pH > 6.0 has been observed for activated carbon composites.43 Such domain for the optimum pH for the adsorption of As(V) has been recently identified by Wei et al.44 using as adsorbent an amine-functionalized acrylic fiber. An optimum pH in the range 6.0–7.0, for the adsorption of As(V), has been reported for aminoalkyl-organo-silane-treated sand.45 Dudek and Kolodynska also observed the increase of As(V) adsorption with the increase of pH up to 5.0 and a steady decrease after that.16
Figure 2b shows that the synthesis strategy of the composite sorbent influenced the point of zero charge (PZC). Thus, the values of PZC obtained by plotting the streaming potential as a function of pH are as follows: 6.65 for AMOX1, 6.47 for AMOX3, and 6.23 for AMOX2, and indicate that the surface of all sorbents was positively charged at pH < 5.0, which was found to be optimum for the adsorption of As(V). The shape of the titration curves was similar for all sorbents, the order of PZC values being AMOX1 > AMOX3 > AMOX2. This order would indicate that the incorporation of a higher amount of PAN conducted to a composite with a higher amount of amidoxime after the amidoximation reaction (the pristine composite SiO2/PAN for the synthesis of AMOX1 and AMOX2 sorbents was containing 22% PAN and 15% PAN, respectively). The fact that the PZC value of the sorbent AMOX3 is located in between the values corresponding to AMOX1 and AMOX2 is connected with the amount of amidoxime incorporated in silica pores, which was 20.35% for AMOX1, 14.15% for AMOX2, and 17.34% for AMOX3 (information acquired from the TG analysis, Figure S1). At pH < PZC, the negatively charged species of As(V) ions are electrostatically attracted to the protonated surface of amidoxime sorbent. Therefore, pH 5 was kept as optimum for the next sorption experiments of As(V).
Figure 2a shows that, unlike As(V), the optimum pH for the adsorption of As(III) anions was located at 8.0, where all sorbents are negatively charged. Up to pH 8, As(III) is present in water only as H3AsO3, the pKa1 value being 9.29.45 The decreasing trend in As(V) and increasing trend in As(III) sorption with the increase of pH value by different metal-based adsorbents has been attributed to the formation of surface species. In contrast to As(V), at high pH, As(III) is present as H2AsO3– ions, while at pH < 9.24, the noncharged As(OH)3 is dominant, which explain the nonadsorption of As(III) at low pH.40,43 However, at pH > 9.24, the negatively charged species of As(III) are rejected by the negatively charged surface of the sorbents. Furthermore, the RE was much higher in the case of As(III) than for As(V) and that is a very interesting characteristic of these amidoxime-based composite sorbents.
3.2.2. Effect of Contact Time
The adsorption kinetics of As(III) and As(V) onto the AMOX composites are presented in Figure 2c,d, respectively. As can be observed, the sorption of As(III) was fast and reached the equilibrium in about 3 h (Figure 2c). The adsorption of As(V) was slower and reached equilibrium in about 4 h (Figure 2d). The adsorption kinetics was carefully examined to get information about the sorption mechanism and on the rate-controlling steps of the adsorption process.7 Some kinetics models were fitted on the experimental data, that is, pseudo-first-order (PFO),46 pseudo-second-order (PSO),47 and Elovich48 models, whose equations are included in Table S1. The kinetic parameters in Table S1 show that the qe values calculated by the PFO kinetic model were closer to those given by the experimental data. Furthermore, the coefficients of determination (R2) were higher in the case of PFO kinetic model than in the case of PSO model, and this shows that the kinetic process is the best described by PFO model, as found for other systems.7,8,41
The Elovich kinetic model reveals the role of adsorbent surface heterogeneity and supports chemisorption as the possible mechanism of sorption. However, Table S1 shows that the values of R2 are <0.9 in the case of Elovich model, and this indicates that the adsorption mechanism of arsenic is not of the chemisorption type.16,41
The contribution of film diffusion and intraparticle diffusion (IPD) was clarified by fitting the IPD model.49 The plots of qt versus t0.5 for the sorption of As(III) and As(V) onto the AMOX composite sorbents are presented in Figure 2e,f, respectively. It is obvious that the adsorption process is multi-stage, indicating that the IPD was not the only rate-limiting step as assessed for the adsorption of arsenic onto other sorbents.7,24,45 The first straight line could be attributed to the diffusion of arsenic through the boundary layer (film diffusion) and the second one, with lower constants, could be assigned to the internal diffusion stage. The last step is connected to the saturation of the binding sites. The values of kid, Ci, and R2 for the first and the second steps in Figure 2e,f are presented in Table S1. The high values of R2 for the first step (R2 > 0.99) would indicate the applicability of the IPD model for the sorption of arsenic onto these composite sorbents. For the first step, the values of kid are almost similar, while the values of Ci were low and increased from AMOX1 to AMOX3, being always higher for As(III) than for As(V). These results are in agreement with those obtained by fitting the PFO kinetic model and would indicate that the adsorption is more favorable on AMOX3 than on the first two sorbents. The Ci values give indication about the contribution of the boundary layer thickness, which means the larger the intercept, the greater the contribution of the film diffusion in the rate-limiting step.31,45 The higher values of Ci reveal a larger contribution of the boundary layer in the adsorption process of As(III) than of As(V).
3.2.3. Adsorption of As(III) and As(V) at Equilibrium
By the investigation of adsorption at equilibrium, useful information is obtained, which help in the identification of the interactions between the adsorbate and the adsorbent.39 The experimental adsorption isotherms for As(III) and As(V) are plotted in Figure 3a,b, respectively.
Figure 3.
Sorption isotherms of As(III) (a) and As(V) (b) onto AMOX sorbents and influence of co-existing anions on the adsorption of As(III) (c) and As(V) (d) onto AMOX3 sorbent.
The experimental data of the adsorption at equilibrium were analyzed by fitting four isotherm models, Langmuir, Freundlich, Sips, and Dubinin–Radushkevich.50−53 The isotherm equations and the obtained parameters are presented in Table 2. The Langmuir isotherm model presumes the adsorption occurs onto specific sites energetically equivalent, as a monolayer, with no interactions between the adsorbed molecules. The Freundlich isotherm is used to model the adsorption process onto heterogeneous surfaces, assuming that the binding sites are not equivalent and interactions between the adsorbed molecules are workable. This empirical model is not suitable for the experimental isotherms, which present a saturation plateau.
Table 2. Isotherm Parameters of Langmuir, Freundlich, Sips, and Dubinin–Radushkevich Models for the Sorption of As(III) and As(V) onto the AMOX-Type Sorbents, at 22 °C.
| AMOX1 |
AMOX2 |
AMOX3 |
|||||
|---|---|---|---|---|---|---|---|
| isotherm parameters | As(III) | As(V) | As(III) | As(V) | As(III) | As(V) | |
| Langmuir Model: | |||||||
| qm, mmol g−1 | 4.3108 | 3.934 | 4.5454 | 4.3926 | 4.68 | 4.6376 | |
| KL, L mmol–1 | 1.852 | 0.7782 | 2.3527 | 0.9878 | 3.6455 | 1.4076 | |
| R2 | 0.9286 | 0.8571 | 0.9387 | 0.8953 | 0.9761 | 0.9155 | |
| χ2 | 0.14 | 0.21 | 0.14 | 0.2 | 0.06 | 0.2 | |
| Freundlich Model: | |||||||
| KF, mmol1–1/n·L1/n·g–1 | 2.2598 | 1.54 | 2.522 | 1.882 | 2.867 | 2.2357 | |
| 1/n | 0.302 | 0.3843 | 0.2871 | 0.363 | 0.2612 | 0.331 | |
| R2 | 0.7309 | 0.6919 | 0.7384 | 0.7195 | 0.8023 | 0.7311 | |
| χ2 | 0.53 | 0.46 | 0.6 | 0.53 | 0.5 | 0.6 | |
| Sips Model: | |||||||
| qm, mmol g–1 | 3.803 | 3.1287 | 4.068 | 3.6491 | 4.3835 | 3.98 | |
| aS | 7.01 | 2.581 | 10.856 | 2.996 | 9.13 | 5.575 | |
| 1/n | 1.997 | 2.846 | 2.011 | 2.464 | 1.484 | 2.302 | |
| R2 | 0.9952 | 0.9879 | 0.9972 | 0.9971 | 0.9895 | 0.9947 | |
| χ2 | 0.01 | 0.02 | 0.006 | 0.01 | 0.027 | 0.02 | |
| Dubinin–Radushkevich Model: | |||||||
| qDR, mol g–1 | 0.00739 | 0.00767 | 0.00766 | 0.00734 | 0.00809 | 0.00826 | |
| β, mol2 J–2 | 4.015 × 10–9 | 3.734 × 10–9 | 3.250 × 10–9 | 5.432 × 10–9 | 5.034 × 10–9 | 4.458 × 10–9 | |
| E, kJ mol–1 | 11.16 | 11.57 | 12.4 | 9.59 | 9.97 | 10.59 | |
| R2 | 0.7937 | 0.8021 | 0.8653 | 0.7952 | 0.8173 | 0.8287 | |
| χ2 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |
The Sips isotherm equation fuses the Langmuir and Freundlich isotherms in a three-parameter isotherm, suitable to model the experimental data which show a saturation plateau. As can be seen in Table 2, the highest values of R2 and the lowest values of χ2 were obtained for the Sips isotherm, and this indicates this isotherm as the most suitable model to describe the sorption process of As(III) and As(V) onto AMOX sorbents. Furthermore, the values of qe were very close to the experimental values, while the Langmuir isotherm overestimated the values of qe. These results would indicate that the sorption process of As(III) and As(V) probably occurred by multisite interactions, well described by the three-parameter isotherms such as the Sips isotherm model.52−54 As it was expected, the Freundlich isotherm was not appropriate to describe the experimental data, the values R2 being the lowest and those of χ2 the highest. The favorable adsorption process is supported by the values of 1/n in the Freundlich isotherm, which are in the range 0–1, for all pairs AMOX sorbent/sorbate (Table 2). It would have been expected as the AMOX1 composite, having the highest content of amidoxime and the highest PZC (Figure 2b), to adsorb the highest amount of arsenic ions. However, the highest values of qm resulted by modeling the experimental data with the Sips isotherm and with the Langmuir isotherm were obtained in the case of AMOX3 composite, both for As(III) and As(V). The enhance of qm values could be attributed to the presence of two amidoxime networks inside the silica pores, which ensure a better accessibility of arsenic to the amidoxime groups. The specific surface area of the AMOX3 composite (Table 1) is much higher than that of AMOX1, and this difference could also contribute to the increase of the sorption performances. The values of qm were always higher for As(III) than for As(V) (Table 2), and this fact could be associated with the adsorption of As(V) as oxoanions (ion exchange) and of As(III) by physisorption.
Information about the nature of interactions between the functional groups of sorbent and the sorbate species could be drawn by the evaluation of mean free energy of adsorption, E (kJ mol–1), defined as the free energy when 1 mol of ion is transferred from infinity in solution to the surface of a solid, which is related to the Dubinin–Radushkevich isotherm constant, β, by eq 4
| 4 |
Recently, it was reported that to gain reliable values of the Dubinin–Radushkevich isotherm constant, the concentration term must be dimensionless, and for this reason, the term should be (1 + Co/Ce), where Co = 1 mol L–1 represents the standard state of the solution, and the units of Ce must be mol L–1.55−57 Therefore, the values of β were acquired by nonlinear fitting of the Dubinin–Radushkevich isotherm on the experimental equilibrium data plotted in Figure S3 with concentration in mol/L. As can be observed in Table 2, the Dubinin–Radushkevich isotherm does not fit so well the experimental data, the R2 values being in the range 0.8–0.86. Therefore, the values of E calculated with eq 4, by using the values of the D–R equilibrium constant (β) resulted by fitting the Dubinin–Radushkevich isotherm on the experimental data, should be considered only as an order of magnitude (Figure S3).57 Nevertheless, the values of E in the range 10–12 kJ/mol and the fact that the kinetic data were fitted the best by the PFO kinetic model could be associated with physical sorption as the most probable mechanism for adsorption of As(III) and As(V) onto AMOX sorbents.
The sorption capacity of the AMOX3 composite against As(III) and As(V) is compared in Table 3 with the values reported in the literature for other sorbents developed for the removal of arsenic species, along with the testing conditions.
Table 3. Comparison of Maximum Equilibrium Sorption Capacity of As(III) and As(V) Ions onto Different Recently Reported Sorbents.
| sorption conditions |
qm, mg/g |
||||
|---|---|---|---|---|---|
| sorbent | T, K | sorbent dose, g/L | initial pH | As(III) | As(V) |
| layered double hydroxides embedded into alginate/PVA beads5 | 303 | 30 | 8 | 1.73 | |
| calix[4]pyrrole15 | 298 | 1 | 6.3 | 14.29 | 15.28 |
| hydrous TiO2 nanoconfined in the pores of anion exchangers21 | 298 | 0.5 | 2 | 26.6 | |
| hydrous Fe2O3 nanoparticles embedded in anion exchangers22 | 298 | 0.5 | 7 | 31.6 | |
| chitin-g-amidoxime35 | 303 | 15 | 6.5 | 19.72 | |
| ion-exchange resins containing N-methyl-d-glucamine39 | 303 | 5 | 6.0 | 237 | |
| 9.0 | 392 | ||||
| ceramic alumina coated with chitosan40 | 298 | 10 | 4 | 56.5 | 96.46 |
| iron-impregnated granular-activated carbon43 | 298 | 1 | 4.8 | 98.4 | 125 |
| tetraethylenepentamine-functionalized acrylic fiber44 | 293 | 1 | 7.0 | 40.5 | 270.3 |
| silica polyamine composites59 | 298 | 10 | 4 | 98 | |
| 6 | 56 | ||||
| iron–chitosan composites58 | 298 | 5 | 7 | 16.15 | 22.47 |
| MWCNTs@PANI@TiO2 nanocomposites60 | 298 | 5 | 5 | 57.37 | |
| amidoxime resin hosted by mesoporous silica (AMOX3) (this work) | 295 | 1 | 8 | 328.7 | |
| 5 | 298.6 | ||||
Based on the data presented in Table 3, it can be inferred that the AMOX3 composite investigated in this work displays great potential in the removal of both As(III) and As(V). It should be pointed out that our AMOX3 composite exhibited considerably higher qm values for the removal of As(III) than other reported materials such as calix[4]pyrrole,13 ceramic alumina coated with chitosan,40 iron-impregnated granular-activated carbon,43 tetraethylenepentamine-functionalized acrylic fiber,44 iron–chitosan composites,58 and MWCNTs@PANI@TiO2 nanocomposites.60
3.2.4. Interference with Co-existing Anions
The investigation of the influence of the co-existing anions present in water on the sorbent performances in the removal of arsenic species has a great significance for practical applications. Therefore, the influence of four co-existing anions (SO42–, NO3–, HCO3–, and H2PO4–), with concentrations up to 10–1 M, on the sorption capacity of the composite AMOX3 for As(III) and As(V), was deeply investigated. Figure 3c,d indicates an obvious decline of the adsorption capacity for arsenic, as much as the concentration of competing anions increased from 10–3 to 10–1 M, the ratio between the concentration of competing anions and arsenic species increasing from 1 mM co-ion: 1.33 mM As up to 100 mM co-ion: 1.33 mM As. By the exploration in detail of the influence of the competitive anions on the adsorption of As(III) onto the AMOX3 composite (Figure 3c), it can be observed that H2PO4– anions caused the highest decrease of the arsenic adsorption, the influence of HCO3– anions being comparable with that of H2PO4–, similar to the results recently reported in the literature.22,61 However, the H2PO4– anions could not completely inhibit the adsorption of As(III), even at a ratio of 100 mM co-ion: 1.33 mM As. The final order concerning the adverse effect of the competing anions on the adsorption of As(III), obvious in Figure 3c, is as follows: H2PO4– > HCO3– > NO3– > SO42–. For the adsorption of As(V) oxoanions (Figure 3d), the order was similar to that observed for As(III), but the decline in the presence of H2PO4– anions was more consistent. This reduction was attributed to the competition between the interfering anions (H2PO4–) and HAsO42– for the adsorption sites of the composite sorbent. Wei et al. observed a similar order for the adsorption of As(V) oxoanions onto an amine sorbent: PO43– > SO42– > NO3–.44 The highest interference observed in the case of PO43– anions is explained by the similarity of the chemical structure of this anion with that of the arsenic anion, which, therefore, could desorb As(V) oxoanions.61,62 It is also obvious that, for the same concentration of the competitive anions, the negative influence of HCO3– was less dramatic in the case of As(V) (Figure 3d) than in the case of As(III) (Figure 3c), while the decline in the adsorption of As(V) oxoanions was more dramatic when H2PO4– was the competitive anion.63 The adverse effect of HCO3– anions was also associated with the probability as these anions to form inner-sphere complexes similar to H2PO4–.61 The decline of the adsorption capacity of AMOX3 for both As(III) and As(V), in the presence of SO42– and NO3– anions, was not so dramatic, probably because these anions could be only bound by outer-sphere complexation.22,61,63,64
3.2.5. Structural Changes after Arsenic Adsorption
Information about the structural changes of the AMOX composites after the sorption of As(III) and As(V) was obtained by the analysis of the FTIR spectra of the sorbents loaded with arsenic (Figure 4) (the AMOX3 sorbent was taken as an example).
Figure 4.
FTIR spectra of the AMOX sorbent before and after loading with As(III) and As(V).
The characteristic bands arising from the stretching vibration of the As–O bond are present at 899 cm–1, for AMOX3+As(V), and at 883 cm–1 for AMOX3+As(III) as previously reported.8,44,65 The bands at 899 cm–1 in the FTIR spectra of AMOX3, after the adsorption of arsenate, would indicate the stretching frequencies of As–O bands in the H2AsO4– group.58
3.2.6. Thermodynamics
Because the Sips isotherm fitted the best the experimental equilibrium data, the equilibrium constants obtained by fitting the Sips model onto the experimental isotherms, with concentrations expressed in mmol L–1, at four temperatures (295, 303, 313, and 323 K, Figure 5a,b,c), were considered as the thermodynamic equilibrium constants and were used to evaluate the change of the Gibbs free energy (ΔG0, kJ mol–1) according to eq 5
| 5 |
where T—the absolute temperature (K) and R—the universal gas constant (8.314 J mol–1 K–1).
Figure 5.
(a, b, c) Experimental sorption isotherms for the adsorption of As(III) and As(V) fitted by Sips isotherm. (d) Plot of ln Ko vs 1/T for the sorption of As(III) and arsenate oxyanions onto AMOX sorbents.
The units of Ko resulted by the nonlinear fitting of the Sips isotherm are L mmol–1. To transform Ko in a dimensionless parameter, eq 6 was used57,66
| 6 |
where KSips is the equilibrium constant given by the model (Sips isotherm, L mmol–1, transformed in L mol–1 by multiplying with 103), CAso (mol L–1) is the standard concentration of As(III) or As(V), and γAs (dimensionless) is the activity coefficient of As(III) and As(V) (∼1, in dilute solutions).
The evaluation of the enthalpy change, ΔHo (kJ mol–1), and the entropy change, ΔSo (J mol–1 K–1), was carried out by using the Van’t Hoff equation (eq 7), plotted in Figure 5d
| 7 |
The calculated values of the thermodynamic parameters for As(III) and As(V) adsorption onto the three sorbents are given in Tables 4 and 5, respectively.
Table 4. Thermodynamic Parameters for the Adsorption of As(III) onto AMOX Sorbents.
| ΔH°, kJ mol–1 | ΔS°, J mol–1 K–1 | ΔG°, kJ mol–1 |
||||
|---|---|---|---|---|---|---|
| sorbent | 295 | 303 | 313 | 323 | ||
| AMOX1 | 25.75 | 160 | –21.73 | –22.77 | –24.8 | –26.1 |
| AMOX2 | 13.97 | 124 | –22.78 | –23.93 | –24.99 | –26.34 |
| AMOX3 | 25.89 | 163 | –22.34 | –23.81 | –25.32 | –26.96 |
Table 5. Thermodynamic Parameters for the Adsorption of As(V) Oxoanions.
| ΔG°, kJ mol–1 |
||||||
|---|---|---|---|---|---|---|
| sorbent | ΔH°, kJ mol–1 | ΔS°, J mol–1 K–1 | 295 | 303 | 313 | 323 |
| AMOX1 | 20.43 | 134 | –19.27 | –20.74 | –21.78 | –23.16 |
| AMOX2 | 20.20 | 135 | –19.63 | –20.92 | –22.27 | –23.42 |
| AMOX3 | 21.52 | 144 | –21.14 | –22.52 | –23.91 | –25.22 |
The positive values of the enthalpy change (ΔH°) demonstrate that the process of As(III) and As(V) adsorption onto the sorbents is endothermic. The positive values of the entropy change (ΔS°) suggested an increase in disorder at the solid/solution interface during the arsenic adsorption process. The negative values of the Gibbs free energy (ΔGo) observed at all temperatures indicate that the adsorption process of both As(III) and As(V) onto the three sorbents was spontaneous and favorable. The increase of the negative values of ΔG0 with the increase of temperature indicates the increase of the degree of spontaneity of adsorption process.
3.2.7. Reusability
The adsorption performances of a sorbent toward solutes also refer to the regeneration and reuse in as many as possible successive sorption/desorption cycles.5,37,41,53 A suitable desorption process should be used to re-establish the sorption capacity close to the initial performances.37 Because arsenic acid is a weak acid, when acid reagents are used as eluents, the arsenic monovalent and divalent anions attached to the resin (H2AsO4– and HAsO42–) are transformed into the noncharged molecule of H3AsO4, which can be leached from the sorbent.40,41 Regeneration of the AMOX sorbents with 0.1 M NaOH proved to be a convenient way to recuperate the sorption abilities of AMOX sorbents.
As Figure 6a shows, the RE values for the sorption of As(III) onto AMOX1, AMOX2, and AMOX3 sorbents decreased from 83.22, 85.61, and 89.46%, in the first cycle, down to 65.82, 67.29, and 71.84%, in the fifth cycle, respectively. The removal of As(V) by AMOX1, AMOX2, and AMOX3 was found to be 65.37, 69.42, and 76.19% in the first cycle and decreased down to 47.59, 48.18, and 58.36% in the fifth cycle, respectively (Figure 6b).
Figure 6.
Reusability of AMOX composites in the removal of As(III) (a) and As(V) (b) as a function of sorption/desorption cycle number: sorption conditions: sorbent dose 1 g/L, pH 8, for As(III), and pH 5 for As(V), temperature 22 °C, contact time 8 h; desorption conditions: elution with 0.2 M HCl (8 h), regeneration with 0.1 M NaOH (6 h).
The RE values decreased with about 18% from the first to the fifth cycle, and this shows that the AMOX sorbents could be regenerated and reused in the arsenic removal in multiple cycles. The same sequence of eluent and regeneration agent (0.2 M HCl and 0.1 M NaOH) proved to be effective for other sorbents.41 These results clearly demonstrate promising possibilities for practical application of the AMOX sorbents owing to their effective arsenate removal.
4. Conclusions
Composite sorbents consisting of amidoxime resin entrapped into the pores of a mesoporous silica were prepared and tested for their performances in the removal of As(III) and As(V) as a function of the amidoxime content and textural characteristics of the composites. The removal capacity of AMOX composites toward As(III) and As(V) from water was found to be strongly dependent on the initial pH (the optimum pH for the removal of As(V) was 5.0, while for As(III), the optimum pH was 8.0), initial concentration of arsenic anions, and the presence of interfering anions. The adsorption mechanism was the most probable physisorption, as indicated by the sorption kinetics (fitted the best by the PFO kinetic model), and by the values of the mean free energy of adsorption (E), whose values were 10–12 kJ/mol. Among the competitive anions, sulfate ions had moderate adverse effects, while the most significant interference occurred in the presence of phosphate and bicarbonate anions. The AMOX sorbents could be regenerated and reused up to five cycles, with an average loss of the RE of about 18%, for both arsenic species. The main advantage of the AMOX sorbents investigated in the present study is their applicability for the removal of both As(III) and As(V), being well known that usually As(III) and As(V) co-exist in groundwater.
Acknowledgments
The authors are grateful to the Romanian Academy for the support in the elaboration of this article.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c03140.
TG (a) and DTG (b) curves of the AMOX composite sorbents; pore size distribution for AMOX composites; experimental isotherms of As(III) and As(V) adsorption onto AMOX composite sorbents fitted by the Dubinin–Radushkevich isotherm model; and kinetic parameters for the adsorption of As(III) And As(V) onto AMOX composite sorbents (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Hao L.; Liu M.; Wang N.; Li G. A Critical Review on Arsenic Removal from Water Using Iron-Based Adsorbents. RSC Adv. 2018, 8, 39545–39560. 10.1039/c8ra08512a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia L. C.; Soares L. C.; Alves Gurgel L. V. A. A Review on the Use of Lignocellulosic Materials for Arsenic Adsorption. J. Environ. Manage. 2021, 288, 112397. 10.1016/j.jenvman.2021.112397. [DOI] [PubMed] [Google Scholar]
- Rathi B. S.; Kumar P. S. A Review on Sources, Identification and Treatment Strategies for the Removal of Toxic Arsenic from Water System. J. Hazard. Mater. 2021, 418, 126299. 10.1016/j.jhazmat.2021.126299. [DOI] [PubMed] [Google Scholar]
- Yao R.; Yang H. An Overview on As(V) Removal from Water by Adsorption Technology. Ann. Muscoskel. Med. 2020, 4, 015–020. 10.17352/amm.000022. [DOI] [Google Scholar]
- Ha H. N. N.; Phuong N. T. K.; An T. B.; Tho T. M.; Thang T. N.; Minh B. Q.; Du C. V. Arsenate Removal by Layered Double Hydroxides Embedded into Spherical Polymer Beads: Batch and Column Studies. J. Environ. Sci. Health, Part A: Environ. Sci. Eng. 2016, 51, 403–413. 10.1080/10934529.2015.1120526. [DOI] [PubMed] [Google Scholar]
- Mohan D.; Pittman C. U. Jr. Arsenic Removal from Water/Wastewater Using Adsorbents - A Critical Review. J. Hazard. Mater. 2007, 142, 1–53. 10.1016/j.jhazmat.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Sharma S.; Balasubramanian K.; Arora R. Adsorption of Arsenic(V) Ions onto Cellulosic-Ferric Oxide System: Kinetics and Isotherm Studies. Desalin. Water Treat. 2015, 57, 9420–9436. 10.1080/19443994.2015.1042066. [DOI] [Google Scholar]
- Karakurt S.; Pehlivan E.; Karakurt S. Removal of Carcinogenic Arsenic from Drinking Water by the Application of Ion Exchange Resins. Oncogen 2019, 2, 1–8. 10.35702/onc.10005. [DOI] [Google Scholar]
- Weerasundara L.; Ok Y. S.; Bundschuh J. Selective Removal of Arsenic in Water: A Critical Review. Environ. Pollut. 2021, 268, 115668. 10.1016/j.envpol.2020.115668. [DOI] [PubMed] [Google Scholar]
- Elcik H.; Celik S. O.; Cakmakci M.; Özkaya B. Performance of Nanofiltration and Reverse Osmosis Membranes for Arsenic Removal from Drinking Water. Desalin. Water Treat. 2016, 57, 20422–20429. 10.1080/19443994.2015.1111812. [DOI] [Google Scholar]
- Nidheesh P. V.; Singh T. S. Arsenic Removal by Electrocoagulation Process: Recent Trends and Removal Mechanism. Chemosphere 2017, 181, 418–432. 10.1016/j.chemosphere.2017.04.082. [DOI] [PubMed] [Google Scholar]
- Li Y.; Qi X.; Li G.; Wang H. Double-Pathway Arsenic Removal and Immobilization from High Arsenic-Bearing Wastewater by Using Nature Pyrite as in situ Fe and S Donator. Chem. Eng. J. 2021, 410, 128303. 10.1016/j.cej.2020.128303. [DOI] [Google Scholar]
- Çermikli E.; Şen F.; Altıok E.; Wolska J.; Cyganowski P.; Kabay N.; Bryjak M.; Arda M.; Yüksel M. Performances of Novel Chelating Ion Exchange Resins for Boron and Arsenic Removal from Saline Geothermal Water Using Adsorption-Membrane Filtration Hybrid Process. Desalination 2020, 491, 114504. 10.1016/j.desal.2020.114504. [DOI] [Google Scholar]
- Chiavola A.; D’Amato E.; Gavasci R.; Sirini P. Arsenic Removal from Groundwater by Ion Exchange and Adsorption Processes: Comparison of Two Different Materials. Water Sci. Technol.: Water Supply 2015, 15, 981–989. 10.2166/ws.2015.054. [DOI] [Google Scholar]
- de Namor A. F.; Hakawati A. A.; Hamdan W. A.; Soualhi R.; Korfali S.; Valiente L. Calix[4]Pyrrole for the Removal of Arsenic (III) and Arsenic (V) from Water. J. Hazard. Mater. 2017, 326, 61–68. 10.1016/j.jhazmat.2016.11.066. [DOI] [PubMed] [Google Scholar]
- Dudek S.; Kołodyńska D. Arsenate Removal on the Iron Oxide Ion Exchanger Modified with Neodynium(III) Ions. J. Environ. Manage. 2022, 307, 114551. 10.1016/j.jenvman.2022.114551. [DOI] [PubMed] [Google Scholar]
- Hubicki Z.; Koodynsk D.. Selective removal of heavy metal ions from water and waste waters using ion exchange methods. Ion Exchange Technologies; Intechopen, 2012; pp 193–240. [Google Scholar]
- Zhang Q.; Pan B.; Zhang W.; Pan B.; Zhang Q.; Ren H. Arsenate Removal from Aqueous Media by Nanosized Hydrated Ferric Oxide (HFO)-Loaded Polymeric Sorbents: Effect of HFO Loadings. Ind. Eng. Chem. Res. 2008, 47, 3957–3962. 10.1021/ie800275k. [DOI] [Google Scholar]
- Dax D.; Chávez M. S.; Xu C.; Willför S.; Mendonça R. T.; Sánchez J. Cationic Hemicellulose-Based Hydrogels for Arsenic and Chromium Removal from Aqueous Solutions. Carbohydr. Polym. 2014, 111, 797–805. 10.1016/j.carbpol.2014.05.045. [DOI] [PubMed] [Google Scholar]
- Rios-Saldaña L. E.; Pérez-Rodríguez F.; Vence-Alvarez E.; Nieto-Delgado C.; Rangel-Mendez J. R. Synthesis of a Granular Composite Based on Polyvinyl Alcohol-Fe:Ce Bimetallic Oxide Particles for the Selective Adsorption of As(V) from Water. J. Water Proc. Eng. 2022, 46, 102621. 10.1016/j.jwpe.2022.102621. [DOI] [Google Scholar]
- Du Y.; Qiu S.; Zhang X.; Nie G. Nanoconfined Hydrous Titanium Oxides with Excellent Acid Stability for Selective and Efficient Removal of As(V) from Acidic Wastewater. Chem. Eng. J. 2020, 400, 125907. 10.1016/j.cej.2020.125907. [DOI] [Google Scholar]
- Li H.; Shan C.; Zhang Y.; Cai J.; Zhang W.; Pan B. Arsenate Adsorption by Hydrous Ferric Oxide Nanoparticles Embedded in Cross-Linked Anion Exchanger: Effect of the Host Pore Structure. ACS Appl. Mater. Interfaces 2016, 8, 3012–3020. 10.1021/acsami.5b09832. [DOI] [PubMed] [Google Scholar]
- Saiz J.; Bringas E.; Ortiz I. New Functionalized Magnetic Materials for As5+ Removal: Adsorbent Regeneration and Reuse. Ind. Eng. Chem. Res. 2014, 53, 18928–18934. 10.1021/ie500912k. [DOI] [Google Scholar]
- Angotzi M. S.; Mameli V.; Cara C.; Borchert K. B. L.; Steinbach C.; Boldt R.; Schwarz D.; Cannas C. Meso- and Macroporous Silica-Based Arsenic Adsorbents: Effect of Pore Size, Nature of the Active Phase, and Silicon Release. Nanoscale Adv. 2021, 3, 6100–6113. 10.1039/d1na00487e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrynska J. Amine- and Thiol-Functionalized SBA-15: Potential Materials for As(V), Cr(VI) And Se(VI) Removal from Water. Comparative Study. J. Water Proc. Eng. 2021, 40, 101942. 10.1016/j.jwpe.2021.101942. [DOI] [Google Scholar]
- Fan H. T.; Fan X.; Li J.; Guo M.; Zhang D.; Yan F.; Sun T. Selective Removal of Arsenic(V) from Aqueous Solution Using a Surface-Ion Imprinted Amine-Functionalized Silica Gel Sorbent. Ind. Eng. Chem. Res. 2012, 51, 5216–5223. 10.1021/ie202655x. [DOI] [Google Scholar]
- Liu P.; Liang Q.; Luo H.; Fang W.; Geng J. Synthesis of Nano-Scale Zero-Valent Iron-Reduced Graphene Oxide-Silica Nano-Composites for the Efficient Removal of Arsenic from Aqueous Solutions. Environ. Sci. Pollut. Res. 2019, 26, 33507–33516. 10.1007/s11356-019-06320-6. [DOI] [PubMed] [Google Scholar]
- Valdés O.; Marican A.; Mirabal-Gallardo Y.; Santos L. S. Selective and Efficient Arsenic Recovery From Water Through Quaternary Amino-Functionalized Silica. Polymers 2018, 10, 626. 10.3390/polym10060626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragan E. S.; Bucatariu F. Design and Characterization of Anionic Hydrogels Confined in Daisogel Silica Composite Microspheres and Their Application in Sustained Release of Proteins. Colloids Surf., A 2016, 489, 46–56. 10.1016/j.colsurfa.2015.10.029. [DOI] [Google Scholar]
- Dragan E. S.; Dinu M. V. Spectacular Selectivity in the Capture of Methyl Orange by Composite Anion Exchangers with the Organic Part Hosted by Daisogel Microspheres. ACS Appl. Mater. Interfaces 2018, 10, 20499–20511. 10.1021/acsami.8b04498. [DOI] [PubMed] [Google Scholar]
- Dragan E. S.; Humelnicu D.; Ignat M.; Varganici C. D. Superadsorbents for Strontium and Cesium Removal Enriched in Amidoxime by a Homo-IPN Strategy Connected with Porous Silica Texture. ACS Appl. Mater. Interfaces 2020, 12, 44622–44638. 10.1021/acsami.0c10983. [DOI] [PubMed] [Google Scholar]
- Lu S.; Chen L.; Hamza M. F.; He C.; Wang X.; Wei Y.; Guibal E. Amidoxime Functionalization of a Poly(Acrylonitrile)/Silica Composite for the Sorption of Ga(III) – Application to the Treatment of Bayer Liquor. Chem. Eng. J. 2019, 368, 459–473. 10.1016/j.cej.2019.02.094. [DOI] [Google Scholar]
- Elwakeel K. Z.; El-Bindary A. A.; Kouta E. Y.; Guibal E. Functionalization of Polyacrylonitrile/Na-Y-Zeolite Composite with Amidoxime Groups for the Sorption of Cu(II), Cd(II) and Pb(II) Metal Ions. Chem. Eng. J. 2018, 332, 727–736. 10.1016/j.cej.2017.09.091. [DOI] [Google Scholar]
- Alexandratos S. D.; Zhu X.; Florent M.; Sellin R. Polymer-Supported Bifunctional Amidoximes for the Sorption of Uranium from Seawater. Ind. Eng. Chem. Res. 2016, 55, 4208–4216. 10.1021/acs.iecr.5b03742. [DOI] [Google Scholar]
- Hanh T. T.; Huy H. T.; Hien N. Q. Pre-irradiation Grafting of Acrylonitrile onto Chitin for Adsorption of Arsenic in Water. Radiat. Phys. Chem. 2015, 106, 235–241. 10.1016/j.radphyschem.2014.08.004. [DOI] [Google Scholar]
- Dragan E. S. Advances in Interpenetrating Polymer Network Hydrogels and Their Applications. Pure Appl. Chem. 2014, 86, 1707–1721. 10.1515/pac-2014-0713. [DOI] [Google Scholar]
- Corazzari I.; Nisticò R.; Turci F.; Faga M. G.; Franzoso F.; Tabasso S.; Magnacca M. Advanced Physico-Chemical Characterization of Chitosan by Means of TGA Coupled On-Line with FTIR and GCMS: Thermal Degradation and Water Adsorption Capacity. Polym. Degrad. Stab. 2015, 112, 1–9. 10.1016/j.polymdegradstab.2014.12.006. [DOI] [Google Scholar]
- Hong H. J.; Kim B. G.; Ryu J.; Park I. S.; Chung K. S.; Lee S. M.; Lee J. B.; Jeong H. S.; Kim H.; Ryu T. Preparation of Highly Stable Zeolite-Alginate Foam Composite for Strontium (90Sr) Removal from Seawater and Evaluation of Sr Adsorption Performance. J. Environ. Manage. 2018, 205, 192–200. 10.1016/j.jenvman.2017.09.072. [DOI] [PubMed] [Google Scholar]
- Ozkula G.; Urbano B. F.; Rivas B. L.; Kabay N.; Bryjak M. Arsenic Sorption Using Mixtures of Ion Exchange Resins Containing N-Methyl-D-Glucamine and Quaternary Ammonium Groups. J. Chil. Chem. Soc. 2016, 61, 2752–2756. 10.4067/s0717-97072016000100001. [DOI] [Google Scholar]
- Boddu V. M.; Abburi K.; Talbott J. L.; Smith E. D.; Haasch R. Removal of Arsenic (III) and Arsenic (V) from Aqueous Medium Using Chitosan-Coated Biosorbent. Water Res. 2008, 42, 633–642. 10.1016/j.watres.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Lee C. G.; Alvarez P. J. J.; Nam A.; Park S. J.; Do T.; Choi U.; Lee S. Arsenic(V) Removal Using an Amine-Doped Acrylic Ion Exchange Fiber: Kinetic, Equilibrium, and Regeneration Studies. J. Hazard. Mater. 2017, 325, 223–229. 10.1016/j.jhazmat.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Yu X.; Tong S.; Ge M.; Wu L.; Zuo J.; Cao C.; Song W. Synthesis and Characterization of Multi-Amino-Functionalized Cellulose for Arsenic Adsorption. Carbohydr. Polym. 2013, 92, 380–387. 10.1016/j.carbpol.2012.09.050. [DOI] [PubMed] [Google Scholar]
- Raychoudhury T.; Schiperski F.; Scheytt T. Distribution of Iron in Activated Carbon Composites: Assessment of Arsenic Removal Behavior. Water Sci. Technol.: Water Supply 2015, 15, 990–998. 10.2166/ws.2015.057. [DOI] [Google Scholar]
- Wei J.; Shen B.; Ye G.; Wen X.; Song Y.; Wang J.; Meng X. Selenium and Arsenic Removal from Water Using Amine Sorbent, Competitive Adsorption and Regeneration. Environ. Pollut. 2021, 274, 115866. 10.1016/j.envpol.2020.115866. [DOI] [PubMed] [Google Scholar]
- Kumar M.; Mukherjee S.; Thakur A. K.; Raval N.; An A.; Gikas P. Aminoalkyl-organo-silane Treated Sand for the Adsorptive Removal of Arsenic from the Groundwater: Immobilizing the Mobilized Geogenic Contaminants. J. Hazard. Mater. 2022, 425, 127916. 10.1016/j.jhazmat.2021.127916. [DOI] [PubMed] [Google Scholar]
- Lagergren S. Zur Theorie Der Sogenannten Adsorption Geloster Stoffe. K. Sven. Vetenskapsakad. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho Y. S.; Mckay G. The Kinetics of Sorption of Basic Dyes from Aqueous Solution by Sphagnum Moss Peat. Can. J. Chem. Eng. 1998, 76, 822–827. 10.1002/cjce.5450760419. [DOI] [Google Scholar]
- Juang R. S.; Chen M. L. Application of the Elovich Equation to the Kinetics of Metal Sorption with Solvent-Impregnated Resins. Ind. Eng. Chem. Res. 1997, 36, 813–820. 10.1021/ie960351f. [DOI] [Google Scholar]
- Weber J. W. J.; Morris J. C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div., Am. Soc. Civ. Eng. 1963, 89, 31–59. 10.1061/jsedai.0000430. [DOI] [Google Scholar]
- Ayawei N.; Ebelegi A. N.; Wankasi D. Modelling and Interpretation of Adsorption Isotherms. Review Article. J. Chem. 2017, 2017, 3039817. 10.1155/2017/3039817. [DOI] [Google Scholar]
- Foo K. Y.; Hameed B. H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. 10.1016/j.cej.2009.09.013. [DOI] [Google Scholar]
- Dragan E. S.; Humelnicu D.; Dinu M. V. Designing Smart Triple-Network Cationic Cryogels with Outstanding Efficiency and Selectivity for Deep Cleaning of Phosphate. Chem. Eng. J. 2021, 426, 131411. 10.1016/j.cej.2021.131411. [DOI] [Google Scholar]
- Humelnicu D.; Lazar M. M.; Ignat M.; Dinu I. A.; Dragan E. S.; Dinu M. V. Removal of Heavy Metal Ions from Multi-Component Aqueous Solutions by Eco-Friendly and Low-Cost Composite Sorbents with Anisotropic Pores. J. Hazard. Mater. 2020, 381, 120980. 10.1016/j.jhazmat.2019.120980. [DOI] [PubMed] [Google Scholar]
- Vieira T.; Artifon S. E. S.; Cesco C. T.; Vilela P. B.; Becegato V. A.; Paulino A. T. Chitosan-based Hydrogels for the Sorption of Metals and Dyes in Water: Isothermal, Kinetic, and Thermodynamic Evaluations. Colloid Polym. Sci. 2021, 299, 649–662. 10.1007/s00396-020-04786-2. [DOI] [Google Scholar]
- Zhou X. Correction to the Calculation of Polanyi Potential from Dubinin-Radushkevich Equation. J. Hazard. Mater. 2020, 384, 121101. 10.1016/j.jhazmat.2019.121101. [DOI] [PubMed] [Google Scholar]
- Puccia V.; Avena M. J. On the Use of the Dubinin-Radushkevich Equation to Distinguish between Physical and Chemical Adsorption at the Solid-Water Interface. Colloid Interface Sci. Commun. 2021, 41, 100376. 10.1016/j.colcom.2021.100376. [DOI] [Google Scholar]
- Neiber R. R.; Galhoum A. A.; El Sayed I. E. T.; Guibal E.; Xin J.; Lu X. Selective Lead (II) Sorption Using Aminophosphonate-Based Sorbents: Effect of Amine Linker, Characterization and Sorption Performance. Chem. Eng. J. 2022, 442, 136300. 10.1016/j.cej.2022.136300. [DOI] [Google Scholar]
- Gupta A.; Chauhan V. S.; Sankararamakrishnan N. Bimetallic Fe/Ni Nanoparticles Derived from Green Synthesis for the Removal of Arsenic (V) in Mine Wastewater. Preparation and Evaluation of Iron–Chitosan Composites for Removal of As(III) and As(V) from Arsenic Contaminated Real Life Groundwater. Water Res. 2009, 43, 3862–3870. 10.1016/j.watres.2009.05.040. [DOI] [PubMed] [Google Scholar]
- Kailasam V.; Rosenberg E.; Nielsen D. Characterization of Surface-Bound Zr(IV) and Its Application to Removal of As(V) and As(III) from Aqueous Systems Using Phosphonic Acid Modified Nanoporous Silica Polyamine Composites. Ind. Eng. Chem. Res. 2009, 48, 3991–4001. 10.1021/ie8016362. [DOI] [Google Scholar]
- Ouyang L.; Wang Y.; Zhang P.; Wang X.; Yuan S. Heterostructured MWCNTs@ PANI@TiO2 Nanocomposites for Enhanced Adsorption of As(III) from Aqueous Solution: Adsorption and Photocatalytic Oxidation Behaviors. Ind. Eng. Chem. Res. 2020, 59, 11743–11756. 10.1021/acs.iecr.0c01778. [DOI] [Google Scholar]
- Lin Y.; Jin X.; Khan N. I.; Owens G.; Chen Z. Bimetallic Fe/Ni Nanoparticles Derived From Green Synthesis for the Removal of Arsenic (V) in Mine Wastewater. J. Environ. Manage. 2022, 301, 113838. 10.1016/j.jenvman.2021.113838. [DOI] [PubMed] [Google Scholar]
- Tchieda V. K.; D’Amato E.; Chiavola A.; Parisi M.; Chianese A.; Amamra M.; Kanaev A. Removal of Arsenic by Alumina: Effects of Material Size, Additives, and Water Contaminants. Clean: Soil, Air, Water 2016, 44, 496–505. 10.1002/clen.201400599. [DOI] [Google Scholar]
- Yin L.; Liu L.; Lin S.; Owens G.; Chen Z. Synthesis and Characterization of Nanoscale Zero-Valent Iron (nZVI) as an Adsorbent for the Simultaneous Removal of As(III) and As(V) from Groundwater. J. Water Proc. Eng. 2022, 47, 102677. 10.1016/j.jwpe.2022.102677. [DOI] [Google Scholar]
- Gao X.; Wang Y.; Hu Q.; Su C. Effects of Anion Competitive Adsorption on Arsenic Enrichment in Groundwater. J. Environ. Sci. Health, Part A: Environ. Sci. Eng. 2011, 46, 471–479. 10.1080/10934529.2011.551726. [DOI] [PubMed] [Google Scholar]
- Myneni S.; Traina S. J.; Waychunas G. A.; Logan T. J. Experimental and Theoretical Vibrational Spectroscopic Evaluation of Arsenate Coordination in Aqueous Solutions, Solids, and at Mineral-Water Interfaces. Geochim. Cosmochim. Acta 1998, 62, 3285–3300. 10.1016/s0016-7037(98)00222-1. [DOI] [Google Scholar]
- Tran H. N.; Lima E. C.; Juang R. S.; Bollinger J. C.; Chao H. P. Thermodynamic Parameters of Liquid-Phase Adsorption Process Calculated from Different Equilibrium Constants Related to Adsorption Isotherms: A Comparison Study. J. Environ. Chem. Eng. 2021, 9, 106674. 10.1016/j.jece.2021.106674. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.